Abstract
Vancomycin and daptomycin MIC results for 1,800 randomly selected oxacillin (methicillin [meticillin])-resistant Staphylococcus aureus (MRSA) bloodstream isolates from nine U.S. hospitals (collected from 2002 to 2006) were determined by a reference broth microdilution (BMD) method using frozen-form panels with precise incremental dilutions and by the Etest technique. The Etest provided vancomycin and daptomycin MIC results that were consistently higher (0.5 to 1.5 log2 dilution steps) than those provided by the reference BMD method. The dominant MRSA population (91.2% of MRSA isolates) would be categorized as vancomycin nonsusceptible by the MIC results from the Etest method if the susceptibility breakpoint was adjusted downward to ≤1 μg/ml, as suggested by clinical outcome studies.
Vancomycin is still used extensively for the treatment of oxacillin (methicillin [meticillin])-resistant Staphylococcus aureus (MRSA) bacteremia, as well as other less serious MRSA infections. However, vancomycin treatment failure is not uncommon, even when the MRSA strains are fully susceptible to vancomycin according to the criterion (breakpoint MIC, ≤2 μg/ml) used by the Clinical and Laboratory Standards Institute (CLSI). A reduction in the efficacy of vancomycin against MRSA strains for which vancomycin MICs are elevated (to 1 to 2 μg/ml) has been widely reported, suggesting that modest elevations in MICs may explain some suboptimal clinical outcomes (10, 13).
The vast majority of clinical laboratories use automated systems and a distinct minority use the disk diffusion method as the routine susceptibility testing technique. However, the disk diffusion method and some automated systems do not accurately detect vancomycin-intermediate S. aureus (7). In addition, there have been a growing number of reports showing discrepancies between in vitro susceptibility test results for vancomycin and clinical outcomes of MRSA infections treated with this glycopeptide (6, 11). Thus, many laboratories are being requested to assess exact MICs by reference or alternative methods, such as that of Etest (AB Biodisk, Solna, Sweden).
Although daptomycin remains very active against S. aureus, an association between reduced susceptibility to vancomycin and reduced susceptibility to daptomycin has been reported by some investigators (1). S. aureus isolates classified as daptomycin nonsusceptible according to MICs (≥2 μg/ml) are still rare, and no definitive resistance mechanism has been identified. However, genetic mutations and increases in daptomycin MICs after prolonged vancomycin and/or daptomycin treatment of infections associated with septic arthritis, osteomyelitis, septic thrombophlebitis, and endocarditis, especially in the presence of intravenous catheters and other prosthetic devices, have been reported previously (1). Thus, microbiology laboratories may be required to perform proper daptomycin susceptibility testing. Since a daptomycin disk diffusion test has not been developed, daptomycin susceptibility has to be evaluated by reference methods (2, 3) or the Etest method. In the present study, we evaluated the correlation between vancomycin and daptomycin MICs obtained by the Etest technique and the CLSI reference broth microdilution (BMD) method.
(This study was presented in part at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy-46th Infectious Diseases Society of America meeting, Washington, DC, 25 to 28 October 2008.)
A total of 1,800 MRSA strains from nine hospitals, one in each of the U.S. census regions, were tested. Each medical center contributed 200 MRSA strains collected from patients with bloodstream infections from 2002 through 2006 (target, 40 strains per year per center). The participant centers were as follows: Tufts Medical Center, Boston, MA; New York Hospital Queens, New York, NY; Ochsner Clinic Foundation, New Orleans, LA; University of Colorado Denver, Aurora, CO; University of Nebraska Medical Center, Omaha, NE; University of Washington, Seattle, WA; University of Alabama at Birmingham, Birmingham, AL; Wayne State University, Detroit, MI; and the Medical University of South Carolina, Charleston, SC.
MICs of daptomycin and vancomycin were determined by the reference BMD method (using frozen-form panels), with the appropriate medium variation (the addition of 50 mg/liter of calcium) for testing daptomycin (2). Thirty-six precise incremental dilutions of vancomycin between 64 and 0.06 μg/ml (64, 32, 16, 8, 4, 3, 2.5, 2, 1.875, 1.75, 1.625, 1.5, 1.375, 1.25, 1.125, 1, 0.938, 0.875, 0.813, 0.75, 0.688, 0.625, 0.563, 0.5, 0.469, 0.438, 0.406, 0.375, 0.344, 0.313, 0.281, 0.25, 0.188, 0.12, 0.094, and 0.06 μg/ml) were tested, and 20 dilutions of daptomycin between 16 and 0.06 μg/ml (16, 8, 4, 2, 1.5, 1, 0.875, 0.75, 0.625, 0.5, 0.438, 0.375, 0.313, 0.25, 0.219, 0.188, 0.156, 0.12, 0.094, and 0.06 μg/ml) were tested. Isolates were also evaluated by Etest for susceptibilities to daptomycin and vancomycin according to the recommendations of the Etest manufacturer (AB Biodisk). To compare the results obtained with the BMD method and those obtained with the Etest technique, BMD results were rounded up to the next dilution provided for the Etest method. Resistance to oxacillin was confirmed by the reference BMD method (dilution range tested, 0.5 to 4 μg/ml) and cefoxitin susceptibilities tested by disk diffusion (2, 3). Quality control strains S. aureus ATCC 25923 and Enterococcus faecalis ATCC 29212 were evaluated concurrently with every set of tests.
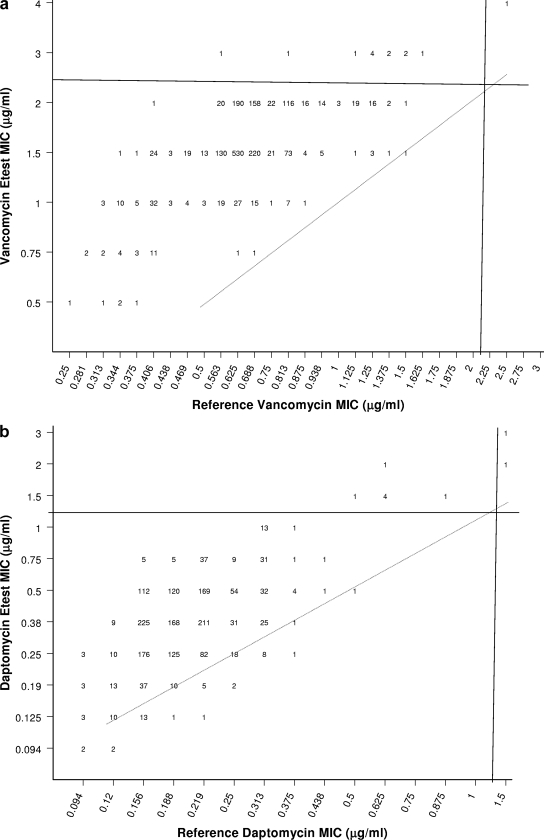
MICs of vancomycin obtained by the Etest method were consistently higher (+0.5 to 1.5 log2 dilutions) than those obtained by the BMD method (Fig. 1a). For strains from all centers combined, the overall vancomycin MIC mode determined by the BMD method was 0.75 μg/ml (corresponding to 75.3% of values, including those rounded up from measured 0.563-, 0.625-, and 0.688-μg/ml MICs) and 96.9% of strains exhibited vancomycin MIC results of ≤1 μg/ml. In contrast, when tested by the Etest, MICs for 58.3 and 32.1% of MRSA strains were 1.5 and 2 μg/ml, respectively (Fig. 1a).
FIG. 1.
Scattergrams showing the correlations between vancomycin (a) and daptomycin (b) MICs obtained by the BMD method and the Etest. Solid lines indicate CLSI susceptibility breakpoints, and broken lines indicate complete agreement between results from the two methods.
For all strains except one, the Etest yielded higher vancomycin MICs than the reference method. Among 1,628 strains for which the Etest MIC was 1.5 μg/ml (1,050 strains) or 2 μg/ml (578 strains), only 44 (2.7%) had BMD MIC results of >1 μg/ml. Furthermore, among 13 strains considered to be nonsusceptible (intermediate) to vancomycin (MIC, 3 or 4 μg/ml) according to the Etest results, only 1 had a vancomycin MIC result of >2 μg/ml by the reference method, demonstrating a positive predictive value of only 7.7%.
Etest MICs of daptomycin were also slightly elevated (+0.5 to 1 log2 dilution) compared to BMD MICs (Fig. 1b). When tested by the BMD method, the daptomycin MIC mode was 0.19 μg/ml (corresponding to 55.4% of values, including MICs of 0.156 and 0.188 μg/ml) and 92.9% of strains exhibited daptomycin MIC results of ≤0.25 μg/ml. In contrast, when tested by the Etest, the MIC mode was 0.38 μg/ml (corresponding to 37.2% of values), which is 1 doubling dilution higher than that obtained by the BMD method. Among nine strains considered to be daptomycin nonsusceptible according to the Etest results, only two had a MIC result of >1 μg/ml when tested by the reference BMD method, demonstrating a positive predictive value of 22.2% (Fig. 1b).
Although the emergence of vancomycin-intermediate S. aureus strains and that of vancomycin-resistant S. aureus strains are reasons for concern, these organisms still are extremely rare (12). Nevertheless, a number of studies have demonstrated increased clinical failure with MRSA isolates for which vancomycin MICs are increased (>1 μg/ml) but still within the CLSI-defined susceptibility range (≤2 μg/ml) (3, 11, 13). In addition, there are reports of the increase of vancomycin MICs over time (designated “MIC creep”) at individual institutions, although this trend has not been validated by large, multi-institutional studies (14). Thus, it is becoming clear to physicians that an “S” result for vancomycin is not sufficient to appropriately guide therapy and that the clinical laboratory should provide accurate and reliable MICs.
Since most clinical laboratories use automated systems to perform susceptibility testing and these systems do not provide a precise vancomycin MIC, many laboratories are using alternative methods for testing vancomycin in selected cases (6). The Etest method is an attractive option for alternative vancomycin testing since it is easy to perform and cost-effective for testing only one drug-bug combination. However, the results of the present study clearly show that the Etest provides vancomycin and daptomycin MIC results consistently higher (by 0.5 to 1.5 log2 dilutions) than those provided by precisely performed reference BMD tests. Furthermore, the fact that the higher vancomycin MIC results provided by the Etest appear to be more reliable in predicting vancomycin treatment responses (6) indicates that the vancomycin susceptibility breakpoint should be reevaluated.
The susceptibility testing method used may have a large impact on physician decisions for vancomycin and daptomycin treatment of MRSA infections. According to the information presented here, the dominant MRSA population (91.2% of strains) would be categorized as vancomycin nonsusceptible by the Etest method if the susceptibility breakpoint was adjusted to ≤1 μg/ml, as suggested by some published clinical outcome studies on recognizing isolates for which vancomycin MICs are 2 μg/ml (4, 5, 8-10, 14). Daptomycin MICs were also affected by the method used, but to a lesser degree (MICs were found to be 0.5 to 1 dilution step higher by the Etest method than by the reference method, with 0.4% false-nonsusceptible results), and may also suffer from the perception of declining daptomycin potency.
Acknowledgments
This study was funded in part by a research grant from Cubist Pharmaceuticals.
We thank the following investigators for kindly providing strains for the study: Paul D. Fey, University of Nebraska Medical Center (Omaha, NE); Douglas Fish, University of Colorado Denver (Aurora, CO); Ajit Limaye, University of Washington (Seattle, WA); George Pankey, Ochsner Clinic Foundation (New Orleans, LA); James Rahal, New York Hospital Queens (New York, NY); Michael Rybak, Wayne State University (Detroit, MI); David Snydman, Tufts Medical Center (Boston, MA); Lisa L. Steed, Medical University of South Carolina (Charleston, SC); and Ken Waites, University of Alabama at Birmingham (Birmingham, AL).
Footnotes
Published ahead of print on 27 April 2009.
REFERENCES
- 1.Boucher, H. W., and G. Sakoulas. 2007. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin. Infect. Dis. 45:601-608. [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Approved standard M07-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Hidayat, L. K., D. I. Hsu, R. Quist, K. A. Shriner, and A. Wong-Beringer. 2006. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch. Int. Med. 166:2138-2144. [DOI] [PubMed] [Google Scholar]
- 5.Howden, B. P., P. B. Ward, P. G. Charles, T. M. Korman, A. Fuller, P. du Cros, E. A. Grabsch, S. A. Roberts, J. Robson, K. Read, N. Bak, J. Hurley, P. D. Johnson, A. J. Morris, B. C. Mayall, and M. L. Grayson. 2004. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin. Infect. Dis. 38:521-528. [DOI] [PubMed] [Google Scholar]
- 6.Hsu, D. I., L. K. Hidayat, R. Quist, J. Hindler, A. Karlsson, A. Yusof, and A. Wong-Beringer. 2008. Comparison of method-specific vancomycin minimum inhibitory concentration values and their predictability for treatment outcome of meticillin-resistant Staphylococcus aureus (MRSA) infections. Int. J. Antimicrob. Agents 32:378-385. [DOI] [PubMed] [Google Scholar]
- 7.Jones, R. N. 2006. Microbiological features of vancomycin in the 21st century: minimum inhibitory concentration creep, bactericidal/static activity, and applied breakpoints to predict clinical outcomes or detect resistant strains. Clin. Infect. Dis. 42(Suppl. 1):S13-S24. [DOI] [PubMed] [Google Scholar]
- 8.Lodise, T. P., B. Lomaestro, J. Graves, and G. L. Drusano. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob. Agents Chemother. 52:1330-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moise-Broder, P. A., A. Forrest, M. C. Birmingham, and J. J. Schentag. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43:925-942. [DOI] [PubMed] [Google Scholar]
- 10.Neoh, H. M., S. Hori, M. Komatsu, T. Oguri, F. Takeuchi, L. Cui, and K. Hiramatsu. 2007. Impact of reduced vancomycin susceptibility on the therapeutic outcome of MRSA bloodstream infections. Ann. Clin. Microbiol. Antimicrob. 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakoulas, G., P. A. Moise-Broder, J. Schentag, A. Forrest, R. C. Moellering, Jr., and G. M. Eliopoulos. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42:2398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sievert, D. M., J. T. Rudrik, J. B. Patel, L. C. McDonald, M. J. Wilkins, and J. C. Hageman. 2008. Vancomycin-resistant Staphylococcus aureus in the United States, 2002-2006. Clin. Infect. Dis. 46:668-674. [DOI] [PubMed] [Google Scholar]
- 13.Soriano, A., F. Marco, J. A. Martinez, E. Pisos, M. Almela, V. P. Dimova, D. Alamo, M. Ortega, J. Lopez, and J. Mensa. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46:193-200. [DOI] [PubMed] [Google Scholar]
- 14.Steinkraus, G., R. White, and L. Friedrich. 2007. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001-05. J. Antimicrob. Chemother. 60:788-794. [DOI] [PubMed] [Google Scholar]