Abstract
A population pharmacokinetic model for efavirenz has been developed from therapeutic drug monitoring data in human immunodeficiency virus (HIV)-positive patients by using a nonlinear mixed-effect model. The efavirenz plasma concentrations (n = 375) of 131 patients were analyzed using high-performance liquid chromatography with UV detection. Pharmacokinetic parameters were estimated according to a one-compartment model. The effects of sex, age, total body weight, height, body mass index, and HIV treatment were analyzed. In a subgroup of 32 patients, genetic polymorphisms of the cytochrome P450 2B6 gene (CYP2B6), CYP3A4, and MDR1 were also investigated. Efavirenz oral clearance and the apparent volume of distribution were 9.50 liters/h and 311 liters, respectively. The model included only the effect of CYP2B6 polymorphisms on efavirenz clearance; this covariate reduced the intersubject variability of clearance by about 27%. Patients showing G/T and T/T CYP2B6 polymorphisms exhibited efavirenz clearances that were about 50% and 75% lower than those observed in the patients without these polymorphisms (G/G). Accordingly, to obtain EFV steady-state concentrations within the therapeutic range (1 to 4 mg/liter), it would be advisable to implement a gradual reduction in dose to 400 or 200 mg/day for patients that are intermediate or poor metabolizers, respectively. However, the remaining interindividual variability observed in the pharmacokinetic parameters of the model highlights the need for dose individualization to avoid inadequate exposure to efavirenz and suggests that these recommended doses be used with caution and confirmed by therapeutic drug monitoring and clinical efficacy. The population model can be implemented in pharmacokinetic clinical software for dosage optimization by using the Bayesian approach.
Efavirenz (EFV) is a nonnucleoside reverse transcriptase inhibitor that has demonstrated appropriate efficacy and safety in the treatment of human immunodeficiency virus type 1 (HIV-1) infection in several clinical studies. It is therefore often used in highly active antiretroviral therapy (HAART) implemented for the treatment of both naïve and experienced patients (25).
The metabolism of EFV includes hydroxylation by cytochrome P450 2B6 (CYP2B6) and, to a lesser extent, by CYP3A4 isoenzymes, followed by glucuronidation. Accordingly, the interindividual variability in its clearance, and hence, its steady-state concentrations, will be related to variability in the activity of these cytochromes. These isoenzymes may undergo induction or inhibition, which must be taken into account when coadministering other drugs that act as substrates for these particular isoforms. Autoinduction is also seen for both of them; this is completed in approximately 28 days and elicits a significant decrease in the half-life of EFV (1). Furthermore, the pharmacokinetic (PK) variability may be partly explained in terms of the polymorphisms present in these isoenzymes, as well as the proteins involved in drug transport (19), both of which affect its activity. The MDR1 gene (multidrug resistance) encodes glycoprotein P, which is important in the transport of different substrates, including some antiretroviral drugs, although the influence of polymorphisms in this gene on plasma EFV levels has not been clearly established and remains under debate (16, 48).
The therapeutic drug monitoring (TDM) approach for the optimization and individualization of drug administration is of huge interest in the treatment of HIV because it seems able to increase the efficiency of treatment and, possibly, lower the adverse effects of antiretrovirals. EFV fulfils many of the criteria for TDM, including a possible correlation between its plasma concentrations and the pharmacological effect, as measured by CD4 cell counts and viral load, as well as toxicity (11, 27, 30). This correlation is better with toxicity than with efficacy (12). Additionally, many acceptable analytical assays are currently available for this drug, and its kinetic disposition displays high interpatient and low intrapatient variabilities (11). TDM is also considered a good tool for estimating adherence. Nonadherence is one of the main problems associated with the emergence of resistant viruses (22, 44, 47) and has a high incidence, 33 to 60% (13, 38, 43).
Nevertheless, viral mutations for resistance to HAART, which lead to poorly defined therapeutic ranges, are one of the main problems hampering the TDM of these drugs. In fact, relatively well-defined therapeutic ranges have only been established for naïve patients. Accordingly, the accepted therapeutic range for EFV (1 to 4 mg/liter [3 to 13 μmol/liter] [11, 27]) must be interpreted with caution because it may change depending on patient status (combination regimens with other antiretrovirals, previous exposure to antiretrovirals or not, resistance mutations, etc.). Indeed, some investigators (40) have suggested that the lower limit for the therapeutic range should be raised to 2.3 mg/liter (7 μmol/liter). Despite this limitation, there is great interest in TDM as a rational approach for improving HAART, and many trials have assessed the feasibility of concentration-controlled therapy studies, evaluating its impact and confirming its benefits (2, 8, 14, 18, 43). In fact, the TDM of antiretrovirals has been included as part of the diagnostic set-up for HIV-infected patients in the national guidelines of different countries, such as France, the United Kingdom, and The Netherlands. Recently, Dahri and Ensom (12) advised the use of a previously published decision-making algorithm (15) to determine if TDM is warranted for antiretrovirals. However, further studies in the clinical setting will need to be conducted before such an approach can be recommended for widespread use.
Population PK enables the estimation of PK parameters from sparse data, such those from TDM, and hence, permits information about the population of interest to be collected with a minimum of blood sampling from each patient. This strategy also permits the identification of the sources of variability able to explain interpatient differences, a key factor in this kind of drug therapy; demographics, gene expression, clinical status, and concomitant therapy are the main factors to be investigated. In this sense, population PK modeling has become a valuable tool for identifying and quantifying variability in the exposure to antiretrovirals (5).
Although several population PK analyses of EFV in HIV-positive patients have been published to date (11, 24, 25, 31, 33, 34), only some of them have analyzed the influence of the genotype of CYP2B6 (11, 31), the main enzyme involved in EFV metabolism. The aim of the present study was to develop a population PK model of EFV from sparse data (collected with TDM) for HIV-positive patients by using nonlinear mixed-effect modeling (NONMEM). This methodology was used to evaluate the effects of age; total body weight (TBW); height; sex; body mass index (BMI); and the CYP2B6, CYP3A4, and MDR1 genotypes on the PK profile of EFV.
MATERIALS AND METHODS
Patients and treatment.
The analysis was conducted with data for 131 HIV-infected subjects treated in the outpatient unit of the Pharmacy Service of the University Hospital of Salamanca, Salamanca, Spain, from October 2005 to April 2007.
The inclusion criteria were (i) confirmed HIV infection; (ii) treatment with EFV initiated at least 3 months before patient inclusion in the study (unchanged dosage for at least 1 month); (iii) adherence to the treatment regimen of more than 90%; (iv) age equal to or more than 18 years; and (v) no comedication with known drug inducers.
Adherence was assessed by using dispensing records, the Simplified Medication Adherence Questionnaire (26), and coefficients of variation (CV) of the mean EFV plasma concentration/dose ratio in each patient of less than 30%, according to previously observed intrapatient variability (11).
Most patients received EFV at 600 mg/day, with the exception of some who received 400 or 800 mg/day once daily (mean ± standard deviation, 597.2 ± 33.3 mg/day) as part of their HAART regimen, and all of them had at least two EFV plasma concentrations for analysis. The demographic and clinical characteristics of patients included in the study are shown in Table 1.
TABLE 1.
Demographic and clinical characteristics of the patients included in the study
| Variable | Value (mean ± SD or as indicated) |
|---|---|
| No. of patients (male/female) | 131 (86/45) |
| No. of EFV plasma concns analyzed | 375 |
| Ethnicity (Caucasian/black) | 129/2 |
| Age (yr) | 41.8 ± 9.1 |
| Weight (kg) | 64.9 ± 12.2 |
| Body mass index (kg2/cm) | 22.4 ± 3.4 |
| Daily dose (mg/day) | 597.2 ± 33.3 |
| EFV plasma concn (mg/liter) | 3.30 ± 2.05 |
| No. of plasma concns per patient | 2.9 ± 1.3 |
| Frequency (%) of indicated CYP2B6 | |
| polymorphism in patients genotypeda | |
| G/G | 53.1 |
| G/T | 28.1 |
| T/T | 18.8 |
Thirty-two of 131 patients (24.4%) were genotyped.
The study was subjected to approval by the ethics committee of the University Hospital of Salamanca, and the patients gave written informed consent for genetic testing.
All patients were enrolled in a Pharmaceutical Care Program and were receiving EFV associated with two nucleoside reverse transcriptase inhibitors, and a protease inhibitor, boosted or not with ritonavir, was added in only three cases.
Sampling, analytical assays, and genetic analyses.
Patients were included in a TDM program, and one blood sample was obtained during each visit to the hospital. Plasma samples for measuring drug concentrations were collected at steady-state (more than 4 weeks after the initiation of EFV treatment), usually at the midpoint of the dosage interval. The times after ingestion (mean ± standard deviation, 10.3 ± 1.8 h) were recorded, and a mean of 2.9 samples per patient was obtained (ranging from 2 to 7).
EFV concentrations were assessed quantitatively with high-performance liquid chromatography with UV detection (9). This method was validated over a range of 0.5 to 10 mg/liter using 600 μl of plasma. The recovery of EFV from human plasma was 107.4%. Within- and between-day precision values, expressed as CV, were always <5.7% for all the internal quality controls (0.5, 2.0, and 10.0 mg/liter). The limit of quantification was 0.25 mg/liter, and the specificity of the 21 drugs most used in HIV patients was tested. Our analysis laboratory participates in the International Interlaboratory Quality Control Program for Therapeutic Drug Monitoring in HIV infection (Dutch association for Quality assessment in Therapeutic Drug Monitoring and Clinical Toxicology [KKGT]), and successful results have been obtained.
In order to characterize the genetic polymorphisms of CYP2B6, CYP3A4, and MDR1, the patients’ blood samples were analyzed by using PHARMACHIP, developed by Progenika, whose methodology is based on specific allele oligonucleotide probes imprinted on a glass support (42).
Covariates.
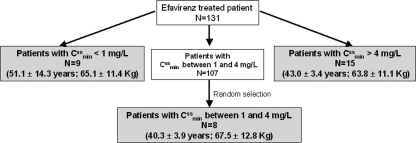
To identify possible correlations between EFV oral clearance (CL/F) and the demographic and treatment characteristics of the patients, the following covariates were collected: sex, age, weight, height, and HIV treatment. In addition, the genetic polymorphisms of CYP2B6, CYP3A4, and MDR1 were analyzed. Because of the high cost of this, only 32 patients of the total group analyzed (n = 131), chosen according the magnitude of their estimated EFV trough steady-state plasma concentrations (Cssmin), were genotyped, including all patients with high (Cssmin > 4 mg/liter; n = 15) and low (Cssmin < 1 mg/liter; n = 9) concentrations and only 8 patients with concentrations within the therapeutic range (Cssmin, 1 to 4 mg/liter), the latter chosen randomly from the 107 remaining patients. Figure 1 shows this selection process and the mean age and weight of the genotyped patients, most of whom were Caucasians. The total number of EFV plasma concentrations obtained for this subset of patients was 158.
FIG. 1.
Patient selection for genetic analysis.
Population PK analysis.
A PK population model was developed by using the NONMEM program (version VI, University of California, San Francisco, CA), which is described elsewhere. A one-compartment open kinetic model with first-order absorption and elimination (specified to NONMEM by the ADVAN2 and TRANS2 routines) was assumed. Thus, the fixed-effect PK parameters estimated directly with the specifications of this model were CL/F and the apparent distribution volume (V/F). The absorption rate constant (ka) was fixed to 0.3 h−1, following Csajka et al. (11). First-order conditional estimation with Laplace approximation was used throughout.
Both additive (θj = θ′ + ηθj) and exponential (θj = θ′exp ηθj) error models were tested to describe interindividual variability, where θj is the estimate for a PK parameter in the jth individual as predicted by the model; θ′ is the population mean of the PK parameter, and ηθj represents the random variable with zero mean and variance ω2. Covariance was also estimated. It should be noted that the first-order method used in this analysis approximates the exponential error model as a proportional error model. The terms for interindividual variability were included only for CL/F and V/F. Additionally, additive (Cij = C′ij + ɛi) and exponential (Cij = C′ijexp ɛi) error models were tested to estimate residual variability, where Cij and C′ij are the observed and predicted EFV concentrations for the jth individual at time i, respectively, and ɛ is the additive error (with zero mean and variance σ2). To elucidate the preliminary relationships between a PK parameter obtained by using a Bayesian maximum a posteriori estimation (POSTHOC option in NOMEM) and covariates, a graphic approach to exploratory data analysis and the stepwise generalized additive model (GAM) implemented in Xpose were used (23). The inclusion of a fixed-effect parameter in the basic model quantifies the relationship between a PK parameter and a covariate and allows it to be known whether that covariate significantly improves the ability of the model to predict the observed concentration-time profile. The objective function value (OFV) difference between two hierarchical models is asymptotically χ2 distributed, with degrees of freedom (df) equal to the difference in the number of parameters between the two models, and it should be at least 3.84 (if df = 1) in order to achieve the desired level of significance of α = 0.05. Other diagnostic criteria for the retention of a covariate in the model were a reduction in unexplained interindividual variability for the associated PK parameter; an improvement in the graphic diagnostic model, evaluated by randomly distributed weighted residuals; a closer relationship between the predicted and observed concentrations; and that the 95% confidence interval (CI), estimated using standard errors (SEs), should not include a zero value. A further criterion was that the percentage estimation errors (EEs) of fixed and random parameters should not be higher than 25 and 50%, respectively (3). The full model thus generated was then subjected to backwards elimination, where each model parameter was fixed to a zero value, using a more stringent criterion of statistical significance (α = 0.01).
Model validation.
The suitability of the final model was evaluated by using pseudoresiduals, a validation approach proposed by Mesnil et al. (28). Monte Carlo simulation conducted with NONMEM was applied to mimic the mean EFV concentrations in adult patients receiving standard doses of EFV (600 mg/day) by assigning the individual characteristics included in the final model (CYP2B6 genotypes) to the simulated population. One thousand random concentrations were generated for each simulated patient, and the pseudoresiduals were computed as described by Comets et al. (10). Statistical analyses were performed with SPSS (version 15, SPSS, Inc., Chicago, IL).
RESULTS
In the basic model, both interindividual and residual variabilities were best described by proportional structures. In this EFV model without covariates, the mean population estimates for CL/F and V/F were 9.22 liters/h and 295.0 liters, and their interindividual variabilities, expressed as CVCL/F and CVV/F, were 50.30%, and 79.75%, respectively. The CV of residual variability was 19.70%.
Graphic exploratory analysis of the relationship between the individual Bayesian CL/F and V/F estimated with NONMEM (POSTHOC option) using the covariates analyzed by GAM revealed that TBW and BMI were weakly correlated with them. Additionally, the patients showing G/T and T/T CYP2B6 polymorphisms exhibited drug CL/F values of around 50% and 75% lower, respectively, than patients without these polymorphisms. These results were confirmed when this discrete covariate—CYP2B6 polymorphisms—was included in the full population model, as well as when the model was developed only for the 32 genotyped patients. According to this exploratory analysis, no other covariate was found to significantly correlate with the individual Bayesian PK parameters. In fact, when age, sex, CYP3A4, MDR1, and HIV treatment were included in the model, the magnitude of the estimates was negligible and the percentages of associated SEs were greater than 100%, indicating the lack of statistical and clinical significance of these covariates. However, the effects of sex; BMI; TBW; height; and CYP2B6, CYP3A4, and MDR1 polymorphisms were analyzed in the different population models assayed.
Table 2 summarizes the statistical analysis of some covariates tested with NONMEM according to this preliminary analysis and shows the differences in the OFV with respect to the basic model and the variations in the interindividual variabilities of CL/F and V/F expressed as CV (%). The influence of TBW and BMI on either CL/F or V/F did not contribute significantly to the goodness of fit, and indeed, the OFV increased with respect to the basic model when they were included in the model. Only the effect of CYP2B6 genotypes on CL/F contributed to a significant decrease in OFV. Thus, the final regression model, whose values are summarized in Table 3, can be defined as follows: CL/F (liters/h) = θ1 × e−θ3×CYP2B6 and V/F (liters) = θ2, where θ1 and θ2 are the estimated coefficients for CL/F and V/F, respectively, and θ3 is the fixed parameter relative to CYP2B6 polymorphisms (which took values 0, 1, or 2 when the patients had wild-type [G/G], heterozygote [G/T], and homozygote [T/T] genotypes, respectively). In this model, the estimate of the CV for interindividual variability in CL/F was 36.47%, versus 50.30% when the covariates were not incorporated into the model (basic model). Regarding V/F, although none of the covariates could be included in the final model, its CVV/F was reduced from 79.75% in the basic model to 55.14% in the final one. Residual variability was decreased by about 25% from that of the basic model. Additionally, scrutiny of the scatterplot of weighted residuals versus predicted concentrations obtained from the final model revealed a significant improvement in its pattern (random distribution) with respect to the basic one, in agreement with the OFV decrease (difference of OFV = 175.438; P < 0.01). Fixed-effect parameters were estimated with a SE of less than 20%. All the random effects had SE values of less than 35%.
TABLE 2.
Summary of the analysis of the influence of the covariates explored in the study of the PK parameters of the model
| Covariates analyzed | OFV (DOFVa) | CVCL/F (%) | CVV/F (%) | σ (%) | Results and comments |
|---|---|---|---|---|---|
| Basic model | 340.477 | 50.30 | 79.75 | 19.70 | Model without covariates |
| Does TBW influence CL/F? | 409.108 (68.631) | 47.64 | 106.77 | 21.33 | No |
| Does BMI influence CL/F? | 366.623 (26.146) | 47.96 | 93.49 | 18.14 | No |
| Does TBW influence V/F? | 360.713 (20.236) | 44.27 | 77.65 | 19.25 | No |
| Does sex influence CL/F? | 382.694 (42.217) | 67.75 | 71.90 | 16.58 | No; unacceptable EE for the fixed parameter associated with the covariate (95% CI includes zero) |
| Does sex influence V/F? | 382.359 (41.882) | 37.02 | 216.33 | 15.62 | No; the parameter associated with the covariate is negligible (0.98) |
| Does CYP2B6 polymorphism influence CL/F? | 165.039 (−175.438) | 36.47 | 55.14 | 14.70 | Yes |
| Does CYP3A4 polymorphism influence CL/F? | 162.376 (−2.663) | 38.08 | 65.04 | 14.63 | No; unacceptable EE for the parameter associated with the covariate (95% CI includes zero) |
| Does MDR1 polymorphism influence CL/F? | 161.375 (−3.664) | 37.81 | 60.99 | 15.26 | No; unacceptable EE for the parameter associated with the covariate (95% CI includes zero) |
DOFV, difference of OFV. TBW, BMI, sex, and CYP2B6 polymorphism covariate DOFVs are the difference from the OFV of the basic model. CYP3A4 and MDR1 polymorphism covariate DOFVs are the difference from the OFV of the CYP2B6 polymorphism model.
TABLE 3.
Parameter estimates and their standard errors for the final population model proposeda
| Parameter | Estimate | SE (%) |
|---|---|---|
| θ1 (liters/h) | 9.50 | 4.06 |
| θ2 (liters) | 311 | 10.76 |
| θ3 | 0.638 | 15.35 |
| CVCL/F (%) | 36.47 | 15.58 |
| CVV/F (%) | 55.14 | 34.74 |
| σ (%) | 14.70 | 15.71 |
Final model: CL/F = θ1 × e−θ3×CYP2B6 and V/F = θ2.
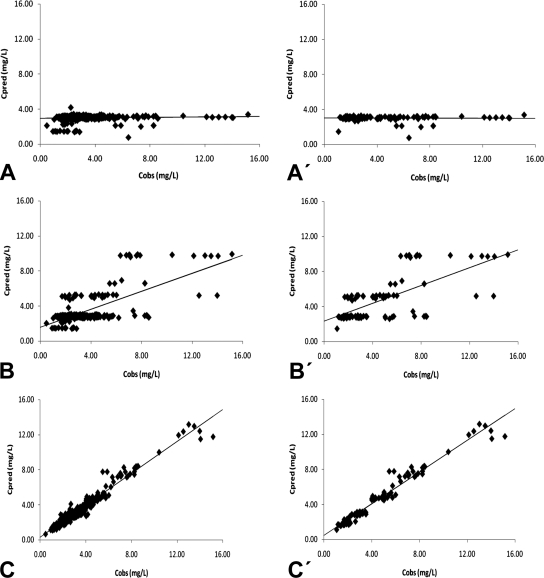
Figure 2 shows the scatterplots of the measured EFV concentrations versus those predicted by the basic and the final models, obtained with the full population (n = 131) and with the genotyped subgroup (n = 32). These plots show the improvement in fit obtained with the final model in both situations, reflected as a tighter and more-random scatter around the identity line. Furthermore, the correlation coefficients of the linear regression between the observed versus fitted concentrations with the final model were 0.690 (full population; P < 0.05) and 0.687 (genotyped population; P < 0.05); i.e., significantly better than the 0.07 and 0.03 calculated when EFV plasma concentrations were estimated with the basic model.
FIG. 2.
Observed (Cobs) versus predicted plasma concentrations of EFV (Cpred) in the whole sample (A, B, and C) and in the genotyped sample (A′, B′, and C′). A, A′, population concentrations predicted with basic model; B, B′, population concentrations predicted with final model; C, C′, individual concentrations predicted with final model. L, liters.
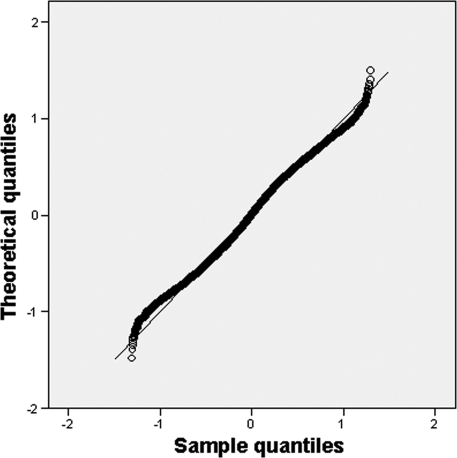
In order to evaluate the final model, pseudoresiduals were computed using 1,000 samples per individual simulated by the Monte Carlo method. The quantile-quantile plot is shown in Fig. 3, which depicts the quantiles of the normalized pseudoresiduals versus the theoretical quantiles from a normal distribution; the assumption of this distribution appears reasonable, since deviations from the identity line (x = y) show minimal departures from the expected distribution. A P value of 0.494 was found, which is higher than the empirical one (0.0037), and hence, the model tested can be considered adequate.
FIG. 3.
Quantile-quantile plot of the pseudoresiduals for the simulated patients versus the uniform distribution: the observed values are plotted against the theoretical quantiles for a uniform distribution over [0,1].
DISCUSSION
The clinical usefulness of patient-specific PK data for antiretrovirals has been evaluated in an effort to develop strategies for monitoring drug exposure in HIV-infected patients (29). The goal of the present study was to investigate the population PK parameters of EFV estimated from TDM data in order to implement them in clinical PK software for dosage optimization.
Although the kinetics of EFV seems to be better described by a two-compartment model (4, 24, 45), in most studies (11, 25, 31, 33, 34), a one-compartment model has been assumed. In view of this, and of the poor design (data from TDM), here a one-compartment model that appears to describe the data adequately was used. Although sampling was only performed at certain discrete and previously defined times (sparse data), CL/F and V/F could be estimated with acceptable SEs.
Although the literature reports very different values for ka (from 0.18 to 1.39 h−1) (31, 33), a ka value of 0.3 h−1 was fixed in the model in agreement with Csajka et al. (11), which is the most consistent study estimating ka, owing to the number of patients and sampling times used. However, being aware of the limitations of fixing this parameter, we tested other models in which ka was fixed at other values found in the literature, but no significant differences in the estimation of CL/F were observed. These results are consistent with those reported by Wade et al. (46), who found that misspecification of ka does not markedly affect the ability to adequately estimate the CL/F, which is the main PK parameter for drug dosage optimization. Meal composition also seems to affect EFV absorption (32), but in the present study, the effect of a high-fat diet could not be taken into account because of the different dietary habits of the patients and the impossibility of establishing a protocol for its analysis. In any case, the effect of diet was minimized since in most patients, drug administration was performed at least 2 h after the evening meal (in agreement with the recommendations of the Pharmaceutical Care Program).
Knowledge of the different factors affecting the PK of a given drug is critical in decisions regarding dosage. TBW and BMI, which are representative indices of body size and which are usually well correlated with drug PK parameters, were not seen to exert any significant influence on the CL/F and V/F of EFV, despite the weak relation observed in the preliminary graphic analysis. In fact, the OFV was significantly increased (>30 units) with respect to that of the basic model when these covariates were included in the model (Table 2). Most PK studies carried out for EFV to date have failed to demonstrate a relationship between TBW and the PK parameters (11, 24, 25, 31, 33), except for a recent publication (41) in which this covariate was included as a factor influencing EFV plasma concentrations. The relatively narrow age range of the group in our study (41.8 ± 9.1 years), of whom most were adults (only four were >65 years old), was probably the reason why age could not be included in the model.
Although both sexes were relatively well represented in our population (86 males/45 females), sex does not appear in the final model either, since the statistical criteria required for its inclusion were not fulfilled. Previously published results for this covariate are contradictory. Thus, whereas some investigators have reported a decrease in the CL/F in women (4, 31), other authors have failed to find such a difference (11, 25, 33); therefore, studies with large numbers of women are required to attempt to establish the true influence of sex, since most studies have included a significantly lower percentage of women than men.
Other studies using the same population approach (NONMEM) have also failed to reveal any significant relationship between demographic covariates (age, sex, and TBW) and the PK parameters of EFV (11, 24, 25, 33). The mean estimated values for CL/F found by those authors ranged from 8.82 to 11.70 liters/h, similar to those observed in our population (9.50 liters/h). Another recent population model developed for black patients with a low number of EFV plasma concentrations analyzed also afforded a similar value of CL/F (9.4 liters/h), although this model included the patient's sex as a covariate (31).
The estimated value for V/F, 311 liters in the proposed model, is similar to the values reported by Pfister et al. (282 liters [33]) and Csajka et al. (252 liters [11]), although other studies have reported widely varying values, ranging from 150 to 421 liters (31, 34). This broad range of values could be explained in terms of a probable degree of uncertainty in the estimation of V/F owing to the nature of the data (sparse data) analyzed in most studies. In fact, in our study, V/F was also estimated from a limited sampling at a single time point; this is why we tried to fix this parameter to the bibliographic values. However, poorer fits were obtained and there was greater uncertainty in CL/F estimation.
It is foreseeable from our results and those of other studies that genetic factors (20, 34) contribute significantly to interindividual variability. Differences in PK due to the CYP2B6 polymorphisms have been reported for several drugs, including EFV, that are mainly metabolized by this isoenzyme (7, 37, 39).
Of the 15 patients with abnormally elevated EFV levels, 12 (80%) had the CYP2B6 G516T polymorphism (6 T/T and 6 G/T) and only 20% did not have it. Although only 32 patients were genotyped (24.4% from the total population studied), which is the main limitation of this study, these findings again support the important effect of the genotypes of this isoenzyme on EFV clearance. Thus, the G/T (intermediate metabolizers) and T/T (poor metabolizers) genotypes modified CL/F by factors of 0.53 (95% CI, 0.44 to 0.65) and 0.28 (95% CI, 0.22 to 0.35), respectively, with respect to the G/G (extensive metabolizers) genotype. Accordingly, CYP2B6 genotypes could partly account for the large interindividual variability in EFV PK and identify individuals at risk of extremely elevated EFV plasma levels.
Other researchers have observed the effect of CYP2B6 genotypes on the CL/F of EFV, but using the same methodology, the quantitative influence of this PK parameter has only been identified by Nyakutira et al. (31) for black patients. This author found ratios of EFV clearance, with respect to those of patients who were extensive metabolizers, of 0.77 (95% CI, 0.46 to 1.1) and 0.42 (95% CI, 0.21 to 0.63) for patients who were intermediate and poor metabolizers, respectively. These values are higher than those found by us, although our mean values lie within the 95% CI proposed by this author. However, the higher clearances estimated in this study could be justified in terms of the use of concomitant medication, including the inducer rifampin (rifampicin), a first-line drug in tuberculosis coinfection, which was not recorded in that study, even though this coinfection affected more than 60% of the patients analyzed. Thus, rifampin was probably present in these patients, since this antibiotic has been shown to elicit increases of more than 30% in EFV clearance because of its induction effect on the CYP2B6 and CYP3A4 enzymes (6).
Since only four patients had the CYP3A4*1B polymorphism, three of them heterozygous (*1/*1B) and one of them homozygous (*1B/*1B), it was not possible to establish a relationship between this polymorphism and PK parameters in the final model. The low rate of the presence of the CYP3A4*1B polymorphism and its minor role in EFV metabolism could account for this. Previous studies aimed at evaluating the effects of CYP3A4*1B, CYP3A5*3, and CYP3A5*6 on EFV PK have also been unable to detect any influence (16, 21).
On the basis of statistical criteria, described in Materials and Methods and shown in Table 2, polymorphisms in MDR1 could not be included in the final model, although a slight increase in EFV CL/F was observed in the preliminary graphic analysis. In this sense, previous studies addressing the relationship between the C3435T MDR1 gene and EFV plasma concentrations have afforded contradictory results (35, 36).
Although the incorporation of the CYP2B6 polymorphism covariate in the final model reduced the interindividual variabilities of the CL/F and V/F parameters, these were still seen to have significant values (36.47% and 55.14%, respectively). It is difficult to compare these findings with those obtained in other, similar studies because the variabilities observed were attributed to different PK parameters of the model. Thus, Nyakutira et al. estimated an interindividual variability for CL/F of 76% (31), significantly higher than that observed in our study and in other publications (11, 24). Nevertheless, all PK variability was attributed to this parameter, and the variability in other PK parameters, such as V/F, was ignored. Another example is the study of Csajka et al. (11), who analyzed sparse data and attributed all the PK variability (54.6%) to bioavailability and ignored the probable variability of the remaining kinetic parameters estimated. Accordingly, although the results for interindividual variability in the PK parameters show some differences, all the above-mentioned studies point to a relatively broad variability in the disposition kinetics of EFV.
The residual variability, expressed as CV, decreased from 19.70% to 14.70% from the basic to the final model, which indicates low variability within patients, an essential prerequisite for TDM. This residual variability was lower than that established by some other authors (11, 24) but similar to that observed by Nyakutira et al. (18% [31]). This low value could be attributed to a closer follow-up of our patients, who were included within a Pharmaceutical Care program that addressed not only their adherence (assessed and considered as an exclusion criterion) but also their drug administration times with respect to the ingestion of food. In light of these results, in the future, a CV of the mean EFV plasma concentration/dose ratio of 20% in each patient could be used for our patients as a new adherence criterion instead of the 30% considered initially; this more-restrictive criterion would possibly contribute to better knowledge about adherence, which is essential to achieve success in antiretroviral therapy.
All parameters of the population model were estimated with acceptable precision, since the SEs were less than 20% and 35% for the fixed-effect and random parameters, respectively.
Although the results have only been compared with those obtained in studies using NONMEM (11, 24, 25, 31, 33), in general, the estimated PK parameter values are in agreement with the results from earlier published studies, regardless of the methodology employed (34). Additionally, the results obtained with Monte Carlo simulation support the suitability of the model. Nevertheless, it would be desirable to perform an external validation study in a new group of patients with similar characteristics, although the absence of consensus concerning the usefulness of TDM of antiretroviral agents makes the collection of these data a slow process.
The final model proposed, although valid, has some limitations that should be noted. (i) In most cases, a standard fixed dose of 600 mg/day was used. (ii) A limited number of EFV concentrations per patient (range, 2 to 7) was used, usually collected at the midpoint of the dosage interval (sparse data from TDM); this is why mixed-effect models were used to adequately characterize the population PK. (iii) The assumption of a one-compartment PK model, because this model is the one most widely used for this drug (11, 25, 31, 33, 34), and the nature of the data prevent the use of more-complex models. (iv) Owing to the high costs of genetic analysis and the fact that this tool has not yet been introduced routinely in clinical practice, only 25% of the patients were genotyped.
According to the PK parameters of the population model and bearing in mind the effects of CYP2B6 genotypes on CL/F, it would be advisable to implement a gradual decrease in the dose to 400 or 200 mg/day for patients who are intermediate or poor metabolizers, respectively, in order to obtain EFV steady-state concentrations close to the mean value of the therapeutic range (1 to 4 mg/liter). These low doses have also been proposed and their efficacy has been demonstrated in another study carried out with Japanese patients (17). However, the remaining interindividual variability in the PK profile observed in this study suggests that these recommended doses should be used with caution and confirmed by TDM and clinical efficacy. This is why we recommend implementing our PK population model with clinical software which, by using Bayesian algorithms, permits the EFV dosage to be optimized with a minimum number of drug plasma levels once adherence has been ensured. Thus, pharmacogenetics and PK combined with TDM should be used to guide EFV dosages. However, since genotyping has not been introduced into routine clinical practice, clinicians should initially be guided by the phenotype assessed through the plasma EFV concentrations obtained in TDM, which may also be used as a selection criterion concerning the patients to be genotyped in order to confirm that the cause of supratherapeutic concentrations is genetic and not attributable to other factors. Additionally, genetic information may prove to be useful for the a priori dosing of drugs whose kinetic profile is governed by isoenzymes and carriers encoded by genes susceptible to polymorphism. Finally, to confirm these preliminary results quantifying the influence of genetic factors, further prospective studies with larger data sets should be carried out.
Acknowledgments
EFV, as a pure compound, was kindly provided by Bristol Myers Squibb Laboratories. This substance was used as both the standard for the validation of the analytical technique and the standard in all quantitative determinations.
This research was supported by funding granted by project FIS PI070714 of the Ministry of Health and Consumption of Spain, in the frame of the National Plan of I+D+I 2004-2007.
Footnotes
Published ahead of print on 11 May 2009.
REFERENCES
- 1.Adkins, J. C., and S. Noble. 1998. Efavirenz. Drugs 56:1055-1064. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, C. S., J. J. Asselin, L. S. Ting, J. S. Montaner, R. S. Hogg, B. Yip, M. V. O'Shaughnessy, and P. R. Harrigan. 2003. Antiretroviral concentrations in untimed plasma samples predict therapy outcome in a population with advanced disease. J. Infect. Dis. 188:541-548. [DOI] [PubMed] [Google Scholar]
- 3.Barret, J. S. 2002. Population pharmacokinetics, p. 315-356. In R. D. Schoenwald (ed.), Pharmacokinetics in drug discovery and development. CRC Press LLC, Boca Raton, FL.
- 4.Barrett, J. S., A. S. Joshi, M. Chai, T. M. Ludden, W. D. Fiske, and H. J. Pieniaszek, Jr. 2002. Population pharmacokinetic meta-analysis with efavirenz. Int. J. Clin. Pharmacol. Ther. 40:507-519. [DOI] [PubMed] [Google Scholar]
- 5.Barrett, J. S., L. Labbé, and M. Pfister. 2005. Application and impact of population pharmacokinetics in the assessment of antiretroviral pharmacotherapy. Clin. Pharmacokinet. 44:591-625. [DOI] [PubMed] [Google Scholar]
- 6.Cabrera, S., M. Cordero, A. Iglesias, M. P. Valverde, A. Domínguez-Gil, and M. J. García. 2008. Efavirenz-rifampicin interaction: therapeutic drug monitoring to efavirenz dosage optimization in HIV/TBC patients. AIDS 22:2549-2551. [DOI] [PubMed] [Google Scholar]
- 7.Christians, U., W. Jacobsen, L. Z. Benet, and A. Lampen. 2002. Mechanisms of clinically relevant drug interactions associated with tacrolimus. Clin. Pharmacokinet. 41:813-851. [DOI] [PubMed] [Google Scholar]
- 8.Clevenbergh, P., S. Mouly, P. Sellier, E. Badsi, J. Cervoni, V. Vincent, H. Trout, and J. F. Bergmann. 2004. Improving HIV infection management using antiretroviral plasma drug levels monitoring: a clinician's point of view. Curr. HIV Res. 2:309-321. [DOI] [PubMed] [Google Scholar]
- 9.Colombo, S., N. Guignard, C. Marzolini, A. Telenti, J. Biollaz, and L. A. Decosterd. 2004. Determination of the new HIV-protease inhibitor atazanavir by liquid chromatography after solid-phase extraction. J. Chromatogr. B 810:25-34. [DOI] [PubMed] [Google Scholar]
- 10.Comets, E., K. Ikeda, P. Hoff, P. Fumoleau, J. Wanders, and Y. Tanigawara. 2003. Comparison of the pharmacokinetics of S-1, an oral anticancer agent, in Western and Japanese patients. J. Pharmacokinet. Pharmacodyn. 30:257-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csajka, C., C. Marzolini, K. Fattinger, L. A. Décosterd, J. Fellay, A. Telenti, J. Biollaz, and T. Buclin. 2003. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin. Pharmacol. Ther. 73:20-30. [DOI] [PubMed] [Google Scholar]
- 12.Dahri, K., and M. H. Ensom. 2007. Efavirenz and nevirapine in HIV-1 infection: is there a role for clinical pharmacokinetic monitoring? Clin. Pharmacokinet. 46:109-132. [DOI] [PubMed] [Google Scholar]
- 13.Dasgupta, A., and P. C. Okhuysen. 2001. Pharmacokinetic and other drug interactions in patients with AIDS. Ther. Drug Monit. 23:591-605. [DOI] [PubMed] [Google Scholar]
- 14.Duong, M., A. Golzi, G. Peytavin, L. Piroth, M. Froidure, M. Grappin, M. Buisson, E. Kohli, P. Chavanet, and H. Portier. 2004. Usefulness of therapeutic drug monitoring of antiretrovirals in routine clinical practice. HIV Clin. Trials 5:216-223. [DOI] [PubMed] [Google Scholar]
- 15.Ensom, M. H., G. A. Davis, C. D. Cropp, and R. J. Ensom. 1998. Clinical pharmacokinetics in the 21st century. Does the evidence support definitive outcomes? Clin. Pharmacokinet. 34:265-279. [DOI] [PubMed] [Google Scholar]
- 16.Fellay, J., C. Marzolini, E. R. Meaden, D. J. Back, T. Buclin, J. P. Chave, L. A. Decosterd, H. Furrer, M. Opravil, G. Pantaleo, D. Retelska, L. Ruiz, A. H. Schinkel, P. Vernazza, C. B. Eap, A. Telenti, and Swiss HIV Cohort Study. 2002. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet 359:30-36. [DOI] [PubMed] [Google Scholar]
- 17.Gatanaga, H., T. Hayashida, K. Tsuchiya, M. Yoshino, T. Kuwahara, H. Tsukada, K. Fujimoto, I. Sato, M. Ueda, M. Horiba, M. Hamaguchi, M. Yamamoto, N. Takata, A. Kimura, T. Koike, F. Gejyo, S. Matsushita, T. Shirasaka, S. Kimura, and S. Oka. 2007. Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6 *6 and *26. Clin. Infect. Dis. 45:1230-1237. [DOI] [PubMed] [Google Scholar]
- 18.González de Requena, D., S. Bonora, S. Garazzino, M. Sciandra, A. D'Avolio, R. Raiteri, R. Marrone, M. Boffito, F. G. De Rosa, A. Sinicco, and G. Di Perri. 2005. Nevirapine plasma exposure affects both durability of viral suppression and selection of nevirapine primary resistance mutations in a clinical setting. Antimicrob. Agents Chemother. 49:3966-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas, D. W. 2005. Will pharmacogenomic discoveries improve HIV therapeutics? Top. HIV Med. 13:90-95. [PubMed] [Google Scholar]
- 20.Haas, D. W., H. J. Ribaudo, R. B. Kim, C. Tierney, G. R. Wilkinson, R. M. Gulick, D. B. Clifford, T. Hulgan, C. Marzolini, and E. P. Acosta. 2004. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS 18:2391-2400. [PubMed] [Google Scholar]
- 21.Haas, D. W., L. M. Smeaton, R. W. Shafer, G. K. Robbins, G. D. Morse, L. Labbe, G. R. Wilkinson, D. B. Clifford, R. T. D'Aquila, V. De Gruttola, R. B. Pollard, T. C. Merigan, M. S. Hirsch, A. L. George, Jr., J. P. Donahue, and R. B. Kim. 2005. Pharmacogenetics of long-term responses to antiretroviral regimens containing efavirenz and/or nelfinavir: an Adult AIDS Clinical Trials Group study. J. Infect. Dis. 192:1931-1942. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch, M., S. Hirsch, B. Conway, R. T. D'Aquila, V. A. Johnson, F. Brun-Vézinet, B. Clotet, L. M. Demeter, S. M. Hammer, D. M. Jacobsen, D. R. Kuritzkes, C. Loveday, J. W. Mellors, S. Vella, D. D. Richman, et al. 1998. Antiretroviral drug resistance testing in adults with HIV infection: implications for clinical management. JAMA 279:1984-1991. [DOI] [PubMed] [Google Scholar]
- 23.Jonsson, E. N., and M. O. Karlsson. 1999. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 58:51-64. [DOI] [PubMed] [Google Scholar]
- 24.Kappelhoff, B. S., A. D. Huitema, Z. Yalvaç, J. M. Prins, J. W. Mulder, P. L. Meenhorst, and J. H. Beijnen. 2005. Population pharmacokinetics of efavirenz in an unselected cohort of HIV-1-infected individuals. Clin. Pharmacokinet. 44:849-861. [DOI] [PubMed] [Google Scholar]
- 25.Kappelhoff, B. S., F. van Leth, T. R. MacGregor, J. Lange, J. H. Beijnen, A. D. Huitema, and 2NN Study Group. 2005. Nevirapine and efavirenz pharmacokinetics and covariate analysis in the 2NN study. Antivir. Ther. 10:145-155. [PubMed] [Google Scholar]
- 26.Knobel, H., J. Alonso, J. L. Casado, J. Collazos, J. González, I. Ruiz, J. M. Kindelan, A. Carmona, J. Juega, A. Ocampo, and GEEMA Study Group. 2002. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: the GEEMA Study. AIDS 16:605-613. [DOI] [PubMed] [Google Scholar]
- 27.Marzolini, C., A. Telenti, L. A. Decosterd, G. Greub, J. Biollaz, and T. Buclin. 2001. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15:71-75. [DOI] [PubMed] [Google Scholar]
- 28.Mesnil, F., F. Mentré, C. Dubruc, J. P. Thénot, and A. Mallet. 1998. Population pharmacokinetic analysis of mizolastine and validation from sparse data on patients using the nonparametric maximum likelihood method. J. Pharmacokinet. Biopharm. 26:133-161. [DOI] [PubMed] [Google Scholar]
- 29.Noormohamed, S. E., W. K. Henry, F. S. Rhame, C. V. Balfour, Jr., and H. H. Fletcher. 1995. Strategies for control of zidovudine concentrations in serum. Antimicrob. Agents Chemother. 39:2792-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Núñez, M., D. González de Requena, L. Gallego, I. Jiménez-Nácher, J. González-Lahoz, and V. Soriano. 2001. Higher efavirenz plasma levels correlate with development of insomnia. J. Acquir. Immune Defic. Syndr. 28:399-400. [DOI] [PubMed] [Google Scholar]
- 31.Nyakutira, C., D. Röshammar, E. Chigutsa, P. Chonzi, M. Ashton, C. Nhachi, and C. Masimirembwa. 2008. High prevalence of the CYP2B6 516G→T(*6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur. J. Clin. Pharmacol. 64:357-365. [DOI] [PubMed] [Google Scholar]
- 32.Perry, C. M., and J. A. Balfour. 1996. Didanosine. An update on its antiviral activity, pharmacokinetic properties and therapeutic efficacy in the management of HIV disease. Drugs 52:928-962. [DOI] [PubMed] [Google Scholar]
- 33.Pfister, M., L. Labbé, S. M. Hammer, J. Mellors, K. K. Bennett, S. Rosenkranz, L. B. Sheiner, and Adult AIDS Clinical Trial Group study 398. 2003. Population pharmacokinetics and pharmacodynamics of efavirenz, nelfinavir, and indinavir: Adult AIDS Clinical Trial Group study 398. Antimicrob. Agents Chemother. 47:130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribaudo, H. J., D. W. Haas, C. Tierney, R. B. Kim, G. R. Wilkinson, R. M. Gulick, D. B. Clifford, C. Marzolini, C. V. Fletcher, K. T. Tashima, D. R. Kuritzkes, E. P. Acosta, and Adult AIDS Clinical Trials Group study. 2006. Pharmacogenetics of plasma efavirenz exposure after treatment discontinuation: an Adult AIDS Clinical Trials Group study. Clin. Infect. Dis. 42:401-407. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Nóvoa, S., P. Barreiro, I. Jiménez-Nácher, and V. Soriano. 2006. Overview of the pharmacogenetics of HIV therapy. Pharmacogenomics J. 6:234-245. [DOI] [PubMed] [Google Scholar]
- 36.Saitoh, A., K. K. Singh, C. A. Powell, T. Fenton, C. V. Fletcher, R. Brundage, S. Starr, and S. A. Spector. 2005. An MDR1-3435 variant is associated with higher plasma nelfinavir levels and more rapid virologic response in HIV-1 infected children. AIDS 19:371-380. [DOI] [PubMed] [Google Scholar]
- 37.Shimada, T., H. Yamazaki, M. Mimura, Y. Inui, and F. P. Guengerich. 1994. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharmacol. Exp. Ther. 270:414-423. [PubMed] [Google Scholar]
- 38.Soldin, O. P., R. J. Elin, and S. J. Soldin. 2003. Therapeutic drug monitoring in human immunodeficiency virus/acquired immunodeficiency syndrome. Quo vadis? Arch. Pathol. Lab. Med. 127:102-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sowunmi, A., T. J. Rashid, O. O. Akinyinka, and A. G. Renwick. 1995. Ethnic differences in nifedipine kinetics: comparisons between Nigerians, Caucasians and South Asians. Br. J. Clin. Pharmacol. 40:489-493. [PMC free article] [PubMed] [Google Scholar]
- 40.Stahle, L., L. Moberg, J. O. Svensson, and A. Sönnerborg. 2004. Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther. Drug Monit. 26:267-270. [DOI] [PubMed] [Google Scholar]
- 41.Stöhr, W., D. Back, D. Dunn, C. Sabin, A. Winston, R. Gilson, D. Pillay, T. Hill, J. Ainsworth, A. Pozniak, C. Leen, L. Bansi, M. Fisher, C. Orkin, J. Anderson, M. Johnson, P. Easterbrook, S. Gibbons, S. Khoo, Liverpool TDM Database, and UK CHIC study. 2008. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir. Ther. 13:675-685. [PubMed] [Google Scholar]
- 42.Tejedor, D., S. Castillo, P. Mozas, E. Jiménez, M. López, M. T. Tejedor, M. Artieda, R. Alonso, P. Mata, L. Simón, A. Martínez, M. Pocoví, and Spanish FH Group. 2005. Reliable low-density DNA array based on allele-specific probes for detection of 118 mutations causing familial hypercholesterolemia. Clin. Chem. 51:1137-1144. [DOI] [PubMed] [Google Scholar]
- 43.Van Heeswijk, R. P. 2002. Critical issues in therapeutic drug monitoring of antiretroviral drugs. Ther. Drug Monit. 24:323-331. [DOI] [PubMed] [Google Scholar]
- 44.Vanhove, G. F., J. M. Schapiro, M. A. Winters, T. C. Merigan, and T. F. Blaschke. 1996. Patient compliance and drug failure in protease inhibitor monotherapy. JAMA 276:1955-1956. [PubMed] [Google Scholar]
- 45.Villani, P., M. B. Regazzi, F. Castelli, P. Viale, C. Torti, E. Seminari, and R. Maserati. 1999. Pharmacokinetics of efavirenz (EFV) alone and in combination therapy with nelfinavir (NFV) in HIV-1 infected patients. Br. J. Clin. Pharmacol. 48:712-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wade, J. R., A. W. Kelman, C. A. Howie, and B. Whiting. 1993. Effect of misspecification of the absorption process on subsequent parameter estimation in population analysis. J. Pharmacokinet. Biopharm. 21:209-222. [DOI] [PubMed] [Google Scholar]
- 47.Wainberg, M. A., and G. Friedland. 1998. Public health implications of antiretroviral therapy and HIV drug resistance. JAMA 279:1977-1983. [DOI] [PubMed] [Google Scholar]
- 48.Winzer, R., P. Langmann, M. Zilly, F. Tollmann, J. Schubert, H. Klinker, and B. Weissbrich. 2003. No influence of the P-glycoprotein genotype (MDR1 C3435T) on plasma levels of lopinavir and efavirenz during antiretroviral treatment. Eur. J. Med. Res. 8:531-534. [PubMed] [Google Scholar]