Abstract
In 1999, heteroresistance to triazoles was reported in Cryptococcus neoformans strains isolated from an azole therapy failure case of cryptococcosis in an AIDS patient and in a diagnostic strain from a non-AIDS patient. In this study, we analyzed 130 strains of C. neoformans isolated from clinical and environmental sources before 1979, prior to the advent of triazoles, and 16 fluconazole (FLC)-resistant strains isolated from AIDS patients undergoing FLC maintenance therapy during 1990 to 2000. All strains isolated prior to 1979 manifested heteroresistance (subset of a population that grows in the presence of FLC) at concentrations between 4 and 64 μg/ml, and all 16 FLC-resistant AIDS isolates manifested heteroresistance at concentrations between 16 and 128 μg/ml. Upon exposure to stepwise increases in the concentration of FLC, subpopulations that could grow at higher concentrations emerged. Repeated transfer on drug-free media caused the highly resistant subpopulations to revert to the original level of heteroresistance. The reversion pattern fell into four categories based on the number of transfers required. The strains heteroresistant at ≥32 μg/ml were significantly more resistant to other xenobiotics and were also more virulent in mice than were those heteroresistant at ≤8 μg/ml. During FLC treatment of mice infected by strains with low levels of heteroresistance, subpopulations exhibiting higher levels of heteroresistance emerged after a certain period of time. The ABC transporter AFR1, known to efflux FLC, was unrelated to the heteroresistance mechanism. Our study showed that heteroresistance to azole is universal and suggests that heteroresistance contributes to relapse of cryptococcosis during azole maintenance therapy.
Cryptococcus neoformans causes life-threatening infection primarily in immunocompromised patients worldwide. Cryptococcal meningoencephalitis, the most common clinical manifestation of cryptococcosis, is fatal unless treated, and the fatality rate is high even with the most efficient antimycotic treatments (11, 22).
Due to its minimal toxic effects and ready penetration into the central nervous system, fluconazole (FLC) has been widely used for treatment of cryptococcosis in AIDS as well as non-AIDS patients (8, 36). Since azoles are fungistatic drugs, strains repeatedly exposed to the drugs eventually adapt to the stress exerted by these drugs and become azole resistant (38).
To date, most advances regarding the mechanism of resistance to azole drugs in pathogenic fungi have been made in Candida species (3, 9, 20, 39-41, 44, 47, 48, 51, 52) and, to a lesser extent, in Aspergillus fumigatus (16-18). The most common mechanisms of resistance to azoles in these pathogens include an increased production of multidrug transporters or the target enzyme Erg11p (12, 15, 25) and mutations in the target gene, ERG11 (26, 39). In addition, the formation of an isochromosome bearing ERG11 and a transcriptional regulator of efflux pumps (42, 43) as well as the activation of Hsp90 that facilitates modification of ergosterol biosynthesis genes (13, 14) has been reported to confer azole resistance in Candida albicans.
Controversy exists as to whether acquired azole resistance occurs in C. neoformans during azole therapy, even though the occurrence of FLC-resistant C. neoformans strains has been reported mostly in AIDS patients undergoing FLC maintenance therapy (2, 5, 21, 30, 33, 55, 56). Little is known about the molecular mechanisms responsible for cryptococcal resistance to azoles except for the case of an ERG11 mutation reported in the strain isolated from an AIDS patient who had recurrent cryptococcal meningoencephalitis (37).
In 1999, we reported the occurrence of azole resistance in strains of C. neoformans that was described as heteroresistance (30). The pattern we described as heteroresistance was observed in C. neoformans strains of serotypes D and A isolated from patients in Italy and Israel, respectively. Both strains produced cultures with heterogeneous populations in which more than 90% of the cells were susceptible (≤16 μg/ml), but the cells highly resistant to FLC (MICs of ≥64 μg/ml) were present as a subpopulation. Interestingly, while the highly resistant subclones demonstrated a homogeneous population of resistant cells on medium containing azole concentrations equal to their MIC, susceptible clones could never be purified to contain only susceptible cells. The highly resistant clones lost resistance upon repeated transfer in drug-free media, while consistently maintaining a small fraction of the resistant subpopulation. Serial isolates from the recurrent infection during maintenance therapy in the Italian patient, however, exhibited an increase in the number of subpopulations that were resistant to drug concentration levels in subsequent episodes. Since molecular strain typing determined the first episode as well as the five subsequent episodes that were caused by the same strain, it indicated an increase in the level of FLC heteroresistance of the strain during therapy.
Heteroresistance in C. neoformans, therefore, can be defined as follows. (i) A heteroresistant subpopulation of cells will have variable growth at a certain concentration of an antimicrobial agent which inhibits a majority of the cells; when exposed to the drug, individual cells may either grow well, slowly, or not at all. (ii) The resistant subpopulation can adapt in a stepwise manner to higher concentrations of the drug. (iii) The resistant subpopulation yields a pure culture of resistant cells, while the susceptible population cannot be purified to contain only susceptible cells. (iv) The resistance in the subpopulation is a reversible adaptive response to the presence of the drug.
In the present study, we analyzed over 100 clinical and environmental strains of C. neoformans isolated before 1979 in order to determine the nature of heteroresistance, an adaptive resistance. Since these strains were isolated at least a decade before the availability of azoles, they were deemed suitable to determine whether heteroresistance of C. neoformans to FLC is intrinsic or the consequence of drug exposure in the environment. To our surprise, heteroresistance to FLC was a common phenomenon in all the strains tested. Moreover, both the frequency and the level of resistance in the heteroresistant clones varied significantly between strains. Interestingly, the levels of FLC at which the strains manifested heteroresistance generally correlated with the observed degree of virulence in mice. Furthermore, the level of heteroresistance was also associated with the ability of C. neoformans yeast cells to survive on media containing toxins and antibiotics produced by soil microorganisms. These findings indicate that heteroresistance to FLC in C. neoformans is intrinsic, and the mechanisms that control the level of heteroresistance to azoles in each C. neoformans strain also contribute to the fungus' ability to respond to various other environmental stresses.
MATERIALS AND METHODS
Strains and media.
A total of 130 strains of C. neoformans (102 of serotype A and 28 of serotype D) isolated prior to 1979 were included in this study. Ninety-seven strains were isolated from cryptococcosis patients, and 33 were isolated from the environment (see Tables S1 and S2 in the supplemental material). These strains have been maintained as lyophilized stock in our laboratory. In addition, we included 16 C. neoformans serotype A strains isolated from AIDS patients who were undergoing FLC maintenance therapy between 1990 and 2000. These isolates had been determined to be FLC resistant (MIC > 16 μg/ml) by reference laboratories (see Table S3 in the supplemental material). Yeast peptone dextrose (YPD) agar (1% yeast extract, 2% peptone, 2% dextrose) was used as the basal medium for growth.
FLC.
FLC powder was provided by Pfizer Global Research & Development (Groton, CT). Stock solutions were prepared in the solvent dimethyl sulfoxide (Sigma) at a concentration of 50 mg/ml. Diflucan was purchased from Pfizer, Roerig (New York, NY) for the experimental animal study.
Susceptibility testing.
Etests (AB Biodisk, Solna, Sweden) as well as spot tests on YPD agar supplemented with different concentrations of FLC were performed for all 146 strains (130 strains isolated before 1979 and 16 FLC-resistant strains isolated during 1990 to 2000 from AIDS patients) to determine their susceptibility and heteroresistance to FLC.
Initial screening for heteroresistance.
Cell suspensions (1 × 103 to 4 × 103 CFU/ml) of all strains suspended in sterile saline were plated onto YPD plates containing various concentrations of FLC (4 to 128 μg/ml). The growth pattern was read after 72 h of incubation at 30°C. The isolates were regarded as possibly heteroresistant when only a fraction of the population grew on plates containing a concentration that is higher than the concentration which allowed 100% of the viable cells to grow. To isolate the highly resistant subclones, the heteroresistant clones that grew on different concentrations of FLC were isolated and passaged on YPD agar containing stepwise (twofold) increases in the concentrations of FLC (up to 256 μg/ml). The culture plates of each passage were incubated at 30°C for 72 to 96 h.
Stability of FLC resistance in vitro.
Highly resistant (≥64 μg/ml) subclones from strains originally manifesting heteroresistance at ≥16 μg/ml FLC were used to study the stability of their resistance phenotype. Cells of each clone were suspended in YPD broth. Fifty microliters of the suspension was then transferred into 5 ml of fresh drug-free YPD broth and incubated at 30°C for 24 h. Such transfers were carried out daily for each isolate for up to 45 days. The proportion of subpopulations resistant to FLC (16 to 128 μg/ml) was determined periodically by plating 100 μl each of subcultures onto YPD agar and YPD plus FLC agar and incubating them at 30°C for 72 h before counting the number of colonies.
Sterol analysis.
We have chosen the H99 strain, a genome sequencing strain of serotype A widely used for pathobiological studies, and isolates from its derived subpopulations resistant to FLC for sterol analysis. The cells of H99 and those resistant at 64 μg/ml FLC (H99R64) were grown overnight in YPD broth at 30°C. Each culture was adjusted to an optical density at 600 nm of 0.2 and divided into two samples—one supplemented with 64 μg/ml FLC, and the other with no drug. All four samples were grown at 30°C for 3 h, and the cells were harvested by centrifugation and washed once with sterile distilled water. The pellets were resuspended in 9 ml methanol; 4.5 ml 60% (wt/vol) KOH was added together with 5 μg cholesterol (used as an internal recovery standard). Cell suspensions were heated to 75°C in a water bath for 2 h to complete the saponification, and the resulting mixtures were allowed to cool to room temperature. The sterols were then extracted twice with 2 ml hexane, using vigorous vortex mixing for 2 min. The upper hexane layers containing the sterols were removed, washed twice with water, and dried over anhydrous sodium sulfate. The hexane solutions containing the sterols were analyzed by gas chromatography (GC) with an Agilent 6850 gas chromatograph with an HP-1 fused silica column and a flame ionization detector. Cholesterol and ergosterol were identified by comparison with standards. For identification, a GC-mass spectrometry (MS) instrument (Polaris Q, from Thermo Electron, coupled to a Focus GC) was used to obtain mass spectral data in the electron impact mode for each of the GC peaks. This GC-MS instrument used a Restek 5MS fused silica column (30-m length, 0.25-mm inside diameter, 25-μm film thickness) at a program temperature from 200°C (1 min) to 300°C at a rate of 10°C/min. The mass spectral data used in combination with a database (NIST/EPA/NIH Mass Spectral Library version 2.0) and published spectra (31) allowed the identification of several peaks for which there were no standards available.
Disruption and reintroduction of the AFR1 gene in the strain H99.
The AFR1 gene was disrupted by homologous recombination. In a deletion construct, the open reading frame of AFR1 was replaced with the NEO gene, using overlap PCR. The native AFR1 gene was deleted by biolistic transformation of the strain H99 with the deletion construct (46). Southern analysis was used to confirm the deletion of AFR1 among the putative afr1Δ clones screened by PCR. The afr1Δ strain was reconstituted with the wild-type AFR1 gene cloned from H99 by electroporative transformation of the afr1Δ strain with the reconstitution construct containing the nourseothricin (NAT) resistance gene as a selectable marker.
Quantitative RT-PCR.
Expression of the AFR1 gene was quantitatively assessed with real-time reverse transcription (RT)-PCR. The yeast cells of wild-type (H99) and resistant (H99R64) strains were grown overnight to logarithmic phase (∼2 × 108 cells/ml) in YPD liquid medium at 30°C with constant shaking (225 rpm). Each culture was then divided into two samples—one supplemented with 64 μg/ml FLC and the other with no drug. All four samples were incubated for 1 h at 30°C; the cells were harvested by centrifugation and were washed with sterile H2O. Cell pellets were frozen on dry ice, lyophilized for 15 h, and then lysed by vortexing with glass beads. Total RNA was isolated using the RNA TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. RNA was purified with the RNeasy MinElute cleanup kit (Qiagen, Valencia, CA) and treated with RNase-free DNase (Qiagen, Valencia, CA) to remove genomic DNA. cDNA was synthesized using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). RT-PCR was performed with TaqMan universal PCR master mix (Applied Biosystems, Branchburg, NJ) and the Applied Biosystems 7500 real-time PCR system. Each reaction was run in triplicate, and data were expressed relative to the transcript level of the glyceraldehyde-3-phosphate dehydrogenase gene (GPD) as an endogenous control. Quantitation of gene expression was performed using the relative standard curve method, and the results were expressed as relative change. The AFR1 primers used for RT-PCR were AFR-RTfw, CCCACTTTGCCATACTTTTGG, and AFR-RTrev, AACTGTGGAGACAAGACCACTGATAA, and the probe was TGGATGTGGCCTCTCGACCCTTTC. The primers for GPD used in RT-PCR were GPD-RTfw, TGGTCGTCAAGGTTGGAATCA, and GPD-RTrev, CTCGATGGCGTTCCTGAGA, and the probe was CGGTTTCGGTCGTATTGGTCGAATTG.
Resistance to other xenobiotics.
Five strains of serotype A that were heteroresistant at ≥32 μg/ml FLC and five strains that were heteroresistant at ≤8 μg/ml (see Table 4; also see Fig. S2 in the supplemental material) were chosen to study their growth on YPD agar containing various antibiotics and l-canavanine, a nonproteinogenic amino acid. The strains were first inoculated into YPD broth and incubated overnight at 30°C, washed, serially diluted (1 to 104 dilutions) in sterile saline, and spotted (3 μl) onto YPD agar plates containing different xenobiotics. The compounds tested were trichostatin A (32 and 64 μg/ml), rhizoxin (0.05 and 0.1 μg/ml), gliotoxin (0.8 and 1.6 μg/ml), and l-canavanine (30 μg/ml). Unless specified, all compounds were obtained from Sigma (Sigma-Aldrich, St. Louis, MO). Cells of the 10 strains were spotted onto YPD agar and incubated at 30, 37, and 40°C for 72 h to compare their growth rates.
TABLE 4.
List of 10 isolates of C. neoformans selected for characterization of heteroresistance in vitro and in vivo
| Isolate | Source | Year of isolation | FLC heteroresistance level (μg/ml) |
|---|---|---|---|
| H99 | Clinical | 1978 | 32 |
| B4548 | Clinical | 1998 | 64 |
| NIH10 | Clinical | 1963 | 32 |
| NIH157 | Clinical | 1966 | 32 |
| NIH376 | Environmental | 1969 | 32 |
| NIH306 | Clinical | 1969 | 8 |
| NIH382 | Clinical | 1969 | 8 |
| NIH355 | Clinical | 1968 | 8 |
| NIH38 | Clinical | 1962 | 4 |
| NIH404 | Environmental | 1970 | 4 |
Virulence study.
All animal studies were approved by the institutional animal care and use committee. To compare the virulence among different strains, a murine model of pulmonary cryptococcosis was established in female BALB/c mice (weight, 20 g) by intranasal inoculation of serotype A strains. Five strains heteroresistant at ≥32 μg/ml and five strains heteroresistant at ≤8 μg/ml FLC, which had been used for the comparison of xenobiotic tolerance, were used in this study. The strains used were also comparable in production of capsule, melanin, and urease at 37°C. The growth rates of these strains at 37°C, measured by the colony size (100 to 150 colonies/plate) on YPD agar after 72 h of incubation, were similar, except for that of NIH38 (heteroresistant at 4 μg/ml), which showed slightly smaller colonies. Although the strain NIH38 had a lower growth rate at 37°C, it was chosen along with the strain NIH404 to represent the group which manifested heteroresistance at 4 μg/ml because of its higher cell viability than that of other strains in the same group. Mice were anesthetized with isoflurane, and a 20-μl droplet containing 5 × 107 cells was placed on the nares of each mouse while the diaphragm was compressed. The diaphragm was released to allow each mouse to inhale the droplet. Ten animals were used for each strain. The survival of mice was recorded daily for a total of 60 days.
FLC therapy for experimental animals.
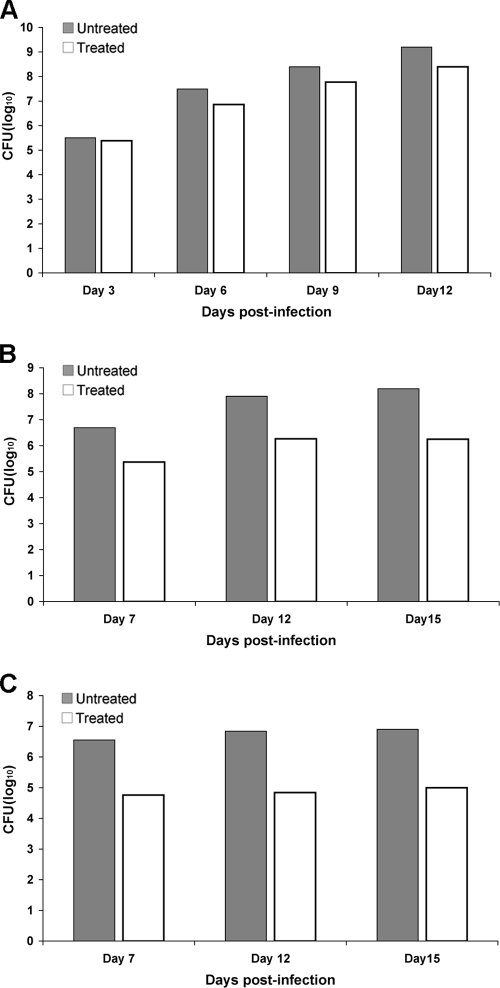
For the FLC efficacy study, a selected group of isolates with different levels of heteroresistance were tested in mice by using the systemic model of cryptococcosis. The mice were challenged intravenously with inoculums ranging from 5 × 104 to 5 × 106 CFU/mouse. FLC was administered intraperitoneally at a concentration of 10 mg/kg/day. Treatment was begun 24 h after infection and was continued for 14 days. The mice were observed through day 40, and deaths were recorded daily. Two mice in each group were used for the analysis of fungal burden in the brain at the indicated days postinfection (see Fig. 7 and 8). Mice were euthanized by CO2-induced asphyxia, and the number of viable CFU was determined by quantitative plating of the brain homogenates onto YPD agar plates with or without FLC at 8, 16, or 32 μg/ml. There were eight mice per group in the survival study and two mice each per group in the analysis of fungal tissue burden.
FIG. 7.
Effect of FLC treatment on brain tissue fungal burdens in mice infected intravenously with strains H99 (A), NIH136 (B), or NIH38 (C). Organ homogenates were obtained from two mice per group that had been sacrificed and necropsied on various days postinfection. Data represent the mean CFU per gram of tissue obtained from two different mice in each group.
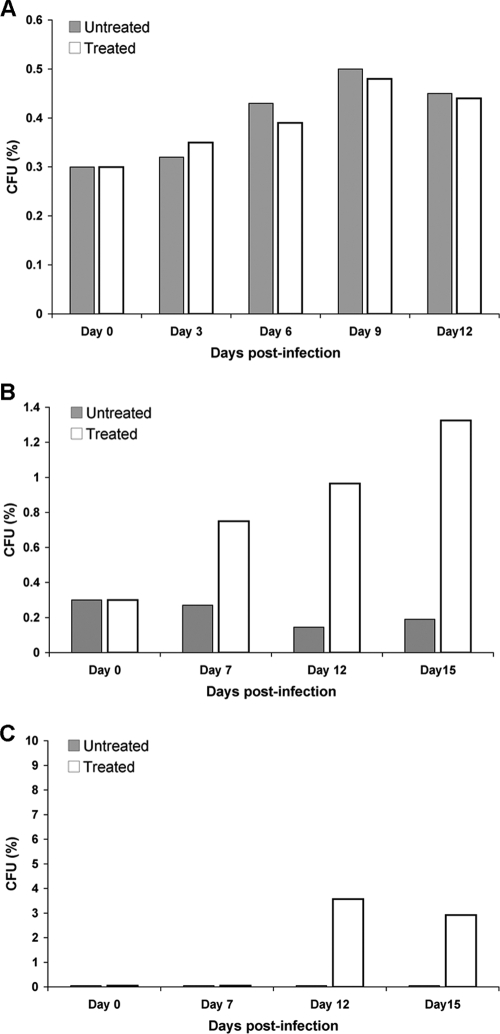
FIG. 8.
Percentage of resistant subpopulations in the brain tissue of mice treated with FLC. The brain homogenates of mice infected with H99 (A), NIH136 (B), or NIH38 (C) were plated onto YPD agar plates containing FLC (8 to 32 μg/ml) and incubated at 30°C, and colonies were counted after 48 h.
Statistics.
Survival data from the animal experiments were analyzed using the log rank test. An unpaired t test was used in evaluating the CFU from the tissue burden studies. A P value of less than 0.05 was considered to be significant.
RESULTS
Heteroresistance to FLC in C. neoformans is intrinsic.
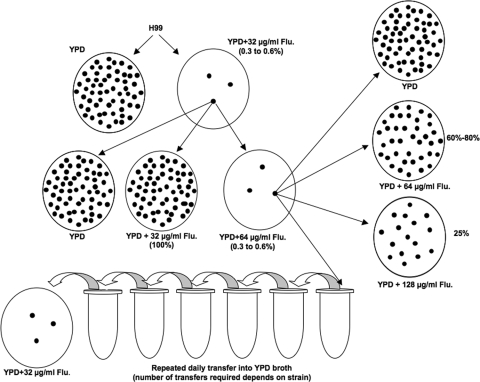
The serotype A reference strain, H99, whose genome has been sequenced (http://www.broad.mit.edu/annotation/genome/cryptococcus_neoformans/MultiHome.html), was used in our study to establish the representative pattern of heteroresistance (Fig. 1). While no inhibition in the growth of H99 cells was observed at 16 μg/ml FLC, only 0.3 to 0.6% of the cells grew as an initial minor subpopulation at 32 μg/ml FLC within 72 h. We designated the level of FLC heteroresistance in H99 as 32 μg/ml. When the cells grown at 32 μg/ml FLC were transferred to fresh YPD agar containing 32 μg/ml FLC, all the cells grew. Upon transfer to medium containing 64 μg/ml FLC, however, only 0.3 to 0.6% grew again and formed colonies within 72 h. Among these cells, now resistant at 64 μg/ml FLC, only 25% of the subpopulation could grow on agar medium containing 128 μg/ml FLC. When subjected to repeated transfer in drug-free broth, the cells of these highly resistant subclones reverted back to a frequency between 0.3 and 0.6% on 32 μg/ml FLC.
FIG. 1.
Diagram of azole heteroresistance in C. neoformans strain H99. The strain showed a heterogeneous composition in which most of the cells were susceptible but some (0.3 to 0.6%) were observed to be resistant to 32 μg of FLC per ml. The resistant colonies grown on medium with the same concentration of FLC (32 μg/ml) generated a homogeneous population of resistant cells. Upon stepwise exposure to increasing concentrations of FLC, subpopulations are observed to adapt to higher concentrations of the drug. Upon repeated transfers in drug-free medium, a majority of the cells in these highly resistant subclones revert back to the stable initial heteroresistant phenotype. Flu, fluconazole.
To our surprise, all 130 strains of C. neoformans that had been isolated before 1979 (see Tables S1 and S2 in the supplemental material), at least 10 to 20 years prior to the advent of azole therapy, manifested heteroresistance at specific concentrations of FLC (Tables 1 and 2). Fifty-seven strains (51 of serotype A and six of serotype D) exhibited subpopulations resistant to FLC at a concentration of ≥16 μg/ml; the remaining 73 strains were more susceptible and produced heteroresistant colonies at an FLC concentration of ≤8 μg/ml (Tables 1 and 2).
TABLE 1.
The 102 isolates of C. neoformans serotype A screened on FLC-containing medium
| Source of isolates collected before 1979 | FLC resistance category (μg/ml)a | No. of isolates tested (%)b | % Heteroresistance |
|---|---|---|---|
| Clinical | 4 | 6 (7.6) | 100 |
| 8 | 32 (40.5) | ||
| 16 | 21 (26.6) | ||
| ≥32 | 20 (25.3) | ||
| Environmental | 4 | 2 (8.7) | 100 |
| 8 | 11 (47.8) | ||
| 16 | 6 (26.1) | ||
| ≥32 | 4 (17.4) |
FLC heteroresistance levels were determined as described in Materials and Methods.
The total number of clinical isolates is 79, and the total number of environmental isolates tested is 23.
TABLE 2.
The 28 isolates of C. neoformans serotype D screened on FLC-containing medium
| Source of isolates collected before 1979 | FLC resistance category (μg/ml)a | No. of isolates tested (%)b | % Heteroresistance |
|---|---|---|---|
| Clinical | 4 | 5 (35.8) | 100 |
| 8 | 7 (50) | ||
| 16 | 1 (7.1) | ||
| 32 | 1 (7.1) | ||
| Environmental | 4 | 4 (28.6) | 100 |
| 8 | 6 (42.8) | ||
| 16 | 4 (28.6) | ||
| 32 | 0 |
FLC heteroresistance levels were determined as described in Materials and Methods.
The total number of clinical isolates is 14, and the total number of environmental isolates tested is 14.
We also analyzed 16 clinical strains isolated from AIDS patients undergoing FLC maintenance therapy between 1990 and 2000 and that had been identified as FLC resistant by the conventional microdilution test (MIC range, 16 to 64 μg/ml) for heteroresistance. Interestingly, all these strains also displayed heteroresistance at FLC levels ranging from 16 to 128 μg/ml (Table 3). Furthermore, all the strains tested showed resistant subpopulations at a frequency of 0.3 to 10% at each specified drug concentration. These data indicate that heteroresistance to FLC in C. neoformans is intrinsic and the resistance in the majority of FLC-resistant isolates recovered from AIDS patients during FLC maintenance therapy is attributed to heteroresistance.
TABLE 3.
The 16 clinical strains of C. neoformans isolated after 1990 screened on FLC-containing medium
| Isolate source | FLC resistance category (μg/ml)a | No. of isolates tested (%)b | % Heteroresistance |
|---|---|---|---|
| Clinical, isolated since 1990 | ≤8.0 | 0 | 100 |
| 16 | 3 (18.8) | ||
| 32 | 2 (12.5) | ||
| 64 | 4 (25) | ||
| 128 | 7 (43.7) |
FLC heteroresistance levels were determined as described in Materials and Methods.
The total number of clinical isolates is 16.
Stepwise increase of FLC-resistant levels in resistant subpopulations.
A minor subpopulation in each strain capable of growth on YPD at a certain concentration of FLC yielded homogenous resistant colonies when subcultured on medium with the same concentration of FLC. This indicated that once the subpopulations had adapted to a certain concentration of FLC, the majority of cells in the clonal population became resistant to the drug at that concentration. The subpopulations of strains heteroresistant at FLC concentrations of 4 to 16 μg/ml were exposed in a stepwise manner to increasing concentrations of up to 64 μg/ml. All such isolates successfully acquired resistance at 64 μg/ml. The clones highly resistant to FLC (initially selected on 32 to 128 μg/ml) could tolerate FLC concentrations up to 256 μg/ml. This indicated that the level of FLC resistance in the heteroresistant subpopulations could be increased in a stepwise manner and that the maximum concentration of FLC to which each strain could adapt varied depending on the strain.
Reversibility of resistance in vitro.
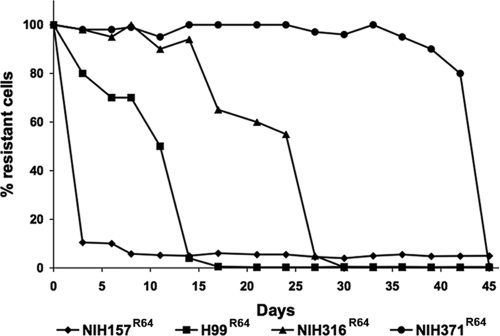
We demonstrated that the FLC resistance levels of the subpopulations for all C. neoformans strains could be increased in a stepwise manner by exposing them to higher concentrations of the drug. We randomly selected 57 strains with original levels of heteroresistance between 16 and 128 μg/ml FLC that had been isolated before 1979 and all 16 FLC-resistant strains isolated from AIDS patients undergoing FLC maintenance therapy between 1990 and 2000 to study the reversibility of resistance. Clones from the stepwise selection at higher drug concentrations (64 to 128 μg/ml) of the 73 strains were repeatedly transferred in drug-free YPD broth. Cultures were periodically plated onto YPD agar with 16 to 128 μg/ml FLC and evaluated for the frequency of the population that formed colonies on the medium at the given drug concentration. All strains isolated before 1979 and the 16 strains, except for one, isolated from AIDS patients with recurrent cryptococcosis during FLC maintenance therapy lost resistance at 64 to 128 μg/ml and reverted back to the original level of heteroresistance by day 45. Interestingly, based on the duration required for reversion, regardless of their original level of heteroresistance, we were able to classify these strains into four different classes. The first class reverted abruptly after only three transfers, while the remaining three classes required at least 16, 26, and 45 transfers, respectively. One of the 16 AIDS isolates, once it acquired resistance to FLC, showed no signs of reversion even by day 45. To show the reversion pattern of the four classes, we selected four strains of serotype A that manifested heteroresistance at 32 μg/ml and analyzed the reversibility of their resistance after selecting subpopulations that could withstand 64 μg/ml FLC. Figure 2 shows reversibility of the four strains representing the four classes among serotype A strains. Subclones resistant at 64 μg/ml FLC were isolated and transferred daily into new YPD drug-free broth for 45 days. The NIH157 strain, which reverted after three transfers, yielded a 10% subpopulation that grew at 32 μg/ml FLC and represented the first class. H99, the reference strain of serotype A, belonged to the second class, which required 16 daily transfers before completely reverting to the original heteroresistance phenotype—growth of 0.3 to 0.6% subpopulations at 32 μg/ml FLC. The frequency of these heteroresistant subpopulations remained unchanged for an additional 29 transfers. The strains NIH316 and NIH371 belonged to the third and fourth classes, respectively.
FIG. 2.
Stability of FLC resistance after daily transfers in drug-free medium at 30°C. Four strains represented four different classes based on the number of daily transfers required prior to reversion regardless of their original level of FLC heteroresistance. Subclones resistant at 64 μg/ml FLC were isolated and transferred daily into new YPD drug-free broth for 45 days. The first class reverted abruptly after three transfers (NIH157R64 strain) while the remaining three classes, represented by isolates H99R64, NIH316R64, and NIH371R64, required at least 16, 26, and 45 transfers, respectively.
Sterol content of H99 and its heteroresistant subpopulations.
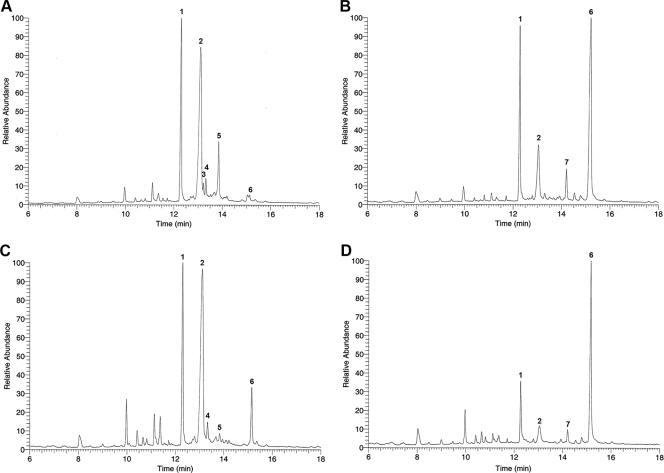
It is possible that the FLC-resistant subpopulation reflected changes in the sterol content of cells, allowing them to survive in high concentrations of FLC. The sterol profile of H99 was compared with that of H99R64, a strain resistant to 64 μg/ml FLC generated in vitro by exposing H99 cells to 64 μg/ml FLC. Upon exposure of H99 cells to 64 μg/ml FLC, the ergosterol content in the cells was greatly reduced, and its major sterol was eburicol, which is consistent with the fact that FLC blocks the C14 demethylation step (31) (Fig. 3A and B). When both H99 and H99R64 strains were treated with FLC, their ergosterol levels were similar, although the eburicol level was higher in the H99R64 strain (Fig. 3B and D). The sterol profile of H99R64 without FLC treatment was similar to that of H99, except that the eburicol level was higher and the ergost-7-enol level was lower than those of the H99 strain (Fig. 3A and C). This difference was likely caused by the fact that the H99R64 strain was maintained on an FLC-containing medium. Upon daily transfers (three times) of the H99R64 cells in drug-free medium, the eburicol and ergost-7-enol levels were restored to those of H99 (data not shown). These results indicated that there were no significant differences in the sterol profiles between the isolates in the presence or absence of FLC. Thus, we excluded the mutation in ergosterol biosynthesis as the cause of resistance in the H99R64 strain in addition to the fact that its resistance is reversible.
FIG. 3.
GC-MS analysis of the sterol profile. The cells of H99 and its resistant subpopulations (H99R64) were grown without FLC (A and C) or with the presence of 64 μg/ml FLC (B and D). Total sterols were extracted from each sample and analyzed by GC-MS. The numbers associated with the GC peaks are as follows: 1, cholesterol; 2, ergosterol; 3, ergost-7,22-enol; 4, neoergosterol; 5, ergost-7-enol; 6, eburicol; 7, unidentified sterol with a molecular weight of 424.
AFR1, an ABC transporter, is not required for heteroresistance.
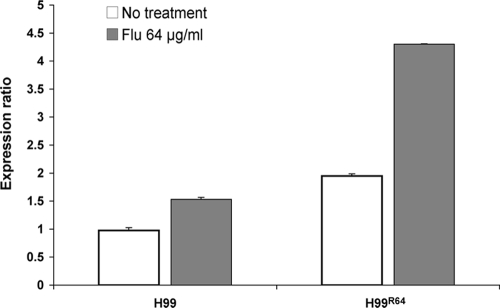
To determine the role of AFR1 in heteroresistance, the only ATP binding cassette transporter thus far known to be involved in C. neoformans FLC efflux, we disrupted the AFR1 gene in the H99 strain. The MIC to FLC of the afr1Δ mutant was reduced >60-fold compared to that of H99 (0.38 versus 24 μg/ml by Etest; see Fig. S1 in the supplemental material). The susceptibility of the reconstituted strain was similar to that of H99, with FLC MICs with Etest ranging from 24 to 32 μg/ml. These results confirmed that the AFR1 gene is involved in FLC resistance in C. neoformans. To assess the role of AFR1 in heteroresistance, cells of the afr1Δ strain were plated onto YPD agar containing various concentrations of FLC. Unlike H99, cells of the afr1Δ strain were not able to form colonies on media containing higher than 1 μg/ml FLC. On media containing 1 μg/ml FLC, 0.3% of cells formed colonies. This indicated the FLC concentration at which the afr1Δ strain that exhibited heteroresistance was reduced 32-fold without losing the frequency of heteroresistant subpopulation. Cells of the subpopulation growing on 1 μg/ml FLC when further exposed to increasing concentrations of the drug in a stepwise manner yielded homogeneous growth on FLC concentrations up to 8 μg/ml. These cells grown at 8 μg/ml FLC (afr1ΔR8 strain cells) were transferred daily into drug-free medium to evaluate stability of the resistance. After 26 transfers in YPD at 30°C, cells of the afr1ΔR8 strain reverted back to the initial heteroresistant phenotype (0.3% at 1 μg/ml), indicating that AFR1 is involved in FLC resistance but is unrelated to the heteroresistant phenotype in C. neoformans. Furthermore, quantitative RT-PCR analysis revealed that the AFR1 expression level in H99R64 was twofold higher than that of the wild-type strain (Fig. 4). A twofold increase in the expression of AFR1 in strain H99R64 confirms the role of AFR1 in FLC resistance.
FIG. 4.
Expression of AFR1 in C. neoformans strains H99 (wild type) and H99R64 (resistant strain) grown in vitro without FLC or with 64 μg/ml FLC. Quantification of the relative transcript levels was performed by real-time RT-PCR analysis as described in Materials and Methods. Data are reported as increases (n-fold) in GPD-normalized expression levels.
Relationship between FLC heteroresistance and resistance to other xenobiotics.
In order to determine the relationship between heteroresistance to FLC and resistance to other xenobiotics, we chose five highly resistant strains (≥32 μg/ml) and five strains expressing heteroresistance at a low concentration of FLC (≤8 μg/ml) (Table 4; see Fig. S2 in the supplemental material) and compared their resistance levels to compounds unrelated to FLC. The compounds chosen for testing are secondary metabolites produced by soil microorganisms, including molds, bacteria, and actinomycetes which are likely to cohabit with C. neoformans in nature. These include gliotoxin (produced by molds such as Aspergillus and Penicillium), rhizoxin (produced by Burkholderia species), and trichostatin A (produced by species of Streptomyces) and l-canavanine (found in legume seeds). l-Canavanine, a nonproteinogenic amino acid (34), was chosen for two reasons. (i) Feral pigeons eat various kinds of legume seeds and pigeon droppings, the major environmental source of C. neoformans, and may contain this arginine analog. It is also a component of the canavanine-glycine-bromothymol blue medium, which is used to distinguish strains of C. neoformans from those of Cryptococcus gattii (23). The highly FLC-heteroresistant strains (≥32 μg/ml) generally exhibited a higher resistance to the four compounds than did the strains with heteroresistance at a lower concentration (≤8 μg/ml) of FLC (Table 5). It is clear, however, that the tolerance to these xenobiotics cannot be defined as heteroresistance, since the tolerance did not increase upon serial passages in higher concentrations of the inhibitors and it could not be reversed in their absence (data not shown).
TABLE 5.
Resistance to antibiotics, toxins, or nonproteinogenic amino acidsa
| Origin | Compound | Source | Strains heteroresistant at indicated concn of FLC (μg/ml)b
|
|
|---|---|---|---|---|
| ≥32 | ≤8 | |||
| Fungal | Gliotoxin | A. fumigatus | +++ | +/− |
| Bacterial | Rhizoxin | Burkholderia sp. | +++ | +c |
| Actinomycete | Trichostatin A | Streptomyces platensis | ++ | +/− |
| Nonproteinogenic amino acid | l-Canavanine | Seeds of legume | +++ | +d |
Each C. neoformans strain (see Table 4) was grown in YPD medium, 10-fold serially diluted (1 to 104 dilutions), and 3 μl of each cell suspension was spotted onto YPD agar plates containing different biological compounds.
++, FLC-resistant strain B4548 was more sensitive to trichostatin A (32 to 64 μg/ml) and the growth was similar to that of FLC-sensitive isolates; +++, all tested strains exhibited resistance to the compound; +/−, all tested strains showed increased susceptibility to the compounds compared to the strains heteroresistant at ≥32 μg/ml FLC.
Strains NIH306 and NIH404 were less sensitive to 0.05 μg/ml rhizoxin and the growth was similar to that of FLC-resistant strains.
Strain NIH382 was less sensitive to l-canavanine (30 μg/ml) and the growth was similar to that of FLC-resistant strains.
Heteroresistance and virulence in experimental animal models.
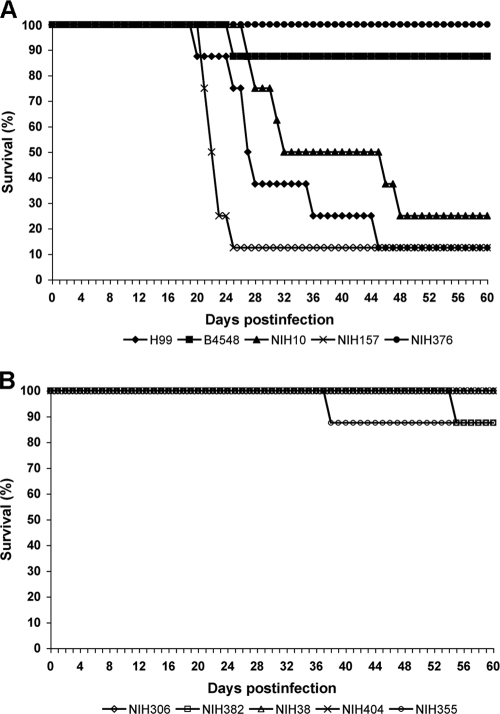
The virulence of strains expressing heteroresistance at different concentrations of FLC was studied in mice, using intranasal inoculation. Three of five strains (H99, NIH157, NIH10) showing heteroresistence at high FLC levels (≥32 μg/ml) exhibited increased virulence causing 80 to 90% mortality within 60 days in comparison to animals infected with low-level heteroresistant strains (≤8 μg/ml), which produced 0 to 10% mortality during the same period. Figure 5 shows the significant differences in survival of mice infected with the two groups (P < 0.0001). These data indicated a correlation between virulence of C. neoformans strains and their level of heteroresistance to FLC in vitro.
FIG. 5.
Virulence of C. neoformans strains expressing different levels of heteroresistance. Anesthetized mice were intranasally inoculated with 5 × 107 viable yeast cells. (A) Five strains heteroresistant at ≥32 μg/ml FLC. (B) Five strains heteroresistant at ≤8 μg/ml FLC. Survival rates (percentages) are plotted against the number of days after inoculation.
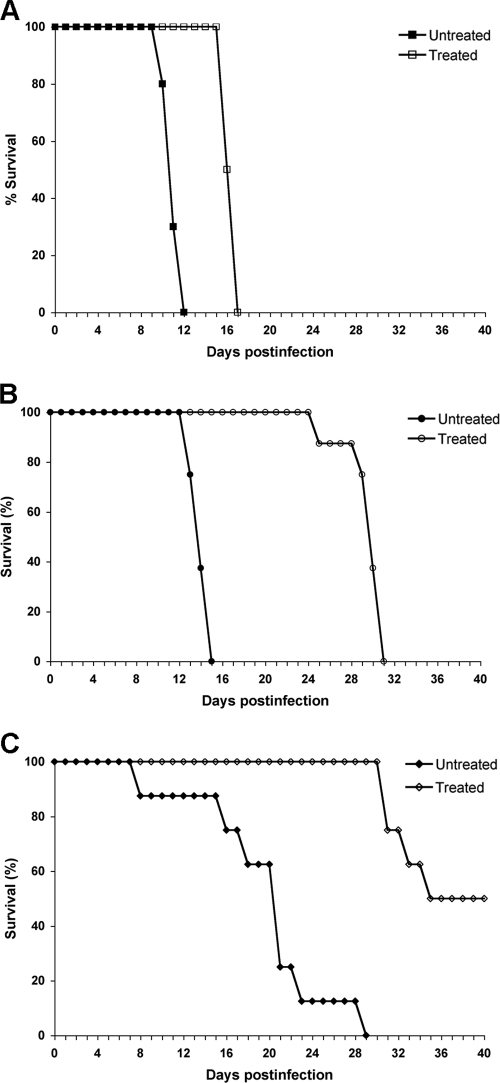
To evaluate the efficacy of FLC therapy, 10 mice each from three groups were inoculated intravenously with strains H99, NIH136, and NIH38 (MICs of FLC by Etest were 24, 2, and 1 μg/ml, respectively) expressing FLC heteroresistance levels of 32, 8, and 4 μg/ml, respectively. The mice were treated with 10 mg FLC/kg/day for 2 weeks starting 24 h after infection. The control mice infected with H99 received only vehicle for 2 weeks. The inoculum of H99 was 100-fold less than that of the other strains, since H99 is significantly more virulent. H99 caused 100% mortality in both the control (no treatment) and the FLC-treated group by day 17, with a marginal difference in the survival rate (Fig. 6A). In the groups infected with NIH136 and NIH38, however, FLC-treated mice survived considerably longer than did those of the untreated controls (P < 0.01) (Fig. 6B and C). The FLC therapy efficacy was most pronounced in mice infected with NIH38 that exhibited heteroresistance at the lowest level (4 μg/ml). While 100% of the control mice died by day 29, only 50% of the mice treated with FLC had died by day 40, the termination date of the experiment (Fig. 6C). To investigate the progression of infection and the possibility of FLC-derived enrichment in resistant subpopulations, fungal burdens in the brain were determined. In the H99 (heteroresistant at 32 μg/ml)-infected animals, there were no clear differences in the brain fungal burdens between treated and control subgroups (Fig. 7A). In contrast, in the groups infected with the strains NIH136 (heteroresistance at 8 μg/ml) and NIH38 (heteroresistance at 4 μg/ml), fungal burdens were lower, as expected, in the FLC-treated animals than in the control mice (1.5 and 2 logs less in NIH136- and NIH38-infected mice, respectively) (Fig. 7B and C). However, when the brain homogenate of the same animal was plated onto YPD plates containing FLC, the number of resistant subpopulations was significantly higher in the treated mice than that in the control mice. No difference in the proportions of the FLC resistance subpopulation at 32 μg/ml was observed between the FLC-treated and untreated H99-infected mice (Fig. 8A). Since the strain NIH136 expressed heteroresistance at 8 μg/ml, the proportion of the FLC-resistant subpopulation was analyzed on YPD containing 8 μg/ml FLC. In the group infected with this strain, the frequency of the resistant subpopulation at 8 μg/ml of FLC increased from 0.3% on day 0 (inoculum) to 1.32% on day 15 in the brain of treated mice, and the frequency of the resistant subclones decreased from 0.3 to 0.19% in untreated animals (Fig. 8B). Interestingly, in animals infected with NIH38 (heteroresistant at 4 μg/ml), the subpopulation resistant at 8 μg/ml was found only in FLC-treated mice that increased from 0% (inoculum) to 3.57% by day 12 of treatment (Fig. 8C). These results demonstrate that FLC treatment has little efficacy in the cases of infection caused by strains expressing high heteroresistance levels (≥32 μg/ml). However, FLC treatment induces the emergence of subclones which can tolerate higher concentrations of FLC if the infecting strain has a low level of heteroresistance (≤8 μg/ml). These results also demonstrate that one can predict FLC therapy efficacy by evaluating the intrinsic level of heteroresistance in the pretreatment strain isolated at the time of diagnosis.
FIG. 6.
Effects of FLC treatment on the survival of mice infected intravenously with strains H99 (5 × 104 cells/mouse) (A), NIH136 (5 × 106 cells/mouse) (B), or NIH38 (5 × 106 cells/mouse) (C) expressing heteroresistance levels at 32, 8, and 4 μg/ml, respectively. FLC was administered intraperitoneally for 2 weeks at 10 mg/kg/day. Survival rates (percentages) for FLC-treated animals and untreated controls are plotted against the number of days after inoculation.
DISCUSSION
Triazoles are the largest class of antifungal drugs in clinical use for the past 2 decades. Long-term usage of FLC, the most widely used triazole drug, for the management of cryptococcosis in AIDS patients, led to the emergence of FLC-resistant strains (2, 4, 19, 33, 50). However, the isolation of resistant strains during long-term azole therapy for cryptococcosis has been much less frequently reported than has been that of other pathogenic yeasts, such as Candida species (7, 15, 41, 47, 48). As a result, relatively little attention has been paid to the mechanism of azole resistance in C. neoformans.
When we first described the heteroresistance to azoles in C. neoformans (30), we excluded the possibility of mutations in ergosterol biosynthetic genes or efflux pumps, since the frequency of resistant subpopulations was too high and all resistant clones reverted back to the original susceptible phenotype. There was, however, a question whether this type of unusual resistance to azoles was intrinsic or acquired through exposure to azole drugs since they were isolated during the 1990s. In the present study, we found FLC heteroresistance in all strains of C. neoformans isolated long before the advent of azole drugs, indicating that heteroresistance in this pathogen is intrinsic. The pattern of heteroresistance was not different between clinical isolates and environmental isolates, further supporting the intrinsic nature of heteroresistance.
Contrary to our observation, Yamazumi et al. (56) reported the heteroresistant phenotype only in 5 of 107 C. neoformans clinical strains. This rate being lower than our results can be explained by the differences in media and screening methods used. We screened the heteroresistant phenotype by plating cells onto YPD agar supplemented with a wide range of FLC concentrations (4 to 128 μg/ml) while disregarding the MIC of each strain. Yamazumi et al. (56), on the other hand, regarded strains to be heteroresistant only when isolates produced resistant subpopulations on potato dextrose agar plates containing FLC concentrations that are four to eight times higher than the MICs of each strain. Besides the differences in the media used, the latter screening method would have excluded all the strains heteroresistant at low concentrations (≤8 μg/ml) of FLC.
Heteroresistance was found to be unrelated to the serotypes of C. neoformans since strains of both serotypes A and D exhibited heteroresistance. However, a majority of the serotype D strains (79%) isolated before 1979 manifested heteroresistance at lower concentrations of FLC (≤8 μg/ml) than did serotype A strains (50%). Moreover, 3.7% of serotype D strains versus 23.5% of serotype A strains from this collection yielded subpopulations resistant at the FLC concentration of ≥32 μg/ml (Tables 1 and 2). It appears, therefore, that a considerably higher percentage of the serotype A strains possess a higher potential to withstand azole stress than do serotype D strains. It is a well-established fact that serotype A strains are predominant in nature as well as in clinical cases worldwide (11, 22). It is interesting to note that the level of FLC concentration at which strains manifest heteroresistance is associated with their ability to tolerate other xenobiotics produced by soil microorganisms and plants. Unlike numerous other soil fungi, C. neoformans does not produce antimicrobial secondary metabolites as a defense mechanism. C. neoformans cells produce a large polysaccharide capsule which can protect them from dehydration, reactive oxidative species, and phagocytosis by soil amoeba (45, 57). It also produces various hydrolytic enzymes, such as protease, urease, and phospholipase (11). However, these may not be sufficient to defend itself and thrive in harsh environmental conditions such as pigeon droppings and soil. It is conceivable that the mechanism which controls heteroresistance to azoles in C. neoformans may also be involved in the regulation of the fungal response to other environmental stresses.
It is also significant that the strains that exhibited a higher level of heteroresistance were also more virulent in the animal model than were strains that expressed a lower level of heteroresistance.
A previous report of the emergence of “mutant” clones resistant to FLC in 21 C. neoformans strains (54) appears to suggest that the rate of “mutation” may actually represent the rate at which subpopulations express heteroresistance at 8 μg/ml FLC. However, this possibility remains to be tested. The gene mutations that result in the FLC resistance appear to be rare in C. neoformans, which may explain the existing controversy as to whether the acquired azole resistance occurs in the species. There has been only one documented clinical case in which a gene mutation was attributed to FLC resistance in a strain isolated from recurrent infection during maintenance therapy. Protein sequence comparisons between Erg11p of the isolate susceptible to FLC and an FLC-resistant strain isolated from a recurrent episode in the same patient revealed an amino acid substitution, G484S, in the resistant strain (37). While numerous genes in the genome of C. neoformans have been annotated as efflux pumps (24), only one ABC transporter, AFR1, to date has been attributed to efflux of FLC (35). Though AFR1 mutants have not been isolated from patients, a laboratory-constructed afr1Δ strain manifested increased susceptibility to FLC in a serotype D strain of C. neoformans (35). Indeed, deletion of AFR1 in the serotype A strain H99 drastically reduced FLC resistance, while resistance in the reconstituted strain with the wild-type AFR1 gene was restored to the original level. Unlike H99, the afr1Δ strain exhibited heteroresistance at 1 μg/ml FLC, which could only be raised to 8 μg/ml FLC by stepwise adaptation. Furthermore, 26 daily transfers in drug-free media were required for the afr1ΔR8 subclones to revert back to the original phenotype (heteroresistance at 1 μg/ml). These results indicated that AFR1 plays a major role in FLC resistance without being involved in the mechanism of heteroresistance.
Our findings underscore the clinical importance of heteroresistance in recurrent cryptococcosis during FLC maintenance therapy. All 16 of the FLC-resistant strains isolated during 1990 to 2000 from therapy failure cases manifested heteroresistance at 16 to 128 μg/ml and yielded clones able to tolerate up to 256 to 400 μg/ml upon stepwise exposure to higher concentrations of FLC. Clones resistant to the highest concentrations of FLC from all but one strain reverted back to their original heteroresistance phenotype by the 45th consecutive transfer in drug-free media. We did not continue daily transfers of the clinical strain that maintained high resistance to FLC through 45 transfers. This strain may require more than 45 daily transfers before reverting back to original heteroresistance levels at 128 μg/ml FLC. On the other hand, this strain may have a mutation in the gene(s) affecting azole resistance in addition to heteroresistance at high levels of FLC.
The reversibility of resistance in almost all FLC-resistant strains isolated from clinical cases may have caused controversy as to whether acquired azole resistance occurs in C. neoformans during azole therapy, even though FLC-resistant C. neoformans strains have been isolated mostly from AIDS patients undergoing FLC maintenance therapy.
It is not surprising to observe that the efficacy of FLC therapy is much higher for infecting strains that have low levels of heteroresistance (≤8 μg/ml) since the maximal level of FLC in cerebrospinal fluid is approximately 8 μg/ml (6, 53). As expected, increases in the frequency of subpopulations resistant to 32 μg/ml FLC were not observed in the brains of H99-infected mice treated with FLC. These results suggest that at least 23.5% of the serotype A strains can cause infection in which FLC therapy may not be efficacious even if resistant subpopulations do not emerge during therapy. FLC therapy significantly prolonged the survival of mice infected with strains that exhibited heteroresistance at ≤8 μg/ml, and at least 50% of serotype A strains isolated before 1979 belonged to this category. However, analysis of CFU in the brains of mice infected with strains of low-level heteroresistance (4 μg/ml and 8 μg/ml) revealed that FLC therapy caused the emergence of subpopulations resistant at 8 μg/ml in both groups. The analysis of CFU from the brains of untreated mice infected with a strain heteroresistant at 4 μg/ml FLC showed no emergence of subpopulations that could tolerate higher levels of FLC throughout the treatment. The brains from treated mice, however, yielded subpopulations resistant at 8 μg/ml FLC by day 12 of treatment. Since FLC is a fungistatic drug, it is apparent that growth arrested in a majority of the fungal cells sensitive to 8 μg/ml FLC while the heteroresistant subpopulation slowly accumulated by day 12 of treatment. Since the present study and previous reports have showed a correlation between the in vitro susceptibility of C. neoformans strains and the in vivo response to FLC (1, 49), heteroresistance may well be a critical factor contributing to the FLC treatment failure of cryptococcosis. Our study suggests that the determination of each strain's heteroresistance level prior to the initiation of FLC therapy is warranted for the better management of cryptococcosis treatment.
Heterogeneous resistance can also develop in Candida albicans after only short exposures to FLC, both in vitro and in vivo (10, 29, 32). This rapid development of resistance has been observed in C. albicans strains that cause disseminated infection in patients after receiving bone marrow transplantation (27, 29). In these clinical isolates, resistance to the azole drugs was transient, as the strains became susceptible after serial transfers in the absence of the drugs (27, 28). A recent study by Selmecki et al. (42) revealed an association between aneuploidy and azole resistance in C. albicans. In the resistant strains, the presence of extra left arms of chromosome 5 was detected, and the gain and loss of this isochromosome were strongly associated with increased and decreased azole resistance. It remains to be elucidated whether a similar mechanism is responsible for the heteroresistance in C. neoformans.
In conclusion, FLC heteroresistance was found to be universal in C. neoformans strains tested and represents a novel intrinsic adaptive mechanism for survival under the azole stress. Not all strains heteroresistant at high levels of FLC were hypervirulent, but all highly virulent strains manifested heteroresistance at high concentrations of FLC. This indicates that determination of the heteroresistance level for the diagnostic strain of each cryptococcosis case will offer helpful insights into the management of long-term FLC therapy. Further studies are necessary to elucidate the molecular mechanisms of heteroresistance.
Supplementary Material
Acknowledgments
We thank John E. Bennett for kindly providing the C. neoformans cultures in his collection deposited before 1979 and for his expert assistance during the course of this study. We are grateful to Ashok Varma for critical reading of the manuscript.
This study was supported by funds from the intramural program of the National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
Published ahead of print on 4 May 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Aller, A. I., E. Martin-Mazuelos, F. Lozano, J. Gomez-Mateos, L. Steele-Moore, W. J. Holloway, M. J. Gutierrez, F. J. Recio, and A. Espinel-Ingroff. 2000. Correlation of fluconazole MICs with clinical outcome in cryptococcal infection. Antimicrob. Agents Chemother. 44:1544-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armengou, A., C. Porcar, J. Mascaro, and F. Garcia-Bragado. 1996. Possible development of resistance to fluconazole during suppressive therapy for AIDS-associated cryptococcal meningitis. Clin. Infect. Dis. 23:1337-1338. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, J. E., K. Izumikawa, and K. A. Marr. 2004. Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob. Agents Chemother. 48:1773-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, J., C. J. Clancy, and M. H. Nguyen. 1998. The hidden danger of primary fluconazole prophylaxis for patients with AIDS. Clin. Infect. Dis. 26:186-187. [DOI] [PubMed] [Google Scholar]
- 5.Bicanic, T., T. Harrison, A. Niepieklo, N. Dyakopu, and G. Meintjes. 2006. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin. Infect. Dis. 43:1069-1073. [DOI] [PubMed] [Google Scholar]
- 6.Brammer, K. W., P. R. Farrow, and J. K. Faulkner. 1990. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev. Infect. Dis. 12(Suppl. 3):S318-S326. [DOI] [PubMed] [Google Scholar]
- 7.Brandt, M. E., M. A. Pfaller, R. A. Hajjeh, E. A. Graviss, J. Rees, E. D. Spitzer, R. W. Pinner, L. W. Mayer, et al. 1996. Molecular subtypes and antifungal susceptibilities of serial Cryptococcus neoformans isolates in human immunodeficiency virus-associated cryptococcosis. J. Infect. Dis. 174:812-820. [DOI] [PubMed] [Google Scholar]
- 8.Brandt, M. E., M. A. Pfaller, R. A. Hajjeh, R. J. Hamill, P. G. Pappas, A. L. Reingold, D. Rimland, and D. W. Warnock. 2001. Trends in antifungal drug susceptibility of Cryptococcus neoformans isolates in the United States: 1992 to 1994 and 1996 to 1998. Antimicrob. Agents Chemother. 45:3065-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brun, S., T. Berges, P. Poupard, C. Vauzelle-Moreau, G. Renier, D. Chabasse, and J. P. Bouchara. 2004. Mechanisms of azole resistance in petite mutants of Candida glabrata. Antimicrob. Agents Chemother. 48:1788-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvet, H. M., M. R. Yeaman, and S. G. Filler. 1997. Reversible fluconazole resistance in Candida albicans: a potential in vitro model. Antimicrob. Agents Chemother. 41:535-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, DC.
- 12.Cowen, L. E., J. B. Anderson, and L. M. Kohn. 2002. Evolution of drug resistance in Candida albicans. Annu. Rev. Microbiol. 56:139-165. [DOI] [PubMed] [Google Scholar]
- 13.Cowen, L. E., A. E. Carpenter, O. Matangkasombut, G. R. Fink, and S. Lindquist. 2006. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot. Cell 5:2184-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowen, L. E., and S. Lindquist. 2005. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309:2185-2189. [DOI] [PubMed] [Google Scholar]
- 15.Cowen, L. E., and W. J. Steinbach. 2008. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryot. Cell 7:747-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva Ferreira, M. E., J. L. Capellaro, E. dos Reis Marques, I. Malavazi, D. Perlin, S. Park, J. B. Anderson, A. L. Colombo, B. A. Arthington-Skaggs, M. H. Goldman, and G. H. Goldman. 2004. In vitro evolution of itraconazole resistance in Aspergillus fumigatus involves multiple mechanisms of resistance. Antimicrob. Agents Chemother. 48:4405-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denning, D. W., K. Venkateswarlu, K. L. Oakley, M. J. Anderson, N. J. Manning, D. A. Stevens, D. W. Warnock, and S. L. Kelly. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz-Guerra, T. M., E. Mellado, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2003. A point mutation in the 14α-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 47:1120-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friese, G., T. Discher, R. Fussle, A. Schmalreck, and J. Lohmeyer. 2001. Development of azole resistance during fluconazole maintenance therapy for AIDS-associated cryptococcal disease. AIDS 15:2344-2345. [DOI] [PubMed] [Google Scholar]
- 20.Helmerhorst, E. J., C. Venuleo, D. Sanglard, and F. G. Oppenheim. 2006. Roles of cellular respiration, CgCDR1, and CgCDR2 in Candida glabrata resistance to histatin 5. Antimicrob. Agents Chemother. 50:1100-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph-Horne, T., D. Hollomon, R. S. Loeffler, and S. L. Kelly. 1995. Cross-resistance to polyene and azole drugs in Cryptococcus neoformans. Antimicrob. Agents Chemother. 39:1526-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon-Chung, K. J., and J. E. Bennett. 1992. Medical mycology. Lea & Febiger, Philadelphia, PA.
- 23.Kwon-Chung, K. J., I. Polacheck, and J. E. Bennett. 1982. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C). J. Clin. Microbiol. 15:535-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loftus, B. J., E. Fung, P. Roncaglia, D. Rowley, P. Amedeo, D. Bruno, J. Vamathevan, M. Miranda, I. J. Anderson, J. A. Fraser, J. E. Allen, I. E. Bosdet, M. R. Brent, R. Chiu, T. L. Doering, M. J. Donlin, C. A. D'Souza, D. S. Fox, V. Grinberg, J. Fu, M. Fukushima, B. J. Haas, J. C. Huang, G. Janbon, S. J. Jones, H. L. Koo, M. I. Krzywinski, J. K. Kwon-Chung, K. B. Lengeler, R. Maiti, M. A. Marra, R. E. Marra, C. A. Mathewson, T. G. Mitchell, M. Pertea, F. R. Riggs, S. L. Salzberg, J. E. Schein, A. Shvartsbeyn, H. Shin, M. Shumway, C. A. Specht, B. B. Suh, A. Tenney, T. R. Utterback, B. L. Wickes, J. R. Wortman, N. H. Wye, J. W. Kronstad, J. K. Lodge, J. Heitman, R. W. Davis, C. M. Fraser, and R. W. Hyman. 2005. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307:1321-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lupetti, A., R. Danesi, M. Campa, M. Del Tacca, and S. Kelly. 2002. Molecular basis of resistance to azole antifungals. Trends Mol. Med. 8:76-81. [DOI] [PubMed] [Google Scholar]
- 26.Marichal, P., L. Koymans, S. Willemsens, D. Bellens, P. Verhasselt, W. Luyten, M. Borgers, F. C. Ramaekers, F. C. Odds, and H. V. Bossche. 1999. Contribution of mutations in the cytochrome P450 14alpha-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology (United Kingdom) 10:2701-2713. [DOI] [PubMed] [Google Scholar]
- 27.Marr, K. A., C. N. Lyons, K. Ha, T. R. Rustad, and T. C. White. 2001. Inducible azole resistance associated with a heterogeneous phenotype in Candida albicans. Antimicrob. Agents Chemother. 45:52-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marr, K. A., C. N. Lyons, T. R. Rustad, R. A. Bowden, and T. C. White. 1998. Rapid, transient fluconazole resistance in Candida albicans is associated with increased mRNA levels of CDR. Antimicrob. Agents Chemother. 42:2584-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marr, K. A., T. C. White, J. A. van Burik, and R. A. Bowden. 1997. Development of fluconazole resistance in Candida albicans causing disseminated infection in a patient undergoing marrow transplantation. Clin. Infect. Dis. 25:908-910. [DOI] [PubMed] [Google Scholar]
- 30.Mondon, P., R. Petter, G. Amalfitano, R. Luzzati, E. Concia, I. Polacheck, and K. J. Kwon-Chung. 1999. Heteroresistance to fluconazole and voriconazole in Cryptococcus neoformans. Antimicrob. Agents Chemother. 43:1856-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nes, W. D., W. Zhou, K. Ganapathy, J. Liu, R. Vatsyayan, S. Chamala, K. Hernandez, and M. Miranda. 2009. Sterol 24-C-methyltransferase: an enzymatic target for the disruption of ergosterol biosynthesis and homeostasis in Cryptococcus neoformans. Arch. Biochem. Biophys. 481:210-218. [DOI] [PubMed] [Google Scholar]
- 32.Nolte, F. S., T. Parkinson, D. J. Falconer, S. Dix, J. Williams, C. Gilmore, R. Geller, and J. R. Wingard. 1997. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob. Agents Chemother. 41:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paugam, A., J. Dupouy-Camet, P. Blanche, J. P. Gangneux, C. Tourte-Schaefer, and D. Sicard. 1994. Increased fluconazole resistance of Cryptococcus neoformans isolated from a patient with AIDS and recurrent meningitis. Clin. Infect. Dis. 19:975-976. [DOI] [PubMed] [Google Scholar]
- 34.Polacheck, I., and K. J. Kwon-Chung. 1986. Canavanine resistance in Cryptococcus neoformans. Antimicrob. Agents Chemother. 29:468-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Posteraro, B., M. Sanguinetti, D. Sanglard, M. La Sorda, S. Boccia, L. Romano, G. Morace, and G. Fadda. 2003. Identification and characterization of a Cryptococcus neoformans ATP binding cassette (ABC) transporter-encoding gene, CnAFR1, involved in the resistance to fluconazole. Mol. Microbiol. 47:357-371. [DOI] [PubMed] [Google Scholar]
- 36.Powderly, W. G. 1996. Cryptococcosis. J. Int. Assoc. Physicians AIDS Care 2:28-31. [PubMed] [Google Scholar]
- 37.Rodero, L., E. Mellado, A. C. Rodriguez, A. Salve, L. Guelfand, P. Cahn, M. Cuenca-Estrella, G. Davel, and J. L. Rodriguez-Tudela. 2003. G484S amino acid substitution in lanosterol 14-α demethylase (ERG11) is related to fluconazole resistance in a recurrent Cryptococcus neoformans clinical isolate. Antimicrob. Agents Chemother. 47:3653-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanglard, D. 2002. Resistance of human fungal pathogens to antifungal drugs. Curr. Opin. Microbiol. 5:379-385. [DOI] [PubMed] [Google Scholar]
- 39.Sanglard, D., F. Ischer, D. Calabrese, M. Micheli, and J. Bille. 1998. Multiple resistance mechanisms to azole antifungals in yeast clinical isolates. Drug Resist. Updat. 1:255-265. [DOI] [PubMed] [Google Scholar]
- 40.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanglard, D., and F. C. Odds. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73-85. [DOI] [PubMed] [Google Scholar]
- 42.Selmecki, A., A. Forche, and J. Berman. 2006. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 313:367-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selmecki, A., M. Gerami-Nejad, C. Paulson, A. Forche, and J. Berman. 2008. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol. Microbiol. 68:624-641. [DOI] [PubMed] [Google Scholar]
- 44.Silver, P. M., B. G. Oliver, and T. C. White. 2004. Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism. Eukaryot. Cell 3:1391-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steenbergen, J. N., H. A. Shuman, and A. Casadevall. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 98:15245-15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toffaletti, D. L., T. H. Rude, S. A. Johnston, D. T. Durack, and J. R. Perfect. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai, H. F., M. Bard, K. Izumikawa, A. A. Krol, A. M. Sturm, N. T. Culbertson, C. A. Pierson, and J. E. Bennett. 2004. Candida glabrata erg1 mutant with increased sensitivity to azoles and to low oxygen tension. Antimicrob. Agents Chemother. 48:2483-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai, H. F., A. A. Krol, K. E. Sarti, and J. E. Bennett. 2006. Candida glabrata PDR1, a transcriptional regulator of a pleiotropic drug resistance network, mediates azole resistance in clinical isolates and petite mutants. Antimicrob. Agents Chemother. 50:1384-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velez, J. D., R. Allendoerfer, M. Luther, M. G. Rinaldi, and J. R. Graybill. 1993. Correlation of in vitro azole susceptibility with in vivo response in a murine model of cryptococcal meningitis. J. Infect. Dis. 168:508-510. [DOI] [PubMed] [Google Scholar]
- 50.Venkateswarlu, K., M. Taylor, N. J. Manning, M. G. Rinaldi, and S. L. Kelly. 1997. Fluconazole tolerance in clinical isolates of Cryptococcus neoformans. Antimicrob. Agents Chemother. 41:748-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White, T. C., S. Holleman, F. Dy, L. F. Mirels, and D. A. Stevens. 2002. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 46:1704-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White, T. C., M. A. Pfaller, M. G. Rinaldi, J. Smith, and S. W. Redding. 1997. Stable azole drug resistance associated with a substrain of Candida albicans from an HIV-infected patient. Oral Dis. 3(Suppl. 1):S102-S109. [DOI] [PubMed] [Google Scholar]
- 53.Wildfeuer, A., H. Laufen, A. F. Schmalreck, R. A. Yeates, and T. Zimmermann. 1997. Fluconazole: comparison of pharmacokinetics, therapy and in vitro susceptibility. Mycoses 40:259-265. [DOI] [PubMed] [Google Scholar]
- 54.Xu, J., C. Onyewu, H. J. Yoell, R. Y. Ali, R. J. Vilgalys, and T. G. Mitchell. 2001. Dynamic and heterogeneous mutations to fluconazole resistance in Cryptococcus neoformans. Antimicrob. Agents Chemother. 45:420-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamazumi, T., M. A. Pfaller, S. A. Messer, A. Houston, R. J. Hollis, and R. N. Jones. 2000. In vitro activities of ravuconazole (BMS-207147) against 541 clinical isolates of Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:2883-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamazumi, T., M. A. Pfaller, S. A. Messer, A. K. Houston, L. Boyken, R. J. Hollis, I. Furuta, and R. N. Jones. 2003. Characterization of heteroresistance to fluconazole among clinical isolates of Cryptococcus neoformans. J. Clin. Microbiol. 41:267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaragoza, O., C. J. Chrisman, M. V. Castelli, S. Frases, M. Cuenca-Estrella, J. L. Rodriguez-Tudela, and A. Casadevall. 2008. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell. Microbiol. 10:2043-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.