Abstract
Tigecycline is a glycylcycline with activity against Enterobacteriaceae, including multidrug-resistant isolates of Klebsiella pneumoniae and Escherichia coli producing extended-spectrum beta-lactamase (ESBL) and carbapenemases. Herein, we used an in vivo murine thigh model to characterize the pharmacodynamic profile of tigecycline against genotypically and phenotypically diverse K. pneumoniae and E. coli isolates. Doses of 3.125 to 300 mg/kg, divided 1 to 6 times daily, were administered subcutaneously against six (two nonresistant, one carbapenemase, and three ESBL producing) K. pneumoniae strains and five (two nonresistant and three ESBL producing) E. coli strains. The phenotypic profile (reported tigecycline MIC) for all isolates ranged from 0.125 to 2 μg/ml. Mean correlation coefficients of free (f) drug exposures (percentage of the dosing interval that free drug concentration remained above the MIC [fT>MIC], the ratio of the free drug area under the concentration-time curve/MIC [fAUC/MIC], and the ratio of maximum concentration of free drug in serum/MIC) for all 11 isolates were 0.595, 0.969, and 0.897, respectively. The fAUC/MIC was the pharmacodynamic parameter that best described the efficacy of tigecycline against both E. coli and K. pneumoniae. Interestingly, reductions in the number of CFU were noted even though doses achieved an fT>MIC of 0%. With respect to fAUC/MIC in the neutropenic model, the cumulative 80% and 50% effective pharmacodynamic indexes (EI80 and EI50) for all 11 isolates were 8.4 and 4.7, respectively. An experiment in nonneutropenic mice infected with an ESBL-producing E. coli and K. pneumoniae isolate resulted in the lowest tigecycline fAUC/MIC EI80 and EI50 values at 1.8 and 1.0 for E. coli and 1.7 and 1.6 for K. pneumoniae. While the phenotypic profile of tigecycline appeared to drive efficacy irrespective of ESBL or carbapenemase production, the presence of a competent immune system markedly reduced this required exposure.
With the recent worldwide emergence of carbapenemase-producing Klebsiella pneumoniae and the steadily increasing prevalence of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae (11, 17, 24), the need for more antibiotics in the rapidly deteriorating armamentarium becomes even more important (18).
Tigecycline, a glycylcycline derived from minocycline, is a novel agent displaying activity against ESBL and carbapenemase-producing K. pneumoniae and Escherichia coli (2, 4, 9). Currently, tigecycline is FDA approved for complicated intra-abdominal infections (cIAI) and complicated skin-skin structure infections (15). With high susceptibilities demonstrated in surveillance studies (2) and positive clinical outcomes shown in trial data from subpopulations infected with ESBL-producing Enterobacteriaceae (6, 23), tigecycline has been increasingly utilized as a treatment option. Given these occurrences and the few available studies describing the exposure-response relationship for the treatment of gram-negative organisms (15, 19, 21), it seems reasonable to uncover the pharmacodynamics of tigecycline.
Herein, we described the pharmacodynamic profile of tigecycline and the magnitude of its efficacy against a diverse group of E. coli and K. pneumoniae isolates in the mouse thigh model.
MATERIALS AND METHODS
Antimicrobial agents.
Tigecycline analytical-grade powder (Wyeth Pharmaceuticals, Inc., Madison, NJ) was reconstituted as recommended by the manufacturer in sterile 0.9% normal saline (NS) within 30 min of injection into the animal.
Bacterial isolates and susceptibility.
Experiments were performed on five (two nonresistant and three ESBL producing) E. coli and six (two nonresistant, three ESBL, and one carbapenemase producing) K. pneumoniae isolates. The resistance mechanisms (genotypic profile) were characterized previously for seven of the isolates; two of these were supplied by S. Jenkins at Mount Sinai Hospital, New York City, NY (one ESBL-producing and one carbapenemase-producing K. pneumoniae isolate), and five ESBLs were supplied by J. Quinn at John Stroger Hospital, Chicago, IL. The phenotypic profile for each isolate was reported by the modal tigecycline MIC. Tigecycline MICs were determined in triplicate by broth microdilution method as per CLSI guidelines (3) or by Etest method. Mueller-Hinton broth was prepared fresh <12 h prior to testing (1).
Thigh infection model.
Pathogen-free, female, CD-1/ICR mice (Harlan-Sprague-Dawley Inc., Indianapolis, IN) weighing ∼25 g were used throughout the experiment. The mice were maintained and utilized as per the guidelines of the Hartford Hospital (Hartford, CT) Institutional Animal Care and Use Committee and were provided food and water ad libitum. Mice were rendered neutropenic by intraperitoneal injection of cyclophosphamide (Bristol-Myers Squibb, Princeton, NJ) at 150 mg/kg of body weight at 4 days and 100 mg/kg at 1 day prior to inoculation.
Prior to use, all isolates were grown on Trypticase soy agar medium with 5% sheep blood at 35°C for 18 to 24 h in ambient air. A suspension of each isolate was freshly prepared from a second subculture of the organism that had been diluted in NS to achieve a final inoculum of 107 CFU/ml. The thigh infection was produced by a single 0.1-ml intramuscular injection of the inoculum into each mouse thigh.
Two hours after inoculation, the mice were randomly divided into cohorts to receive subcutaneous injections at a volume of 0.2 ml containing either tigecycline (treatment group) or NS (control group). The treatment groups received either single or multiple doses of tigecycline at 3.125, 6.25, 12.5, 25, and 50 mg/kg to eclipse a total daily dose range of 3.125 to 200 mg/kg/day for E. coli and 6.25 to 300 mg/kg/day for K. pneumoniae. In order to achieve total daily doses above 50 mg/kg/day, we administered multiple doses of 25 mg/kg for 75 to 100 mg/kg/day and 50 mg/kg for ≥100 mg/kg/day. Two control groups were included in the study of each isolate; the 0-h group was euthanized concurrent with the start of dosing; meanwhile, the 24-h control group was administered NS subcutaneously in accordance with the most frequently dosed treatment group. The groups (three mice per group) were euthanized with CO2 inhalation, followed by cervical dislocation. Immediately following sacrifice, each of the thighs was removed and individually homogenized in 5 ml of NS. Thigh homogenate was serially diluted with a range of dilutions and spiral plated onto agar medium for CFU/ml determination.
Immunocompetent mouse thigh infection model.
Groups of ICR mice underwent the same procedure as the neutropenic mice but without the use of cyclophosphamide prior to infection with an inoculum of ∼108 CFU/ml. The two isolates used for these studies were treated with either single or multiple tigecycline daily dose regimens of 3.125 to 50 mg/kg/day for E. coli isolate 315 (EC 315) and 12.5 to 150 mg/kg/day for K. pneumoniae isolate 320 (KP 320).
Pharmacokinetic studies.
We utilized the pharmacokinetic data obtained from a previous tigecycline neutropenic murine thigh infection model performed at our laboratory (5). In brief, the pharmacokinetic portion of the study used single 0.2-ml subcutaneous doses of tigecycline at 6.25, 12.5, 25, and 50 mg/kg. Mice were sacrificed, and blood samples were collected at 8 to 12 time points ranging from 0.5 to 24 h after tigecycline administration (six animals per time point). These single doses were used to derive the pharmacokinetic parameters by way of first-order infusion and elimination, using a nonlinear least-square technique in WinNonlin, version 5.0.1 (Pharsight Corporation, Mountain View, CA). The concentration-time profile for 3.125 mg/kg was derived from the simulated pharmacokinetic parameters of the 6.25 mg/kg dose.
As best described by the two-compartment model, linearity was displayed across the dose range, with a mean half-life of 9.9 h (range, 7.4 to 11.8). The resulting peak concentrations (Cmax) ranged from 1.7 to 12.2 μg/ml. The area under the concentration-time curve (AUC) between 0 and 24 h for the single doses, determined by using the trapezoidal rule, ranged from 4.8 to 49.2 μg·h/ml. In order to establish the free drug concentrations (f), concentration-dependent protein binding as described in the previous analysis was utilized (5). As such, all time points within each concentration-time profile were equally adjusted based on the percentage of free drug determined by the Cmax. Based on these calculations, the resulting fAUCs of doses used in the bacterial density studies ranged from 0.45 to 23.1 μg·h/ml.
Data analysis.
Efficacy was measured by the arithmetic mean change in log10 CFU/ml of the 24-h control or treatment group from the 0-h control mouse thigh (2 h after inoculation). For each pharmacodynamic parameter, which included the percentage of time the concentration was above the MIC (T>MIC), AUC/MIC, and Cmax/MIC, the free drug was utilized. The pharmacodynamic parameter that best correlated with tigecycline efficacy was chosen based on the highest reported correlation coefficient (r2) using the sigmoidal maximal effect (Emax) model. Moreover, the Emax model was used to uncover the exposure index (EI) required for 80% (EI80) and 50% (EI50) of maximum effectiveness and bacteriostasis for each individual isolate and the three composite curves (5 E. coli, 6 K. pneumoniae, and all 11 enterobacteria isolates).
RESULTS
In vitro susceptibility.
The resistance mechanisms and MICs of tigecycline for each of the E. coli and K. pneumoniae isolates are displayed in Table 1. The MICs of the respective organisms ranged from 0.125 to 0.5 μg/ml and 0.5 to 2 μg/ml.
TABLE 1.
The phenotypes (tigecycline MIC modal values) and genotypes (resistance mechanisms) of E. coli and K. pneumoniae test isolates
| Isolatea | MIC (μg/ml) | Resistance mechanism |
|---|---|---|
| EC 54 | 0.25 | Nonresistant |
| EC 120 | 0.125 | Nonresistant |
| EC 315 | 0.5 | Producing ESBL |
| EC 321 | 0.25 | Producing ESBL |
| EC 322 | 0.25 | Producing ESBL |
| KP 134 | 0.5 | Nonresistant |
| KP 135 | 0.5 | Nonresistant |
| KP 255 | 2 | Producing ESBL |
| KP 266 | 0.5 | Producing ESBL |
| KP 320 | 2 | Producing ESBL |
| KP 321 | 0.5 | Producing carbapenemase |
Internal strain designation.
Bacterial density assessment.
In the untreated mice, the mean bacterial density for 0 h and 24 h was 5.71 (range, 5.55 to 5.92) and 8.69 (range, 6.56 to 9.73) log10 CFU/ml, respectively. The mean bacterial density after 24 h in treated animals was 3.80 (range, 3.01 to 4.51) log10 CFU/ml, resulting in a 1.93 (range, 1.10 to 2.91) mean maximal log10 CFU/ml reduction. Similar results were observed in the estimated mean maximal log10 CFU/ml reductions at 24 h, as shown in Table 2.
TABLE 2.
Dose-response relationship of tigecycline against E. coli and K. pneumoniae test isolates in the neutropenic mouse thigh model
| Isolatea | Efficacy of tigecycline as determined by:
|
|||
|---|---|---|---|---|
| EI80 (fAUC/MIC) | EI50 (fAUC/MIC) | Static exposure (fAUC/MIC) | Maximum Δlog10 (CFU/ml) | |
| EC 54 | 7.70 | 5.19 | 5.96 | −2.05 |
| EC 120 | 7.30 | 3.70 | 4.76 | −2.30 |
| EC 315b | 4.46 | 3.71 | 3.88 | −2.00 |
| EC 321b | 9.07 | 6.40 | 6.83 | −2.54 |
| EC 322b | 10.64 | 6.27 | 7.50 | −2.40 |
| KP 134 | 10.04 | 5.91 | 6.53 | −1.42 |
| KP 135 | 7.78 | 3.62 | 5.40 | −1.51 |
| KP 255b | 4.81 | 2.64 | 2.02 | −3.15 |
| KP 266b | 7.15 | 5.37 | 5.13 | −1.34 |
| KP 320b | 3.27 | 2.01 | 3.09 | −1.12 |
| KP 321c | 7.48 | 4.53 | 5.47 | −1.46 |
| Mean (SD) | 7.25 (2.30) | 4.49 (1.47) | 5.14 (1.64) | −1.93 (0.56) |
| Composite | 8.40 | 4.74 | 5.32 | −1.94 |
Internal strain designation.
ESBL-producing isolate.
Carbapenemase-producing isolate.
Determination of pharmacodynamic indices.
The mean (and standard deviation) r2 values for fCmax/MIC, fAUC/MIC, and fT>MIC were 0.90 (0.06), 0.97 (0.02), and 0.59 (0.37), respectively. Interestingly, two isolates (KP 255 and KP 320) with a MIC of 2 μg/ml had r2 values that could not be calculated for fT>MIC, so they were determined to be a value of zero. When the composite r2 values for all 11 isolates were determined for fCmax/MIC, fAUC/MIC, and fT>MIC, the respective parameters were 0.55, 0.81, and 0.45. Based on these data, as depicted in Fig. 1, fAUC/MIC was the pharmacodynamic index most predictive of efficacy. Figure 1 also illustrates a frequent scenario when isolate KP 320 exhibited CFU/ml reductions even though it did not achieve free drug concentrations above the MIC at any time during the dosing interval (fT>MIC of 0%).
FIG. 1.
Comparing the free tigecycline concentration activity in three pharmacodynamic parameters using dose-response curves in K. pneumoniae isolate KP 320 (MIC = 2 μg/ml). (A) fT>MIC. (B) fCmax/MIC. (C) fAUC/MIC.
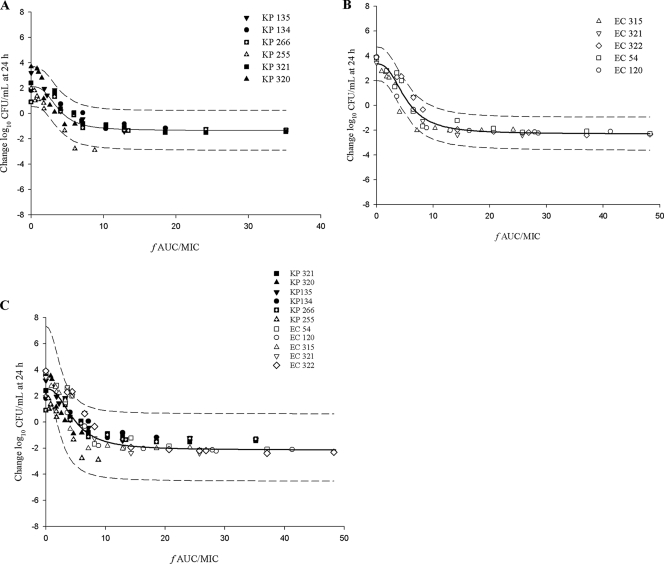
Using the pharmacodynamic index fAUC/MIC, the mean values for EI80, EI50, and bacteriostasis for all isolates tested were 7.25, 4.49, and 5.14, respectively (Table 2). When the respective mean values were separated between the two enterobacteria, the EI80, EI50, and bacteriostasis values were 6.75, 4.01, and 4.60 in K. pneumoniae and 7.83, 5.05, and 5.79 in E. coli. Despite the slight disparity between the two organisms and among all the isolates, these differences seemed inconsequential based on the composite curves (Fig. 2). The composite curve for all 11 bacterial isolates (Fig. 2C) exhibited EI80, EI50, and bacteriostasis values that were similar to the mean bacterial isolate values at 8.4, 4.7, and 5.3, respectively.
FIG. 2.
Composite Emax model and 95% confidence interval of fAUC/MIC as a function of change in bacterial density for diverse isolates. (A) K. pneumoniae. (B) E. coli. (C) Isolates of both types of enterobacteria.
Immunocompetence studies.
The mean initial bacterial loads for isolates EC 315 and KP 320 at 0 h in control mice were 6.98 and 7.18 log10 CFU/ml, respectively. After 24 h, despite a competent immune system, the untreated control mice displayed an increased mean change in bacterial density of 1.3 log10 CFU/ml for both isolates. The mean observed maximal log10 CFU/ml reductions after 24 h in tigecycline-treated animals for isolates EC 315 and KP 320 were 2.41 and 1.91. Compared to the EI80, EI50, and bacteriostasis values in the neutropenic model (4.46, 3.71, 3.88 in EC 315 and 3.27, 2.01, and 3.09 in KP 320), the respective values (expressed as fAUC/MIC) in the immunocompetent animal model were reduced (1.81, 1.00, and 0.72 in EC 315 and 1.70, 1.59, and 1.59 in KP 320). Overall, the data for the immunocompetent animals suggest that tigecycline exposures required to produce sustained antibacterial effects are markedly reduced compared to the neutropenic state (Fig. 3).
FIG. 3.
Two fAUC/MIC exposure response curves comparing immunocompetent (IC) and neutropenic (NP) murine thigh infection models in two isolates. (A) ESBL-producing E. coli isolate EC 315. (B) ESBL-producing K. pneumoniae isolate KP 320.
DISCUSSION
With little on the antibacterial horizon to use against gram-negative organisms and the antibiotic aggregate slowly dwindling, the need for better utilization of available treatment options becomes ever more vital. One way of gaining this insight is by knowing the pharmacodynamic index and the target exposures of the antibiotic to the infecting organism. The aim of this study was to characterize the exposure-response relationship of tigecycline, a relatively newer agent, against two commonly found members of the Enterobacteriaceae, K. pneumoniae and E. coli. By using a neutropenic thigh infection model to determine the pharmacodynamic indices of tigecycline, we found the fAUC/MIC to be the most highly correlated parameter to describe tigecycline efficacy. Similarly, other reports (14, 16) and a human pharmacokinetic/pharmacodynamic analysis study (19) have suggested that the AUC/MIC is most predictive of efficacy because tigecycline has shown time-dependent effects, a long half-life, and a moderately long postantibiotic effect. In contrast, another neutropenic murine thigh infection pharmacodynamic model observed that tigecycline efficacy was best depicted by fT>MIC using a small subset of bacterial isolates with MICs of ≤0.5 μg/ml; interestingly, fT>MIC was the least predictive pharmacodynamic index (r2 = 0.59) in our study. The index discordance could possibly be explained by the observation that Van Ogthrop et al. obtained serum samples only during the pharmacokinetic time points that ranged from 0.25 to 7 h (21). Moreover, this reason may effectively explain why Van Ogthrop et al. found that tigecycline pharmacokinetic parameters were best described by a one-compartment model and that tigecycline exhibited a half-life that was approximately five times shorter than our pharmacokinetic data indicate (5).
In order to better compare and contrast the magnitude of the pharmacodynamic index required for in vivo efficacy with the other exposure-response target data based on studies in humans (19), we first used the composite EI80 and EI50 values derived from our neutropenic animals. Based on the surrogate markers found using the parameter fAUC/MIC, we established that our targets of 8.4 and 4.7 were not consistent with the human pharmacodynamic study (19). In the study by Passarell et al. (19), the total AUC/MIC target of 6.96, when adjusted for a mean protein binding of 79% (16), equated to an approximate fAUC/MIC target of 1.5. In the aforementioned human pharmacodynamic study, target exposures for both clinical and microbiological responses were established with classification and regression tree analysis by applying clinical trial data of cIAI patients infected mostly with E. coli. Although our neutropenic model overpredicted the required exposures relative to the exposure reported in humans, we found that the magnitude in immunocompetent animals appeared more analogous with the nonneutropenic cIAI clinical trial. This was apparent from the mean EI50 and EI80 values from the two enterobacteria isolates (EC 315 and KP 320), which were 1.3 and 1.8, respectively. Consequently, these values represented an approximate 1 (EI50) to 1.5 (EI80) log kill CFU/ml.
Given the standard tigecycline daily dose regimen (100 mg loading dose plus 50 mg every 12 h), if we assumed an AUC in infected humans of 6.37 μg·h/liter (22) and corrected for a mean protein binding of 79% (16), the target range reported of 1.3 to 1.8 in K. pneumoniae or E. coli infections would be readily attainable at a tigecycline MIC of ≤1 μg/ml. Similarly, a tigecycline study utilizing Monte Carlo simulation (12) determined that the likelihood of achieving the apparent pharmacodynamic exposures with the given total AUC/MIC target of 6.96 appeared poor at a MIC beyond 1 μg/ml. In correspondence with these assessments, a systemic review analyzed 10 studies of multidrug-resistant enterobacteria infections. In the review, positive outcomes were reported in a subset of patients (13 of 18) that were treated exclusively with tigecycline (MIC of ≤1 μg/ml) for their initial infection with either multidrug-resistant organism (K. pneumoniae or E. coli) (10). Based on the data from the systemic review and the MIC distribution at 90% in the 2008 U.S. tigecycline surveillance data (E. coli MIC of 0.25 μg/ml and K. pneumoniae MIC of 1 μg/ml) (8), the target exposures reported in our immunocompetent animal model are capable of being achieved in patients infected with either organism.
Interestingly, even though the resistance mechanisms of six ESBL- and one carbapenemase-producing organism(s) were included in our study, these mechanisms appeared to have no bearing on our observed target exposures. Similar results have been observed in other tigecycline in vitro and surveillance studies showing that activity does not appear to be affected by porin loss, ESBL, AmpC, or carbapenemase production or a combination thereof (2, 4, 9). In this context, Vasilev et al. (23) assessed the in vitro and clinical data of multidrug-resistant enterobacteria and evaluated the effectiveness of tigecycline in relation to the microbiological and clinical outcomes in these organisms. In the noncomparative study (23), the microbiological eradication and clinical cure rates at test of cure in the microbiologically evaluable population were 6 of 6 and 5 of 6 in patients infected with K. pneumoniae and 4 of 6 and 4 of 9 in patients infected with E. coli, respectively. In the study, it is important to note that three cIAI patients infected with E. coli were removed from the microbiologically evaluable population when it was discovered that the initial surgical intervention for cIAI was inadequate.
A subinhibitory (sub-MIC) effect was observed in our in vivo study. This characteristic was displayed in all tested isolates with MICs of ≥0.5 μg/ml, whereby a reduction of bacterial density occurred despite an fT>MIC of 0%. Recently, similar effects were observed with an in vitro time kill study (20) that performed a tigecycline concentration escalation against a multidrug-resistant Acinetobacter baumannii isolate at a tigecycline MIC of 1 μg/ml. In the study, concentrations as low as 0.8×the MIC appeared to resemble the maximal bacterial density reductions and limited 24-h regrowth posed by concentrations at 4× the MIC. Although the reasons for sub-MIC effects with tigecycline are unclear, further observation would be needed to elucidate any findings.
Overall, the best pharmacodynamic index to describe tigecycline in treating phenotypically diverse E. coli and K. pneumoniae isolates was fAUC/MIC. While data generated in neutropenic animals defined the pharmacodynamic parameters, the magnitude of the parameter relative to the human pharmacodynamic targets was best defined by the immunocompetent animal model. Although resistance mechanisms did not affect the exposure-response target, the MICs did appear to be the driving force behind tigecycline's efficacy. These data further support the role of the antibiotic tigecycline in the therapy of tigecycline-susceptible multidrug-resistant organisms.
Acknowledgments
We acknowledge the following for their assistance with this study (listed alphabetically): Mary Anne Banevicius, Catharine Bulik, Henry Christensen, Jennifer Hull, David Levitz, Aryun Kim, Debora Santini, Christina Sutherland, Pamela Tessier, and Lindsay Tuttle for their assistance with the conduct of the animal experimentation and the analytical determinations of tigecycline. We also thank Stephen Jenkins and John Quinn for providing us with the test isolates.
This work was supported by a grant from Wyeth Research, Madison, NJ.
Footnotes
Published ahead of print on 13 April 2009.
REFERENCES
- 1.Bradford, P. A., P. J. Petersen, M. Young, C. H. Jones, M. Tischler, and J. O'Connell. 2005. Tigecycline MIC testing by broth dilution requires use of fresh medium or addition of the biocatalytic oxygen-reducing reagent oxyrase to standardize the test method. Antimicrob. Agents Chemother. 49:3903-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castanheira, M., H. S. Sader, L. M. Deshpande, T. R. Fritsche, and R. N. Jones. 2008. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-β-lactamase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 52:570-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical Laboratory Standard Institute. 2007. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. CLSI M7-A7. Wayne, PA.
- 4.Conejo, M. C., J. R. Hernández, and A. Pascual. 2008. Effect of porin loss on the activity of tigecycline against Klebsiella pneumoniae producing extended-spectrum beta-lactamases or plasmid-mediated AmpC-type beta-lactamases. Diagn. Microbiol. Infect. Dis. 61:343-345. [DOI] [PubMed] [Google Scholar]
- 5.Crandon, J. L., M. A. Banevicius, and D. P. Nicolau. 2009. Pharmacodynamics of tigecycline against phenotypically diverse Staphylococcus aureus isolates in a murine thigh model. Antimicrob. Agents Chemother. 53:1165-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curcio, D., F. Fernández, A. Cané, L. Barcelona, and D. Stamboulian. 2008. Indications of a new antibiotic in clinical practice: results of the tigecycline initial use registry. Braz. J. Infect. Dis. 12:198-201. [DOI] [PubMed] [Google Scholar]
- 7.DiPersio, J. R., and M. J. Dowzicky. 2007. Regional variations in multidrug resistance among Enterobacteriaceae in the USA and comparative activity of tigecycline, a new glycylcycline antimicrobial. Int. J. Antimicrob. Agents 29:518-527. [DOI] [PubMed] [Google Scholar]
- 8.Gales, A. C., H. S. Sader, and T. R. Fritsche. 2008. Tigecycline activity tested against 11,808 bacterial pathogens recently collected from US medical centers. Diagn. Microbiol. Infect. Dis. 60:421-427. [DOI] [PubMed] [Google Scholar]
- 9.Hope, R., M. Warner, N. A. C. Potz, E. J. Fagan, D. James, and D. M. Livermore. 2006. Activity of tigecycline against ESBL-producing and AmpC-hyperproducing Enterobacteriaceae from south-east England. J. Antimicrob. Chemother. 58:1312-1314. [DOI] [PubMed] [Google Scholar]
- 10.Kelesidis, T., D. E. Karageorgopoulos, I. Kelesidis, and M. E. Falagas. 2008. Tigecycline for the treatment of multidrug-resistant Enterobacteriaceae: a systematic review of the evidence from microbiological and clinical studies. J. Antimicrob. Chemother. 62:895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ku, Y. H., Y. C. Chuang, and W. L. Yu. 2008. In vitro activity of tigecycline against clinical isolates of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae, Serratia marcescens and Enterobacter cloacae. J. Microbiol. Immunol. Infect. 41:332-336. [PubMed] [Google Scholar]
- 12.Kuti, J. L., M. Dowzicky, and D. P. Nicolau. 2008. A pharmacodynamic simulation to assess tigecycline efficacy for hospital-acquired pneumonia compared with other common intravenous antibiotics. J. Chemother. 20:69-76. [DOI] [PubMed] [Google Scholar]
- 13.Li, C., C. A. Sutherland, C. H. Nightingale, and D. P. Nicolau. 2004. Quantitation of tigecycline, a novel glycylcycline [corrected] by liquid chromatography. J. Chromatogr. B 811:225-229. [DOI] [PubMed] [Google Scholar]
- 14.Meagher, A. K., P. G. Ambrose, T. H. Grasela, and E. J. Ellis-Grosse. 2005. The pharmacokinetic and pharmacodynamic profile of tigecycline. Clin. Infect. Dis. 41:S333-340. [DOI] [PubMed] [Google Scholar]
- 15.Meagher, A. K., J. A. Passarell, B. B. Cirincione, S. A. Van Wart, K. Liolios, T. Babinchak, E. J. Ellis-Grosse, and P. G. Ambrose. 2007. Exposure-response analyses of tigecycline efficacy in patients with complicated skin and skin-structure infections. Antimicrob. Agents Chemother. 51:1939-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muralidharan, G., M. Micalizzi, J. Speth, D. Raible, and S. Troy. 2005. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob. Agents Chemother. 49:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naesens, R., J. P. Ursi, J. Van Schaeren, and A. Jeurissen. 2009. In vitro activity of tigecycline against multidrug-resistant Enterobacteriaceae isolates from a Belgian hospital. Eur. J. Clin. Microbiol. Infect. Dis. 28:381-384. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 18.Nicasio, A. M., J. L. Kuti, and D. P. Nicolau. 2008. The current state of multidrug-resistant gram-negative bacilli in North America. Pharmacotherapy 28:235-249. [DOI] [PubMed] [Google Scholar]
- 19.Passarell, J. A., A. K. Meagher, K. Liolios, B. B. Cirincione, S. A. Van Wart, T. Babinchak, E. J. Ellis-Grosse, and P. G. Ambrose. 2008. Exposure-response analyses of tigecycline efficacy in patients with complicated intra-abdominal infections. Antimicrob. Agents Chemother. 52:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheetz, M. H., C. Qi, J. R. Warren, M. J. Postelnick, T. Zembower, A. Obias, and G. A. Noskin. 2007. In vitro activities of various antimicrobials alone and in combination with tigecycline against carbapenem-intermediate or -resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:1621-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Ogtrop, M. L., D. Andes, T. J. Stamstad, B. Conklin, W. J. Weiss, W. A. Craig, and O. Vesga. 2000. In vivo pharmacodynamic activities of two glycylcyclines (GAR-936 and WAY 152,288) against various gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 44:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Wart, S. A., J. S. Owen, E. A. Ludwig, A. K. Meagher, J. M. Korth-Bradley, and B. B. Cirincione. 2006. Population pharmacokinetics of tigecycline in patients with complicated intra-abdominal or skin and skin structure infections. Antimicrob. Agents Chemother. 50:3701-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasilev, K., G. Reshedko, R. Orasan, M. Sanchez, J. Teras, T. Babinchak, G. Dukart, A. Cooper, N. Dartois, H. Gandjini, R. Orrico, and E. Ellis-Grosse on behalf of the 309 Study Group. 2008. A phase 3, open-label, non-comparative study of tigecycline in the treatment of patients with selected serious infections due to resistant gram-negative organisms including Enterobacter species, Acinetobacter baumannii and Klebsiella pneumoniae. J. Antimicrob. Chemother. 62:i29-40. [DOI] [PubMed] [Google Scholar]
- 24.Woodford, N., J. Zhang, M. Warner, M. E. Kaufmann, J. Matos, A. Macdonald, D. Brudney, D. Sompolinsky, S. Navon-Venezia, and D. M. Livermore. 23 September 2008. Arrival of Klebsiella pneumoniae producing KPC carbapenemase in the United Kingdom. J. Antimicrob. Chemother. 62:1261-1264. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]