Abstract
Acyclovir is effective in the prevention and treatment of herpes simplex virus (HSV) and varicella-zoster virus (VZV) infections. The aim of this study was to characterize the population pharmacokinetics of acyclovir observed following treatment with intravenous acyclovir and oral valacyclovir (valaciclovir) in young people with malignancy. Plasma acyclovir concentration-time data were collected from 43 patients (age range, 9 months to 20 years) who had been given multiple doses of acyclovir (5 mg/kg of body weight) and/or valacyclovir (10 mg/kg). Nonlinear mixed-effect modeling was employed to analyze acyclovir population pharmacokinetics and identify influential covariates. Simulations (n = 1,000) were conducted to explore the ability of the current doses to maintain acyclovir concentrations above the recommended 50% inhibitory concentration for HSV or VZV (0.56 mg/liter or 1.125 mg/liter, respectively) for more than 12 h. A one-compartment pharmacokinetic model with first-order elimination best described the acyclovir concentration-time data. The population mean estimates for clearance (CL), volume of distribution (V), absorption rate (ka), and bioavailability (F) were 3.55 liters/h, 7.36 liters, 0.63 h−1, and 0.60, respectively. Inclusion of body weight and estimated creatinine CL (CLCR) in the final model reduced the interindividual variabilities in CL and V from 61% to 24% and from 75% to 36%, respectively. Simulations revealed that with the use of the current doses, maximal efficacy can be achieved in over 45% of patients weighing 25 to 50 kg and with CLCR levels of 2.0 to 4.0 liters/h/m2, but only in a much smaller proportion of patients, with low weights (10 kg) and high CLCRs (5.5 liters/h/m2), suggesting that higher doses are required for this subgroup. This validated population pharmacokinetic model for acyclovir may be used to develop dosing guidelines for safe and effective antiviral therapy in young people with malignancy.
Acyclovir {9-[(2-hydroxyethoxy)-methyl]guanine} is an antiviral agent which has been shown to be effective in the prevention and treatment of herpes simplex virus (HSV) (33) and varicella-zoster virus (VZV) infections (3). It is also used for prophylaxis of cytomegalovirus infection following solid organ transplantation (1). Acyclovir exhibits a selective inhibition of herpes virus replication and has potent clinical antiviral activity against HSV and VZV, which can cause significant morbidity and mortality due to primary infection or to reactivation following long-term latency in pediatric oncology and pediatric bone marrow/stem cell transplantation (16, 24). Children with malignant diseases who experience prolonged periods of myelosuppression due to cytotoxic chemotherapy or hematopoietic stem cell transplant are highly susceptible to invasive viral infections (16). The use of indwelling vascular catheters further increases the risk of development of invasive viral infections among immunosuppressed children (20).
The efficacy of oral acyclovir may be limited by its low oral bioavailability (F), which is between 15% and 30% (17). Valacyclovir (valaciclovir), the l-valyl ester prodrug of acyclovir, is rapidly and extensively converted to acyclovir after oral administration (42) and can increase the oral F value of acyclovir up to 50% (37).
Following administration of acyclovir or valacyclovir, approximately 10% of its dose is metabolized in the liver (17). Acyclovir is predominately eliminated by renal excretion via glomerular filtration and tubular secretion (17, 23). A patient's renal function significantly influences the pharmacokinetics of acyclovir, with the mean systemic clearances (CLs) of acyclovir being 327 ± 80, 248 ± 62, and 190 ± 62 ml/min/1.73 m2 in adult patients, with estimated creatinine CLs (CLCRs) of >80, 50 to 80, and 15 to 50 ml/min/1.73 m2, respectively (8).
Moreover, renal impairment is a commonly reported adverse effect of acyclovir (approved product information for acyclovir; Mayne Pharma Pty., Ltd., Victoria, Australia [http://mims.hcn.net.au]). For intravenous dosing, acyclovir must be infused over 1 hour to avoid high concentrations, as acyclovir sodium can precipitate as crystals in the renal tubules, with the risk of tubular damage (approved product information for acyclovir; Mayne Pharma Pty., Ltd.). Hence, the dosage of acyclovir or valacyclovir may need to be reduced, and the dosing interval may need to be increased, in patients with renal impairment to reduce the risk of acyclovir-induced neurotoxicity due to drug accumulation (15, 18).
Acyclovir demonstrates high interindividual variability (IIV) in its treatment response (2, 27, 29), with a wide range of adverse reactions. While the current dosage recommendations for acyclovir and valacyclovir are based on body weight (5 mg/kg and 10 mg/kg of body weight, respectively), there are no specific recommendations for reduction of the acyclovir dose in pediatric patients with renal impairment, with the exception of some data for neonates (10). Valacyclovir is used in the clinical setting because of its significantly high F value compared with that of oral acyclovir (37). However, information regarding its optimal use in children is limited because pharmacokinetic data are lacking and the safety and effectiveness of valacyclovir in children have not been established (approved product information for valacyclovir; GlaxoSmithKline Pty., Ltd., Victoria, Australia [http://mims.hcn.net.au]).
The aims of this study were to characterize the pharmacokinetics of acyclovir following intravenous infusion of acyclovir and oral administration of valacyclovir, to investigate patient factors which contribute to the wide IIV in acyclovir pharmacokinetics, and to evaluate the current dose regimens by using a simulation approach for children and young people with malignancy.
MATERIALS AND METHODS
Patients.
A total of 43 children and young people with malignancy, aged between 9.2 months and 19.9 years, were recruited into this prospective, single-center observational investigation of pharmacokinetics of acyclovir and valacyclovir. This study was approved by the Ethics Committee at the Children's Hospital at Westmead, and written informed consent was provided by the parents of all the children. The characteristics of the children and young people who participated in this study are summarized in Table 1.
TABLE 1.
Characteristics of children and young people receiving acyclovir and valacyclovir
| Characteristic | Median (range) |
|---|---|
| No. of patients | 43 |
| No. receiving intravenous dosing only | 25 |
| No. receiving oral dosing only | 7 |
| No. receiving intravenous and oral dosing | 11 |
| No. of observations | 1,216 |
| Comedication (MMF) | |
| No. with | 9 |
| No. without | 34 |
| No. of males | 25 |
| No. of females | 18 |
| Age (yr) | 6.3 (0.8-19.9) |
| Wt (kg) | 19.6 (7.3-70.2) |
| BSA (m2)a | 0.77 (0.37-1.7) |
| BMI (kg/m2) | 17.7 (10.5-24.8) |
| Height (cm) | 111.0 (69.0-173.5) |
| CLCR (liters/h/m2)b | 3.7 (2.0-5.7) |
| GFR (liters/h)c | 4.0 (1.4-10.1) |
| No. of patients with diagnosis | |
| Acute lymphoblastic leukemia | 16 |
| Acute myeloid leukemia | 6 |
| Neuroblastoma | 5 |
| Wiskott Aldrich syndrome | 3 |
| Fanconi's anemia | 2 |
| Other diseases | 11 |
Drug administration and blood sampling.
Acyclovir (Mayne Pharma Pty., Ltd., Victoria, Australia) was diluted in 0.9% saline and administered as a 1-h intravenous infusion at a dose of 5 mg/kg three times daily. Valacyclovir (GlaxoSmithKline Pty., Ltd., Victoria, Australia) was given as an oral dose of 10 mg/kg twice daily. Prophylactic treatment was typically prolonged, often lasting many months. Out of the 43 patients, 25 received intravenous acyclovir and 7 received oral valacyclovir. The remaining 11 patients received both acyclovir and valacyclovir at different times during their treatment.
Children and young people weighing more than 20 kg and receiving intravenous acyclovir or oral valacyclovir had a series of 12 to 14 blood samples (3 ml each) drawn over an 8-h or 12-h time period within one dosing interval. The blood samples were collected from the patients' central venous cannula. For intravenous acyclovir, blood sampling times were prior to the dose; then at the end of the infusion; and 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, and 8 h after the end of the infusion. For oral valacyclovir, the blood sampling times were prior to the dose and then at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 h after the dose. Fewer samples were collected from children who weighed less than 20 kg. These samples typically included a sample prior to the dose; then at the end of the infusion; and 0.5, 1, 3, 5, and 7 h after the dose. The remaining children had randomly timed but accurately recorded blood samples collected. Blood samples were not collected during the infusion, because a central line was used and there was a possibility of contamination. Plasma was separated by centrifugation at 1,200 × g for 10 min at 4°C (Beckman CS-15R; Beckman Instruments, Fullerton, CA) and was stored at −40°C until analysis. All samples were analyzed within 1 month of collection.
Acyclovir assay.
Acyclovir concentrations in plasma samples were measured using a previously published high-performance liquid chromatography assay which was established and validated in our laboratory (43). Analyte recovery was 101%, and the assay response was linear over the acyclovir concentration range of 0.1 to 60 mg/liter. Intra- and interday accuracy and precision levels were less than 7% over this range. The limit of detection and the limit of quantitation were 0.033 mg/liter and 0.1 mg/liter, respectively. A comparison of numerous plasma samples from patients receiving a range of medicines revealed no interfering peaks in the acyclovir assay.
Population pharmacokinetic analysis.
Data were analyzed using the nonlinear mixed-effect modeling program NONMEM, version V, level 1.1 (GloboMax, Hanover, MD). Wings for NONMEM, version 405 (http://wfn.sourceforge.net/), was used as a front-end processor. Compaq Visual Fortran, version 6.1A (Compaq Computer Corporation, Houston, TX), was used to perform the Fortran compilation. Graphical outputs of NONMEM were undertaken using CrossGraphs, version 2.3 (PPD Development, Cambridge, MA). The first-order-conditional-estimation-with-interaction method was used throughout the model-building and evaluation procedure. Model selection was based on goodness of fit, in addition to criteria of statistical significance, model plausibility, and stability. Models were compared statistically by using a likelihood ratio test on the differences in the NONMEM-derived objective function value (OFV). Statistical significance was set at P values of <0.05 (ΔOFV = 3.84; P < 0.05). Plots of residuals and weighted residuals, standard errors of parameter estimates, and changes in estimates of IIV, interoccasion variability (IOV), and residual variability were also examined (4, 11, 12, 14, 40).
Base model building.
Preliminary analyses focused on selection of the structural and statistical models, without consideration of covariate effects. The plots of observed concentration-time data of acyclovir were examined first. One- and two-compartment pharmacokinetic models with first-order elimination were compared to investigate the best fit for the concentration-time data. The NONMEM subroutines ADVAN2 TRANS2 and ADVAN4 TRANS4 were employed in the analyses (4). An exponential error model was employed to model IIV for the pharmacokinetic parameters, defined as θi = θ̃ × EXP(ηi), where θi represents the value of the pharmacokinetic parameter for the ith individual, θ̃ is the typical value of the pharmacokinetic parameter θ in the population (e.g., the population mean), and ηi quantifies the deviation of θi from θ̃ with a normal distribution (0, ω2). The difference between the jth observed concentration (Y) in the ith individual and its respective prediction (|AtY) was modeled with a combined exponential and additive error model, expressed as Y = |AtY × EXP(ɛ1) + ɛ2, where ɛ1 and ɛ2 are random effects quantifying the errors between Y and |AtY with a normal distribution (0, σ2). IOV for the parameter θ was evaluated as an additional level of random effect and was expressed as θ = θ̃ × EXP(η + k1 × OCC1 + k2 × OCC2 + … + kn × OCCn), where OCCn has the value of 1 for the nth occasion and 0 otherwise; k1 to kn are random variables assumed to be normally distributed, with a mean of 0; and variance is denoted by π2 (22). In the modeling analysis, each “occasion” was defined as 7 days for patients who were administered acyclovir or valacyclovir daily. The effect of IOV was tested on CL and volume of distribution (V) by using NONMEM's BLOCK SAME option (approved product information for valacyclovir; GlaxoSmithKline Pty., Ltd.), with the assumption that IOV was the same for all occasions.
Covariate analysis.
The covariates screened for their possible influence on acyclovir pharmacokinetic parameters included sex, body weight, height, age, body surface area (BSA), body mass index (BMI), comedication with mycophenolate mofetil (MMF), glomerular filtration rate (GFR; determined by measuring the plasma CL of 43Tc99-diethylenetriaminepentaacetic acid), and estimated CLCR. CLCR for children and young people was estimated using the Counahan formula (7), expressed as CLCR (ml/min/1.73 m2) = 0.43 × height (cm)/plasma creatinine concentration (mg/dl).
The influence of individual covariates on pharmacokinetic parameters was examined by plotting the empirical Bayesian estimates of individual parameters (derived from the base model) against covariates. Covariates identified as potentially influential were included in the population pharmacokinetic model.
The effects of body weight (wt) on CL and V were assessed with the use of an allometric scaling function (21), expressed as CL = θ1 × (wt/19.6)0.75 and V = θ2 × (wt/19.6), where the population CL and V terms were standardized to 19.6 kg, which represents the median value of weight in this study group.
To assess the effect of renal function independently of body size, the value of estimated CLCR was standardized to the value per m2, and this value was corrected for the median population CLCR of 3.7 liters/h/m2, using CL = θ1 × (CLCR/3.7)FAC. To explore the impacts of different elimination pathways on the CL of acyclovir, we examined the utility of dividing CL into a nonrenal component and a renal component, using CL = CLNR + CLR, where CLNR is the nonrenal CL and CLR is the renal CL, and the effect of renal function on renal CL was also evaluated, using CL = CLNR + CLR × (CLCR/3.7).
Following initial screening of individual covariates, covariates were added cumulatively in a stepwise fashion to the model in the order of their levels of contribution to the reduction in OFV in the preliminary analysis until there was no further reduction in OFV. Next, a backward elimination step, in which the influence of each covariate was removed from the model in descending order of its level of contribution to the change in OFV and the remaining parameters in the model were reestimated, was performed. Statistical significance for forward addition and backward elimination was set at P values of <0.01 (ΔOFV = 6.63; P < 0.01) (4, 35, 41).
Model evaluation.
A nonparametric bootstrap resampling method was performed to evaluate the accuracy and stability of the final population pharmacokinetic model. A total of 1,000 bootstrap samples were generated from the original data set, and the final population model was fitted to the bootstrap data sets by using the Wings for NONMEM program. Parameter estimates for each of the 1,000 data sets were reestimated as previously described by Parke et al. (28). For each parameter, the resulting estimates were sorted, and the 2.5th and 97.5th percentiles were obtained as the lower and upper boundaries for the 95% confidence interval (95% CI) of the parameter estimate (12). The mean parameter estimates obtained by bootstrapping were compared with those obtained by the population model to assess the reliability of the final model estimates (13).
Simulated dose regimens.
The final population pharmacokinetic model was used to simulate acyclovir concentration-time data sets for 1,000 hypothetical patients within the range of this group of children and young people using the current recommended dosing regimens (intravenous acyclovir at 5 mg/kg or oral valacyclovir at 10 mg/kg). Previous published reports suggest that acyclovir's maximal antiviral efficacy is achieved when concentrations remain above the 50% inhibitory concentration (IC50) for more than 12 h during each 24-h dosing interval (32, 38). The periods of time within the dosing interval in which the acyclovir concentrations remained above the IC50 of 0.56 mg/liter for HSV or 1.125 mg/liter for VZV (39) were calculated for hypothetical patients with a range of body weights and CLCRs then used to determine the percentage of children who achieved maximal efficacy by using the current acyclovir and valacyclovir doses.
RESULTS
Concentration data.
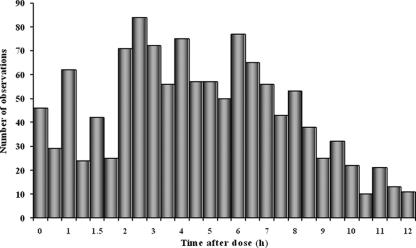
A total of 1,216 concentration-time measurements were collected from 43 children and young people (Table 1). The numbers of acyclovir concentrations per patient ranged from 3 to 50 samples, with a median of 25 samples per patient. A frequency histogram of blood samples collected at specific times during the dose interval is shown in Fig. 1. The acyclovir concentrations observed after intravenous dosing ranged from 0.1 mg/liter to 43.7 mg/liter, with a mean of 5.3 ± 6.9 mg/liter, and the valacyclovir concentrations observed after oral dosing ranged from 0.1 mg/liter to 11.6 mg/liter, with a mean of 2.3 ± 2.6 mg/liter. Samples were drawn between 0 h and 8 h postdose for intravenous acyclovir and between 0 h and 12 h postdose for oral valacyclovir.
FIG. 1.
Frequency histogram of blood samples collected at specific times during the dose interval.
Base model building.
A one-compartment pharmacokinetic model was selected to describe the acyclovir concentration-time data. Although the OFV of the two-compartment model (577.59) was lower than that observed for the one-compartment model (586.66), the pharmacokinetic parameter estimates were almost identical, and the plots of residuals and weighted residuals; standard errors of parameter estimates; and changes in estimates of IIV, IOV, and residual variability were not significantly improved compared with those in the one-compartment model, especially for the key parameter CL. The model evaluation (bootstrapping) analysis revealed that the two-compartment pharmacokinetic model was less stable than the one-compartment model; moreover, the wide 95% CIs for V of the peripheral compartment (V2; 1.12 to 1,120 liters) and random residual errors (σ2; 6.35 × 10−6 to 0.11) indicated that these parameters could not be accurately estimated. On the basis of these observations and comparisons, a one-compartment pharmacokinetic model with first-order elimination for estimating CL, V, ka, and F (with IIV for estimating CL, V, ka, and F and with IOV for estimating CL and V) was utilized in this study. Inclusion of IOV into two fixed-effect parameters, CL and V, provided a significant decrease (81.00 points) in OFV. This model included a combined exponential and additive error model for estimating residual error.
Covariate analysis.
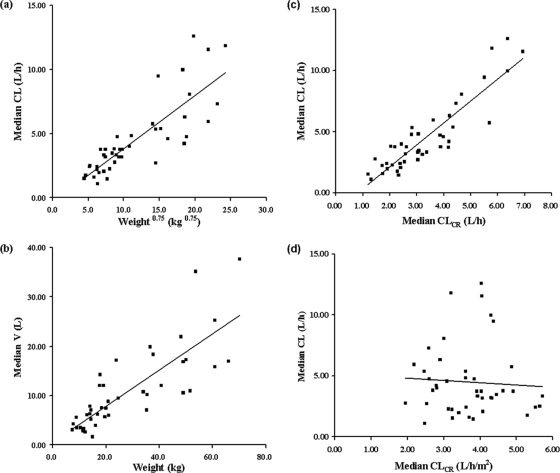
Individual posterior Bayesian estimates of pharmacokinetic parameters generated from the base model were plotted against each covariate. Weight, height, age, BSA, BMI, CLCR, and GFR were found to be potentially influential. Acyclovir CL increased with increasing age (r2 = 0.64), body weight (r2 = 0.69), BSA (r2 = 0.69), CLCR (r2 = 0.81), and GFR (r2 = 0.40). There was also a trend toward increased V with increasing body weight (r2 = 0.66), height (r2 = 0.59), and BSA (r2 = 0.65). Inclusion of these covariates significantly reduced the OFV by the predefined cutoff value of 3.84 (P < 0.05) when tested against the base model.
Age, weight, CLCR, GFR, and BSA were found to have statistically significant effects on CL, whereas height, weight, and BSA were found to significantly affect V. However, weight, height, BSA, and BMI all describe the sizes of the patients, and CLCR and GFR are renal function markers. In the final model, only one of each of these covariate groups was chosen, in accordance with its level of contribution to the reduction of OFV. Two covariates were identified as significant, and these are presented in Fig. 2.
FIG. 2.
Scatter plots of empirical Bayesian estimates versus covariates. (a) CL versus weight with an allometric scaling function. (b) V versus weight. (c) CL versus CLCR (liter/h). (d) CL versus CLCR (liter/h/m2).
The inclusions of weight as a covariate on CL (with an allometric scaling function) and weight alone on V terms were found to be statistically significant, with a decrease in OFV by 56.27 points. Furthermore, the IIVs for CL and V were found to decrease from 61.0% to 28.2% and from 75.3% to 36.1%, respectively. The inclusion of CLCR as a covariate on the parameter CL was found to result in a statistically significant decrease in OFV (by 5.70 points).
The model-building process is summarized in Table 2. Removal of weight and CLCR individually as part of the backward elimination step resulted in increases in OFV of 61.23 and 10.66, respectively.
TABLE 2.
Summary of building steps for the one-compartment covariate model for acyclovir
| Model no. | Covariate | Model | Comparison model no. | OFV | ΔOFV | P |
|---|---|---|---|---|---|---|
| 1 | CL = θ1 × EXP(IIV); V = θ2 × EXP(IIV) | 734.59 | ||||
| 2 | Base model | CL = θ1 × EXP(IIV + IOV); V = θ2 × EXP(IIV + IOV) | 1 | 653.59 | −81.00 | <0.001 |
| 3 | Allometric wt on CL and V | CL = θ1 × (WT/19.6)0.75 × EXP(IIV+IOV); V = θ2 × (wt/19.6) × EXP(IIV + IOV) | 2 | 597.32 | −56.27 | <0.001 |
| 4 | CLCR on CL | CL = θ1 × (wt/19.6)0.75 × (CLCR/3.7)FAC × EXP(IIV + IOV); V = θ2 × (wt/19.6) × EXP(IIV + IOV) | 3 | 586.66 | −10.66 | <0.01 |
The effects of body weight on CL and V and the effect of renal function on CLR were also evaluated. This model yielded typical population values (standard errors) of 1.63 liters/h (33%) for CLNR, 1.90 liters/h (29%) for CLR, 7.35 liters (8%) for V, 0.63 (15%) for ka, and 0.60 (13%) for F. However, the bootstrap replicates for CLNR and CLR had wide 95% CIs, and the model was not used further. The final covariate models for CL and V of acyclovir were CL = θ1 × (wt/19.6)0.75 × (CLCR/3.7)FAC × EXP(IIV + IOV) and V = θ2 × (wt/19.6) × EXP(IIV + IOV), respectively.
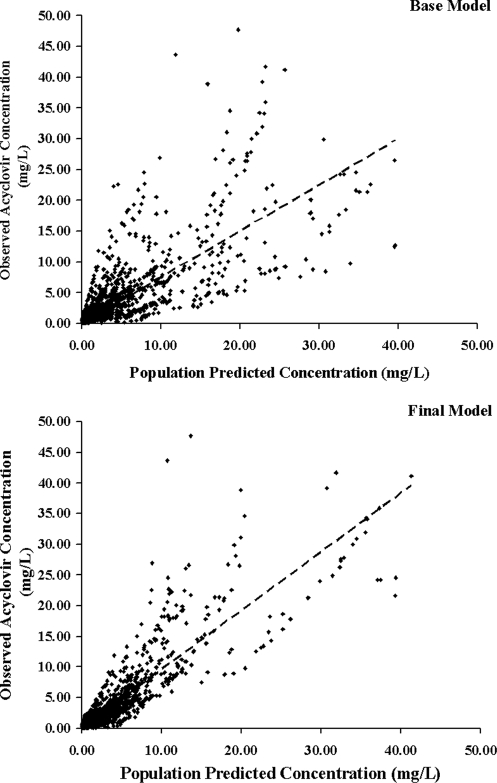
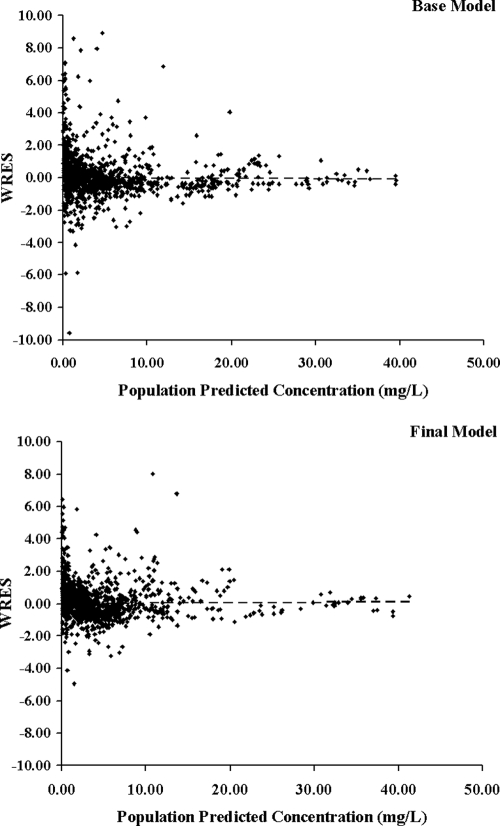
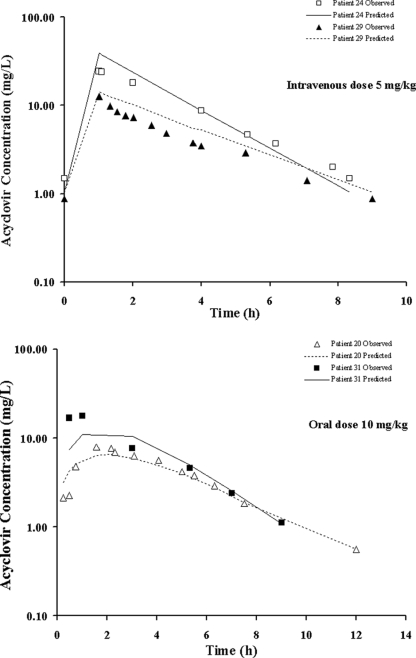
The population pharmacokinetic parameter values estimated from the base and final models are presented in Table 3. The population mean estimates for CL, V, ka, F, and the renal function factor (FAC) from the final model were 3.55 liters/h, 7.36 liters, 0.63 h−1, 0.60, and 0.51, respectively, and the estimates for IIV were 23.6% for CL, 35.9% for V, 58.1% for ka, and 41.8% for F. The IOV estimates were 19.2% and 30.4% for CL and V, respectively. Figures 3 and 4 display the diagnostic plots for the base and final models. The residual variability and the range of weighted residuals in the final model are reduced compared with those in the base model. There was no apparent trend observed in the weighted residual plots, and the population-predicted concentrations were symmetrically distributed around the line of identity, indicating that the model adequately describes the concentration-time profile of acyclovir at the doses studied. There was also no difference in scatter displayed by the plots when the scheduled intensive collection type was compared with the opportunistic random type (data not shown). The representative observed and model-predicted concentration-time profiles for four typical patients treated with intravenous acyclovir at a dose of 5 mg/kg or oral valacyclovir at a dose of 10 mg/kg are shown in Fig. 5 and show an excellent model fit.
TABLE 3.
Population pharmacokinetic parameter estimates derived from the base and final models, based on 1,000 bootstrap replicates
| Parametera | Base model
|
Final model
|
Value for 1,000 bootstraps
|
|||
|---|---|---|---|---|---|---|
| Mean | RSE (%) | Mean | RSE (%) | Mean | 95% CIb | |
| Fixed effects | ||||||
| CL (liters/h) | 3.8 | 11 | 3.55 | 5 | 3.56 | 3.22-3.94 |
| V (liters) | 8.35 | 14 | 7.36 | 8 | 7.39 | 6.25-8.64 |
| ka (liters/h) | 0.67 | 17 | 0.63 | 15 | 0.66 | 0.46-0.95 |
| F | 0.57 | 15 | 0.60 | 13 | 0.61 | 0.45-0.77 |
| RF factor | 0.51 | 27 | 0.49 | 0.19-0.80 | ||
| IIV (CV) (%) | ||||||
| ωCL | 61.0 | 20 | 23.6 | 30 | 23.0 | 13.9-30.1 |
| ωV | 75.3 | 22 | 35.9 | 31 | 34.6 | 21.2-45.8 |
| ωka | 62.5 | 45 | 58.1 | 44 | 57.0 | 27.9-84.4 |
| ωF | 49.4 | 27 | 41.8 | 33 | 39.8 | 20.8-53.6 |
| IOV (CV) (%) | ||||||
| πCL | 19.4 | 32 | 19.2 | 32 | 19.1 | 12.2-25.0 |
| πV | 30.8 | 39 | 30.4 | 38 | 30.8 | 19.1-42.8 |
| Random residual variability | ||||||
| σ1 | 0.26 | 16 | 0.26 | 16 | 0.25 | 0.21-0.30 |
| σ2 | 0.1 | 57 | 0.1 | 58 | 0.09 | 0.03-0.16 |
RSE, relative standard error; RF factor, renal function factor; σ1 and σ2, residual variability measures; CV, coefficient of variation.
Corresponding to parameter estimates at the 2.5th and 97.5th percentiles for bootstrap runs.
FIG. 3.
Observed acyclovir concentrations versus population-predicted concentrations for the base and final population pharmacokinetic models (the dashed line is the line of identity).
FIG. 4.
Weighted residuals (WRES) versus population-predicted acyclovir concentrations for the base and final population pharmacokinetic models.
FIG. 5.
Concentration-time profiles of acyclovir as predicted by the final model for four representative patients, following treatment with intravenous acyclovir (patients 24 [body weight, 19.1 kg; CLCR, 3.9 liters/h/m2; CL, 4.69 liters/h] and 29 [body weight, 70.2 kg; CLCR, 3.2 liters/h/m2; CL, 11.68 liters/h]) or oral valacyclovir (patients 20 [body weight, 50.1 kg; CLCR, 2.6 liters/h/m2; CL, 5.73 liters/h] and 31 [body weight, 18.5 kg; CLCR, 3.9 liters/h/m2; CL, 3.40 liters/h]).
Model evaluation.
From the original data set, 1,000 replicate data sets were generated using a bootstrapping approach and used for evaluation of the stability of the final model and the accuracy of the parameter estimates. The mean values for all fixed-effect parameters and the mean values for random-effect parameters were within ±15% of those obtained with the NONMEM final model, indicating the good reliability of this model. Table 3 presents the results of the bootstrap procedure with parameters (presented as means ± 95% CIs).
Simulation.
Simulations (n = 1,000) suggest that the maximal efficacies for HSV and VZV are achieved in 54.0% to 93.5% and 37.5% to 88.3% of children and young people, respectively, receiving 5 mg/kg acyclovir intravenously (Table 4) and in 38.2% to 85.6% and 24.0% to 76.5% of children and young people, respectively, receiving 10 mg/kg valacyclovir orally (Table 5). With the current dosing regimens, only a small proportion (24.0 to 54.0%) of light patients (10 kg) with higher CLCRs (5.5 liters/h/m2) achieved maximal efficacy against HSV and VZV.
TABLE 4.
Lengths of time that acyclovir concentration remained above the IC50 threshold for HSV or VZV at steady state within a 24-h interval and percentages of patients achieving maximal efficacy after treatment with 5 mg/kg intravenous acyclovir three times dailya
| Wt (kg) | Median time (h) for indicated CLCR (5th-95th %ile) (% of patientsb)
|
|||||
|---|---|---|---|---|---|---|
| 2.0 liters/h/m2
|
4.0 liters/h/m2
|
5.5 liters/h/m2
|
||||
| HSV | VZV | HSV | VZV | HSV | VZV | |
| 10 | 21.8 (7.2-24.0) (81.5) | 16.4 (5.8-24.0) (69.5) | 15.2 (5.1-24.0) (64.7) | 11.5 (4.1-24.0) (47.4) | 12.9 (4.3-24.0) (54.0) | 9.8 (3.5-24.0) (37.5) |
| 25 | 24.0 (9.1-24.0) (90.2) | 20.6 (7.3-24.0) (80.6) | 19.2 (6.4-24.0) (75.9) | 14.5 (5.1-24.0) (62.8) | 16.3 (5.4-24.0) (68.0) | 12.3 (4.4-24.0) (51.9) |
| 50 | 24.0 (10.8-24.0) (93.5) | 24.0 (8.7-24.0) (88.3) | 22.8 (7.6-24.0) (83.8) | 17.2 (6.1-24.0) (71.7) | 19.3 (6.4-24.0) (76.7) | 14.6 (5.2-24.0) (63.2) |
Maximal efficiency was calculated based on 1,000 simulations for each child with a given body weight and CLCR. The IC50 thresholds for HSV and VZV are 0.56 mg/liter and 1.125 mg/liter, respectively.
Percentage of patients achieving maximal efficacy for HSV or VZV.
TABLE 5.
Lengths of time that acyclovir concentration remained above the IC50 threshold for HSV or VZV at steady state within a 24-h interval and percentages of patients achieving maximal efficacy after treatment with 10 mg/kg oral valacyclovir twice dailya
| Wt (kg) | Median time (h) for indicated CLCR (5th-95th %ile) (% of patientsb)
|
|||||
|---|---|---|---|---|---|---|
| 2.0 liters/h/m2
|
4.0 liters/h/m2
|
5.5 liters/h/m2
|
||||
| HSV | VZV | HSV | VZV | HSV | VZV | |
| 10 | 16.1 (5.2-24.0) (67.6) | 12.6 (4.3-24.0) (53.5) | 11.3 (3.6-24.0) (47.2) | 8.8 (3.0-24.0) (32.3) | 9.6 (3.1-24.0) (38.2) | 7.5 (2.6-21.8) (24.0) |
| 25 | 20.3 (6.5-24.0) (78.3) | 15.9 (5.5-24.0) (67.3) | 14.2 (4.6-24.0) (59.5) | 11.1 (3.8-24.0) (45.8) | 12.1 (3.9-24.0) (50.2) | 9.4 (3.2-24.0) (37.1) |
| 50 | 24 (7.7-24.0) (85.6) | 18.9 (6.5-24.0) (76.5) | 16.9 (5.4-24.0) (69.9) | 13.2 (4.5-24.0) (56.8) | 14.3 (4.6-24.0) (60.4) | 11.2 (3.9-24.0) (46.6) |
Maximal efficiency was calculated based on 1,000 simulations for each child with a given body weight and CLCR. The IC50 thresholds for HSV and VZV are 0.56 mg/liter and 1.125 mg/liter, respectively.
Percentage of patients achieving maximal efficacy for HSV or VZV.
DISCUSSION
To our knowledge, this study is the first investigation in which a population pharmacokinetic modeling approach was applied to assess the pharmacokinetics of both acyclovir and valacyclovir in children and young people with malignancy. This is one of the few studies that have estimated the oral F value of acyclovir after valacyclovir in patients receiving the drug as part of their therapy. The pharmacokinetic parameter estimates derived from the final model were compared with those derived from prior pharmacokinetic studies of children (Table 6). The estimations of CL and V in the present study are notably lower than those reported in the conventional studies (9, 26, 36). CL is presented in units corrected for body weight (per kg) (Table 6) and can be affected by renal function, while some of the children in the present study had lower CLCRs than those in previous studies. The acyclovir V at steady state approximates total body water content, which is about 0.6 liters/kg (31). The value for V in the present study (0.4 liters/kg) is more consistent with this extent of distribution, whereas the results from previous studies seem higher than the total body water content. The reasons for the differences in pharmacokinetic observation between the present study and previous reports are unclear; however, this study represents a larger set of observational data, with results for 43 children and young people, than other study cohorts (Table 6).
TABLE 6.
Comparison of pharmacokinetic parameter estimates of acyclovir for children and young people obtained using the final model and estimates previously reported in the literature
| Paremeter | Valuea for present study
|
Valuea for conventional study
|
||||
|---|---|---|---|---|---|---|
| Total group | i.v. group | Oral Group | i.v. groupb | Oral groupc | Oral groupd | |
| Diagnosis group | Children and young people with malignancy | Children and young people with malignancy | Children and young people with malignancy | Immunocompromised children | Immunocompromised children | Epstein-Barr virus infection |
| No. of children | 43 | 36 | 18 | 18 | 8 | 8 |
| Age (yr) | 6.3 (0.8-19.9) | 5.8 (0.8-19.9) | 7.7 (2.4-18.6) | 6.9 (1.4-18.1) | 6.5 (5.0-9.0) | 8-17 |
| Wt (kg) | 19.6 (7.3-70.2) | 19.4 (7.3-70.2) | 20.9 (10.5-53.7) | 19.0 (11.0-60.0) | 21.7 (18.8-26.5) | 30-71 |
| CLCR (liters/h/m2) | 3.7 (2.0-5.7) | 3.8 (2.0-5.6) | 3.9 (2.0-5.7) | NAe | 8.4 (6.9-12.6) | 3.6-4.8 |
| Dose | 5 mg/kg/8 h | 10 mg/kg/12 h | 10.5 mg/kg (4.9-23.3 mg/kg) | 250 mg/12 h | 500 mg/8 h | |
| Data analysis program | NONMEM (v 5.1.1) | NONMEM (v 5.1.1) | NONMEM (v 5.1.1) | PC-NONLIN (v 2.0) | WinNonlin Pro (v 3.0; Pharsight) | Noncompartmental methods |
| CL (liters/h/kg) | 0.2 (0.1-0.3) | 0.2 (0.1-0.3) | 0.2 (0.1-0.2) | 0.5 (0.2-0.8) | 0.5 (0.2-0.6) | 1.2 ± 0.4f |
| V (liters/kg) | 0.4 (0.1-0.8) | 0.4 (0.1-0.8) | 0.4 (0.2-0.7) | 1.2 (0.5-3.3) | 1.3 (0.8-1.9) | 3.0 ± 1.3g |
| Half-life (h) | 1.5 (0.8-3.6) | 1.5 (0.8-3.6) | 1.4 (0.9-3.4) | 1.9 (1.2-3.3) | 2.1 (1.7-2.7) | 1.7 ± 0.4h |
| F (%) | 60 | 48 | ||||
All values are median (range) except where otherwise noted.
Eksborg et al. (9).
Nadal et al. (26).
Simon et al. (36). Ranges are given alone, as medians are unavailable.
NA, not applicable (all children had normal renal function [GFR > 3.1 liters/h/m2]).
CL/F (mean ± standard deviation).
V/F (mean ± standard deviation).
Mean ± standard deviation.
Considerable IIV was observed in the pharmacokinetics of acyclovir in this patient population. After a range of patient characteristics and clinical factors was evaluated, it was found that weight (with an allometric scaling function) and CLCR had significant influences on CL but that only weight influenced V of acyclovir in the final model. Inclusion of these factors, which described IIV for these parameters, significantly improved the population pharmacokinetic model performance based on the likelihood ratio test. The findings of the importance of these covariates are in agreement with those for a previous pharmacokinetic study of acyclovir (39). A considerable amount of pharmacokinetic variability still remains unaccounted for and may impact on the predictive ability of the final model.
Among children, CL and V are commonly weight related, and acyclovir dosage recommendations for children and young people are based on weight. The present study cohort of children and young people had a broad weight range (7.3 to 70.2 kg), and a clear trend toward increased acyclovir CL with increasing body weight was evident. A similar trend toward increased V with increasing body weight was also observed in this group of patients. The inclusion of weight in the population model resulted in large reductions in IIV of CL and V (32.8% and 39.2%, respectively).
Renal excretion is known to be an important elimination pathway for acyclovir (23), and hence, the influence of renal function on the acyclovir CL observed in this study is both physiologically plausible and expected. In the present study, a lower CLCR (liters/h) was associated with a decreased acyclovir CL. This finding is consistent with previous studies which showed reduction of acyclovir neurotoxicity (15) and improved clinical treatment outcome with reduced acyclovir doses (18) in patients who have impaired renal function.
We tested concomitant administration of MMF as a possible influential covariate on acyclovir CL but found no significant effects. Previous reports have shown conflicting results. Shah et al. (34) found no significant changes in either acyclovir or mycophenolic acid pharmacokinetics when these drugs were coadministered, relative to what was found when each was given alone, for 12 healthy subjects given single doses of 800 mg oral acyclovir and 1 g MMF. In another study, coadministration of valacyclovir and MMF did not lead to significantly altered values for acyclovir pharmacokinetic parameters, except for time to maximum concentration of drug in serum, which was about 0.5 h shorter with MMF (19). In contrast, Bullingham et al. (5) reported that maximum concentration of drug in serum and area under the curve (AUC) for acyclovir were increased by 18% when this drug was coadministered with MMF in healthy subjects, while Gimenez et al. (19) reported that the maximum concentration of drug in serum, time to maximum concentration of drug in serum, and AUC for acyclovir were significantly increased (by 40%, 0.38 h, and 31%, respectively) following coadministration of acyclovir and MMF in healthy subjects.
Population pharmacokinetic studies assist in evaluating the influence of significant patient factors on pharmacokinetic parameters to account for interpatient variability. With the use of this data analysis approach, concentration information from both intensive sampling and sparse sampling, which is common for pediatric patients with malignancy because of ethical, logistical, and medical considerations, can be utilized. This approach also provides valuable knowledge regarding population distribution of pharmacokinetic parameter values and factors influencing the variability of these values.
The only previously reported population pharmacokinetic analysis of acyclovir was performed by Tod et al. (39), with 79 children younger than 2 years of age (one to five samples per patient) administered an acyclovir oral suspension. The best pharmacokinetic model for describing oral acyclovir concentration-time data was a one-compartment model with first-order elimination. The apparent population mean CL was 25.5 liters/h, that for V was 37.0 liters, and that for ka was 0.28 h−1. Tod et al. also reported a two-compartment model for describing acyclovir disposition after intravenous dosing for 18 older children (nine samples per patient) and attempted to combine acyclovir oral and intravenous concentration-time data by using a two-compartment model to estimate the oral F value of acyclovir, which was found to be 0.118. As previously discussed (39), a possible limitation of their approach was that data for the intravenous dose were mainly from older children whereas data for the oral dose were mainly from younger children: the F values for these two groups may be different.
To investigate the suitability of acyclovir dosing regimens, a simulation study using the final population pharmacokinetic model was employed. The efficacy of acyclovir is dependent on the daily dose, the number of doses per day, and IC50 for the type of virus. Tod et al. (39) utilized clinical data (30) and other findings (6) to support the assumption that the length of time that acyclovir concentration remains above a given threshold (IC50) is an important criterion for efficacy. It has also been suggested that maximal efficacy is reached when the length of time that the acyclovir concentration remains above the IC50 is greater than 12 h in each 24-h dosing period (32, 38). For infections with VZV, a higher acyclovir AUC is required because of the higher acyclovir IC50 for VZV isolates (39). On the basis of these considerations, 0.56 mg/liter and 1.125 mg/liter, which correspond to a worst-case IC50 for HSV strains and a bad-case IC50 for VZV strains (39), respectively, were used as the IC50 thresholds in the present simulation study. The simulation results showed that the current dosing regimen (intravenous dosing at 5 mg/kg three times daily or oral dosing at 10 mg/kg twice daily) appears not to be appropriate for treatment of HSV and VZV infections in all children and young people, according to these criteria (body weight and renal function). Acyclovir exposure (the percentage of children who achieved maximal efficacy for HSV and VZV) was relatively low in lighter children with higher CLCRs, suggesting that lighter children with high CLCRs are relatively underdosed on 5 mg/kg intravenous acyclovir or 10 mg/kg oral valacyclovir and may have a greater chance of developing viral infections. Similarly, heavier children with low CLCRs have relatively high levels of exposure to acyclovir and may be relatively overdosed and have a greater chance of developing adverse reactions. Rational dosing strategies for acyclovir and valacyclovir antiviral therapy need to be considered for children and young people on the basis of body weight and renal function.
In conclusion, a one-compartment pharmacokinetic model describing the concentration-time profile of acyclovir observed after intravenous infusion dosing of acyclovir and oral dosing of valacyclovir has been developed and evaluated. Body weight and renal function were found to influence acyclovir CL, whereas the drug's V was influenced by body weight. Simulations performed with this population pharmacokinetic model suggest that patients with good renal function and/or lower body weight have a relatively high chance of being underdosed. Further studies developing new dosing schedules are required to ensure that the maximal antiviral efficacy of acyclovir is achieved in children and young people of various body weights and renal function levels.
Acknowledgments
Lihua Zeng is supported, in part, by NHMRC project grant 396702. Christa E. Nath is supported by the Leukemia Research Support Fund of the Children's Hospital at Westmead and by NHMRC project grant 396702.
We thank the nurses and allied clinical staff of the Children's Hospital at Westmead for taking blood samples and for patient care and the patients and their families for taking part in this study.
Footnotes
Published ahead of print on 4 May 2009.
REFERENCES
- 1.Balfour, H. H., Jr., B. A. Chace, J. T. Stapleton, R. L. Simmons, and D. S. Fryd. 1989. A randomized, placebo-controlled trial of oral acyclovir for the prevention of cytomegalovirus disease in recipients of renal allografts. N. Engl. J. Med. 320:1381-1387. [DOI] [PubMed] [Google Scholar]
- 2.Blum, M. R., S. H. Liao, and P. de Miranda. 1982. Overview of acyclovir pharmacokinetic disposition in adults and children. Am. J. Med. 73:186-192. [DOI] [PubMed] [Google Scholar]
- 3.Boeckh, M. 2006. Prevention of VZV infection in immunosuppressed patients using antiviral agents. Herpes 13:60-65. [PubMed] [Google Scholar]
- 4.Boeckmann, A. J., L. B. Sheiner, and S. L. Beal. 1994. NONMEM version 5.1.1 user's guide. GloboMax, Hanover, MD.
- 5.Bullingham, R. E., A. J. Nicholls, and B. R. Kamm. 1998. Clinical pharmacokinetics of mycophenolate mofetil. Clin. Pharmacokinet. 34:429-455. [DOI] [PubMed] [Google Scholar]
- 6.Bye, A. 1997. A mechanistic approach to understanding the response to antiinfectives in the population approach: measuring and managing variability in response, concentration and dose, p. 105-113. In L. Aarons (ed.), The population approach: measuring and managing variability in response, concentration and dose. COST B1 Medicine, European cooperation in the field of scientific and technical research, European Commission, Brussels, Belgium.
- 7.Counahan, R., C. Chantler, S. Ghazali, B. Kirkwood, F. Rose, and T. M. Barratt. 1976. Estimation of glomerular filtration rate from plasma creatinine concentration in children. Arch. Dis. Child. 51:875-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Miranda, P., and M. R. Blum. 1983. Pharmacokinetics of acyclovir after intravenous and oral administration. J. Antimicrob. Chemother. 12(Suppl. B):S29-S37. [DOI] [PubMed] [Google Scholar]
- 9.Eksborg, S., N. Pal, M. Kalin, C. Palm, and S. Soderhall. 2002. Pharmacokinetics of acyclovir in immunocompromized [sic] children with leukopenia and mucositis after chemotherapy: can intravenous acyclovir be substituted by oral valacyclovir? Med. Pediatr. Oncol. 38:240-246. [DOI] [PubMed] [Google Scholar]
- 10.Englund, J. A., C. V. Fletcher, and H. H. Balfour, Jr. 1991. Acyclovir therapy in neonates. J. Pediatr. 119:129-135. [DOI] [PubMed] [Google Scholar]
- 11.Ette, E. I., P. J. Williams, and J. R. Lane. 2004. Population pharmacokinetics III: design, analysis, and application of population pharmacokinetic studies. Ann. Pharmacother. 38:2136-2144. [DOI] [PubMed] [Google Scholar]
- 12.Ette, E. I., P. J. Williams, Y. H. Kim, J. R. Lane, M. J. Liu, and E. V. Capparelli. 2003. Model appropriateness and population pharmacokinetic modeling. J. Clin. Pharmacol. 43:610-623. [PubMed] [Google Scholar]
- 13.Ette, E. I. 1997. Stability and performance of a population pharmacokinetic model. J. Clin. Pharmacol. 37:486-495. [DOI] [PubMed] [Google Scholar]
- 14.Ette, E. I., and T. M. Ludden. 1995. Population pharmacokinetic modeling: the importance of informative graphics. Pharm. Res. 12:1845-1855. [DOI] [PubMed] [Google Scholar]
- 15.Feldman, S., J. Rodman, and B. Gregory. 1988. Excessive serum concentrations of acyclovir and neurotoxicity. J. Infect. Dis. 157:385-388. [DOI] [PubMed] [Google Scholar]
- 16.Feldman, S., and L. Lott. 1987. Varicella in children with cancer: impact of antiviral therapy and prophylaxis. Pediatrics 80:465-472. [PubMed] [Google Scholar]
- 17.Fletcher, C., and B. Bean. 1985. Evaluation of oral acyclovir therapy. Drug Intell. Clin. Pharm. 19:518-524. [DOI] [PubMed] [Google Scholar]
- 18.Gill, M. J., and E. Burgess. 1990. Neurotoxicity of acyclovir in end stage renal disease. J. Antimicrob. Chemother. 25:300-301. [DOI] [PubMed] [Google Scholar]
- 19.Gimenez, F., E. Foeillet, O. Bourdon, S. Weller, C. Garret, R. Bidault, and E. Singlas. 2004. Evaluation of pharmacokinetic interactions after oral administration of mycophenolate mofetil and valacyclovir or acyclovir to healthy subjects. Clin. Pharmacokinet. 43:685-692. [DOI] [PubMed] [Google Scholar]
- 20.Groeger, J. S., A. B. Lucas, H. T. Thaler, H. Friedlander-Klar, A. E. Brown, T. E. Kiehn, and D. Armstrong. 1993. Infectious morbidity associated with long-term use of venous access devices in patients with cancer. Ann. Intern. Med. 119:1168-1174. [DOI] [PubMed] [Google Scholar]
- 21.Holford, N. H. G. 1996. A size standard for pharmacokinetics. Clin. Pharmacokinet. 30:329-332. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson, M. O., and L. B. Sheiner. 1993. The importance of modelling interoccasion variability in population pharmacokinetic analyses. J. Pharmacokinet. Biopharm. 21:735-750. [DOI] [PubMed] [Google Scholar]
- 23.Laskin, O. L. 1983. Clinical pharmacokinetics of acyclovir. Clin. Pharmacokinet. 8:187-201. [DOI] [PubMed] [Google Scholar]
- 24.Ljungman, P., B. Lonnqvist, O. Ringden, P. Skinhoj, G. Gahrton, et al. 1989. A randomized trial of oral versus intravenous acyclovir for treatment of herpes zoster in bone marrow transplant recipients. Bone Marrow Transplant. 4:613-615. [PubMed] [Google Scholar]
- 25.Mosteller, R. D. 1987. Simplified calculation of body-surface area. N. Engl. J. Med. 317:1098. [DOI] [PubMed] [Google Scholar]
- 26.Nadal, D., G. Leverger, E. M. Sokal, D. Floret, Y. Perel, K. Leibundgut, and S. Weller. 2002. An investigation of the steady-state pharmacokinetics of oral valacyclovir in immunocompromised children. J. Infect. Dis. 186(Suppl. A):S123-S130. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien, J. J., and D. M. Campoli-Richards. 1989. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 37:233-309. [DOI] [PubMed] [Google Scholar]
- 28.Parke, J., N. H. Holford, and B. G. Charles. 1999. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput. Methods Programs Biomed. 59:19-29. [DOI] [PubMed] [Google Scholar]
- 29.Perry, C. M., and D. Faulds. 1996. Valaciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in herpesvirus infections. Drugs 52:754-772. [DOI] [PubMed] [Google Scholar]
- 30.Reitano, M., S. Tyring, W. Lang, C. Thoming, A. M. Worm, S. Borelli, L. O. Chambers, J. M. Robinson, L. Corey, and the International Valaciclovir HSV Study Group. 1998. Valaciclovir for the suppression of recurrent genital HSV infection: a large-scale dose range-finding study. J. Infect. Dis. 178:603-610. [DOI] [PubMed] [Google Scholar]
- 31.Rubin, J. 1987. Overdose with acyclovir in a CAPD patient. Perit. Dial. Int. 7:42-43. [Google Scholar]
- 32.Saiag, P., D. Praindhui, C. Chastang, and the Genival Study Group. 1999. A double-blind randomized study for assessing the equivalence of valaciclovir 1000 mg once daily versus 500 mg twice daily in the early treatment of recurrent genital herpes. J. Antimicrob. Chemother. 44:525-531. [DOI] [PubMed] [Google Scholar]
- 33.Saral, R., R. F. Ambinder, W. H. Burns, C. M. Angelopulos, D. E. Griffin, P. J. Burke, and P. S. Lietman. 1983. Acyclovir prophylaxis against herpes simplex virus infection in patients with leukemia. A randomized, double-blind, placebo-controlled study. Ann. Intern. Med. 99:773-776. [DOI] [PubMed] [Google Scholar]
- 34.Shah, J., D. Juan, and R. Bullingham. 1994. A single dose drug interaction study of mycophenolate mofetil and acyclovir in normal subjects. J. Clin. Pharmacol. 34:1029. [Google Scholar]
- 35.Sheiner, L. B. 1986. Analysis of pharmacokinetic data using parametric models. III. Hypothesis tests and confidence intervals. J. Pharmacokinet. Biopharm. 14:539-555. [DOI] [PubMed] [Google Scholar]
- 36.Simon, M. W., D. N. Fish, and R. G. Deeter. 2002. Pharmacokinetics and safety of valaciclovir in children with Epstein-Barr virus illness. Drugs R. D. 3:365-373. [DOI] [PubMed] [Google Scholar]
- 37.Soul-Lawton, J., E. Seaber, N. On, R. Wootton, P. Rolan, and J. Posner. 1995. Absolute bioavailability and metabolic disposition of valaciclovir, the l-valyl ester of aciclovir, following oral administration to humans. Antimicrob. Agents Chemother. 39:2759-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spruance, S. L., S. K. Tyring, B. de Gregoris, C. Miller, K. Beutner, and the Valaciclovir HSV Study Group. 1996. A large scale, placebo-controlled, dose ranging trial of peroral valaciclovir for episodic treatment of recurrent herpes genitalis. Arch. Intern. Med. 156:1729-1735. [PubMed] [Google Scholar]
- 39.Tod, M., F. Lokiec, R. Bidault, F. de Bony, O. Petitjean, and Y. Aujard. 2001. Pharmacokinetics of oral acyclovir in neonates and in infants: a population analysis. Antimicrob. Agents Chemother. 45:150-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wahlby, U., E. N. Jonsson, and M. O. Karlsson. 2002. Comparison of stepwise covariate model building strategies in population pharmacokinetic-pharmacodynamic analysis. AAPS PharmSci 4:E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wahlby, U., E. N. Jonsson, and M. O. Karlsson. 2001. Assessment of actual significance levels for covariate effects in NONMEM. J. Pharmacokinet. Pharmacodyn. 28:231-252. [DOI] [PubMed] [Google Scholar]
- 42.Weller, S., M. R. Blum, M. Doucette, T. Burnette, D. M. Cederberg, P. de Miranda, and M. L. Smiley. 1993. Pharmacokinetics of the acyclovir pro-drug valacyclovir after escalating single- and multiple-dose administration to normal volunteers. Clin. Pharmacol. Ther. 54:595-605. [DOI] [PubMed] [Google Scholar]
- 43.Zeng, L., C. E. Nath, P. J. Shaw, J. W. Earl, and A. J. McLachlan. 2008. HPLC-fluorescence assay for acyclovir in children. Biomed. Chromatogr. 22:879-887. [DOI] [PubMed] [Google Scholar]