Abstract
LeuO, a LysR family transcription factor, exists in a wide variety of bacteria of the family Enterobacteriaceae and is involved in the regulation of as yet unidentified genes affecting the stress response and pathogenesis expression. Using genomic screening by systematic evolution of ligands by exponential enrichment (SELEX) in vitro, a total of 106 DNA sequences were isolated from 12 different regions of the Escherichia coli genome. All of the SELEX fragments formed complexes in vitro with purified LeuO. After Northern blot analysis of the putative target genes located downstream of the respective LeuO-binding sequence, a total of nine genes were found to be activated by LeuO, while three genes were repressed by LeuO. The LeuO target gene collection included several multidrug resistance genes. A phenotype microarray assay was conducted to identify the gene(s) responsible for drug resistance and the drug species that are under the control of the LeuO target gene(s). The results described herein indicate that the yjcRQP operon, one of the LeuO targets, is involved in sensitivity control against sulfa drugs. We propose to rename the yjcRQP genes the sdsRQP genes (sulfa drug sensitivity determinant).
LeuO, a LysR-type transcriptional regulator of Escherichia coli, was originally identified as an activator of the leuABCD leucine synthesis operon (22). Later, LeuO was found to be involved in activation of bglGFB (utilization of β-d-glucoside) (58) and in repression of cadCBA (lysine decarboxylation) (50) and dsrA (encoding a regulatory small RNA for translational control of rpoS and hns) (47). Recently, LeuO was also found to regulate the yjjQ-bglJ operon, coding for a LuxR-type transcription factor (54), indicating that the genes under the control of YjjQ and BglJ are also regulated indirectly by LeuO. The accumulated data indicate that LeuO is not a specific regulator of the leu operon but a global regulator of apparently unrelated various genes. In Salmonella, LeuO activates ompS1 (encoding an outer membrane protein) (16) and ompS2 (encoding a pathogenicity determinant) (21), both influencing virulence in the mouse model (28, 48, 56, 61). Proteomic analysis of Salmonella showed that LeuO activates assT and the uncharacterized STY3070 gene and represses ompX and tpx (21). In Vibrio cholerae, LeuO is involved in control of biofilm formation (32) and in the stringent response (31). These observations altogether support the prediction that LeuO is a global regulator controlling a number of genes, including the stress response genes and virulence-related genes. In agreement with these predictions, the expression of leuO is low in exponential growth phase under steady-state growth conditions but is enhanced during transition into stationary phase (15).
The only LeuO target in E. coli identified so far is the intergenic sequence between the divergently organized yjjP and yjjQ-bglJ operons, where LeuO is involved in counteraction against silencing by H-NS (54). H-NS, one of the abundant nucleoid proteins, is now recognized as a global repressor, often called a silencer, controlling a number of cell process-related genes (13, 14, 34), including the genes for pathogenesis and the stress response and genes acquired by horizontal transfer (19, 23, 35, 41). A physiological role of LeuO has since been supposed to be counteraction against H-NS-mediated silencing (11, 53, 54). Except for this target, however, the whole set of genes under the direct control of LeuO has been left unidentified. To gain insight into the molecular basis of LeuO action, it is first necessary to identify the genes under its direct control. A shortcut approach is to search the entire E. coli genome for DNA recognition sequences of LeuO. For this purpose, we performed genomic screening by systematic evolution of ligands by exponential enrichment (SELEX), using LeuO purified from E. coli K-12 strain W3350.
A total of 12 LeuO-binding sequences have been identified, including the intergenic sequence between yjjP and yjjQ-bglJ, which is the only target hitherto identified (54). Each of the putative LeuO targets identified by genomic SELEX was analyzed by gel shift assays in vitro and by Northern blot analysis in vivo. Remarkably, some of these LeuO target genes are involved in the formation and/or function of multidrug efflux pumps. We then examined the possible influence of leuO disruption or LeuO overproduction on sensitivity to 240 species of drugs, using phenotype microarray (PM) analysis. As a result, LeuO expression was found to increase resistance against all seven sulfa drug species tested. A mutant lacking the yjcRQP operon, one of the LeuO targets, encoding a putative multidrug efflux pump, showed increased sensitivity to these sulfa drugs, even in the presence of LeuO expression, suggesting that YjcRQP is a sulfa drug efflux pump. We thus propose to rename the yjcRQP genes the sdsRQP genes (sulfa drug sensitivity determinant).
MATERIALS AND METHODS
Bacterial strains and plasmids.
Escherichia coli BW25113 (W3110 lacIq rrnBT14 ΔlacZWJ16 hsdR514 ΔaraBADAH33 ΔrhaBADLD78), leuO disruptant JW0075, hns disruptant JW1225, acrE disruptant JW3233, ygcL disruptant JW2730, and yjcR disruptant JW4043 were obtained from the Keio collection (3) via the E. coli Stock Center (National Institute of Genetics, Mishima, Japan). For the Northern blotting and Western blotting assays, cells were grown at 37°C under aeration in LB medium. Cell growth was monitored by measuring the turbidity at 600 nm.
For large-scale purification of LeuO, the expression plasmid pLeuO was constructed as follows. A DNA fragment corresponding to the LeuO coding sequence was amplified by PCR, using E. coli W3110 genome DNA as the template and a pair of primers designed to hybridize upstream or downstream of the LeuO coding sequence. After digestion with NdeI and NotI (note that the restriction enzyme sites were included within the primer sequences), the product was cloned into pET21a(+) (Novagen) between NdeI and NotI sites. The plasmid construct was confirmed by DNA sequencing.
For testing of LeuO transcriptional regulation of its target genes in vivo, the LeuO expression plasmid pHM109, with a chloramphenicol resistance marker gene, was isolated from the ASKA library. For maintenance of pHM109, chloramphenicol was added at a final concentration of 30 μg/ml, while for induction of LeuO expression, isopropyl-β-d-thiogalactopyranoside (IPTG) was added at 0.5 mM for 30 min.
Purification of LeuO protein.
An E. coli BL21(DE3) transformant with the LeuO expression plasmid pLeuO was grown in LB broth, and LeuO expression was induced by adding 1 mM IPTG. After 3 h of induction, cells were harvested and subjected to LeuO purification. Protein purification was carried out according to the standard procedure used in our laboratory (39, 40, 52). In brief, lysozyme-treated cells were sonicated in the presence of 100 mM phenylmethylsulfonyl fluoride. After centrifugation of cell lysate (30 ml) at 15,000 rpm for 60 min at 4°C, the resulting supernatant was mixed with 2 ml of 50% Ni-nitrilotriacetic acid (Ni-NTA) agarose solution (Qiagen) and loaded onto a column. After being washed with 10 ml of lysis buffer, the column was washed with 10 ml of washing buffer (50 mM Tris-HCl, pH 8.0, at 4°C, 100 mM NaCl). Proteins were then eluted with 2 ml of an elution buffer (200 mM imidazole, 50 mM Tris-HCl, pH 8.0, at 4°C, 100 mM NaCl) and dialyzed against a storage buffer (50 mM Tris-HCl, pH 7.6, 200 mM KCl, 10 mM MgCl2, 0.1 mM EDTA, 1 mM dithiothreitol, and 50% glycerol). The LeuO used throughout this study was >95% pure, as analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
SELEX search for LeuO-binding sequences.
The substrate DNA for SELEX screening was prepared by PCR amplification of 200- to 300-bp E. coli DNA fragments, using a plasmid library as the template, with each plasmid carrying an E. coli genome fragment of 200 to 300 bp in length (51, 52). For SELEX screening, 5 pmol of DNA fragments and 10 pmol of His-tagged LeuO were mixed in a binding buffer (10 mM Tris-HCl, pH 7.8, at 4°C, 3 mM magnesium acetate, 150 mM NaCl, 1.25 mg/ml bovine serum albumin) and incubated for 30 min at 37°C. The mixture was applied to a Ni-NTA column, and after washing of unbound DNA with binding buffer containing 10 mM imidazole, DNA-LeuO complexes were eluted with an elution buffer containing 200 mM imidazole. DNA fragments recovered from the complexes were PCR amplified as described above. After two cycles of SELEX, several discrete bands were identified by PAGE. For sequencing of LeuO-bound DNA fragments, PCR products were cloned into pT7 Blue-T vector (Novagen) and transformed into E. coli DH5α. Sequencing was carried out using the T7 primer (5′-TAATACGACTCACTATAGGG-3′).
Gel mobility shift assay.
Gel shift assays were performed as described previously (39, 40, 52). In brief, probes were generated by PCR amplification of LeuO-binding sequences obtained by SELEX, using a pair of primers, 5′-fluorescein isothiocyanate (FITC)-labeled T7-F primer (5′-TAATACGACTACTATAGGG-3′) and T7-R primer (5′-GGTTTTCCCAGTCACACGACG-3′), plasmids containing the respective LeuO recognition sequences as templates, and Ex Taq DNA polymerase (Takara). FITC-labeled PCR products were purified by PAGE. For gel shift assays, 0.3 pmol of each FITC-labeled probe was incubated at 37°C for 30 min with various amounts of LeuO in 12 ml of gel shift buffer consisting of 10 mM Tris-HCl, pH 7.8, at 4°C, 150 mM NaCl, and 3 mM magnesium acetate. After addition of a DNA dye solution, the mixture was directly subjected to 6% PAGE. Fluorescently labeled DNA in gels was detected using a Pharos FX Plus system (Bio-Rad).
Northern blot analysis.
Total RNAs were extracted from exponentially growing E. coli cells (optical density at 600 nm [OD600] = 0.5) by the hot phenol method (1). RNA purity was checked by electrophoresis on a 2% agarose gel in the presence of formaldehyde, followed by staining with ethidium bromide. Digoxigenin (DIG)-labeled probes were prepared by PCR amplification, using W3110 genomic DNA as a template, DIG-11-dUTP (Roche) and deoxynucleoside triphosphates as substrates, gene-specific forward and reverse primers (see Table S1 in the supplemental material for primer sequences), and Ex Taq DNA polymerase (Takara, Japan). Total RNAs (4 μg) were incubated in formaldehyde-MOPS (morpholinepropanesulfonic acid) gel loading buffer for 10 min at 65°C for denaturation, subjected to electrophoresis on a formaldehyde-containing 2% agarose gel, and then transferred to a nylon membrane (Roche). Hybridization was performed with the DIG Easy Hyb system (Roche) at 50°C overnight with a DIG-labeled probe. For detection of the DIG-labeled probe, the membrane was treated with anti-DIG-AP Fab fragments and CDP-Star (Roche), and the image was scanned with an LAS-4000 IR multicolor scanner (Fuji Film). The product size of RNA was estimated on the basis of migration of RNA markers (Toyobo).
Western blot analysis.
Antibodies against LeuO were raised in rabbits by injection with the purified LeuO protein. Western blot analysis was carried out by a standard method, as described previously (26). In brief, E. coli cells grown in 0.5 ml of LB medium were harvested by centrifugation and resuspended in 0.2 ml of lysis buffer (50 mM Tris-HCl, pH 7.5, 50 mM NaCl, 5% glycerol, and 1 mM dithiothreitol), and then lysozyme was added to a final concentration of 20 μg/ml. After sonication, 5 μg of cell extract was subjected to 10% SDS-PAGE and blotted onto a polyvinylidene difluoride membrane by use of a semidry transfer apparatus. Membranes were first subjected to immunodetection with anti-LeuO and then developed with an enhanced chemiluminescence kit (Amersham Pharmacia Biotech). The image was analyzed with an LAS-4000 IR multicolor scanner (Fuji Film).
PM assay for drug sensitivity test.
A PM assay was performed essentially according to previously published methods (5, 64), using Biolog PM plates (Biolog Inc., CA). E. coli BW25113 cells transformed with either the LeuO expression plasmid pHM109 or vector pCA24 were grown overnight at 37°C in M9-glucose (0.4%) medium containing chloramphenicol. Cells were washed with IF-0 GN Base inoculating fluid (Biolog Inc., CA) and then resuspended in IF-10 GN Base inoculating fluid (Biolog Inc., CA) containing 2.0 g of tryptone, 1.0 g of yeast extract, and 1.0 g of NaCl per liter at a density corresponding to 85% transmittance (OD420, ∼0.12), using a 20-mm-diameter tube. Tetrazolium violet, IPTG, and chloramphenicol were added at final concentrations of 0.01%, 25 mM, and 30 mg/ml, respectively. The suspensions were then inoculated into the appropriate microplates (PM11 to -20) for a sensitivity test with various chemicals (Biolog Inc., CA), using a volume of 100 μl/well. Cell growth was monitored by measuring the respiration-dependent color change of tetrazolium violet in each well. The microplates were thus placed in an OmniLog instrument at 37°C, and light transmittance was monitored with an OmniLog reader (Biolog Inc., CA) at 15-min intervals for 36 h. The OmniLog-PM software generates a time course for tetrazolium color formation (respiration) for cells with and without the LeuO expression plasmid and calculates differences in the growth rates between the control without LeuO expression and the test sample with LeuO expression. The units are arbitrary. Positive values indicate that the cells with the LeuO expression plasmid pMH109 showed greater rates of respiration than the control cells with the vector plasmid pCA24. Negative values indicate that the control cells in the absence of LeuO expression showed greater rates of respiration than the test samples with LeuO expression. The PM assay was performed twice.
RESULTS AND DISCUSSION
Isolation of LeuO-binding sequences by genomic SELEX.
To gain insights into the entire network of transcription regulation of the E. coli genome by the transcription factor LeuO, an attempt was made to identify the whole set of target genes under the direct control of LeuO. For this purpose, we performed a systematic search of LeuO recognition sequences by using the genomic SELEX system, as successfully employed for searches of regulation targets of Cra (51), RutR (52), RstA (39), PdhR (40), NemA (59), AllR (20), and CitB (63). A mixture of E. coli genome DNA fragments of 200 to 300 bp in length was mixed with purified His-tagged LeuO protein, and DNA-LeuO complexes were affinity isolated with the use of Ni-NTA agarose. After two cycles of SELEX, LeuO-bound DNA fragments formed several sharp bands on PAGE gels (data not shown). After sequencing of isolated SELEX fragments, a total of 106 independent sequences were identified, and these were located in 12 regions on the E. coli genome (see Table 1 and Fig. 1 for gene organization; see Table S2 in the supplemental material for locations on the genome). All 12 regions are located within intergenic spacers, where transcription regulation signals, such as promoters and transcription factor-binding sites, are located. One of the SELEX fragments was located at the intergenic region between yjjP, encoding a predicted membrane protein, and the yjjQ-bglJ operon, encoding two LuxR-type transcriptional regulators for pathogenesis control. After analysis of in vivo transcription, Stratmann et al. (54) indicated that this LeuO site controls transcription of the yjjQ and bglJ genes. BglJ is believed to regulate the bgl operon for glucoside metabolism. If this is the case, then the genes under the control of the YjjQ and BglJ transcription factors should be regulated indirectly by LeuO, together forming LeuO-YjjQ and LeuO-BglJ hierarchies in the regulation network. The other 11 regions with LeuO-binding sequences are newly identified in this study (Table 1 and Fig. 1).
TABLE 1.
LeuO-binding DNA fragments isolated by genomic SELEXa
| No. of clones | Left gene | Gene function | Right gene | Gene function | Presence of H-NS site |
|---|---|---|---|---|---|
| 53 | envR (←) | Transcriptional regulator | (→) acrE | Multidrug efflux pump | + |
| 17 | ybdO (←) | Transcriptional regulator | (←) dsbG | Disulfide isomerase | + |
| 12 | ygcL (←) | Unknown | (←) ygcB | Unknown | + |
| 11 | yjjP (←) | Unknown | (→) yjjQ | Transcriptional regulator | + |
| 3 | yjcR (←) | Sulfa drug efflux pump | (←) yjcS | Unknown | + |
| 2 | ybbP (→) | Transporter | (→) rhsD | Unknown | + |
| 2 | citC (←) | Citrate lyase synthesis | (→) dpiB | Two-component system | + |
| 2 | yjiY (←) | Membrane protein | (→) tsr | Serine chemoreceptor | + |
| 1 | entF (→) | Enterobactin synthase | (→) fepE | O-antigen polymerization | + |
| 1 | ygdH (→) | Unknown | (→) sdaC | l-Serine degradation | − |
| 1 | uxaC (←) | d-Galacturonate degradation | (→) exuT | Hexuronate transporter | − |
| 1 | gltD (→) | Glutamate synthase | (→) gltF | Periplasmic protein | + |
A total of 106 DNA fragments were isolated by genomic SELEX screening. After being sequenced, these fragments were found to be located in 12 regions on the E. coli genome. Arrows show the direction of gene transcription. Bold type indicates that the gene's location is downstream from the LeuO-binding site. The presence of the H-NS binding site within the indicated LeuO-binding regions is from the work of Oshima et al. (41).
FIG. 1.
Gene organization of operons that form complexes in vitro with purified LeuO protein. (A) Operons that are activated in vivo by LeuO, as detected by Northen blot analysis (see Fig. 3). (B) Operons that are repressed by LeuO. Symbols: closed circles, promoters; open squares, H-NS binding sites (from the work of Oshima et al. [41]).
A total of 53 SELEX clones contained sequences from the intergenic region between the divergently transcribed envR and acrEF genes. The acrEF gene products form a multidrug efflux pump of the RND (major nodulation-cell division) family, while EnvR is predicted to be a repressor of acrEF (36). A total of 12 SELEX clones contained a sequence upstream of the ygcLKJKH-ybgTF operon (Fig. 1), which is under the control of the BaeSR two-component system, the regulator of the yegMNOB (mdtABCD) multidrug transporter genes (4). In addition, LeuO-binding sequences were also identified on the promoter region of another multidrug efflux pump operon, yjcRQP (mdtNOP) (12, 44, 55). In fact, a mutant defective in yjcP (mdtP) showed increased susceptibility to acriflavin, puromycin, and tetraphenylarsonium chloride (54). Taken together, the results show that a set of genes for multidrug resistance seems to be under the control of LeuO, including the AcrEF, YgcLKJIH-YgbTF, and MdtNOP genes.
LeuO-binding sites were identified upstream of genes encoding membrane proteins involved in the response to external stresses (Table 1 and Fig. 1), including yjiY, encoding a predicted inner membrane protein (10); tsr, encoding the serine chemoreceptor for chemotaxis control (2); fepE, encoding a homolog of the Salmonella wzz gene, which controls the genes for ferric enterobactin transporter (42) and the chain length of lipopolysaccharide O antigen, which influences pathogenesis and colonization in humans and mice (6, 33); the sdaCB operon, encoding l-serine deaminase (SdaB) and l-serine transporter (SdaC), a determinant of colicin sensitivity (18, 49); the exuTR operon, encoding hexuronate transporter (ExuT); the divergently transcribed uxaCA operon, encoding enzymes for d-glucuronate degradation (45); and gltF, a member of the glutamate synthase operon, encoding a putative periplasmic protein (7). Likewise, LeuO binding was detected upstream of the rhsB operon, which is induced by sulfate starvation (60). The uncharacterized protein RhsB carries a hydrophilic domain with repetitive Rhs sequence elements and a divergent C-terminal sequence and is supposed to play a role in sensing sulfate availability (17).
It is noteworthy that LeuO binding was also detected upstream of the genes for three transcription factors, including an as yet uncharacterized LysR-type YbdO protein, a two-component system, CitAB, controlling citrate-specific fermentation genes (37, 62, 63), and ExuR, controlling the hexuronate transport operon (45). Thus, LeuO stays at the top of the hierarchy of LeuO-YbdO, LeuO-CitAB, and LeuO-ExuR networks. The signal(s) sensed by LeuO could be expanded through these transcription factor networks.
LeuO binding in vitro to SELEX fragments.
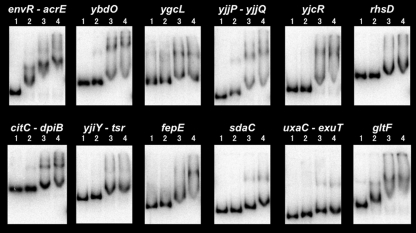
Using genomic SELEX screening, we isolated a total of 106 LeuO-binding sequences from 12 regions of the E. coli genome (Table 1). To confirm the LeuO-binding activity of these sequences, we performed a gel shift assay. For this purpose, we prepared 12 species of FITC-labeled DNA probe of approximately 500 bp in length, each including the sequence upstream from the initiation codon of the putative target gene and the LeuO-binding sequence, by PCR. The gel shift assay was performed using three different concentrations of purified LeuO protein (Fig. 2). Among the 12 SELEX sequences, the binding of LeuO has so far been identified only for the yjjP-yjjQ intergenic sequence (54). In good agreement with the published report, the yjjP-yjjQ probe formed a clear complex band with the LeuO protein. All 11 other probes also formed complexes with LeuO, indicating that the newly identified LeuO recognition sequences indeed harbor binding affinity for LeuO. The number of SELEX isolates correlates with the in vitro binding affinity of the test transcription factor for the target sequences within the SELEX fragments (51, 52, 63). In good agreement with this prediction, the most abundant, envR-acrE intergenic sequence showed the highest affinity to LeuO because the LeuO-probe complexes were formed at the lowest protein concentration used.
FIG. 2.
Gel shift assay of LeuO-SELEX fragment interaction. An 8 nM concentration of each of the indicated fluorescently labeled DNA probes was incubated at 37°C for 15 min in the absence (lanes 1) or presence of 2.0 (lanes 2), 4.0 (lanes 3), and 8.0 nM (lanes 4) purified LeuO and then directly subjected to electrophoresis on a 5% polyacrylamide gel.
The consensus sequence of LeuO binding has not yet been identified, even though a certain level of preference for AT-rich sequences was predicted (9). On the basis of the location of transcription factor-binding sites in the yjjP-yjjQ intergenic region, a hypothesis was proposed that the site of LeuO binding overlaps with that of H-NS and thus that the function of LeuO is a counteraction to H-NS-mediated transcription silencing (21, 54). In agreement with this prediction, H-NS also prefers AT-rich sequences for binding in vitro (57). A recent chromatin immunoprecipitation-oligonucleotide chip analysis indicated the location of H-NS binding in vivo along the E. coli genome (41). It is noteworthy that most of the LeuO target genes identified by SELEX contain the H-NS binding site at their respective promoter regions (Fig. 1).
Intracellular level of LeuO protein.
In general, transcription factors bind to promoter regions of target genes, and the regulation mode between repression and activation correlates with the position of their binding relative to the respective transcription start site (24, 25). Taking this general rule into consideration, we estimated which target genes are under the control of LeuO (see Table 1 for the relationship between the LeuO-binding site and the transcription direction of neighboring genes and Fig. 1 for the gene organization of these putative LeuO target genes). The possible influence of LeuO on transcription of the predicted target genes was then analyzed in vivo by Northern blot analysis. For setup of culture conditions for RNA sampling, we first determined the intracellular level of LeuO protein by quantitative Western blot analysis, using anti-LeuO antibody raised in rabbits against purified LeuO.
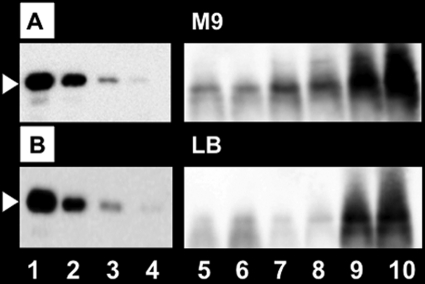
The intracellular level of LeuO in wild-type E. coli was measured for both M9-glucose culture (Fig. 3A) and LB culture (Fig. 3B). In the exponential growth phase, the LeuO level was very low in the poor medium and below the detection level in the rich medium. Upon entry into the stationary phase, the LeuO level increased markedly, in good agreement with published results (8, 15). In the stationary phase, the LeuO level was also higher in the minimal medium culture (Fig. 3A) than in the rich medium culture (Fig. 3B). The leuO gene is repressed by H-NS, a global silencer, and is activated by LeuO itself (8). Even in the absence of H-NS, however, LeuO was hardly detected in log-phase cultures in rich medium (Fig. 4A, lanes 1 to 3).
FIG. 3.
Intracellular level of LeuO protein. Wild-type E. coli was grown at 37°C in either M9-0.4% glucose medium (A) or LB medium (B) for various times and then subjected to quantitative Western blot analysis (26) for determination of the LeuO level. Lanes 1 to 4, 10, 5, 2.5, and 1 ng purified LeuO protein; lanes 5 to 10, extracts of cells harvested at OD600s of 0.15, 0.3, 0.6, 1.2, 5.0 (48 h), and 5.0 (72 h).
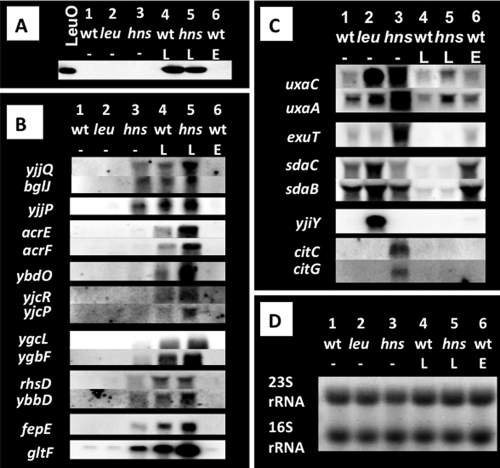
FIG. 4.
Northern blot analysis of LeuO-dependent gene transcripts. Wild-type E. coli BW25113 (lanes 1; wt), leuO deletion mutant JW0075 (lanes 2; leu), hns deletion mutant JW1225 (lanes 3; hns), the wild type with pLeuO (lanes 4; wt), the hns mutant with pLeuO (lanes 5; hns), and the wild type with empty vector (lanes 6; wt) were grown at 37°C in LB medium. (A) Whole-cell extracts were prepared from cells harvested at an OD600 of 0.5 and subjected to Western blot analysis against anti-LeuO protein. (B) Total RNA was isolated from each culture, and 4 μg was fractionated by PAGE in the presence of urea. After blotting of RNAs onto filters, transcripts of the LeuO target genes were detected with the fluorescently labeled probes indicated on the left. (C) Total RNA was subjected to Northern blot analysis as in panel B. Probes used are indicated on the left. (D) Total RNAs used for Northern blot analysis were fractionated by PAGE, and the gel was stained with ethidium bromide for detection of 16S and 23S rRNAs.
Since the expression level of LeuO is very low in exponential growth phase, the influence of LeuO has been examined under LeuO expression conditions by use of a LeuO expression plasmid (11, 21, 54). We next employed an induced LeuO expression system for analysis of the influence of LeuO on in vivo transcription of the target genes identified by SELEX. Wild-type E. coli BW25113, the isogenic leuO mutant JW0075, and the hns mutant JW1225 were transformed with either pMH109 for LeuO expression or pCA24 vector as a control. After Western blot analysis, the expression of LeuO was confirmed for all strains transformed with pMH109 (Fig. 4A, lanes 4 and 5). The expression level of LeuO in pMH109-transformed cells was slightly higher than that in stationary-phase wild-type cells without the expression plasmid (data not shown).
In vivo transcription of putative LeuO target genes.
RNA samples were prepared from each of these strains and subjected to Northern blot analysis (Fig. 4B and C). The amounts of RNA analyzed were almost the same between test samples, as detected by measuring rRNAs (Fig. 4D). The level of RNA was measured for each of the predicted regulation target genes.
(i) Genes activated by LeuO.
The only LeuO targets identified so far are the divergent operons yjjQ-bglJ and yjjP (54). The mRNA levels of these two operons were first determined by using yjjQ, bglJ, and yjjP probes (Fig. 4B). With both the yjjQ and bglJ probes, a single RNA band of approximately 1.5 kb, corresponding to yjjQ-bglJ bicistronic mRNA, was detected in both wild-type and hns mutant cells when LeuO was overexpressed (Fig. 4B, yjjQ and bglJ panels, lanes 4 and 5). In the absence of LeuO expression, this signal was not detected for both the leuO mutant and the wild type (Fig. 4B, yjjQ and bgl panels, lanes 1, 2, and 6), in agreement with the low-level expression of LeuO under the culture conditions employed. In the hns mutant, a significant level of yjjQ-bglJ RNA was detected, but it increased after overproduction of LeuO (Fig. 4B, yjjQ and bglJ panels, lanes 3 and 5). Next, we analyzed transcription in the opposite direction. An approximately 1.0-kb transcript of the divergently organized yjjP gene was detected in the presence of overexpressed LeuO or in the absence of H-NS (Fig. 4B, yjjP panel, lanes 3 to 5), indicating that LeuO activates transcription in both directions. The experimental results are consistent with a previous proposal (54).
Using the same RNA samples and the same reaction conditions, we then performed Northern blot analysis against all other newly identified LeuO target genes. Using acrE and acrF probes, we detected a 4.5-kb RNA signal that corresponds to the predicted size of a full-length transcript of the acrEF operon, encoding a multidrug efflux pump (Fig. 4B). This acrEF RNA was detected in both wild-type and hns mutant cells, only with induced expression of LeuO (Fig. 4B, acrE and acrF panels, lanes 4 and 5). In the presence of LeuO expression, the level of acrEF mRNA was twofold higher in the hns mutant than in the wild type. In the absence of the LeuO expression plasmid, acrEF RNA was not detected in both the wild type and the hns mutant (Fig. 4B, acrE and acrF panels, lanes 1 and 3). In contrast to transcription of the yjjQ-bglJ operon (see above), the low level of LeuO in the wild type is not enough for activation of acrEF, even in the absence of the H-NS silencer. The envR gene, encoding a transcription regulator, is transcribed in the opposite direction from that of the acrEF operon, but envR mRNA was not detected in the presence or absence of LeuO and H-NS (data not shown), implying that LeuO activates only the acrEF operon. Among 12 LeuO-binding spacer sequences analyzed herein, this yjjP--S-yjjQ-bglJ system (where “S” indicates the SELEX fragment described in Table S2) is the only case in which LeuO regulates both divergently transcribed operons.
With the ybdO probe, a single, 1.0-kb-long RNA band for the ybdO gene, encoding an as yet uncharacterized transcription factor, was detected (Fig. 4B). A single, 5.0-kb-long RNA encoding a predicted YjcRQP drug efflux pump was detected with the use of both yjcR and yjcP probes (Fig. 4B). These mRNAs were detected in the same cultures as in the case of acrEF mRNA. Thus, we concluded that both the ybdO and yjcRQP operons are under the control of the LeuO activator and the H-NS repressor.
Seven genes, ygcLKJIH-ygbTF, have been predicted to form a single operon (27) (see Fig. 1 for gene organization). Using the most upstream (ygcL) and the most downstream (ygbF) probes, we detected a single RNA band of about 6.0 kb, which agrees with the predicted size of a ygcLKJIH-ygbTF polycistronic transcript (Fig. 4B). This transcript was detected in both wild-type and hns mutant cells when LeuO was overexpressed, as in the cases of acrEF, ybdO, and yjcRQP. The level of mRNA was again higher in the hns mutant than in the wild type.
The other three RNA species, a 6.0-kb rhsD-ybbC-ylbH-ybbD RNA detected with the rhsD and ybbD probes, a 1.5-kb fepE RNA detected with the fepE probe, and a 1.0-kb gltF RNA detected with the gltF probe (Fig. 4B), could be detected in the hns mutant without the LeuO expression plasmid, and the signal intensity increased by adding the LeuO expression plasmid. The gltF gene is located downstream of gltBD, coding for glutamate synthase, and was thought to form a single operon with gltBD (7). Northern blot analysis, however, indicated that gltF mRNA is approximately 1.0 kb long, suggesting that it is a monocistronic mRNA and that the gltF gene carries its own promoter. In concert with this prediction, there is a long spacer of 560 bp in length between gltBD and gltF. The gltF mRNA was detected in the hns mutant without the LeuO expression plasmid, but its level increased by introducing the LeuO expression plasmid (Fig. 4B, gltF panel, lanes 3 and 5). In the presence of the LeuO expression plasmid, gltF mRNA was detected even in the wild-type strain (Fig. 4B, gltF panel, lane 4).
These results altogether indicate that LeuO activates the transcription of at least nine target operons, one previously identified and eight newly identified, with LeuO-binding sites (the yjjQ-bglJ and yjjP operons share the same LeuO-binding site) (see Fig. 1 for the H-NS site). Transcription activation of these operons was observed in the absence of H-NS and was significantly enhanced after overexpression of LeuO in both wild-type and hns mutant cells; the mode of LeuO action may be attributable to both the interference of silencing by H-NS, as proposed in the counteraction model between H-NS and transcription factors (11, 53, 54), and transcription activation by direct interaction with RNA polymerase, as in the cases of some other LysR family transcription factors (24, 25, 30).
(ii) Genes repressed by LeuO.
Some of the newly identified LeuO target genes were repressed in the presence of LeuO (Fig. 4C; see Fig. 1 for the gene organization). Three RNA species, a 3.9-kb uxaCA RNA detected with the uxaA probe, a 3.0-kb sdaCB mRNA detected with both the sdaC and sadB probes, and a yjiY RNA detected with the yjiY probe, were significantly increased in the leuO mutant compared with the wild type (Fig. 4C), implying that these three operons are repressed by LeuO. Downstream of the yjiY gene, coding for a starvation protein A (cspA) homolog, there are two uncharacterized genes, yjiX and yjiA, with a GTPase motif (see Fig. 1 for the gene organization), but it remains unidentified whether these two genes are cotranscribed with the yjiY gene. mRNA for the tsr gene for the serine sensor receptor, which is transcribed in the opposite direction from yjiY, was not detected irrespective of the presence or absence of LeuO and H-NS (data not shown), leaving it uncertain whether the tsr gene is under the control of LeuO. Downstream of the uxaCA genes is an uncharacterized ygjY gene, coding for a conserved inner membrane protein (see Fig. 1 for the gene organization), but at present, it is not known whether this ygjY gene is cotranscribed with the uxaCA operon. Supporting the repression model, the uxaCA, sdaCB, and yjiY mRNAs all decreased to undetectable levels after overexpression of LeuO (Fig. 4C, lanes 1, 2, 4, and 6). Taking these results together, we concluded that LeuO acts as a repressor for transcription of the uxaCA operon, encoding the enzymes for galacturonate catabolism, the sdaCB operon, encoding serine degradation enzymes, and the uncharacterized yjiY gene. Among these three operons under the repression of LeuO, the level of uxaCA mRNA increased in the hns mutant (Fig. 4C, uxaCA panels, lane 3). On the other hand, both the sdaCB and yjiY mRNAs were not detected, even in the absence of H-NS (Fig. 4C, sdaCB and yjiY panels, lane 3).
The exuTR operon is transcribed in the opposite direction from the uxaCA operon, and H-NS binds in vivo in the spacer region between the uxaCA and exuTR operons (Fig. 1). The level of exuT mRNA was increased in the hns mutant, as in the case of uxaCA mRNA (Fig. 4C). Among the 12 LeuO-binding sequences identified herein, 10 are included in the library of in vivo H-NS-binding sequences on the E. coli genome (Table 1 and Fig. 1) (41). In the case of the divergently transcribed uxaCA and exuTR operons, however, the H-NS binding site was not detected near the LeuO-binding site within the spacer region. Thus, it remains unsolved how H-NS influences transcription of these two operons.
The expression of the uxaCA and exuT operons in the hns mutant was also repressed by LeuO overproduction (Fig. 4C). The activation was not detected with the wild-type strain (Fig. 4B). In contrast, the repression by LeuO was detected with wild-type cells (Fig. 4C). A possible explanation for this disagreement is that transcription activation requires high concentrations of LeuO, while repression takes place even with low concentrations of LeuO. One possible mechanism of transcription activation by LeuO is the interference of silencing of the target promoters by H-NS. For effective competition with H-NS, high levels of LeuO may be needed. It is noteworthy that there is a lack of a detectable binding site for the general silencer H-NS within the repression-type sdaCB, uxaT, and uxaCA promoters (41).
With the use of citC and citG probes, a 6.0-kb RNA of the citCDEFXG operon, encoding citrate lyase, was detected only in the hns mutant strain (Fig. 4C, citC and citG panels, lane 3). Derepression of the cit operon in the hns mutant was cancelled, however, by introduction of the LeuO expression plasmid (Fig. 4C, citC and citG panels, lane 5). Since mRNA for the cit operon was not detected under the culture conditions employed, the effect of LeuO on transcription of the cit operon remains unidentified.
Analysis of role of LeuO in drug resistance by PM assay.
It is noteworthy that the set of operons activated by LeuO includes several genes that are involved in drug resistance, such as acrEF, encoding a multidrug efflux pump, yjcRQP, encoding a putative multidrug efflux pump, and ygcLKJIH-ygbTF, regulated by BaeR, which controls the genes for novobiocin and deoxycholate resistance (4). So far, five families of drug extrusion translocases have been identified based on sequence similarity (43, 46), consisting of the MFS (major facilitator superfamily), SMR (small multidrug resistance), RND (resistance-nodulation-cell division), ABC (ATP-binding cassette), and MATE (multidrug and toxic compound extrusion) families. In E. coli, a total of 37 putative drug transporter genes (encoding 19 MFS, 3 SMR, 7 RND, 7 ABC, and 1 MATE protein) were found by sequence annotation. The major multidrug efflux system has been considered to be composed of the AcrAB-TolC proteins (29). Systematic attempts have since been made to identify the physiological roles of genes related to drug resistance (38, 55), but the roles of other predicted drug efflux systems still remain unsolved. The results described above suggest that LeuO activates at least three operons related to drug sensitivity control. AcrEF belongs to the RND family of drug efflux transporters, while several lines of evidence suggest that the uncharacterized yjcRQP and ygcLKJIH-ygbTF operons are also involved in drug efflux, thus controlling sensitivity against drugs (4, 12, 36).
To reveal the possible involvement of the LeuO-regulated genes in drug resistance and to identify drug species under the control of LeuO, we performed a PM assay under a total of 960 culture conditions (four different concentrations of each of 240 drug chemicals). We measured the growth of wild-type E. coli in the absence and presence of LeuO expression, using the PM assay system. The time course of cell growth was monitored by measuring the cell density-dependent increase in respiration (see Materials and Methods).
The LeuO overexpression strain exhibited slower growth in the presence of 27 drug species (Table 2; the whole set of growth patterns is available upon request). LeuO-induced high-level expression of certain membrane components, including some drug efflux pump proteins, might lead to membrane disintegration, leading to interference with certain membrane functions and to a permeability increase for a group of drugs. In the presence of 14 other drug species, however, the growth rate of the LeuO expression strain was faster than that of the control without LeuO expression (Table 2). LeuO expression was supposed to enhance the expression of a multidrug efflux pump(s) for this group of drugs, leading to an increase in drug resistance.
TABLE 2.
Sensitivity test of wild-type E. coli with LeuO expression plasmid against various chemicalsa
| Test chemical | Growth difference | Mode of action | Plate/well |
|---|---|---|---|
| Chemicals that enhanced growth of wild-type E. coli with LeuO expression plasmid | |||
| 2,2′-Dipyridyl | 41 | Chelator, lipophilic | PM13/B05-08 |
| Furaltadone | 41 | DNA synthesis, nitro-compound | PM14/A05-08 |
| Sulfamethazine | 42 | Folate antagonist | PM12/D05-08 |
| Sulfadiazine | 64 | Folate antagonist | PM12/E05-08 |
| Sulfathiazole | 54 | Folate antagonist | PM12/F05-08 |
| Sulfamethoxazole | 52 | Folate antagonist | PM12/G05-08 |
| Sulfanilamide | 75 | Folate antagonist | PM16/B05-08 |
| Sulfachloropyridazine | 64 | Folate antagonist | PM17/C05-08 |
| Sulfamonomethoxine | 44 | Folate antagonist | PM17/C09-12 |
| d-Serine | 122 | Inhibits 3PGA dehydrogenase | PM17/A01-04 |
| Urea hydrogen peroxide | 85 | Oxidizing agent | PM15/F01-04 |
| Chlortetracycline | 41 | Protein synthesis, tetracycline | PM11/A05-08 |
| Rifamycin SV | 52 | RNA polymerase | PM12/A09-12 |
| Ethylenediamine | 50 | Transport, toxic cation | PM18/F01-04 |
| Chemicals that retarded growth of wild-type E. coli with LeuO expression plasmid | |||
| Fusaric acid | −107 | Chelator, lipophilic | PM14/B05-08 |
| 5,7-Dichloro-8-hydroxyquinaldine | −93 | Chelator, lipophilic | PM15/B09-12 |
| 1,10-Phenanthroline | −82 | Chelator, lipophilic | PM15/C09-12 |
| Caffeine | −105 | Cyclic AMP phosphodiesterase | PM17/H05-08 |
| Promethazine | −96 | Cyclic nucleotide phosphodiesterase | PM14/H05-08 |
| Oxolinic acid | −94 | DNA topoisomerase | PM13/B09-12 |
| Ofloxacin | −131 | DNA topoisomerase, quinolone | PM11/H09-12 |
| Lomefloxacin | −81 | DNA topoisomerase, quinolone | PM11/B09-12 |
| Chloroxylenol | −211 | Fungicide | PM16/H05-08 |
| Dichlofluanid | −97 | Fungicide, phenylsulfamide | PM16/C01-04 |
| Trifluoperazine | −101 | Ion channel, Ca2+ | PM13/G09-12 |
| 2-Phenylphenol | −113 | Membrane | PM18/H05-08 |
| Polymyxin B | −86 | Membrane, outer | PM19/H09-12 |
| Plumbagin | −105 | Oxidizing agent | PM18/H09-12 |
| Lawsone | −104 | Oxidizing agent | PM19/E09-12 |
| Spectinomycin | −90 | Protein synthesis | PM12/G01-04 |
| Neomycin | −99 | Protein synthesis, aminoglycoside | PM11/F09-12 |
| Streptomycin | −80 | Protein synthesis, aminoglycoside | PM12/E01-04 |
| Troleandomycin | −127 | Protein synthesis, macrolide | PM20/H09-12 |
| Iodonitro tetrazolium violet | −105 | Respiration | PM19/D05-08 |
| Tetrazolium violet | −85 | Respiration | PM20/B09-12 |
| Sodium caprylate | −81 | Respiration, ionophore | PM19/F09-12 |
| Sodium nitrite | −81 | Transport, toxic anion | PM14/G09-12 |
| Thallium(I) acetate | −126 | Transport, toxic cation | PM13/F09-12 |
| Ceftriaxone | −109 | Wall, cephalosporin | PM11/G01-04 |
| Aztreonam | −86 | Wall, lactam | PM18/F09-12 |
| Phenethicillin | −80 | Wall, lactam | PM19/F01-04 |
The PM assay was performed twice using the OmniLog system. The growth rate of wild-type E. coli BW25113 was monitored in the presence and absence of the indicated chemicals. Chemicals that led to a growth difference of >+40 or <−80 (in arbitrary units; averages for two independent experiments) are listed. Chemicals shown in boldface represent sulfa drugs.
The collection of 240 drug species examined included seven species of sulfa drug (sulfachloropyridazine, sulfadiazine, sulfamethazine, sulfamethoxazole, sulfamonomethoxine, sulfanilamide, and sulfathiazole). Since LeuO expression led to an increase in sensitivity to all seven drugs, we predicted that LeuO is needed for transcription of the gene(s) involved in the determination of sensitivity to these sulfa drugs. This prediction was examined by a sensitivity test with wild-type and three mutant strains, each lacking the first gene of one of the LeuO-regulated operons encoding three putative drug efflux transporters. The wild type and three deletion strains, the acrE (for the acrEF operon), ygcL (for the ygcLKJIH-ygbTF operon), and yjcR (for the yjcRQP operon) mutants, with either pMH109 plasmid (LeuO expression vector) or pCA24 (the empty vector for control), were grown in the presence of increasing concentrations of the sulfa drugs. Figure 5 shows one example in which cell growth was monitored by cell respiration after 8 h of inoculation in the presence of increasing concentrations of sulfadiazine. The drug concentration was increased twofold stepwise up to 10 μg/ml. In the absence of the LeuO expression plasmid, the growth inhibition of the wild type was observed at sulfadiazine concentrations above 1.25 μg/ml (Fig. 5). In contrast, the growth of the wild type transformed with the LeuO expression vector (Fig. 5) was observed even in the presence of 10 μg/ml sulfadiazine, indicating that resistance against sulfadiazine increases in the presence of LeuO overexpression. We then performed the sulfadiazine sensitivity test with three mutants. Both the acrE and ygcL deletion mutants showed essentially the same sensitivity patterns as the wild type, irrespective of the presence or absence of the LeuO expression plasmid (Fig. 5), indicating that the drug efflux pumps formed by the acrEF and ygcLKJIH-ygbTF gene products are not specifically involved in the efflux of sulfadiazine. On the other hand, the sulfadiazine sensitivity pattern of the yjcR deletion mutant lacking the drug efflux pump encoded by the yjcRQP operon was significantly different from the other three patterns (Fig. 5). In the absence of the LeuO expression plasmid, the mutant was more sensitive to sulfadiazine than the wild type and the other two mutants. Even in the presence of LeuO overexpression, this mutant was more sensitive to sulfadiazine than the other three strains with the LeuO expression plasmid (Fig. 6). Based on these observations, we predicted that the YjcRQP pump is involved in the efflux of sulfadiazine.
FIG. 5.
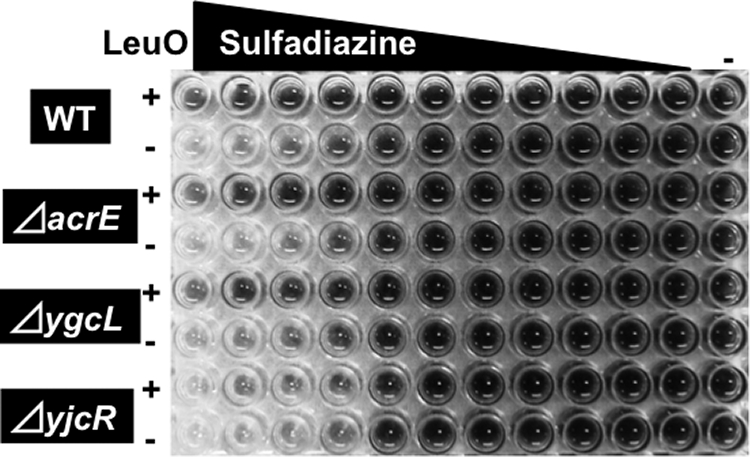
Sulfadiazine sensitivity test with mutants lacking the multidrug pump genes. E. coli BW25113 (WT), the JW0451 acrE mutant (acrE), the JW2730 ygcL mutant (ygcL), and the JW4041 mdtN mutant (yjcR), each with and without the LeuO expression plasmid, were grown in the presence of increasing concentrations of sulfadiazine for 8 h. The concentrations of sulfadiazine were as follows (from left to right): 10 μg/ml in the first column, followed by twofold dilutions to the 11th column; the last column contained no drug. Cell growth was monitored by measuring respiration (tetrazolium color formation).
FIG. 6.
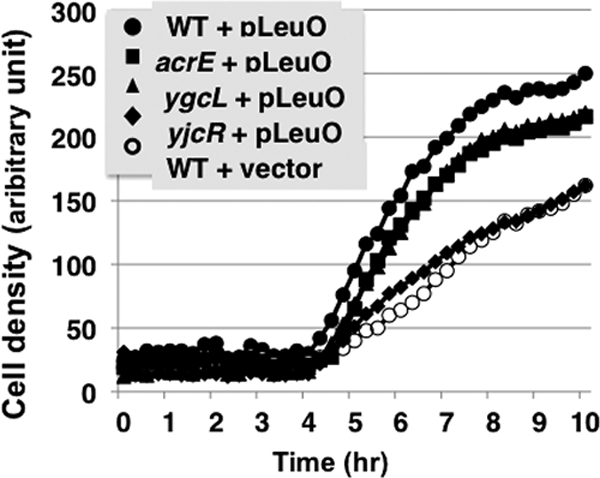
Effect of sulfadiazine on growth. E. coli BW25113 (WT), the JW0451 acrE mutant (acrE), the JW2730 ygcL mutant (ygcL), and the JW4041 mdtN mutant (yjcR), each carrying either the LeuO expression plasmid pLeuO or empty plasmid, were grown in the presence or absence of 5 μg/ml sulfadiazine. Cell growth was monitored by measuring respiration (tetrazolium color formation).
Sulfonamide drugs were the first antimicrobial drugs and paved the way for antibiotic therapy in medicine. Antibacterial sulfonamides act as competitive inhibitors of the enzyme dihydropteroate synthetase, which catalyzes the conversion of para-aminobenzoate to dihydropteroate, a key step in the synthesis of folate, which is used for nucleic acid synthesis. Up to the present, however, the path of sulfonamide transport across E. coli membranes has not been identified. Using the same drug sensitivity test, we then compared the sensitivities of all four test strains against six other sulfa drugs, i.e., sulfachloropyridazine, sulfamethazine, sulfamethoxazole, sulfamonomethoxine, sulfanilamide, and sulfathiazole. The drug concentration-dependent growth patterns were essentially the same as that observed with sulfadiazine (data not shown). Taking all of the data from the drug sensitivity assay together, we concluded that the yjcRQP operon is involved in sensitivity control against sulfa drugs, and thus we propose to rename the yicRQP operon the sdsRQP operon (sulfa drug sensitivity determinant).
Physiological role of LeuO.
In this study, we found that LeuO activates the transcription of at least nine target operons, including one previously identified and eight newly identified operons, through either interference with H-NS silencing or direct interaction with RNA polymerase. Most of the activated genes encode cell surface proteins involved in the transport of chemicals across membranes or in resistance to external stresses. LeuO-induced high-level expression of some membrane components might lead to alteration in the membrane integrity and in membrane functions, including permeability to a group of drugs, including sulfa drugs. In addition to the hitherto characterized AcrEF factor, an RND family drug efflux transporter, the uncharacterized yjcRQP and ygcLKJIH-ygbTF operons were found to be involved in efflux of sulfa drugs.
The expression of LeuO increases in stationary phase (Fig. 3). We then examined the sensitivity of stationary-phase wild-type cells to sulfadiazine (Fig. 7). In the exponential growth phase, the drug sensitivity was essentially the same between the wild-type and the leuO mutant, but upon entry into stationary phase, the leuO mutant became more sensitive to sulfadiazine. Taking the results together, we concluded that the drug efflux pump encoded by the yjcRQP operon is indeed involved in sulfa drug resistance, and thus we propose to rename the yjcRPQ operon the sdsRQP operon (sulfa drug sensitivity determinant). This finding also supports the prediction that the LeuO-induced alteration of membrane integrity contributes to the transformation of gram-negative bacteria into a dormant state, as in the case of sporulation of gram-positive bacteria.
FIG. 7.

Sensitivities of wild-type and leuO mutant E. coli cells to sulfamidazine. Overnight cultures of wild-type and leuO mutant E. coli in M9-0.4% glucose medium at 37°C were transferred into fresh M9-0.4% glucose medium containing the indicated concentrations of sulfamidazine. Growth was monitored by measuring the turbidity (OD600). After 12 h of culture, the relative rates of cell growth in the presence and absence of drug were plotted.
Supplementary Material
Acknowledgments
We thank the National Institute of Genetics E. coli Stock Center for wild-type BW25113 and its gene knockout mutants JW0075, JW1225, JW3233, JW2730, and JW4043.
This work was supported by grants-in-aid (17076016, 18310133, and 21241047 to A.I. and 21710198 to T.S.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by a Nano-Biology Project of the Micro-Nano Technology Research Center of Hosei University.
Footnotes
Published ahead of print on 1 May 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aiba, H., S. Adhya, and B. de Crombrugghe. 1981. Evidence for two functional gal promoters in intact Escherichia coli cells. J. Biol. Chem. 25611905-11910. [PubMed] [Google Scholar]
- 2.Ames, P., and J. S. Parkinson. 1994. Constitutively signaling fragments of Tsr, the Escherichia coli serine chemoreceptor. J. Bacteriol. 1766340-6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed]
- 4.Baranova, N., and H. Nikaido. 2002. The baeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J. Bacteriol. 1844168-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochner, B. R., P. Gadzinki, and E. Panomitros. 2001. Phenotype microarrays for high-throughput phenotypic testing and assay of gene function. Genome Res. 111246-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bravo, D., C. Silva, J. A. Carter, A. Hoare, S. A. Alvarez, C. J. Blondel, M. Zaldivar, M. A. Valvano, and I. Conteras. 2008. Growth-phase regulation of lipopolysaccharide O-antigen chain length influences serum resistance in serovars of Salmonella. J. Med. Microbiol. 57938-946. [DOI] [PubMed] [Google Scholar]
- 7.Castano, I., N. Flores, F. Valle, A. A. Covarrubias, and F. Bolivar. 1992. gltF, a member of the gltBDF operon of Escherichia coli, is involved in nitrogen-regulated gene expression. Mol. Microbiol. 62733-2741. [DOI] [PubMed] [Google Scholar]
- 8.Chen, C. C., M. Fang, A. Majumder, and H. Y. Wu. 2001. A 72-base pair AT-rich DNA sequence element functions as a bacterial gene silencer. J. Biol. Chem. 2769478-9485. [DOI] [PubMed] [Google Scholar]
- 9.Chen, C. C., M. Y. Chou, C. H. Huang, A. Majumder, and H. Y. Wu. 2005. A cis-spreading nucleoprotein filament is responsible for the gene silencing activity found in the promoter relay mechanism. J. Biol. Chem. 2805101-5112. [DOI] [PubMed] [Google Scholar]
- 10.Daley, D. O., M. Rapp, E. Granseth, K. Melen, D. Drew, and G. Von Heijne. 2005. Global topology analysis of the Escherichia coli inner membrane proteome. Science 3081321-1323. [DOI] [PubMed] [Google Scholar]
- 11.De la Cruz, M. A., M. Ferenandez-Mora, C. Gradarrama, M. A. Flores-Valdez, V. H. Bustamante, A. Vazquez, and E. Calva. 2007. LeuO antagonizes H-NS and StpA-dependent repression in Salmonella enterica ompS1. Mol. Microbiol. 66727-743. [DOI] [PubMed] [Google Scholar]
- 12.Dinh, T., I. T. Paulsen, and M. H. Saier, Jr. 1994. A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J. Bacteriol. 1763825-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorman, C. J. 2004. H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2391-400. [DOI] [PubMed] [Google Scholar]
- 14.Dorman, C. J. 2007. H-NS, the genome sentinel. Nat. Rev. Microbiol. 5157-161. [DOI] [PubMed] [Google Scholar]
- 15.Fang, M., A. Majumder, K. J. Tsai, and H. Y. Wu. 2000. ppGpp-dependent leuO expression in bacteria under stress. Biochem. Biophys. Res. Commun. 27664-70. [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Mora, M., J. L. Puente, and E. Calva. 2004. OmpR and LeuO positively regulate the Salmonella enterica serovar Typhi ompS2 porin gene. J. Bacteriol. 1862909-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuelner, G., J. A. Gray, J. A. Kirschman, A. F. Lehner, A. B. Sadosky, D. A. Vlazny, J. Zhang, S. Zhao, and C. W. Hill. 1990. Structure of the rhsA locus from Escherichia coli K-12 and comparison of rhsA with other members of the rhs multigene family. J. Bacteriol. 172446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerard, F., N. Pradel, and L. F. Wu. 2005. Bactericidal activity of colicin V is mediated by an inner membrane protein, SdaC, of Escherichia coli. J. Bacteriol. 1871945-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grainger, D. C., D. Hurd, M. D. Goldberg, and S. J. Busby. 2006. Association of nucleoid proteins with coding and non-coding segments of the Escherichia coli genome. Nucleic Acids Res. 344642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa, A., H. Ogasawara, A. Kori, and A. Ishihama. 2008. AllR is the allantoin/glyoxylate-sensing master regulator of the genes for degradation and reutilization of purines. Microbiology 1543366-3378. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Lucas, I., A. L. Gallego-Hernandez, S. Encarnacion, M. Fernandez-Mora, A. G. Martinez-Batallar, H. Salgado, R. Oropeza, and E. Calva. 2008. The LysR-type transcriptional regulator LeuO controls expression of several genes in Salmonella enterica serovar Typhi. J. Bacteriol. 1901658-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hertzberg, K. M., R. Gemmill, J. Jones, and J. M. Calvo. 1980. Cloning of an EcoRI-generated fragment of the leucine operon of Salmonella typhimurium. Gene 8810-814. [DOI] [PubMed] [Google Scholar]
- 23.Hommais, F., E. Krin, C. Laurent-Winter, O. Soutourina, A. Malpertury, J. P. Le Caer, A. Danchin, and P. Bertin. 2001. Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein, H-NS. Mol. Microbiol. 4020-36. [DOI] [PubMed] [Google Scholar]
- 24.Ishihama, A. 1993. Protein-protein communication within the transcription apparatus. J. Bacteriol. 1752483-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishihama, A. 2000. Functional modulation of Escherichia coli RNA polymerase. Annu. Rev. Microbiol. 54499-518. [DOI] [PubMed] [Google Scholar]
- 26.Jishage, M., A. Iwata, S. Ueda, and A. Ishihama. 1996. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J. Bacteriol. 1785447-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keseler, I. M., J. Collado-Vides, S. Gama-Castro, J. Ingraham, S. Paley, I. T. Paulsen, M. Peralta-Gil, and P. D. Karp. 2005. EcoCys; a comprehensive database resource for Escherichia coli. Nucleic Acids Res. 33D334-D337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawley, T. D., K. Chan, L. J. Thompson, C. C. Kim, G. R. Govoni, and D. M. Monack. 2006. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog. 2e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma, D., D. K. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 1645-55. [DOI] [PubMed] [Google Scholar]
- 30.Maddocks, S. E., and P. C. Oyston. 2008. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 1543609-3623. [DOI] [PubMed] [Google Scholar]
- 31.Majumder, A., M. Fang, K. J. Tsai, C. Ueguchi, T. Mizuno, and H. Y. Wu. 2001. LeuO expression in response to starvation for branched-chain amino acids. J. Biol. Chem. 27619046-19051. [DOI] [PubMed] [Google Scholar]
- 32.Moorthy, S., and P. I. Watnick. 2005. Identification of novel stage-specific genetic requirements through whole genome transcription profiling of Vibrio cholerae biofilm development. Mol. Microbiol. 571623-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray, G. L., S. R. Attridge, and R. Morona. 2003. Regulation of Salmonella typhimurium lipopolysaccharide O antigen chain length is required for virulence; identification of FepE as a second Wzz. Mol. Microbiol. 471395-1406. [DOI] [PubMed] [Google Scholar]
- 34.Navarre, W. W., M. McClelland, S. J. Libby, and F. C. Fang. 2007. Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev. 211456-1471. [DOI] [PubMed] [Google Scholar]
- 35.Navarre, W. W., S. Porwollik, Y. Wang, M. McClelland, S. J. Libby, and F. C. Fang. 2006. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313236-238. [DOI] [PubMed] [Google Scholar]
- 36.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 1785853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nilekani, S., and C. S. Raman. 1983. Purification and properties of citrate lyase from Escherichia coli. Biochemistry 224657-4663. [DOI] [PubMed] [Google Scholar]
- 38.Nishino, K., and A. Yamaguchi. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 1835803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogasawara, H., A. Hasegawa, E. Kanda, T. Miki, K. Yamamoto, and A. Ishihama. 2007. Genomic SELEX search for target genes under the control of PhoQP-RstBA signal relay cascade. J. Bacteriol. 1894791-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogasawara, H., Y. Ishida, K. Yamada, K. Yamamoto, and A. Ishihama. 2007. PdhR (pyruvate dehydrogenase complex regulator) controls the respiratory electron transport system in Escherichia coli. J. Bacteriol. 1895534-5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oshima, T., S. Ishikawa, K. Kurokawa, A. Aiba, and N. Ogasawara. 2006. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res. 13141-153. [DOI] [PubMed] [Google Scholar]
- 42.Ozenberger, B. A., M. S. Nahlik, and M. S. McIntosh. 1987. Genetic organization of multiple fep genes encoding ferric enterobactin transport functions in Escherichia coli. J. Bacteriol. 1693638-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paulsen, I. T., N. K. Sliwinski, and M. H. Saier, Jr. 1998. Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J. Mol. Biol. 277573-592. [DOI] [PubMed] [Google Scholar]
- 44.Persson, B. L., J. Petersson, U. Fristedt, R. Weinander, A. Berhe, and J. Pattison. 1999. Phosphate permeases of Saccharomyces cerevisiae: structure, function and regulation. Biochim. Biophys. Acta 1422255-271. [DOI] [PubMed] [Google Scholar]
- 45.Portalier, R., J. Robert-Baudouy, and F. Stoeber. 1980. Regulation of Escherichia coli K-12 hexuronate system genes: exu regulon. J. Bacteriol. 1431095-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Putman, M., H. W. van Veen, and W. H. Konings. 2000. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64672-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Repolia, F., and S. Gottesman. 2003. Temperature sensing by the dsrA promoter. J. Bacteriol. 1856609-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez-Morales, O., M. Fernandez-Mora, I. Hernandez-Lucas, A. Vazquez, J. L. Puente, and E. Calva. 2006. Salmonella enterica serovar Typhimurium ompS1 and ompS2 mutants are attenuated for virulence in mice. Infect. Immun. 741398-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shao, Z., R. T. Lin, and E. B. Newman. 1994. Sequencing and characterization of the sdaC gene and identification of the sdaCB operon in Escherichia coli K12. Eur. J. Biochem. 222901-907. [DOI] [PubMed] [Google Scholar]
- 50.Shi, X., and G. N. Bennett. 1995. Effects of multicopy LeuO on the expression of the acid-inducible lysine decarboxylase gene in Escherichia coli. J. Bacteriol. 177810-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimada, T., N. Fujita, M. Maeda, and A. Ishihama. 2005. Systematic search for the Cra-binding promoters using genomic SELEX system. Genes Cells 10907-918. [DOI] [PubMed] [Google Scholar]
- 52.Shimada, T., K. Hirao, A. Kori, K. Yamamoto, and A. Ishihama. 2007. RutR is the uracil/thymine-sensing master regulator of a set of genes for synthesis and degradation of pyrimidines. Mol. Microbiol. 66744-757. [DOI] [PubMed] [Google Scholar]
- 53.Stoebel, D. M., A. Free, and C. J. Dorman. 2008. Anti-silencing: overcoming H-NS-mediated repression of transcription in gram-negative enteric bacteria. Microbiology 1542533-2545. [DOI] [PubMed] [Google Scholar]
- 54.Stratmann, T., S. Madhusudan, and K. Schnetz. 2008. Regulation of the yjjQ-bglJ operon, encoding LuxR-type transcription factors, and the divergent yjjP gene by H-NS and LeuO. J. Bacteriol. 190926-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sulavik, M. C., C. Houseweart, C. Cramer, N. Jiwani, N. Murgolo, J. Greene, B. DiDomenico, K. J. Shaw, G. H. Miller, R. Hare, and G. Shimer. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 451126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tenor, J. L., B. A. McCormick, F. M. Ausubel, and A. Aballay. 2004. Caenorhabditis elegans-based screen identifies Salmonella virulence factors required for conserved host-pathogen interactions. Curr. Biol. 141018-1024. [DOI] [PubMed] [Google Scholar]
- 57.Tolstorukov, M. Y., K. M. Virnik, S. Adhya, and V. B. Zhurkin. 2005. A-tract clusters may facilitate DNA packaging in bacterial nucleoid. Nucleic Acids Res. 333907-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ueguchi, C., T. Ohta, C. Seto, T. Suzuki, and T. Mizuno. 1998. The leuO gene product has a latent ability to relieve bgl silencing in Escherichia coli. J. Bacteriol. 180190-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Umezawa, Y., H. Ogasawara, T. Shimada, A. Kori, and A. Ishihama. 2008. The uncharacterized transcription factor YdhM is the regulator of the nemA gene, coding for N-ethylmaleimide reductase. J. Bacteriol. 1905890-5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van der Ploeg, J. R., M. A. Weiss, E. Saller, H. Nashimoto, N. Saito, M. A. Kertesz, and T. Leisinger. 1996. Identification of sulfate starvation-regulated genes in Escherichia coli: a gene cluster involved in the utilization of taurine as a sulfur source. J. Bacteriol. 1785438-5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, Q., J. G. Frye, M. McClelland, and R. M. Harshey. 2004. Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Mol. Microbiol. 52169-187. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto, K., F. Matsumoto, S. Minagawa, T. Oshima, N. Fujita, N. Ogasawara, and A. Ishihama. 2009. Characterization of CitA-CitB signal transduction activating genes involved in anaerobic citrate catabolism in Escherichia coli. Biosci. Biotechnol. Biochem. 73346-350. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto, K., F. Matsumoto, T. Oshima, N. Fujita, N. Ogasawara, and A. Ishihama. 2008. Anaerobic regulation of citrate fermentation by CitAB in Escherichia coli. Biosci. Biotechnol. Biochem. 723011-3014. [DOI] [PubMed] [Google Scholar]
- 64.Zhou, L., X. H. Lei, B. R. Bochner, and B. L. Wanner. 2003. Phenotype microarray analysis of Escherichia coli K-12 mutants with deletions of all two-component systems. J. Bacteriol. 1854956-4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.