Abstract
A qnrS1-positive strain of Escherichia coli was detected among 73 poultry isolates showing ciprofloxacin MICs of ≥0.125 μg/ml. The qnrS1 gene was associated with a Tn3-like transposon, as previously described to occur in a Salmonella enterica serovar Infantis strain of animal origin, but the plasmid scaffold carrying this element resembled that of a plasmid previously identified in Salmonella enterica serovar Dublin. These elements suggest genetic exchanges among Salmonella and E. coli and a potential animal reservoir for the qnr genes.
Three plasmid-mediated quinolone resistance mechanisms have been described so far: Qnr peptides, capable of protecting DNA gyrase and topoisomerase IV from quinolones; Aac(6′)-Ib-cr aminoglycoside acetyltransferase, modifying the quinolones with a piperazinyl substituent (e.g., ciprofloxacin); and the quinolone efflux pump QepA. Plasmid-mediated quinolone resistance is being increasingly recognized in Enterobacteriaceae from human infections but seems very rare in strains of animal origin (13). However, in a recent study from China, 16.9% of the isolates from food-producing animals contained one or more plasmid-mediated quinolone resistance determinants (10). In Europe, qnr-carrying Escherichia coli strains have not yet been described to occur in animals, and Qnr peptides have been reported to occur only in Salmonella enterica serovar Infantis isolates from chicken carcasses in Germany and in Salmonella enterica serovar Bredeney isolates from chicken meat in The Netherlands (9, 14). In this study, the occurrences of qnr, aac(6′)-Ib-cr, and qepA genes in 73 E. coli strains of avian origin were investigated. These 73 strains were all those showing ciprofloxacin MICs of ≥0.125 μg/ml among 113 isolates recovered between April 2003 and December 2006 (18, 25, 27, and 43 isolates collected in 2003, 2004, 2005, and 2006, respectively) during the surveillance activities of the Istituto Zooprofilattico delle Venezie (Legnaro, Italy). The 113 isolates (74 from poultry with colibacillosis and 39 from poultry at slaughter) represented over 10% of all the E. coli isolates from poultry collected during the study period in the Italian region which hosts the greatest number of poultry farms. Of the 73 isolates analyzed, 65 were fully resistant to ciprofloxacin (MIC range, 4 to 32 μg/ml) and 8 showed reduced susceptibility (MIC range, 0.125 to 0.5 μg/ml). MICs were determined by using Etest kits (AB Biodisk, Solna, Sweden) in accordance with the manufacturer's recommendations; the interpretative breakpoints were based on CLSI susceptibility criteria (6, 7). The screening for the qnrA, qnrB, qnrS, aac(6′)-Ib, and qepA genes was carried out by multiplex and simplex PCR amplifications, using primers and conditions previously described (2, 12, 15), and amplicons were sequenced to determine the gene variants. One qnrS1-positive isolate (strain 3963) was detected among the eight isolates showing reduced susceptibility to ciprofloxacin (12.5%); all the other isolates of this collection were negative for the qnr, aac(6′)-Ib, and qepA genes. Strain 3963 was isolated from a regularly slaughtered chicken in 2006. This strain belonged to phylogenetic group D (5) and to multilocus sequence type 398 (http://mlst.ucc.ie/mlst/dbs/Ecoli). Strain 3963 showed resistance to enrofloxacin and reduced susceptibility to nalidixic acid, ciprofloxacin, and levofloxacin (Table 1) (according to references 6 and 7). This strain was also resistant to ampicillin but susceptible to broad-spectrum cephalosporins. No mutations were identified in the quinolone resistance-determining regions of the gyrA, gyrB, and parC genes (16). Strain 3963 also carried the blaTEM-1 gene, as demonstrated by PCR and sequencing using primers and conditions previously described (11).
TABLE 1.
Susceptibilities of the qnrS1 donor (3963), transformant (TOP10-3963), and recipient (E. coli TOP10) strains to selected antibiotics
| Strain | MIC (μg/ml)a
|
||||||
|---|---|---|---|---|---|---|---|
| CIP | LV | NA | ENRO | AMP | CRO | CTX | |
| 3963 | 0.38 | 0.5 | 16 | 2 | ≥256 | 0.032 | 0.047 |
| Recipient E. coli TOP10 | 0.003 | 0.006 | 2 | 0.08 | 6 | 0.064 | 0.064 |
| Transformant TOP10-3963 | 0.25 | 0.25 | 6 | 0.50 | ≥256 | 0.094 | 0.064 |
CIP, ciprofloxacin; LV, levofloxacin; NA, nalidixic acid; ENRO, enrofloxacin; AMP, ampicillin; CRO, ceftriaxone; CTX, cefotaxime.
Plasmid DNA from strain 3963 was extracted (PureLink HiPure plasmid filter midiprep kit; Invitrogen, Milan, Italy) and used to transform competent E. coli TOP10 cells (Invitrogen, Milan, Italy). Transformants were selected on LB agar plates containing 0.06 μg/ml of ciprofloxacin. TOP10-3963 transformants contained both qnrS1 and blaTEM-1 genes and showed resistance to ampicillin and increased MICs for fluoroquinolones (Table 1). Strain 3963 failed to produce transconjugants when rifampin (rifampicin)-resistant E. coli CSH26 was used as the recipient strain.
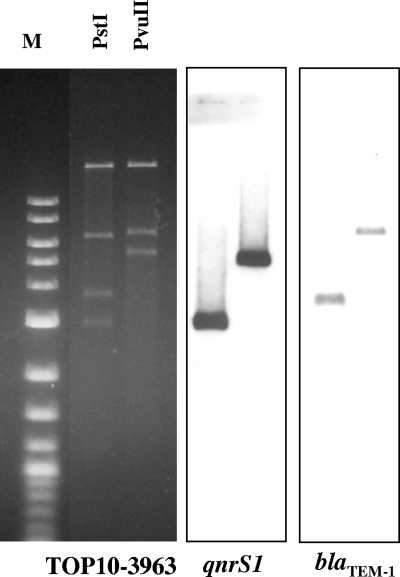
The transferred qnrS1 plasmid of approximately 45 kb was further analyzed by restriction analysis, Southern blot hybridization experiments (Fig. 1), cloning, and DNA sequencing of the regions flanking the qnrS1 gene. In particular, the 3,592- and 2,851-bp PstI fragments containing the qnrS1 and blaTEM-1 genes, respectively, were both cloned into the PstI-pZero-2.1 kanamycin-resistant vector (Invitrogen, Milan, Italy), selecting E. coli DH5α recombinant clones, on LB agar plates containing 100 μg/ml kanamycin and either 0.06 μg/ml ciprofloxacin or 50 μg/ml ampicillin, respectively. The DNA sequences of the cloned PstI fragments perfectly matched the sequence of the resistance region from plasmid pINF5, a qnrS1-positive plasmid previously identified in S. enterica serovar Infantis isolates from chicken carcasses in Germany (EMBL database accession no. AM234722) (9). In particular, the 3,592-bp PstI fragment contained the 3′ end of the tnpA gene of transposon Tn3, the relict of the insertion sequence IS2, and the entire qnrS1 gene. The 2,851-bp PstI fragment contained the blaTEM-1 gene, the resolvase gene of Tn3, and part of the 5′ end of the Tn3 tnpA gene. Since no information is available on the pINF5 plasmid scaffold and the 3963 transformant strain was found untypeable for the 18 incompatibility groups tested by PCR-based replicon typing (1), a further characterization of the 3963 plasmid was performed. Plasmid DNA was digested by Sau3A, producing fragments ranging between 100 and 2,000 bp that were ligated in the BamHI-pZero-2 vector and obtaining a random library. Several recombinant clones were randomly selected and fully sequenced. Three clones provided information on the 3963 plasmid scaffold, since they contained 359-, 759-, and 1,467-bp inserts matching at 95 to 99% with the DNA sequence of the IncX1 virulence plasmid pOU1114 (EMBL database accession no. DQ115387). Plasmid pOU1114 is a 35-kb plasmid previously identified in Salmonella enterica serovar Dublin strain OU7025, isolated in Taiwan (4). The sequenced inserts from plasmid 3963 tagged three regions scattered along a large portion of the pOU1114 scaffold, including the pilX1, pilX2, and pilX4 genes (members of the CagE, TrbE, and VirB families and components of the type IV transporter system, localized at nucleotide [nt] positions 17341 to 21034 in DQ115387), a region encoding the conjugal transfer TrbI-like protein (nt 12061 to 13266), and a region encoding a protein similar to the DNA distortion polypeptide from plasmid R6K of the IncX group (nt 29451 to 29807). These sequence data suggest that the 3963 plasmid scaffold is very similar to that described to occur in the pOU1114 plasmid of S. enterica serovar Dublin, although the latter did not contain the qnrS1 gene.
FIG. 1.
Restriction analysis of plasmid obtained from the transformant TOP10-3963, restricted by PstI and PvuII (New England BioLabs, Inc., Ipswich, MA) and Southern blot hybridization with digoxigenin-labeled qnrS1 and blaTEM-1 probes (Roche Diagnostics GmbH, Mannheim, Germany). M, 1-kb DNA extension ladder (Invitrogen, Milan, Italy).
Our findings indicate that Qnr determinants are present in E. coli isolates from poultry in Europe and cannot be associated with the quinolone resistance-determining region mutations as previously described for other qnrS1-positive Enterobacteriaceae (3). Fluoroquinolones are widely used in poultry production, and qnr-positive E. coli isolates could be selected and transmitted to humans through the food chain (8). The complete Tn3::IS2::qnrS1 transposon and the plasmid scaffold carrying this element harbored by our E. coli isolate were previously described to occur in two different S. enterica strains of animal origin, belonging to serovars Infantis and Dublin, respectively. These data suggest genetic exchanges among Salmonella and E. coli strains of animal origin and open new perspectives on the potential animal reservoirs of qnr genes.
Acknowledgments
We are grateful to Michela Corrò (Istituto Zooprofilattico Sperimentale delle Venezie, Legnaro, Italy) for providing E. coli strains.
This work was partly funded by Med-Vet-Net, Workpackages 21 and 29. Med-Vet-Net is a European Union-funded Network of Excellence.
Footnotes
Published ahead of print on 18 May 2009.
REFERENCES
- 1.Carattoli, A., A. Bertini, L. Villa, V. Falbo, K. L. Hopkins, and E. J. Threlfall. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219-228. [DOI] [PubMed] [Google Scholar]
- 2.Cattoir, V., L. Poirel, V. Rotimi, C. J. Soussy, and P. Nordmann. 2007. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J. Antimicrob. Chemother. 60:394-397. [DOI] [PubMed] [Google Scholar]
- 3.Chenia, H. Y., B. Pillay, and D. Pillay. 2006. Analysis of the mechanisms of fluoroquinolone resistance in urinary tract pathogens. J. Antimicrob. Chemother. 58:1274-1278. [DOI] [PubMed] [Google Scholar]
- 4.Chu, C., Y. Feng, A. C. Chien, S. Hu, C. H. Chu, and C. H. Chiu. 2008. Evolution of genes on the Salmonella virulence plasmid phylogeny revealed from sequencing of the virulence plasmids of S. enterica serotype Dublin and comparative analysis. Genomics 92:339-343. [DOI] [PubMed] [Google Scholar]
- 5.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. CLSI M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard, 3rd ed. CLSI M31-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Fàbrega, A., J. Sánchez-Céspedes, S. Soto, and J. Vila. 2008. Quinolone resistance in the food chain. Int. J. Antimicrob. Agents 31:307-315. [DOI] [PubMed] [Google Scholar]
- 9.Kehrenberg, C., S. Friederichs, A. de Jong, G. B. Michael, and S. Schwarz. 2006. Identification of the plasmid-borne quinolone resistance gene qnrS in Salmonella enterica serovar Infantis. J. Antimicrob. Chemother. 58:18-22. [DOI] [PubMed] [Google Scholar]
- 10.Ma, J., Z. Zeng, Z. Chen, X. Xu, X. Wang, Y. Deng, D. Lü, L. Huang, Y. Zhang, J. Liu, and M. Wang 2009. High prevalence of plasmid-mediated quinolone resistance determinants qnr, aac(6′)-Ib-cr, and qepA among ceftiofur-resistant Enterobacteriaceae isolates from companion and food-producing animals. Antimicrob. Agents Chemother. 53:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mabilat, C., S. Goussard, W. Sougakoff, R. C. Spencer, and P. Courvalin. 1990. Direct sequencing of the amplified structural gene and promoter for the extended-broad-spectrum beta-lactamase TEM-9 (RHH-1) of Klebsiella pneumoniae. Plasmid 23:27-34. [DOI] [PubMed] [Google Scholar]
- 12.Park, C. H., A. Robicsek, G. A. Jacoby, D. Sahm, and D. C. Hooper. 2006. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 50:3953-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robicsek, A., G. A. Jacoby, and D. C. Hooper. 2006. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect. Dis. 6:629-640. [DOI] [PubMed] [Google Scholar]
- 14.Veldman, K., W. van Pelt, and D. Mevius. 2008. First report of qnr in Salmonella in The Netherlands. J. Antimicrob. Chemother. 61:452-453. [DOI] [PubMed] [Google Scholar]
- 15.Yamane, K., J. Wachino, S. Suzuki, and Y. Arakawa. 2008. Plasmid-mediated qepA gene among Escherichia coli clinical isolates from Japan. Antimicrob. Agents Chemother. 52:1564-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang, H., S. Chen, D. G. White, S. Zhao, P. McDermott, R. Walker, and J. Meng. 2004. Characterization of multiple-antimicrobial-resistant Escherichia coli isolates from diseased chickens and swine in China. J. Clin. Microbiol. 42:3483-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]