Abstract
High-throughput screening of 100,000 lead-like compounds led to the identification of nine novel chemical classes of trypanothione reductase (TR) inhibitors worthy of further investigation. Hits from five of these chemical classes have been developed further through different combinations of preliminary structure-activity relationship rate probing and assessment of antiparasitic activity, cytotoxicity, and chemical and in vitro metabolic properties. This has led to the identification of novel TR inhibitor chemotypes that are drug-like and display antiparasitic activity. For one class, a series of analogues have displayed a correlation between TR inhibition and antiparasitic activity. This paper explores the process of identifying, investigating, and evaluating a series of hits from a high-throughput screening campaign.
Parasitic protozoa of the family Trypanosomatidae are the causative agents of many significant tropical diseases, including African trypanosomiasis, Chagas' disease, and leishmaniasis. Human African trypanosomiasis is caused by the two pathogenic parasite subspecies Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense. In the 2004 World Health Report (http://www.who.int/whr/2004/en/index.html), African trypanosomiasis was estimated to cause 48,000 deaths and a disease burden of 1.5 million disability-adjusted life years (DALYs) annually; Chagas' disease, 14,000 deaths and a disease burden of 0.7 million DALYs annually; leishmaniasis, 51,000 deaths and a disease burden of 2.1 million DALYs annually.
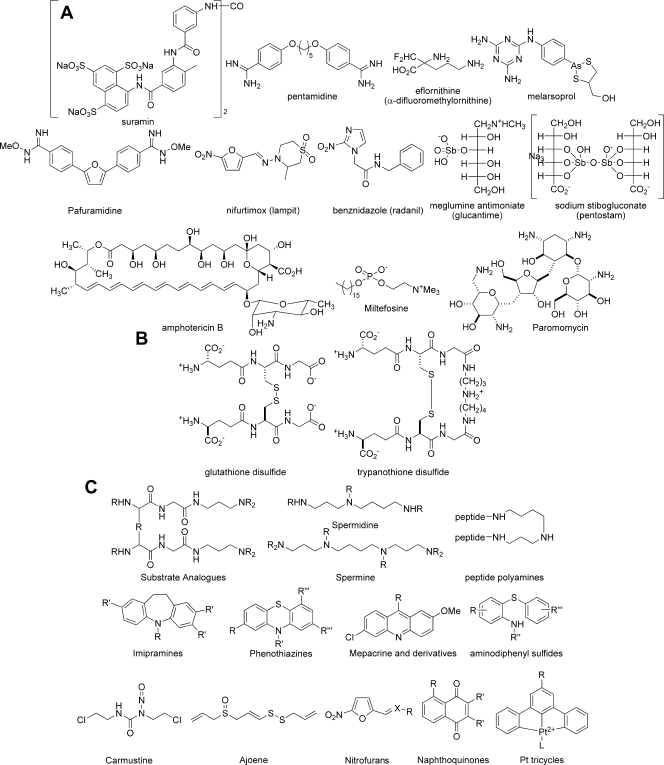
Treatment of African trypanosomiasis is difficult, especially in its advanced stage, when it infects the central nervous system. Only a few effective treatments are available: suramin and pentamidine for the disease in its early stage, and eflornithine and melarsoprol for late-stage treatment (Fig. 1A). These treatments are less than ideal, because they are difficult to administer, toxic, and expensive (14). New treatments in clinical trials include the oral diamidine prodrug pafuramidine (9) and nifurtimox-eflornithine combination therapy (43) (Fig. 1A). The protozoan parasite Trypanosoma cruzi is the causative agent of Chagas' disease, which is found in 18 countries in Latin America. There are currently two significant therapies for Chagas' disease: nifurtimox and benznidazole (Fig. 1A) (21). These drug treatments are ineffective at preventing the development of chronic Chagas' disease and at treating the chronic disease (42), and they induce a number of adverse effects. Leishmaniasis is caused by numerous parasitic protozoan subspecies of the genus Leishmania and is endemic in 88 countries on four continents (www.who.int/tdr/diseases/default.htm). The most common form of leishmaniasis is cutaneous leishmaniasis, which causes multiple self-healing lesions, and the most serious form is visceral leishmaniasis, which is fatal if left untreated. Forms of leishmaniasis other than cutaneous leishmaniasis are very difficult to treat. The most common therapies are the pentavalent antimony drugs meglumine antimoniate and sodium stibogluconate (Fig. 1A), which are difficult to administer and require long treatment regimens. An increase in the incidence of drug resistance has been reported (11), requiring the use of prohibitively expensive drugs, such as liposomal amphotericin B (49) (Fig. 1A). Miltefosine, initially developed as an anticancer agent, is a new therapy against leishmaniasis; it was registered in India in 2002 and in Germany in 2004 as a topical formulation (4) and has recently been licensed in India as an oral treatment (46) (Fig. 1A). Potential problems that could limit its application are its teratogenic effects and high production costs (3). Paromomycin, an aminoglycoside antibiotic, is currently being developed for visceral leishmaniasis in a joint effort by a group of nonprofit organizations (26) (Fig. 1A). Hence, there is an urgent need for the development of new, cost-effective antitrypanosomiasis drugs with minimal side effects.
FIG. 1.
(A) Trypanosomiasis drugs currently on the market. (B) Trypanothione disulfide and glutathione disulfide. (C) Reported TR inhibitors. Pt, platinum.
Trypanosomatids differ from nearly all other eukaryotes and prokaryotes in their specific thiol redox metabolism (17). The intracellular reducing environment is maintained by a unique thiol redox system, where the glutathione-glutathione reductase (GR) couple found in mammalian cells is replaced by the (bis-glutathionyl)spermidine trypanothione-trypanothione reductase (TR) couple. TR, the most thoroughly studied enzyme of the trypanothione redox metabolism (28), is a key enzyme of the parasite antioxidant defense (44), does not occur in the mammalian host, and has been found to be essential for all trypanosomatids currently studied (15, 31, 48). The 3-dimensional structure of TR in free form (24, 30, 54), as well as complexed with substrates (2, 6, 7) and competitive inhibitors (18, 25), has been solved.
TR and human GR have similar catalytic mechanisms; 14 of the 19 amino acid residues close to the binding site are conserved. However, they are specific to their respective disulfide substrates (36) (Fig. 1B). GR has a hydrophilic, positively charged region in its active site that interacts with the glycine carboxylates of glutathione disulfide, while TR has a larger binding site, with a hydrophobic and negatively charged region with which the spermidine moiety of trypanothione disulfide (T[S]2) binds. The absence of TR from the mammalian host and the sensitivity of trypanosomatids to oxidative stress make TR an attractive target for trypanosomiasis therapeutics (24, 29).
Since the identification of TR and its potential application as a target for a new chemotherapeutic approach to trypanosomiasis and leishmaniasis in 1985 (17), a significant number of TR inhibitors have been identified (for reviews, see references 1, 28, 33, 39, 44, and 51). Published TR inhibitors can be loosely classified into five groups: substrate analogues, polyamine and peptide inhibitors, tricyclic compounds, irreversible inhibitors, and subversive substrates (Fig. 1C). Studies of the substrate specificity of TR have found that the active site tolerates noncognate substrate architecture and removal of the disulfide moiety (36), such that a number of nonreducible acyclic and cyclic substrate analogues (13) display TR inhibition. Polyamine- and peptide-based TR inhibitors are generally structurally related to spermine and spermidine and have been shown to be effective competitive inhibitors (32). A series of tricyclic compounds based on the known antidepressant imipramine and the phenothiazine-based neuroleptics, as well as the antiprotozoal drug mepacrine (quinacrine), have been explored. Aminodiphenyl sulfides are structurally related to the phenothiazine-based neuroleptics and have been found to retain TR activity while losing the undesired neuroleptic activity displayed in the tricylic compounds. Dimerization and the introduction of additional hydrophobic side chains have been shown to increase the potency of aminodiphenyl sulfides but also to introduce undesirable ADME (absorption, distribution, metabolism, and excretion) properties (20). A number of irreversible inhibitors, such as carmustine [1,3-bis(2-chloroethyl)-1-nitrosourea], ajoene, and various platinum complexes, have been found to be good inhibitors of TR, yet their activity is often nonselective (18, 40). Trivalent antimony compounds derived from the pentavalent prodrugs Glucantime and Pentostam are also effective inhibitors of TR (12) and play a key role in cell killing (52) (Fig. 1A). Subversive substrates, also known as turncoat inhibitors, such as nitrofurans and naphthoquinones, are good TR inhibitors. However, their redox cycling activity is also nonselective. General trends of TR inhibitors have revealed that the most effective and selective inhibitors are amines that contain hydrophobic groups. Unfortunately, little clear correlation has been found between enzyme inhibition and antiparasitic activity for the TR inhibitors investigated, and prediction of specific absorption rates (SAR) for TR inhibitors has been reported to be difficult. This has been postulated to be due to the extended active site (∼ 22 by 20 by 28 Å), allowing many different binding modes (19), but the matter is also complicated by the possibility of differential transport effects in whole-parasite assays. As a target, TR is also problematic in that competitive inhibition of TR can be reversed by the accumulation of the endogenous substrate, and more than 90% of TR activity must be inhibited before trypanosome growth is stalled (31).
There is still an urgent need for the identification of new, structurally novel TR inhibitors suitable for development into antiparasitic drugs. The objective of this work, therefore, was to identify novel classes of drug-like TR inhibitors with potent and selective trypanocidal activity by high-throughput screening (HTS) of a diverse chemical library.
Here we disclose several new classes of compounds with TR-inhibitory activity at low micromolar concentrations, and we discuss the surprising observations that several of these had unexpected potency and selectivity profiles in whole-cell antiparasitic assays, probably via mechanisms other than those mediated by TR inhibition.
MATERIALS AND METHODS
TR was supplied in a recombinant form (from Trypanosoma cruzi) by Alan Fairlamb's lab at The University of Dundee, Dundee, Scotland. T[S]2 was purchased from Bachem. 5,5′-Dithiobis-(2-nitrobenzoic acid) (DTNB; also known as Ellman's reagent) and NADPH were supplied by Sigma-Aldrich. The assay buffer components HEPES, bovine serum albumin (BSA), and Tween 20 were also purchased from Sigma-Aldrich. EDTA, also used for the buffer, was bought from BDH. Library compounds were preprepared in 50-μl V-bottom MatriCal polypropylene plates. The assay was carried out in 384-well SpectraPlates (clear; flat, square bottom; medium or high binding) from Perkin-Elmer Life Sciences. The plates used as MiniTrak reagent reservoirs were deep-well MatriCal plates and were also purchased from Perkin-Elmer Life Sciences. The MiniTrak IX and Fusion instruments used to automate and read the assays were purchased from Perkin-Elmer Life Sciences.
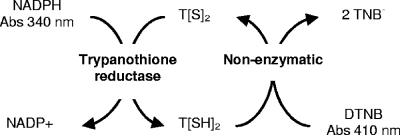
A TR enzyme assay described by Hamilton et al. (22) was adapted for high-throughput TR inhibition studies. The principle of the assay involves the formation of T[SH]2, which is coupled to the reduction of DTNB, forming a yellow thionitrobenzoate (TNB) ion, and the reoxidation of the T[SH]2 product back into the T[S]2 substrate (Fig. 2). This method maintains a constant substrate concentration, saving on reagents, and enables the linearity of the assay for as long as 60 min, with a sensitivity fourfold higher than that of the classical assay method at 340 nm (8). Production of TNB may thus be measured continuously with a microplate absorbance reader. The coupled-enzyme assay was used to measure the kinetics of the TR-catalyzed reaction.
FIG. 2.
Principle of the coupled TR enzyme inhibition assay. Abs, absorbance.
Standard procedures were used to determine kinetic parameters. The apparent Km (Kmapp) and Vmax were determined by a nonlinear regression fit of the data to the Michaelis-Menten equation using XLfit4 (IDBS) software.
The following standard assay protocol was then established. Twenty microliters of a solution containing 20 mU/ml TR (from T. cruzi), 12 μM T[S]2, and 200 μM DTNB in assay buffer (40 mM HEPES, 1 mM EDTA, 0.01% BSA, and 0.05% Tween 20, adjusted to pH 7.5 using KOH) was added to the wells of a 384-well plate. The TR used in the assay was from Alan Fairlamb. Each plate contained a column (column 23) of negative controls that did not contain the TR enzyme. A 0.4-μl portion of compound was then added to the test wells (columns 1 to 22), and 0.4 μl of dimethyl sulfoxide was added to the negative- and positive-control wells (columns 23 and 24). In the primary screen, compounds were tested at a concentration of 25 μM using a Perkin-Elmer Life Sciences MiniTrak IX system with a V&P Scientific pin tool automated liquid-handling system integrated with a Perkin-Elmer Fusion alpha absorbance reader. Compounds were tested in triplicate in either a 5-point titration (fivefold serially diluted, with the highest dose in the assay tested at 100 μM) or an 11-point titration (serially diluted 1 in 3, with the highest dose being 100 μM). The plates were then incubated for at least 15 min at room temperature. Just prior to the reading of each plate, 20 μl of 300 μM NADPH in assay buffer was added to all wells. TR activity was then determined by measuring the increase in the absorbance of the TNB ion at 410 nm kinetically over a 12.5-min period with 2.5-min intervals at room temperature.
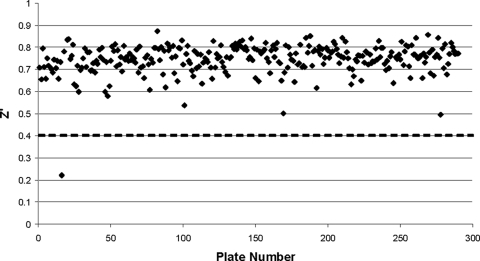
The quality of the assay results was monitored by determination of the Z′ factor for each assay plate (53) (see Fig. 5). The percentage of inhibition of the enzyme by each varying dose of compound was calculated as 100 × {1 − [(x − μ−)/(μ+ − μ−)]}, where x is the slope of enzymatic activity after compound treatment, μ− is the mean slope of enzymatic activity for the negative controls, and μ+ is the mean slope of enzymatic activity for the positive controls.
FIG. 5.
Primary screen quality control. The Z′ parameter was calculated by the method of Zhang et al. (53) from the measurements from the 16 positive and 16 negative control wells located on each assay plate. Assay plates with a Z′ value of <0.4 (dashed line) were rejected, and the assay was repeated.
Fifty percent inhibitory concentrations (IC50s) were then obtained by nonlinear least-squares fitting of these data to the 4-parameter logistic plot equation y = A + {(B − A)/[1 + (C/x)D]}. The activity of all hit compounds was verified by reorder of dry stock. The new batches were retested and were found to have reproducible activity. Their purity was also analyzed by liquid chromatography-mass spectrometry, and all samples were found to be >90% pure. See Holloway et al. (23) for a summary of this screening campaign and details of the discovery of the 2-iminobenzimidazole class of TR inhibitors.
See the supplemental material for details of the in vitro assays conducted at the Swiss Tropical Institute (STI), a discussion of the conjugated-indole and iminobenzimidazole classes of compounds, and chemistry methods for representative synthesis of all compounds.
RESULTS AND DISCUSSION
Development of an assay to identify small-molecule inhibitors of TR.
Our chemical compound library is a collection of approximately 100,000 compounds purchased from commercial vendors. The compounds in this chemical library were selected to provide “lead-like” chemical structures with a guiding philosophy that most successful drug development projects have started from leads that are smaller and more polar than the drug itself. “Lead-like” chemical structures were defined as simple molecular structures that are chemically nonreactive and synthetically accessible and that have “drug-like” properties. The 100,000 compounds in our “lead discovery library” represent a diverse set of molecules as judged by Tanimoto (T) dissimilarity analysis (T ≤ 0.85), and although simple filters based on the Lipinski criteria were not used in the selection process, 89% of the compounds in the library are Lipinski compliant (34) and 81% conform with Oprea's criteria for “lead-likeness” (38).
An automated screening protocol for TR was developed using the photometric assay described by Hamilton et al. (22). An automated TR screening protocol was developed by adaptation of a coupled TR enzyme assay through Ellman's reagent-mediated regeneration of trypanothione. This coupled assay maintains a constant substrate concentration, which not only uses smaller quantities of reagents and increases sensitivity but also eliminates the depletion of the substrate by compounds, which would produce false-positive results.
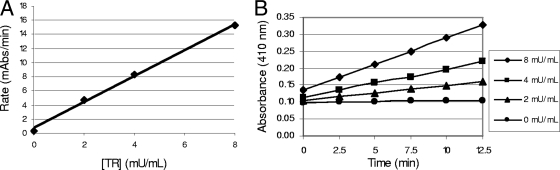
The coupled-enzyme assay was used to measure the kinetics of the TR-catalyzed reaction. It was found that at TR concentrations as high as 8 mU/ml, the rate of absorbance measured was linear for at least 15 min, and a plot of the initial reaction rate (determined from the time course data) versus the enzyme concentration was also linear (Fig. 3). This indicated that the initial rate conditions were being maintained over the time period of the assay.
FIG. 3.
Kinetic characterization of TR. The rate of TNB formation was measured at 412 nm in a 50-μl assay volume at room temperature. Each reaction mixture contained TR at the indicated concentrations, 100 μM DTNB, and 150 μM NADPH in a buffer containing 40 mM HEPES, 1 mM EDTA, 0.01% BSA, and 0.05% Tween 20. Reactions were conducted at 22°C, and reaction progress was followed for 12.5 min. (A) Effect of TR concentration on initial rate; (B) TR reaction time course.
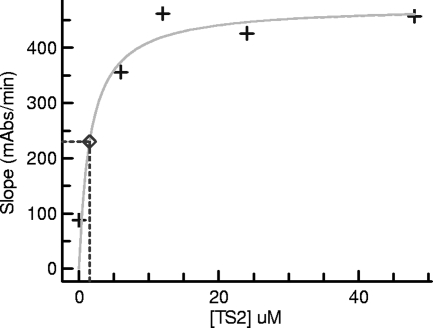
To determine the Kmapp for T[S]2, the rate of TNB product formation catalyzed by TR (8 mU/ml) in a 50-μl reaction mixture over 20 min at room temperature was measured. A Kmapp of 1.5 μM was determined (Fig. 4) for T[S]2 in the presence of 50 μM DTNB and 150 μM NADPH. This result is in reasonable agreement with the Kmapp of 6.5 μM determined previously by Hamilton et al. (22) with 1 mU/ml TR at 27°C.
FIG. 4.
Determination of the Km and Vmax for T[S]2. The Kmapp and Vmax for the TR reaction were determined by fitting the Michaelis-Menten equation to the experimental data using nonlinear regression. The kinetic parameters were determined by measuring the TR-catalyzed rate of TNB formation in 50 μl of a buffer containing 40 mM HEPES, 1 mM EDTA, 0.01% BSA, and 0.05% Tween 20. The assay mixture also contained TR (8 mU/ml) and varying T[S]2 concentrations, and the reaction rate was measured over 20 min at 22°C.
The HTS assay determined the rate of formation of the TNB anion, measured continuously for exactly 12.5 min at 410 nm in a 40-μl assay volume at room temperature. Each reaction mixture contained the following constituents: TR (0.2 mU), T[S]2 (6 μM), DTNB (100 μM), the test compound (25 μM), and NADPH (150 μM). Tween 20 (0.05%) was included in the assay buffer to improve the liquid-handling properties of the assay mixture and to eliminate aggregation as a source of false-positive results (37). The assay was adapted for operation in 384-well microtiter plates and was found to be exceptionally robust and reproducible. The average Z′ and Z values (53) achieved over the entire primary screen were 0.72 and 0.54, respectively (Fig. 5). (See an early publication by this group [23] for more details about the screen.)
An automated screening protocol for GR, based on our TR assay, was also developed. Higher concentrations of GR and glutathione disulfide were required because of their higher Kms. The assay determined the rate of TNB formation, which was measured continuously for 15 min at 410 nm in a 40-μl assay volume at room temperature. Each reaction mixture contained the following constituents: GR (0.5 mU), glutathione disulfide (62.5 μM), DTNB (100 μM), the test compound (25 μM), and NADPH (150 μM).
HTS results.
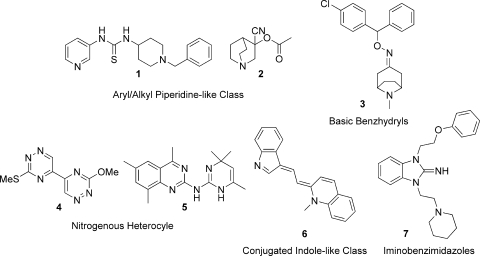
The primary screen of 100,000 compounds identified 120 compounds that inhibited TR activity by 50% or more at a concentration of 25 μM. The potency of the 120 compounds was then determined by assaying compounds as 11-point titrations. These compounds had a potency range (expressed as the IC50) of 1 to 67 μM and contained 12 distinct structural classes. A focus set of compounds was then chosen by considering inhibitory potency, synthetic accessibility, and compound novelty. The focus set consisted of nine structural classes and 44 compounds, including 12 analogues of one structural class, and had a median IC50 of 16 μM. The biological activity of the focus set was further explored by whole-parasite and cytotoxicity assays conducted at the STI (41), and based on their parasiticidal activities, five chemical classes were chosen for further investigation, which included preliminary ADME profiling and SAR probing. These five classes comprised aryl/alkyl piperidines (compounds 1 and 2), basic benzhydryls (compound 3), nitrogenous heterocycles (compounds 4 and 5), conjugated indoles (compound 6), and iminobenzimidazoles (compound 7) (Fig. 6); they are discussed in more detail below.
FIG. 6.
Representatives of the five chemical classes of TR inhibitors chosen for further investigation.
Aryl/alkyl piperidines.
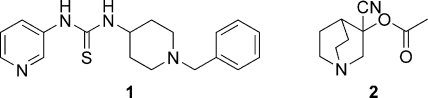
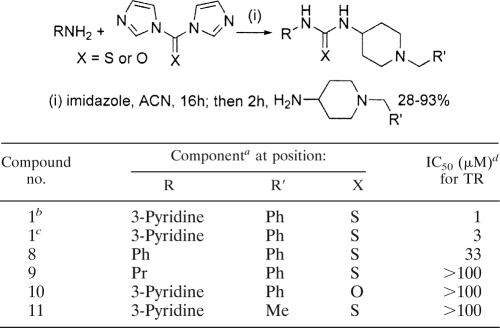
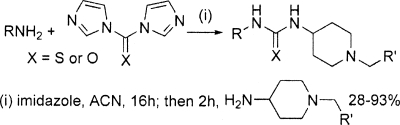
Several compounds that inhibited TR contained an aryl/alkyl piperidine group, and two of these—compounds 1 and 2—were selected for further investigation. Compound 1, an unusual thiourea, stood out as a promising potential lead series based on its TR-inhibitory potency (IC50, 1 μM) and its unique, drug-like structure (Fig. 7). There were no other close representatives of this class either in the lead discovery library or available from commercial vendors. Therefore, a simple and versatile synthetic route was employed to resynthesize the original hit and access a small number of deletion analogues (Table 1). The appropriate 1,1′-diimidazole derivative was utilized to couple two amines through an sp2-hybridized carbon atom (27). Testing of the resynthesized hit (compound 1) by our in-house TR assay gave fairly good agreement with the library compound (IC50s, 3 μM versus 1 μM, respectively [Table 1]). Replacement of the pyridine moiety with a phenyl group (compound 8) led to considerably weaker activity (IC50, 33 μM), and replacement with a propyl group (compound 9) caused the loss of all detectable inhibition. Similarly, the direct urea analogue (compound 10) and a compound in which the phenyl group was replaced with a methyl group (compound 11) had no activity, indicating that the phenyl and sulfur moieties are also essential for activity.
FIG. 7.
Piperidine chemical class (compounds 1 and 2).
TABLE 1.
Synthesis and TR inhibition of the piperidine chemical class
Ph, phenyl group; Pr, propyl group; Me, methyl group.
Library compound.
Synthetic compound.
Isolated from the Brazilian T. cruzi strain X1011.
The whole-cell screening results received from the STI indicated that the strong TR inhibition observed for the parent compound (compound 1) did not translate to strong antiparasitic activity (Table 2). This loss of activity may be due to metabolism in the cell or to transport issues, since significant biomolecular barriers need to be negotiated in order to achieve activity against the T. cruzi amastigote. Additionally, the high concentration of the substrate and the need to inhibit >90% of enzyme activity (31) may preclude manifestation of potent antiparasitic activity. It is also possible that the tautomeric thiol form of compound 1 could react with the TNB anion in the assay through formation of a disulfide link, falsely giving an apparently positive readout for TR inhibition, although the high DTNB concentration (100 μM) makes this seem unlikely. More plausibly, disulfide formation could occur with dihydrotrypanothione (substrate concentration, 6 μM) or enzyme cysteine. The sharply negative SAR suggests that specific engagement with TR may be relevant, which is good from a drug development perspective except for the fact that the reducing environment of the cell in T. cruzi may preclude the possibility of ever observing antiparasitic activity through this mechanism of action. A further complication is the presence of 0.2 mM 2-mercaptoethanol in some antitrypanosome assays, such as the T. brucei assay, which would react with any thiol-reactive compounds, thereby effectively creating a new and different compound. Ultimately, the focus of this research campaign was moved away from this class of compounds.
TABLE 2.
Antiparasitic activities of novel TR inhibitors
| Class | Compound no. | IC50 (μM) for:
|
CC50f | |||||
|---|---|---|---|---|---|---|---|---|
| TR (T. cruzi)a | P. falciparumb | L. donovanic | T. cruzid | T. bruceie | Human GR | |||
| Piperidine | 1 | 2 | 8.6 | >30 | >30 | 15 | >90 | |
| 2 | 52 | 3 | >30 | 19 | 0.9 | 18 | ||
| Basic benzhydryl | 3 | 33 | 8 | >30 | 0.1 | 3 | 35 | |
| Nitrogenous heterocycle | 4 | 27 | 0.2 | 8 | 15 | 5 | 30 | |
| 5 | 17 | 2 | 14 | 6 | 1 | 10 | ||
| 17 | >100 | 4 | 32 | 8 | 27 | 11 | ||
| 20 | >100 | 42 | >64 | 38 | 39 | >64 | ||
| Conjugated indole | 6 | 23 | 0.2 | 3 | 6 | 0.1 | 14 | |
| Iminobenzimidazole | 7 | 9 | 2 | >30 | 19 | 0.6 | >100 | 42 |
Recombinant TR of the Brazilian T. cruzi strain Silvio X10/1.
Strain 3D7, erythrocyclic stages. Chloroquine (IC50, 9.4 nM) was used as a control.
Strain MHOM/ET/67/L82, amastigote stage. Miltefosine (IC50, 0.27 μM) was used as a control.
Tulahuen strain C2C4, amastigote stage. Benznidazole (IC50, 1.8 μM) was used as a control.
Trypanosoma brucei rhodesiense strain STIB 900, bloodstream form. Melarsoprol (IC50, 17 nM) was used as a control.
CC50, 50% cytotoxic concentration, measured against the rat skeletal myoblast line L6 for compounds 1 through 6, 17, and 20 and against the human colon carcinoma cell line HT-29 for compound 7. Podophyllotoxin was used as a control (IC50, 14 nM).
Another compound in the piperidine chemical class that was of some interest is compound 2 (Fig. 7). Although this compound showed very mild TR inhibition (52 μM), it displayed quite potent antiparasitic activity against T. brucei (0.9 μM) (Table 2). This jump in activity may be due to compound 2 acting on a target different from TR. In this context, it is interesting that certain quinuclidines with trypanocidal activity have been reported to act as squalene synthetase inhibitors (10).
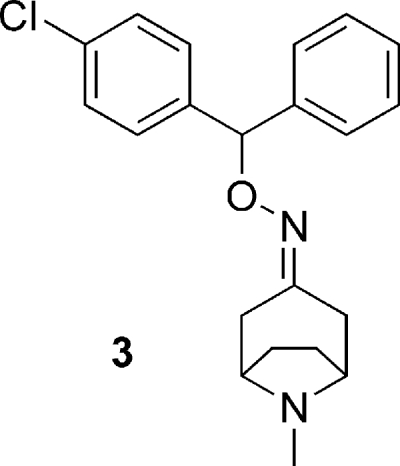
Basic benzyhydryls.
A structurally distinct compound was a basic benzhydryl derivative, compound 3 (Fig. 8). This compound killed T. cruzi both potently and selectively (Table 2), although this killing does not appear to correlate with the relatively weaker TR inhibition. The assessment of early ADME profile and development potential indicated an intermediate rate of degradation in human liver microsomes, consistent with a moderate rate of hepatic clearance in vivo. The microsomal test system provided no evidence for susceptibility to primary glucuronidation (i.e., phase II metabolism) (Table 3). Compound 3 was assessed as having good “drug-like” properties based on a mixture of in silico and experimental methods. In addition, its low polar surface area indicates that compound 3 is a potential candidate for passive permeability through the blood-brain barrier (Table 3). Even though TR seems unlikely to be involved in the trypanocidal activity of this compound, its selective and potent activity against T. cruzi led to the selection of this drug-like compound class for further investigation (J. B. Baell and G. A. Holloway, U.S. patent application 61/092029).
FIG. 8.
Basic benzyhydryl class (compound 3).
TABLE 3.
Physicochemical evaluation and in vitro metabolism in human liver microsomes of the basic benzhydryl and nitrogenous heterocycle classes
The relative loss of the parent compound and the formation of metabolic products following incubation in human liver microsomes were determined by liquid chromatography-mass spectrometry. The concentration of the test compound versus time was fitted to an exponential decay function to determine the first-order rate constant for substrate depletion. This rate constant was used to calculate the in vitro intrinsic clearance (CLint) and the hepatic extraction ratio (EH).
In vitro metabolic stability groupings are as follows: very high, predicted hepatic extraction ratio (EH) of >0.95; high, predicted EH of 0.7 to 0.95; intermediate, predicted EH of 0.3 to 0.7; low, predicted EH of <0.3.
Calculated value.
The two pKa values shown for each form of compound 14 are for the ionization of their pyrimidine and quinazoline sites, respectively.
Measured using a chromatographic elogD method (35).
Measured using nephelometry (5).
Nitrogenous heterocycles.
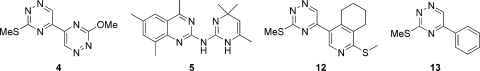
Two nitrogenous heterocycles, a 1,2,4-triazine (compound 4) and a quinazoline (compound 5), were identified for further investigation (Fig. 9). Two distant analogues of the 1,2,4-triazine (compound 4) were purchased (compounds 12 and 13) (Fig. 9) and tested but did not display detectable TR inhibition (Table 4), indicating the importance of the dimer functionality for activity against TR. The 1,2,4-triazine (compound 4) did display antiparasitic activity against T. cruzi (Table 2) but was more potent against T. brucei and Leishmania donovani and was particularly potent against Plasmodium falciparum, without being overtly cytotoxic. As with other classes of TR hits, it seems unlikely that the mechanism of action involves TR.
FIG. 9.
Nitrogenous heterocycle chemical class (compounds 4, 5, 12, and 13).
TABLE 4.
Synthesis and TR inhibition of the nitrogenous heterocycle chemical class
Me, methyl; diMe, dimethyl; Et, ethyl; OEt, ethoxy.
Isolated from the Brazilian Trypanosoma cruzi Silvio strain X10/1.
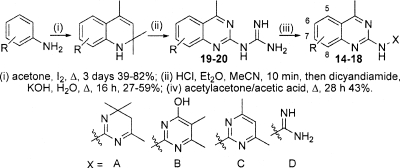
Quinazoline (compound 5) was a mild inhibitor of TR, which corresponded to mild antiparasitic activity in all three whole-cell screens and with a poor selectivity index over nonspecific cytotoxicity (Table 2). The early ADME profile and development potential of the quinazoline series was assessed by the Centre for Drug Candidate Optimisation. The original hit (compound 5) was out of stock from our chemical vendors, and therefore a close dihydropyrimidine-containing analogue (compound 14) (Table 3) was used. The quinazoline (compound 14) was predicted to exhibit a high in vivo hepatic clearance rate. A putative “metabolite” of compound 14 (P+18) was detected in control samples (microsomal samples without NADPH) and the acetonitrile-water stock solutions. This breakdown product was postulated to be caused by the hydrolysis of the imine moiety due to chemical instability. Upon incubation of compound 14 in human liver microsomes with NADPH, multiple putative mono-oxygenated (P+16) and dioxygenated (P+32) metabolites were observed. There was no additional metabolism or change in the metabolite profile when compound 14 was incubated in the presence of both NADPH and UDP-glucuronic acid, suggesting that this compound does not undergo primary glucuronidation (i.e., phase II metabolism). Metabolism issues aside, quinazoline (compound 14) was judged from its physicochemical profile to have good “drug-like” properties based on a mixture of in silico assessment and experimental results, but these results are dependent on the tautomeric form of the compound chosen for evaluation (Table 3). The in silico assessment of the hydrophilic tautomer (Table 3, compound 14, form B) was more consistent with the high experimentally determined solubility at both pH 6.5 and pH 2.0, suggesting that this may be the energetically preferred form, although many factors can influence the tautomeric distribution.
A small number of analogues in the quinazoline class were sourced from the lead discovery library, purchased from chemical vendors, and synthesized in-house (Table 4, selected compounds). Analogues were synthesized by subjection of the relevant aniline to Skraup reaction conditions at a high dilution, which gave the corresponding 1,2-dihydro-2,2,4-trimethylquinoline (16) (Table 4). Conversion to the hydrochloride salts and treatment with dicyanamide gave the substituted 4-methylquinazolyl-2-guanidines (50). Subsequent treatment with acetylacetone gave the corresponding pyrimidine (47); however, nonoptimized treatment of the substituted 4-methylquinazolyl-2-guanidines with mesityl oxide failed to give the corresponding dihydropyrimidine under the conditions specified in the literature (45). Analysis of the activities of the analogues in the quinazoline class against the TR enzyme (Table 4) revealed that some changes in the substitution on the quinazoline ring could be tolerated without significant loss of activity (IC50s, 28 μM for compound 15 and 32 μM for compound 14); however, a significant loss of TR inhibition potency was observed when the dihydropyrimidine moiety (X = A) was replaced by a more stable pyrimidinol/pyrimidinone (X = B) (compound 16), pyrimidine (X = C) (compounds 17 and 18), or guanidine (X = D) (compounds 19 and 20) moiety (Table 4).
The early SAR results suggested that the dihydropyrimidine moiety is essential for TR-inhibitory activity; however, the microsomal metabolism assessment suggests that this is a chemically unstable moiety that is rapidly metabolized. Two of the more stable analogues (compounds 20 and 17) were sent with compound 5 to the STI for testing in the whole-cell parasite assays (Table 2). The guanidine analogue (compound 20) essentially lost antiparasitic activity, but the pyrimidine analogue (compound 17) retained significant activity, indicating that this antiparasitic activity may be occurring through a mode of action different from TR inhibition.
Conjugated-indole and iminobenzimidazole compound classes.
The conjugated-indole class of compounds, exemplified by compound 6, and the iminobenzimidazoles, exemplified by compound 7, exhibited potent activity, in particular against T. brucei (Fig. 6; Table 2). There is an apparent mismatch between the level of this activity and the observed TR-inhibitory activity. Ultimately, these classes were deprioritized, due mainly to concerns about their selectivity profiles. A fuller discussion of these classes, as well as useful predictive ADME data, is contained in the supplemental material.
Significance.
HTS technology was applied for the development and automation of a TR (from Trypanosoma cruzi) assay in order to identify trypanocidal compounds selective for T. cruzi. This effort led to the identification of five unique chemical classes of putative TR inhibitors from the lead discovery library. These compound classes were designated aryl/alkyl piperidines, basic benzyhydryls, nitrogenous heterocycles, conjugated indoles, and iminobenzimidazoles. They were investigated further through a combination of preliminary ADME profiling, SAR probing, and activity profile analysis in the form of antiparasitic activity and cytotoxicity. We found that many of these compounds also displayed potent antiparasitic activity but that this tended to be against T. brucei or P. falciparum rather than T. cruzi. Moreover, the degree of antiparasitic activity was more potent than could be readily explained by the enzyme inhibition assay data. It is difficult or foolhardy to quantify the extrapolation from enzyme inhibition data into expected antiparasitic activity, but as a general rule, the IC50s for the latter would be expected to be several times greater than those for the former, because at the very least, the compound has to contend with transport through membranes and serum protein binding. In the cases reported here, such as that of compound 1, the observed antiparasitic activity was as much as 200-fold greater than the values obtained from the TR inhibition assay. For all these reasons, it is most unlikely that the most potent antiparasitic activities observed are mediated via TR inhibition (8).
However, it should be noted that these outcomes do not in themselves tarnish TR as a valid trypanocidal target. Our HTS library was designed to contain simple, low-molecular-weight compounds that might bind only relatively weakly in the low micromolar range to target proteins but that are also highly optimizable. Screening hits that are on target at the low micromolar level of enzyme inhibition, such as those observed here, may be unlikely to exhibit antiparasitic activity until their enzyme inhibition activity is optimized. Overall, in our experience of many projects at the Walter and Eliza Hall Institute of Medical Research Biotechnology Centre, we have not generally observed target-mediated cell-based activity until molecular potency values reach submicromolar levels. Heavy prioritization in this program was placed on the degree of trypanocidal activity exhibited by nonoptimized screening hits over and above the degree of their TR-inhibitory potency.
We believe that there is room for further discussion around this issue so that target-based drug discovery programs can be constructed optimally to judge target validity as one of the main research outcomes. This would require much earlier medicinal chemistry optimization, prior to exhibition of antiparasitic activity, than is often deemed to be acceptable at present. Without this, the success of the target-based approach may be judged in an unfairly harsh light compared with that of whole-organism screening-based approaches.
An entirely unexpected outcome of this program was that a number of compounds exhibited potent antiparasitic activity. We anticipated that, if anything, nonoptimized screening hits from this campaign would at best manifest as an occasional weakly active compound selective for T. cruzi. Whether the triage of compounds via the TR assay has resulted, for unknown reasons, in an enriched compound set endowed with potent antiparasitic activity independent of TR inhibition remains to be established. Regardless of the mechanism of action, the observation of potent antiparasitic activity is interesting and potentially therapeutically relevant in its own right. We hope that the selectivity profiling, hit-to-lead research, and predictive ADME data that we report here will be of interest and benefit to the neglected-disease community. We note that the basic benzhydryl class has been selected as one of only a few compound classes to undergo lead optimization by the Drugs for Neglected Diseases Initiative-funded Chagas Consortium, which was established in 2008 with key partners in Australia and Brazil.
Supplementary Material
Acknowledgments
This investigation received financial support from the UNICEF/UNDP/World Bank/WHO special program for research and training in tropical diseases (TDR), through WEHI from NHMRC IRIISS grant 361646, and from a Victorian State Government OIS grant.
We acknowledge Ahilan Saravanamuthu for conducting the early TR assay development.
Footnotes
Published ahead of print on 13 April 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Augustyns, K., K. Amssoms, A. Yamani, P. K. Rajan, and A. Haemers. 2001. Trypanothione as a target in the design of antitrypanosomal and antileishmanial agents. Curr. Pharm. Des. 7:1117-1141. [DOI] [PubMed] [Google Scholar]
- 2.Bailey, S., K. Smith, A. H. Fairlamb, and W. N. Hunter. 1993. Substrate interactions between trypanothione reductase and N1-glutathionylspermidine disulphide at 0.28-nm resolution. Eur. J. Biochem. 213:67-75. [DOI] [PubMed] [Google Scholar]
- 3.Berman, J., A. D. M. Bryceson, S. Croft, J. Engel, W. Gutteridge, J. Karbwang, H. Sindermann, J. Soto, S. Sundar, and J. A. Urbina. 2006. Miltefosine: issues to be addressed in the future. Trans. R. Soc. Trop. Med. Hyg. 100:S41-S44. [DOI] [PubMed] [Google Scholar]
- 4.Berman, J. D. 2006. Development of miltefosine for the leishmaniases. Mini Rev. Med. Chem. 6:145-151. [DOI] [PubMed] [Google Scholar]
- 5.Bevan, C. D., and R. S. Lloyd. 2000. A high-throughput screening method for the determination of aqueous drug solubility using laser nephelometry in microtiter plates. Anal. Chem. 72:1781-1787. [DOI] [PubMed] [Google Scholar]
- 6.Bond, C. S., Y. Zhang, M. Berriman, M. L. Cunningham, A. H. Fairlamb, and W. N. Hunter. 1999. Crystal structure of Trypanosoma cruzi trypanothione reductase in complex with trypanothione, and the structure-based discovery of new natural product inhibitors. Structure (London) 7:81-89. [DOI] [PubMed] [Google Scholar]
- 7.Borges, A., M. L. Cunningham, J. Tovar, and A. H. Fairlamb. 1995. Site-directed mutagenesis of the redox-active cysteines of Trypanosoma cruzi trypanothione reductase. Eur. J. Biochem. 228:745-752. [DOI] [PubMed] [Google Scholar]
- 8.Buckner, F. S., C. L. M. J. Verlinde, A. C. la Flamme, and W. C. van Voorhis. 1996. Efficient technique for screening drugs for activity against Trypanosma cruzi using parasites expressing β-galactosidase. Antimicrob. Agents Chemother. 40:2592-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burri, C., S. Bernhard, C. Olson, A. Mpanya Kabeya, J. P. Fina Lubaki, and G. Pohlig. 2007. Phase III trial of pafuramidine maleate (DB289), a novel, oral drug for treatment of first stage sleeping sickness, poster P1.16. R. Soc. Trop. Med. Hyg. Centenary Conf., London, United Kingdom.
- 10.Cammerer, S. B., C. J. S. Jimenez, L. Gros, S. O. Lorente, C. Rodrigues, J. C. F. Rodrigues, A. Caldera, L. M. R. Perez, W. da Souza, M. Kaiser, R. Brun, J. A. Urbina, D. G. Pacanowska, and I. H. Gilbert. 2007. Quinuclidine derivatives as potential antiparasitics. Antimicrob. Agents Chemother. 51:4049-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croft, S. L., S. Sundar, and A. H. Fairlamb. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham, I. 1977. New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J. Protozool. 24:325-329. [DOI] [PubMed] [Google Scholar]
- 13.Czechowicz, J. A., A. K. Wilhelm, M. D. Spalding, A. M. Larson, L. K. Engel, and D. G. Alberg. 2007. The synthesis and inhibitory activity of dethiotrypanothione and analogues against trypanothione reductase. J. Org. Chem. 72:3689-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delespaux, V., and H. P. de Koning. 2007. Drugs and drug resistance in African trypanosomiasis. Drug Resist. Updat. 10:30-50. [DOI] [PubMed] [Google Scholar]
- 15.Dumas, C., M. Ouellette, J. Tovar, M. L. Cunningham, A. H. Fairlamb, S. Tamar, M. Olivier, and B. Papadopoulou. 1997. Disruption of the trypanothione reductase gene of Leishmania decreases its ability to survive oxidative stress in macrophages. EMBO J. 16:2590-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards, J. P., L. Zhi, C. L. F. Pooley, C. M. Tegley, S. J. West, M.-W. Wang, M. M. Gottardis, C. Pathirana, W. T. Schrader, and T. K. Jones. 1998. Preparation, resolution, and biological evaluation of 5-aryl-1,2-dihydro-5H-chromeno[3,4-f]quinolines: potent, orally active, nonsteroidal progesterone receptor agonists. J. Med. Chem. 41:2779-2785. [DOI] [PubMed] [Google Scholar]
- 17.Fairlamb, A. H., P. Blackburn, P. Ulrich, B. T. Chait, and A. Cerami. 1985. Trypanothione: a novel bis(glutathionyl)spermidine cofactor of glutathione reductase in trypanosomatids. Science 227:1485-1487. [DOI] [PubMed] [Google Scholar]
- 18.Gallwitz, H., S. Bonse, A. Martinez-Cruz, I. Schlichting, K. Schumacher, and R. L. Krauth-Siegel. 1999. Ajoene is an inhibitor and subversive substrate of human glutathione reductase and Trypanosoma cruzi trypanothione reductase: crystallographic, kinetic, and spectroscopic studies. J. Med. Chem. 42:364-372. [DOI] [PubMed] [Google Scholar]
- 19.Garforth, J., H. Yin, J. H. McKie, K. T. Douglas, and A. H. Fairlamb. 1997. Rational design of selective ligands for trypanothione reductase from Trypanosoma cruzi. Structural effects on the inhibition by dibenzazepines based on imipramine. J. Enzyme Inhib. 12:161-173. [DOI] [PubMed] [Google Scholar]
- 20.Girault, S., E. Davioud-Charvet, L. Maes, J.-F. Dubremetz, M.-A. Debreu, V. Landry, and C. Sergheraert. 2001. Potent and specific inhibitors of trypanothione reductase from Trypanosoma cruzi: bis(2-aminodiphenylsulfides) for fluorescent labeling studies. Bioorg. Med. Chem. 9:837-846. [DOI] [PubMed] [Google Scholar]
- 21.Guedes, P. M. M., J. L. R. Fietto, M. Lana, and M. T. Bahia. 2006. Advances in Chagas disease chemotherapy. Anti-Infect. Agents Med. Chem. 5:175-186. [Google Scholar]
- 22.Hamilton, C. J., A. Saravanamuthu, I. M. Eggleston, and A. H. Fairlamb. 2003. Ellman's-reagent-mediated regeneration of trypanothione in situ: substrate-economical microplate and time-dependent inhibition assays for trypanothione reductase. Biochem. J. 369:529-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holloway, G. A., J. B. Baell, A. H. Fairlamb, P. M. Novello, J. P. Parisot, J. Richardson, K. G. Watson, and I. P. Street. 2007. Discovery of 2-iminobenzimidazoles as a new class of trypanothione reductase inhibitors by high-throughput chemical screening. Bioorg. Med. Chem. Lett. 17:1422-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter, W. N., S. Bailey, J. Habash, S. J. Harrop, J. R. Helliwell, T. Aboagye-Kwarteng, K. Smith, and A. H. Fairlamb. 1992. Active site of trypanothione reductase. A target for rational drug design. J. Mol. Biol. 227:322-333. [DOI] [PubMed] [Google Scholar]
- 25.Jacoby, E. M., I. Schlichting, C. B. Lantwin, W. Kabsch, and R. L. Krauth-Siegel. 1996. Crystal structure of the Trypanosoma cruzi trypanothione reductase mepacrine complex. Proteins 24:73-80. [DOI] [PubMed] [Google Scholar]
- 26.Klempner, M. S., T. R. Unnasch, and L. T. Hu. 2007. Taking a bite out of vector-transmitted infectious diseases. N. Engl. J. Med. 356:2567-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kling, A., G. Backfisch, J. Delzer, H. Geneste, C. Graef, W. Hornberger, U. E. W. Lange, A. Lauterbach, W. Seitz, and T. Subkowski. 2003. Design and synthesis of 1,5- and 2,5-substituted tetrahydrobenzazepinones as novel potent and selective integrin αvβ3 antagonists. Bioorg. Med. Chem. 11:1319-1341. [DOI] [PubMed] [Google Scholar]
- 28.Krauth-Siegel, R. L., H. Bauer, and R. H. Schirmer. 2005. Dithiol proteins as guardians of the intracellular redox milieu in parasites: old and new drug targets in trypanosomes and malaria-causing plasmodia. Angew. Chem. Int. Ed. Engl. 44:690-715. [DOI] [PubMed] [Google Scholar]
- 29.Krauth-Siegel, R. L., S. K. Meiering, and H. Schmidt. 2003. The parasite-specific trypanothione metabolism of Trypanosoma and Leishmania. Biol. Chem. 384:539-549. [DOI] [PubMed] [Google Scholar]
- 30.Krauth-Siegel, R. L., C. Sticherling, I. Jöst, C. T. Walsh, E. F. Pai, W. Kabsch, and C. B. Lantwin. 1993. Crystallization and preliminary crystallographic analysis of trypanothione reductase from Trypanosoma cruzi, the causative agent of Chagas' disease. FEBS Lett. 317:105-108. [DOI] [PubMed] [Google Scholar]
- 31.Krieger, S., W. Schwarz, M. R. Ariyanayagam, A. H. Fairlamb, R. L. Krauth-Siegel, and C. Clayton. 2000. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Mol. Microbiol. 35:542-552. [DOI] [PubMed] [Google Scholar]
- 32.Li, Z., M. W. Fennie, B. Ganem, M. T. Hancock, M. Kobaslija, D. Rattendi, C. J. Bacchi, and M. C. O'Sullivan. 2001. Polyamines with N-(3-phenylpropyl) substituents are effective competitive inhibitors of trypanothione reductase and trypanocidal agents. Bioorg. Med. Chem. Lett. 11:251-254. [DOI] [PubMed] [Google Scholar]
- 33.Liñares, G. E. G., E. L. Ravaschino, and J. B. Rodriguez. 2006. Progresses in the field of drug design to combat tropical protozoan parasitic diseases. Curr. Med. Chem. 13:335-360. [DOI] [PubMed] [Google Scholar]
- 34.Lipinski, C. A., F. Lombardo, B. W. Dominy, and P. J. Feeney. 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46:3-26. [DOI] [PubMed] [Google Scholar]
- 35.Lombardo, F., M. Shalaeva, K. A. Tupper, and F. Gao. 2001. ElogDoct: a tool for lipophilicity determination in drug discovery. 2. Basic and neutral compounds. J. Med. Chem. 44:2490-2497. [DOI] [PubMed] [Google Scholar]
- 36.Marsh, I. R., and M. Bradley. 1997. Substrate specificity of trypanothione reductase. Eur. J. Biochem. 243:690-694. [DOI] [PubMed] [Google Scholar]
- 37.McGovern, S. L., E. Caselli, N. Grigorieff, and B. K. Shoichet. 2002. A common mechanism underlying promiscuous inhibitors from virtual high-throughput screening. J. Med. Chem. 45:1712-1722. [DOI] [PubMed] [Google Scholar]
- 38.Oprea, T. I., A. M. Davis, S. J. Teague, and P. D. Leeson. 2001. Is there a difference between leads and drugs? A historical perspective. J. Chem. Infect. Comput. Sci. 41:1308-1315. [DOI] [PubMed] [Google Scholar]
- 39.O'Sullivan, M. C. 2005. The battle against trypanosomiasis and leishmaniasis: metal-based and natural product inhibitors of trypanothione reductase. Curr. Med. Chem. Anti-Infect. Agents 4:355-378. [Google Scholar]
- 40.Otero, L., M. Vieites, L. Boiani, A. Denicola, C. Rigol, L. Opazo, C. Olea-Azar, J. D. Maya, A. Morello, R. L. Krauth-Siegel, O. E. Piro, E. Castellano, M. Gonzalez, D. Gambino, and H. Cerecetto. 2006. Novel antitrypanosomal agents based on palladium nitrofurylthiosemicarbazone complexes: DNA and redox metabolism as potential therapeutic targets. J. Med. Chem. 49:3322-3331. [DOI] [PubMed] [Google Scholar]
- 41.Parveen, S., M. O. F. Khan, S. E. Austin, S. L. Croft, V. Yardley, P. Rock, and K. T. Douglas. 2005. Antitrypanosomal, antileishmanial, and antimalarial activities of quaternary arylalkylammonium 2-amino-4-chlorophenyl phenyl sulfides, a new class of trypanothione reductase inhibitor, and of N-acyl derivatives of 2-amino-4-chlorophenyl phenyl sulfide. J. Med. Chem. 48:8087-8097. [DOI] [PubMed] [Google Scholar]
- 42.Paulino, M., F. Iribarne, M. Dubin, S. Aguilera-Morales, O. Tapia, and A. O. M. Stoppani. 2005. The chemotherapy of Chagas' disease: an overview. Mini Rev. Med. Chem. 5:499-519. [DOI] [PubMed] [Google Scholar]
- 43.Priotto, G., S. Kasparian, D. Ngouama, S. Ghorashian, U. Arnold, S. Ghabri, and U. Karunakara. 2007. Nifurtimox-eflornithine combination therapy for second-stage Trypanosoma brucei gambiense sleeping sickness: a randomized clinical trial in Congo. Clin. Infect. Dis. 45:1435-1442. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt, A., and R. Krauth-Siegel. 2002. Enzymes of the trypanothione metabolism as targets for antitrypanosomal drug development. Curr. Top. Med. Chem. 2:1239-1259. [DOI] [PubMed] [Google Scholar]
- 45.Shikhaliev, K. S., A. V. Falaleev, G. I. Ermolova, and A. S. Solov'ev. 2002. 2-Quinazolylguanidines in heterocyclization reactions. 2. Condensation with α,β-unsaturated carbonyl compounds. Chem. Heterocycl. Compounds 38:210-212. [Google Scholar]
- 46.Sindermann, H., and J. Engel. 2006. Development of miltefosine as an oral treatment for leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 100:S17-S20. [DOI] [PubMed] [Google Scholar]
- 47.Söntjens, S. H. M., J. T. Meijer, H. Kooijman, A. L. Spek, M. H. P. van Genderen, R. P. Sijbesma, and E. W. Meijer. 2001. A multiple hydrogen-bond scaffold based on dipyrimidin-2-ylamine. Org. Lett. 3:3887-3889. [DOI] [PubMed] [Google Scholar]
- 48.Tovar, J., S. Wilkinson, J. C. Mottram, and A. H. Fairlamb. 1998. Evidence that trypanothione reductase is an essential enzyme in Leishmania by targeted replacement of the tryA gene locus. Mol. Microbiol. 29:653-660. [DOI] [PubMed] [Google Scholar]
- 49.Vyas, S. P., and S. Gupta. 2006. Optimizing efficacy of amphotericin B through nanomodification. Int. J. Nanomedicine 1:417-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webb, T. R., D. Lvovskiy, S.-A. Kim, X. Ji, N. Melman, J. Linden, and K. A. Jacobson. 2003. Quinazolines as adenosine receptor antagonists: SAR and selectivity for A2B receptors. Bioorg. Med. Chem. 11:77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Werbovetz, K. A. 2000. Target-based drug discovery for malaria, leishmaniasis, and trypanosomiasis. Curr. Med. Chem. 7:835-860. [DOI] [PubMed] [Google Scholar]
- 52.Wyllie, S., M. L. Cunningham, and A. H. Fairlamb. 2004. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J. Biol. Chem. 279:39925-39932. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, J.-H., T. D. Y. Chung, and K. R. Oldenburg. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays J. Biomol. Screen. 4:67-73. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, Y., C. S. Bond, S. Bailey, M. L. Cunningham, A. H. Fairlamb, and W. N. Hunter. 1996. The crystal structure of trypanothione reductase from the human pathogen Trypanosoma cruzi at 2.3 Å resolution. Protein Sci. 5:52-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.