Abstract
Aging reduces the regenerative capacities of many tissues. In this paper, we show a critical role of the glycogen synthase kinase 3β (GSK3β)-cyclin D3 pathway in the loss of the regenerative capacity of the liver. In young animals, high levels of growth hormone (GH) increase expression of GSK3β, which associates with cyclin D3 and triggers degradation of cyclin D3. In livers of old mice, the GSK3β promoter is repressed by C/EBPβ-histone deacetylase 1 (HDAC1) complexes, leading to the reduction of GSK3β. The treatment of old mice with GH increases expression of GSK3β via removal of the C/EBPβ-HDAC1 complexes from the GSK3β promoter. We found that the GSK3β-cyclin D3 pathway is also altered in young GH-deficient Little mice and that treatment of Little mice with GH corrects the GSK3β-cyclin D3 pathway. We present evidence that GSK3β regulates liver proliferation by controlling growth-inhibitory activity of C/EBPα. The downregulation of GSK3β in young mice inhibits liver proliferation after partial hepatectomy via the cyclin D3-C/EBPα pathway, while the elevation of GSK3β in old mice accelerates liver proliferation. Thus, this paper shows that GSK3β is a critical regulator of liver proliferation and that the reduction of GSK3β with age causes the loss of regenerative capacities of the liver.
The decline of the regenerative capacities of tissues leads to the development of many age-related symptoms. Previous studies have identified several candidates which might contribute to the loss of regenerative capacities of tissues. Expression of p16INK4a has been found to be increased with age in many tissues and is involved in the loss of regenerative capacities of some tissues (19). Experiments with parabiotic animals have shown that the circulating systems of young mice contain some systemic factors which are able to rejuvenate progenitor cells in skeletal muscle and in the livers of old mice and correct proliferation of these tissues (7). Particularly, the young systemic environment reduces amounts of the C/EBPα-Brm complex, which is abundant in livers of old mice and which reduces the regenerative capacity of the old livers (16, 34), through mechanisms that do not involve alterations in protein levels of components of the complex (7, 34). Several papers have suggested that growth hormone (GH) might be one of the systemic factors which correct regenerative capacities of tissues, including liver (11, 14, 20). For example, the treatment of old mice with GH corrects liver proliferation in old mice (20) and eliminates the C/EBPα-Brm complex through reduction of cyclin D3 (34).
Cyclin D3 belongs to a family of D-type cyclins which include cyclins D1, D2, and D3. D-type cyclins display significant homology, suggesting that they may perform overlapping functions. However, the expression patterns of the D-type cyclins vary widely, and individual cyclins D showed distinct functions in cell cycle progression and differentiation (2, 5, 17). Cyclin D1 is not detectable in quiescent livers but is induced after partial hepatectomy (PH) and is involved in the promotion of liver proliferation. Overexpression of cyclin D1 alone is sufficient to initiate proliferation in young livers (24). In contrast, cyclin D3 is expressed in a number of quiescent tissues and displays functions that support growth arrest and differentiation status of the tissues (2, 5, 10). Protein levels of cyclin D3 are increased during differentiation of myocytes and are involved in the regulation of the MyoD-mediated program of gene expression (8). Moreover, cyclin D3 is also increased during differentiation of 3T3-L1 cells (26) and contributes to differentiation of adipocytes by activation of peroxisome proliferator-activated receptor γ (28). High levels of cyclin D3 are observed in the quiescent liver (27). We have recently shown that cyclin D3 activates cdk4, which is a key positive regulator of growth-inhibitory activity of C/EBPα, in livers of old mice (33). C/EBPα is expressed in many tissues and is involved in the regulation of differentiation and in growth arrest (18, 25). In old livers, cyclin D3-cdk4 also phosphorylates an RNA binding protein, CUGBP1, and increases interactions of CUGBP1 with translation initiation complex eIF2 (29). This activation of translational activity of CUGBP1 results in the increased translation of several proteins, including transcription factor C/EBPβ and histone deacetylase 1 (HDAC1) (32).
The expression of D-type cyclins is regulated at several levels, including transcription, translation, and posttranslational modifications (1, 21, 22). Cyclin D1 and cyclin D3 have been shown to be targeted for proteasomal degradation by phosphorylation (23). Recent studies have discovered that the stability of cyclin D3 protein is reduced by glycogen synthase kinase 3β (GSK3β) in human B-lymphocyte Reh cells (23) and by p38 in Molts-4 lymphoblastoid cells and COS cells (4) by phosphorylation at Thr283, while protein phosphatase 1 (PP1) dephosphorylates and stabilizes cyclin D3 in lymphoid Reh cells (21). GSK3β is a ubiquitously expressed multifunctional serine/threonine protein kinase originally identified as a regulator of glycogen synthesis (38). Previous investigations of GSK3β have been focused primarily on the ability of GSK3β to phosphorylate and inactivate or degrade specific target substrates (9). It has been shown that stability of cyclin D3 is also regulated by PP1. PP1 is a conserved enzyme which is involved in the regulation of many cellular processes, including glycogen metabolism and cell division (3, 6).
In this study, we found that GSK3β is a key regulator of liver proliferation and that the downregulation of GSK3β in young animals inhibits liver proliferation via the cyclin D3-C/EBPα pathway, while overexpression of GSK3β in old mice reduces growth-inhibitory activity of C/EBPα and corrects proliferation of the liver. We have also shown that the reduction of GSK3β in livers of old mice is caused by epigenetic silencing of the GSK3β promoter by C/EBPβ-HDAC1 complexes. GH upregulates GSK3β in the liver by removing the C/EBPβ-HDAC1 complexes from the GSK3β promoter.
MATERIALS AND METHODS
Antibodies and reagents.
Antibodies to cyclin D3 (C-16), cyclin D1 (H-295), cdc2 (L-19), PCNA (FL-261), GSK3β (H-76), p-GSK3β (Ser9), GSK3α (H-75), PP1 (FL-18), p38 (H-147), C/EBPα (14AA and N19), C/EBPβ (C19), CUGBP1 (3B1), and His-probe (AD1.1.10) were purchased from Santa Cruz Biotechnology. Antibody to phospho-cyclin D1 (Thr286) was from Cell Signaling. Antibodies to acetyl-histone H3 (Lys9) and histone H3 trimethyl Lys9 were from Abcam. Monoclonal anti-β-actin antibody was from Sigma. His6 monoclonal antibody was from Clontech. GSK3β small interfering RNA (siRNA) and control siRNA-A were from Santa Cruz Biotechnology. Cycloheximide, LiCl, SB20380, Z-Leu-Leu-leu-al (MG132), and N-acetyl-Leu-Leu-norleucinal (LLnL) were from Sigma. The bromodeoxyuridine (BrdU) uptake assay kit was from Invitrogen.
Animals and treatments of old mice and Little mice (Ghrhrlit/lit) with GH.
Experiments with animals were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine (protocol AN-1439). In this study, we used young mice (4 to 6 months) and old mice (22 months). Liver, lung, brain, and adipose tissues were harvested and kept at −80°C. For GH treatment of old mice, recombinant mouse GH (rmGH) provided by the National Hormone and Peptide Program was injected into old mice (2 mg/kg) subcutaneously for 3 days. Physiological saline (0.9% NaCl) was used as vehicle for rmGH. The control animals were injected with physiological saline. Vectors expressing wild-type (WT) and T283A mutant His-cyclin D3 were delivered into mice by injections using the “in vivo-jetPEI” transfection reagent (PolyPlus Transfection) at the third day after GH treatment. Protein extracts were isolated from liver, lung, adipose tissues, and brain at 24 h after transfection and examined for expression of the WT and Thr283A mutant cyclin D3 using antibodies to His tag. For GH treatment of Little mice, rmGH was injected into Little mice subcutaneously twice a day (1 mg/kg) for 7 days.
PH.
Vectors expressing His-GSK3β or siGSK3β were delivered into young mice (4 months) or old mice (22 months) by injections using the “in vivo-jetPEI” transfection reagent (PolyPlus Transfection). PH was performed at 24 h after transfection as described in our previous publications (16, 30). Seventy percent of the liver was surgically removed, and regeneration was allowed to proceed for 24, 36, 48, and 72 h. Livers were collected and frozen in liquid nitrogen. Three animals at each time point after PH were examined. Liver proliferation was examined by BrdU uptake and by measuring expression of cell cycle proteins.
Cell culture and transient-transfection analysis.
The human Hep3B2 hepatoma cell lines (ATCC) were cultured in minimal essential medium supplemented with 10% fetal bovine serum (HyClone) and 100 U/ml penicillin-streptomycin (Gibco) at 37°C in a humidified incubator with 5% CO2. Mouse embryonic fibroblast (MEF) cell lines from WT and GSK3β−/− mice were cultured in Dulbecco modified Eagle medium as described previously (15). Since the levels of endogenous cyclin D3 are very low in MEF cells, we transfected His-cyclin D3 into WT and GSK3β knockout (KO) MEFs and then treated them with recombinant GH (rhGH; Cell Science) for 24 to 48 h. Proteins were isolated and used for Western blotting as described below. In experiments with LiCl and SB20380 treatment, the cells were pretreated with LiCl (15 mM) or SB20380 (10 μM) for 15 min before addition of rhGH.
Protein isolation and Western blotting.
Cytoplasmic and nuclear extracts were isolated from livers of young and old mice or from Hep3B2 cells as described in previous papers (29, 30). Inhibitors of phosphatases were included in all buffers used for the isolation of proteins or protein-protein complexes. Proteins (50 to 100 μg) were loaded on gradient (4 to 20% or 8 to 16%; Bio-Rad) polyacrylamide gels, transferred onto membranes, and probed with antibodies against proteins of interest. To verify protein loading, each filter was reprobed with β-actin and/or stained with Coomassie blue.
Coimmunoprecipitation (co-IP).
Cyclin D3 was immunoprecipitated from nuclear extracts of young and old livers or from whole-cell extracts of Hep3B2 cells with polyclonal antibody to cyclin D3. GSK3β, GSK3α, p38, and PP1 in cyclin D3 IPs were examined by Western blotting with antibodies to GSK3β and PP1. To examine the interactions of C/EBPα with cyclin-dependent kinase 2 (cdk2) in livers after PH, C/EBPα was immunoprecipitated with polyclonal antibodies and cdk2 in these antibodies was determined using monoclonal antibodies. We also detected the S193-ph isoform of C/EBPα using IP with antibodies to C/EBPα and Western blotting with antibodies to ph-S193. All co-IP studies were performed with TrueBlot eBioscience reagents.
2D gel Western blotting.
Two-dimensional (2D) gel analysis was performed as described in our previous papers (30, 36). Briefly, cytoplasmic and/or nuclear lysates from young and old livers were separated by 2D gel electrophoresis (Protean IEF; Bio-Rad), transferred onto membranes, and probed with antibodies to total cyclin D3 and to the cyclin D1-Thr286-ph isoform or with antibodies to CUGBP1.
Electrophoretic mobility shift assay (EMSA).
The GSK3β promoter contains several C/EBP sites (see Fig. 3B). In this study, we have used two DNA probes which cover two C/EBP binding sites. The sequences of these probes are as follows: site 1, 5′-GGTCTACGTGGTTTCGGCATATCGGTTG-3′; site 2, 5′-GGAACTCCCGTTTCCACATCATCTAACAAT-3′. The C/EBP consensus sequences are shown in bold. The double-stranded probes were gel purified and labeled by Klenow “fill-in” reaction with radioactive [32P]dCTP. The probes were incubated with nuclear extracts isolated from cells transfected with C/EBPα and C/EBPβ or with nuclear extracts from mouse liver. The binding buffer contained 20 mM Tris-HCl (pH 7.5), 100 mM KCl, 5 mM MgCl2, 5 mM dithiothreitol, 1 μg/10 μl poly(dI-dC), and 10% glycerol. Antibodies were added to the binding reaction mixtures before probe addition. The reaction mixtures were separated by 5% native gel electrophoresis; the gel was dried and exposed with X-ray film.
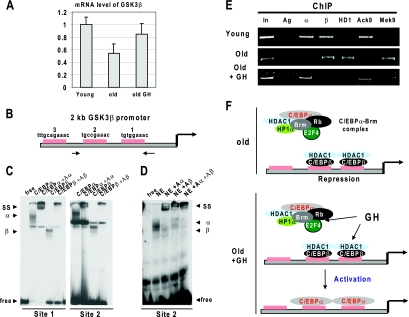
FIG. 3.
GH increases expression of GSK3β in the liver by removal of epigenetic silencing the GSK3β promoter. (A) Treatment of old mice with GH increases expression of GSK3β mRNA. Quantitative reverse transcriptase PCR was performed with RNA isolated from livers of young, old, and GH-treated old mice. Levels of GSK3β mRNA were calculated as ratios to the GAPDH control. Error bars indicate standard deviations. (B) The GSK3β promoter contains three C/EBP sites. The diagram shows the locations of C/EBP sites within the mouse 2-kb GSK3β promoter. Sequences on the top are C/EBP consensus sequences. Arrows show positions of primers used for ChIP. (C) C/EBPα and C/EBPβ bind to the GSK3β promoter. EMSA was performed with nuclear extracts from HEK293 cells transfected with C/EBPα or with C/EBPβ. The left image shows EMSA with a DNA probe covering site 1; the right image shows EMSA with site 2. Antibodies to C/EBPα (Aα) and C/EBPβ (Aβ) were incorporated in the binding reaction mixtures as shown at the top. SS, supershift; α, shift with C/EBPα; β, shift with C/EBPβ. (D) C/EBPα and C/EBPβ bind to the GSK3β promoter. Nuclear extracts from mouse liver (NE) were incubated with a DNA probe covering site 2. Antibodies to C/EBPα and C/EBPβ were added in the binding reaction mixtures as shown at the top. (E) ChIP assay with chromatin solutions from young, old, and GH-treated old mice. C/EBPα (α), C/EBPβ (β), HDAC1 (HD1), AcK9 histone H3 (Ack9), and trimethyl K9 histone H3 (Mek9) were precipitated from chromatin solutions. PCR was performed with primers covering sites 1 and 2 on the GSK3β promoter (see panel B). In, 1/100 of input; Ag, precipitation with agarose beads. (F) A hypothetical model for the epigenetic silencing of the GSK3β promoter in livers of old mice and for the mechanisms by which GH activates the promoter (see text).
ChIP.
Chromatin IP (ChIP) assay was performed as described in our previous papers, using a Chip-It kit (31, 32, 34). Briefly, chromatin solutions were prepared from livers of young, old, and GH-treated old mice. C/EBPα, C/EBPβ, HDAC1, histone H3K9, and histone H3-trimethyl K9 were immunoprecipitated from the solutions. DNA was isolated and used for PCRs with primers covering two C/EBPα sites within the GSK3β promoter (see Fig. 3B). The sequences of these primers are 5′-ATTACAAATTGGCTGCCTGTCGGG-3′ (forward) and 5′-AGATGTCTGTAGAGGCCTGGCAAA-3′ (reverse). PCR mixtures were amplified for one cycle of 95°C (5 min), 58°C (5 min), and 72°C (2 min), and then PCR mixtures were amplified for 31 cycles of 95°C (1 min), 58°C (2 min), and 72°C (1.5 min). PCR products were separated by 2% agarose gel electrophoresis or by 8% polyacrylamide gel electrophoresis.
Real-time quantitative reverse transcriptase PCR.
Total RNA was isolated from mouse livers using Trizol reagent (Invitrogen). The RNA was treated with RNase-free DNase (Invitrogen), and cDNA was synthesized using the oligo(dT) primer and Superscript II reverse transcriptase (Invitrogen). The real-time PCR mixtures contained 1× Brilliant II SYBR green QPCR master mix (Stratagene), 200 nM of each primer, 1× ROX dye (Invitrogen), and the synthesized cDNA. PCR amplification was performed in triplicate in a 96-well plate for each sample on an ABI PRISM 7000 sequence detection system (Applied Biosystems). The relative expression of cyclin D3 and GSK3β was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The sequences of PCR primers are as follows: cyclin D3, 5′-CTATGAACTACCTGGATCGCTACCT-3′ (forward) and 5′-CAGACGGTACCTAGAAGCTGCAA-3′ (reverse), producing a 76-bp fragment; GSK3β, 5′-CGGGACCCAAATGTCAAACT-3′ (forward) and 5′-TCCGAGCATGTGGAGGGATA-3′ (reverse), producing a 121-bp fragment; and GAPDH, 5′-GCACAGTCAAGGCCGAGAAT-3′ (forward) and 5′-GCCTTCTCCATGGTGGTGAA-3′ (reverse), producing a 151-bp fragment.
Colony growth assay.
Examination of growth arrest in HEK293 and Hep3B2 cells was performed as described in our previous papers (16, 31, 35). Briefly, C/EBPα was cloned into the pAdTrack plasmid under control of the cytomegalovirus promoter. This plasmid also expresses green fluorescent protein (GFP), which allows visualization of transfected cells. For cyclin D3 and GSK3β, the plasmids were cotransfected with GFP to visualize cells. The number of single green colonies (growth arrest) and the number of colonies containing two, three, or more cells per colony (proliferation) were counted at days 1, 2, and 3 after transfections. The data in this paper present summaries of counts at day 3 after transfections, with at least three independent experiments. A total of 200 to 300 colonies were calculated in each experiment.
RESULTS
GH stabilizes cyclin D3 in liver, lung, brain, and adipose tissues of old mice.
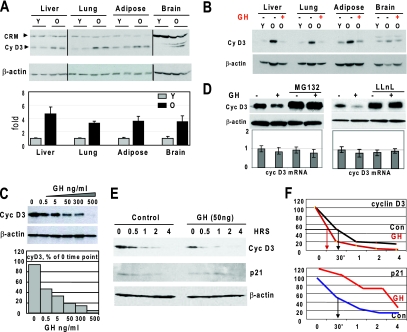
We have previously found that protein levels of cyclin D3 are increased in livers of old mice and that this increase contributes to the age-associated alterations of biological processes in the liver (33). Examination of additional tissues of old mice demonstrated 3.7- to 4.4-fold elevations of cyclin D3 in liver, lung, brain, and adipose tissues (Fig. 1A). Since GH regulates cyclin D3 in the liver, we next asked whether this regulation also takes place in other tissues. We found that treatment of old mice with GH decreases expression of cyclin D3 in all examined tissues to levels which are observed in tissues of young mice (Fig. 1B). Examination of cyclin D3 mRNA, however, showed no significant changes (data not shown), suggesting that GH-mediated reduction of cyclin D3 occurs on the level of protein stability. Therefore, we examined the pathway by which hepatocytes regulate the stability of cyclin D3. We initially determined whether GH regulates cyclin D3 in cultured Hep3B2 cells. As shown in Fig. 1C, exposure of Hep3B2 cells to GH leads to a decrease of cyclin D3 protein. GH-mediated cyclin D3 degradation is proteasome dependent, since the inhibition of proteasome by MG132 and by LLnL blocks the ability of GH to reduce levels of cyclin D3 protein, while levels of cyclin D3 mRNA are not affected (Fig. 1D). We next determined the half-life of cyclin D3 in control cells and in cells treated with GH. The Hep3B2 cells were treated with GH, and protein synthesis was blocked by cycloheximide. In the absence of GH, the half-life of cyclin D3 is approximately 30 min, while treatment of the cells with GH reduces the half-life of cyclin D3 to 15 to 20 min (Fig. 1E and F). To ensure that the GH-mediated destabilization of cyclin D3 is specific, we examined the half-life of another short-lived protein, p21, and found that the half-life of p21 is not reduced in GH-treated cells. Moreover, we detected a slight stabilization of p21 in GH-treated cells (Fig. 1E and F). Thus, these data show that GH specifically increases degradation of cyclin D3 in hepatocytes.
FIG. 1.
The reduction of GH leads to elevation of protein levels of cyclin D3 in liver, lung, brain, and adipose tissues of old mice. (A) Protein levels of cyclin D3 are increased in the liver, lung, adipose, and brain tissues of old mice. Nuclear extracts from tissues of young (Y) and old (O) mice were examined by Western blotting (top image) using antibody against cyclin D3. The membranes were reprobed with β-actin, and cyclin D3 levels were calculated as ratios to β-actin. CRM, cross-reactive molecule. Since the levels of cyclin D3 differ in different tissues, optimal exposures of Western blotting are shown for each tissue. The bar graph presents a summary of three independent experiments. Error bars indicate standard deviations. (B) Treatment of old mice with GH reduces protein levels of cyclin D3 in liver, lung, adipose, and brain tissues. Western blotting was performed with nuclear extracts from tissues of young, old, and GH-treated old mice using antibody to cyclin D3. The membrane was reprobed with antibody to β-actin. (C) GH treatment reduces cyclin D3 protein levels in Hep3B2 cells. Hep3B2 cells were treated with increasing concentrations of GH (shown at the top). Protein extracts were examined by Western blotting with antibody to cyclin D3. Protein levels of cyclin D3 were calculated as ratios to β-actin and are shown by the bar graph. (D) GH reduces cyclin D3 through activation of proteasome-mediated degradation of cyclin D3. Hep3B2 cells were treated with GH in cells with or without inhibitors of proteasome (LLnL and MG132). Protein levels of cyclin D3 were examined by Western blotting. The membranes were reprobed with β-actin. The bar graphs show the levels of mRNA in LLnL- and MG132-treated cells and in control cells. (E) GH increases degradation of cyclin D3. Hep3B2 cells were pretreated with GH (50 ng/ml) for 16 h, and cycloheximide was added to the medium. Proteins were analyzed by immunoblotting with antibodies to cyclin D3, p21, and β-actin. (F) Half-lives of cyclin D3 and p21. The levels of cyclin D3 and p21 were calculated as ratios to β-actin and then as percentage of the values at time zero.
GSK3β is reduced in livers of old mice.
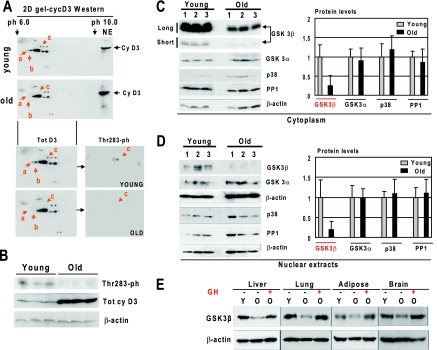
Since phosphorylation of cyclin D3 at Thr283 increases its degradation in cultured cells (4, 23), we examined whether the phosphorylation of cyclin D3 might be involved in the regulation of cyclin D3 in the liver. The 2D-Western blotting approach identified several isoforms of cyclin D3 in livers of young and old mice. Three of these isoforms (a, b, and c) are detected only in livers of young mice (Fig. 2A). The reprobe of the membrane with antibodies that recognize the Thr283-phosphorylation isoform of cyclin D3 showed that isoform c of cyclin D3 is the Thr283-ph isoform. Western blotting with antibodies to total cyclin D3 and with antibodies to Thr283-ph confirmed these data and showed that the Thr283-ph isoform of cyclin D3 is observed only in livers of young mice (Fig. 2B). Next, we searched for kinases/phosphatases that might regulate this phosphorylation. It is known that GSK3β and p38 phosphorylate cyclin D3 at Thr283 (4, 23), while PP1 dephosphorylates and stabilizes cyclin D3 (21). Since another isoform of GSK, GSK3α, has overlapping functions with GSK3β, we have also included this protein in our analysis. We have found that protein levels of GSK3β are 4.1- to 5.1-fold reduced in old livers (Fig. 2C and D). Densitometric analyses of the protein levels as ratios to β-actin showed no significant changes for p38, GSK3α, and PP1 between young and old livers. Since cyclin D3 is elevated in liver, lung, brain, and adipose tissues of old mice, we examined whether these tissues have also changed levels of GSK3β and whether GH treatment might restore expression of GSK3β in tissues of old animals. As can be seen in Fig. 2E, protein levels of GSK3β are reduced with age in all examined tissues, and GH treatment of old mice increases GSK3β in liver, lung, brain, and adipose tissues to the levels observed in tissues of young mice.
FIG. 2.
Hyperphosphorylation of cyclin D3 at Thr283 in livers of young mice correlates with high levels of GSK3β. (A) Hyperphosphorylated isoforms of cyclin D3 are observed only in livers of young mice. Nuclear extracts from livers of young and old mice were separated by 2D gel electrophoresis and probed with antibodies to cyclin D3. Positions of differentially expressed isoforms of cyclin D3 (a, b, and c) are shown by red arrows. For the bottom part, the membrane was reprobed with antibodies to the Thr283 phosphorylated isoform of cyclin D3. (B) The Thr283-ph isoform of cyclin D3 is abundant in young livers. Western blotting was performed with nuclear extracts from young and old mouse livers with antibodies to the Thr283-ph isoform of cyclin D3. The filter was reprobed with antibodies to total cyclin D3 and with antibodies to β-actin. (C and D) Protein levels of GSK3β are reduced in livers of old mice. Cytoplasmic (C) and nuclear (D) extracts from livers of young and old mice were examined by Western blotting with antibodies to GSK3β, GSK3α, PP1, p38, and β-actin. Short (30-s) and long (2-min) exposures of the cyclin D3 membrane are shown. The protein levels of the examined proteins were normalized to signals of β-actin. The bar graphs present a summary of three or four independent experiments. Error bars indicate standard deviations. (E) Treatment of old mice with GH increases protein levels of GSK3β in liver, lung, brain, and adipose tissues. Protein extracts from liver, lung, adipose, and brain tissues of young (Y), old (O), and GH-treated old mice were examined by Western blotting with antibodies against GSK3β. The membranes were reprobed with antibodies to β-actin. Since signals of cyclin D3 are different in the examined tissues, the optimal exposures are shown for the Western blotting with each tissue.
GH increases expression of GSK3β in the liver by removal of epigenetic silencing of the GSK3β promoter.
We have determined mechanisms by which GH increases expression of GSK3β in the liver. Real-time PCR revealed that the levels of GSK3β mRNA are reduced in livers of old mice and that treatment of old animals with GH increases GSK3β mRNA to the levels observed in livers of young mice (Fig. 3A). It has been previously shown that livers of old mice are characterized by significant alterations in the epigenetic control associated with transcriptional activities of C/EBP family proteins. Particularly, it has been shown that the major portion of C/EBPα is recruited into complexes with Brm and that the amounts of free C/EBPα are not sufficient to support full expression of direct targets of C/EBPα (34). Aging liver is also characterized by the elevation of another member of C/EBP family, C/EBPβ (29), and by elevation of HDAC1 (31, 32). Therefore, we examined whether these alterations might be involved in the GH-mediated regulation of the GSK3β promoter. We initially performed a search for E2F and C/EBP consensuses within the 2-kb promoter of the mouse GSK3β gene. We identified three consensus sequences for C/EBP proteins (Fig. 3B), while no consensus sequences for E2F factors were found. To determine whether C/EBP proteins bind to the identified sites, we performed EMSA experiments with DNA probes covering C/EBP sites 1 and 2. C/EBPα and C/EBPβ overexpressed in cultured cells were initially used. Figure 3C shows that both C/EBPα and C/EBPβ bind to these sites. Examination of nuclear extracts from the liver showed that endogenous C/EBP proteins also bind to the GSK3β promoter (Fig. 3D). Incorporation of antibodies to C/EBPα and C/EBPβ into the binding reaction mixtures demonstrated that these two transcription factors are the major proteins which interact with the GSK3β promoter in the liver. We next determined the occupation of the GSK3β promoter by C/EBPα, C/EBPβ, and HDAC1 proteins in young, old, and old GH-treated mice by using a ChIP approach. In young mice, both C/EBPα and C/EBPβ are observed on the GSK3β promoter. In old mice C/EBPα is not detected on the promoter; however, the C/EBPβ-HDAC1 complex is abundant (Fig. 3E). We have previously shown that GH disrupts the C/EBPα-Brm complex and releases C/EBPα (34) and that GH also reduces C/EBPβ-HDAC1 complexes (31, 32). Consistent with these data, the ChIP assay showed that GH treatment leads to the appearance of C/EBPα on the GSK3β promoter and to the removal of C/EBPβ-HDAC1 from the promoter. We next examined modifications of histone H3 associated with the GSK3β promoter. In young liver, histone H3 is acetylated at K9 on the GSK3β promoter, while in old mice, histone H3 is trimethylated at K9. This pattern of histone H3 modifications is consistent with the repression of the promoter in old mice. As one can see in Fig. 3E, GH treatment releases the repression of the GSK3β promoter. Thus, these results are consistent with the hypothesis that the reduction of GSK3β with age is mediated by epigenetic repression of the GSK3β promoter by the C/EBPβ-HDAC1 complex and sequestration of C/EBPα into the C/EBPα-Brm complex. The treatments of old mice with GH disrupt the C/EBPα-Brm complex, leading to association of C/EBPα with the GSK3β promoter and to the increase of GSK3β expression (see Fig. 3F).
Aging decreases the association of cyclin D3 with GSK3β and increases interactions of cyclin D3 with PP1.
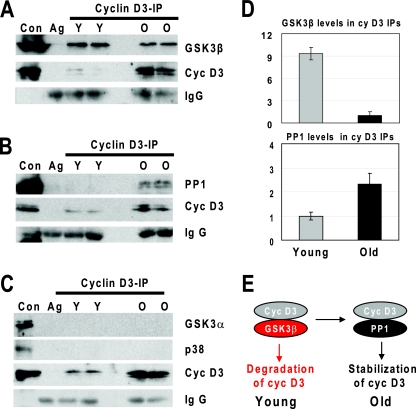
We next determined whether there is a physical association between cyclin D3 and GSK3β in the livers and whether this association is different in the livers of young and old mice. As shown in Fig. 4A, GSK3β is associated with cyclin D3 in both young and old livers; however, the molar ratios of these proteins within the cyclin D3-GSK3β complex differ dramatically. In young animals, this ratio is up to about 9.2-fold higher than that observed in old liver (Fig. 4D). We also performed similar experiments to examine physical associations of cyclin D3 with PP1. We found that PP1 is also detected in cyclin D3 immune complexes in both young and old mouse livers. However, the PP1/cyclin D3 ratio is significantly increased in livers of old mice compared with that in livers of old animals (Fig. 4B). Densitometry calculations showed that the PP1/cyclin D3 ratio in old animals is about 2.3-fold higher than that observed in young animals (Fig. 4D). Although levels of GSK3α and p38 are not changed in livers of old mice, we examined whether these proteins might be associated with cyclin D3. As one can see in Fig. 4C, co-IP experiments did not detect interactions of GSK3α and p38 with cyclin D3 in young and in old mice. These studies demonstrate that aging changes the interactions of cyclin D3 with GSK3β and PP1. These results are consistent with the hypothesis that the increased association of cyclin D3 with PP1 in livers of old mice stabilizes cyclin D3 (Fig. 4E).
FIG. 4.
Association of cyclin D3 with GSK3β is reduced in old livers, while association of cyclin D3 with PP1 is increased. (A to C) Cyclin D3 was immunoprecipitated from nuclear extracts of young (Y) and old (O) livers. The immunoprecipitates were subjected to Western blotting with antibodies against GSK3β and cyclin D3 (A); against PP1 and cyclin D3 (B); and cyclin D3, p38, and GSK3α (C). Heavy chains of immunoglobulin G (IgG) are shown for each IP. Ag, agarose mock control. (D) The amounts of GSK3β or PP1 within cyclin D3 IPs were calculated as ratios to cyclin D3. The bar graphs present a summary of three independent experiments. Error bars indicate standard deviations. (E) Diagram showing a switch of interacting partners of cyclin D3, leading to the stabilization of cyclin D3 by PP1 in livers of old mice.
GSK3β is required for GH-mediated downregulation of cyclin D3.
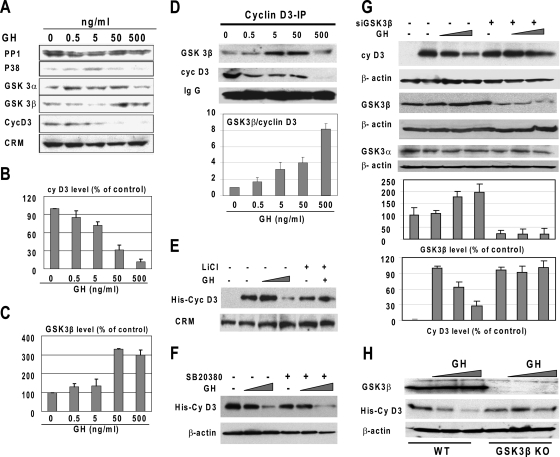
To further test a causal role of GSK3β in the GH-mediated degradation of cyclin D3, we examined expression of GSK3β in GH-treated Hep3B2 cells. As shown in Fig. 5A, GH causes a significant increase in the GSK3β protein level. Densitometric calculations revealed that expression of cyclin D3 is almost completely inhibited with 500 ng/ml of GH (Fig. 5B), while this concentration of GH leads to a threefold elevation of GSK3β (Fig. 5C). We next immunoprecipitated cyclin D3 from control and GH-treated Hep3B2 cells and examined these immunoprecipitates by Western blotting with antibodies to cyclin D3 and GSK3β. As shown in Fig. 5D, the increase of GH in the medium increases the amounts of GSK3β in the cyclin D3 complexes. Calculations of GSK3β/cyclin D3 ratios in the cyclin D3 IPs showed that cyclin D3 complexes contained dose-dependently increased amounts of GSK3β relative to those observed in the untreated cells (Fig. 5D).
FIG. 5.
GSK3β is required for GH-mediated degradation of cyclin D3. (A) GH treatment elevates GSK3β levels and reduces levels of cyclin D3 in Hep3B2 cells. Hep3B2 cells were treated with increasing concentrations of GH. Protein extracts were examined by Western blotting with antibodies to the proteins shown on the left. Cross-reactive molecule (CRM) on the cyclin D3 filter serves as an internal loading control. (B and C) The bar graphs show protein levels of cyclin D3 (B) and GSK3β (C) calculated as ratios to CRM. Error bars indicate standard deviations. (D) GH increases interactions of GSK3β with cyclin D3 in Hep3B2 cells. Cyclin D3 was immunoprecipitated from nuclear extracts and the immunoprecipitates were analyzed by Western blotting with antibodies to cyclin D3 and GSK3β. Heavy chains of immunoglobulin G (IgG) are shown for each IP. Levels of GSK3β in cyclin D3 IPs were calculated as ratios to cyclin D3. The bar graph shows a summary of three independent experiments. (E) Inhibition of GSK3β by LiCl abrogates the GH-induced degradation of cyclin D3. His-cyclin D3 was transfected into Hep3B2 cells. The cells were pretreated with the GSK3β inhibitor LiCl (15 mM), and GH (500 ng/ml) was added to the medium. Proteins were examined by Western blotting. (F) Inhibition of p38 by the specific inhibitor SB20380 does not affect GH-mediated downregulation of cyclin D3. His-cyclin D3 was transfected into Hep3B2 cells. The cells were pretreated with SB20380 (10 μM), and GH (50 and 500 ng/ml) was added to the medium. (G) Inhibition of GSK3β by siRNA blocks the ability of GH to reduce cyclin D3 in Hep3B2 cells. Hep3B2 cells were cotransfected with His-cyclin D3 and GSK3β siRNA. The cells were treated with GH, and protein extracts were isolated. Western blotting was performed with antibodies to cyclin D3, GSK3β, and GSK3α. Each filter was reprobed with antibodies to β-actin. Bar graphs show the levels of GSK3β and cyclin D3 calculated as ratios to β-actin and then as percentages of the ratios in untreated cells. A summary of three independent experiments is shown for each protein. (H) GH fails to reduce cyclin D3 in GSK3β KO MEFs. WT and GSK3β KO MEFs were transfected with His-cyclin D3 and treated with increasing concentrations of GH. The expression of GSK3β and cyclin D3 was examined by Western blotting. The filter was reprobed with β-actin.
To determine whether GSK3β plays a causal role in GH-mediated downregulation of cyclin D3, we examined whether GH is able to reduce cyclin D3 in cells with inhibited expression of GSK3β. Three approaches were applied for these studies: inhibition of GSK3β activity by LiCl, inhibition of GSK3β by siRNA, and examination of GSK3β KO MEFs (15). As shown in Fig. 5E, the treatment of cells with LiCl abolishes the ability of GH to degrade cyclin D3. Since p38 has been shown to trigger degradation of cyclin D3 via phosphorylation at Thr283, we examined whether SB20380, an inhibitor of p38, might affect GH-mediated degradation of cyclin D3. These studies showed that the inhibition of p38 does not affect the ability of GH to downregulate cyclin D3 (Fig. 5F). We next inhibited the expression of GSK3β in Hep3B2 cells by use of specific siRNA and examined the effects of GH on cyclin D3. As one can see in Fig. 5G, GSK3β siRNA significantly reduces the levels of GSK3β. This inhibition is specific, because the levels of GSK3α are not affected by siRNA to GSK3β. Densitometry calculations of the levels of cyclin D3 as ratios to β-actin showed no reduction of cyclin D3 caused by GH treatment in cells with inhibited levels of GSK3β (Fig. 5G). To obtain additional evidence that GSK3β is required for the GH-mediated downregulation of cyclin D3, we utilized GSK3β KO MEF and WT MEF. We transfected His-cyclin D3 into these cells and examined the effects of GH on the stability of cyclin D3. As one can see in Fig. 5H, GH reduces His-cyclin D3 in WT MEFs; however, GH fails to reduce His-cyclin D3 in GSK3β KO cells. Thus, three different approaches revealed that the GH-dependent inhibition of cyclin D3 requires GSK3β.
Thr283 is a key residue which is required for GH-mediated degradation of cyclin D3 in Hep3B2 cells and in tissues of old mice.
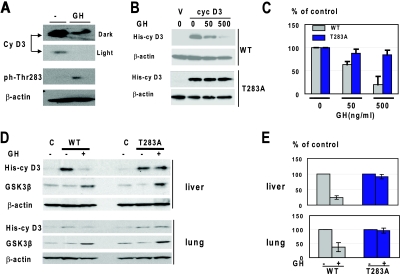
We next examined whether Thr283 is phosphorylated upon addition of GH. Hep3B2 cells were treated with GH, and protein extracts were used for Western blotting with antibodies to total cyclin D3 and to Thr283-ph. As one can see in Fig. 6A, despite the reduction of total levels of cyclin D3 by GH, the amounts of Thr283-ph isoforms are increased in cells treated with GH. We next examined whether Thr283A mutation of cyclin D3 will affect the ability of GH to downregulate cyclin D3. We generated vectors which express WT cyclin D3 and Thr283A-cyclin D3 linked to a His tag. Hep3B2 cells were transfected with vectors expressing WT His-cyclin D3 and Thr283A-His-cyclin D3. The cells were treated with GH and examined for levels of cyclin D3. We found that the expression of WT cyclin D3 was inhibited by GH; however, the levels of Thr283A cyclin D3 remained unchanged after exposure of cells to GH (Fig. 6B and C). These results revealed that Thr283 is required for GH-induced degradation of cyclin D3 in Hep3B2 cells.
FIG. 6.
GH-mediated degradation of cyclin D3 in cultured cells and in mouse tissues requires Thr283. (A) Treatment of Hep3B2 cells with GH phosphorylates cyclin D3 at Thr283. Hep3B2 cells were treated with GH (50 ng/ml), and protein extracts were examined by Western blotting with antibodies to total cyclin D3 and to the Thr283-ph isoform of cyclin D3. Dark and light exposures of total cyclin D3 are shown. The membrane was reprobed with antibodies to β-actin. (B) Mutation of Thr283 to Ala blocks the ability of GH to reduce cyclin D3 in Hep3B2 cells. Hep3B2 cells were transfected with vectors expressing WT His-cyclin D3 and Thr283A-His-cyclin D3. The cells were treated with increasing concentrations of GH as shown. Total cell lysates were analyzed by Western blotting with antibody against the His tag. The filters were reprobed with antibodies to β-actin. (C) Protein levels of cyclin D3 are shown as a summary of three independent experiments. Error bars indicate standard deviations. (D) GH fails to reduce levels of the Thr283A mutant cyclin D3 in livers and lungs of old mice. WT His-cyclin D3 and T283A-His-cyclin D3 were delivered into old mice and into animals pretreated with GH. Nuclear extracts were examined by Western blotting using antibodies against the His tag and GSK3β. (E) Levels of cyclin D3 were calculated as ratios to β-actin. The bar graphs show summaries of two independent experiments with four controls and four cyclin D3-injected mice.
Our investigations have shown that the GH-mediated downregulation of cyclin D3 in mouse tissues (Fig. 1) correlates with the GH-mediated increase of GSK3β in these tissues (Fig. 2). Therefore, we asked whether GSK3β is critical for the GH-mediated downregulation of cyclin D3 in these tissues. WT His-cyclin D3 and Thr283A-His-cyclin D3 were used for these studies. Old mice were treated with GH for 3 days and then transfected with vectors expressing WT His-cyclin D3 and Thr283A-His-cyclin D3. The expression of cyclin D3 was measured by Western blotting with antibodies to the His tag. The levels of WT His-cyclin D3 protein are significantly reduced in the livers and lungs of GH-treated old mice; however, the levels of the Thr283A-cyclin D3 mutant are not affected by GH in these tissues (Fig. 6D and E). Western blotting with antibodies to GSK3β revealed that GSK3β is equally activated in animals injected with WT and with Thr283A-cyclin D3 (Fig. 6D). We were not able to examine brain and adipose tissues, since the expression of injected His-cyclin D3 was not detectable in these tissues. Taken together, these studies showed that GH-mediated reduction of cyclin D3 in the tissues of old mice requires phosphorylation of the Thr283 residue, which is a target of GSK3β kinase.
Young GH-deficient Little mice have an altered GSK3β-cyclin D3 pathway.
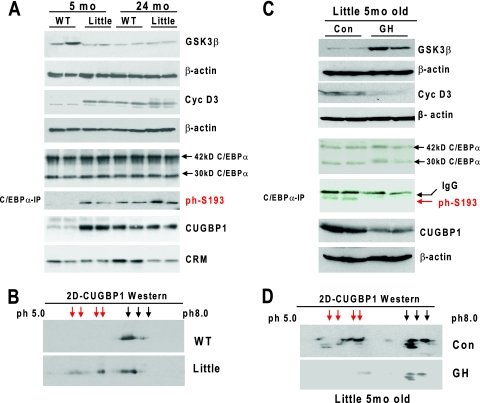
Our data in this paper suggest that the reduction of GH with age causes the elevation of cyclin D3 through the reduction of GSK3β. To directly test this hypothesis in genetically appropriate biological settings, we utilized Little mice (Ghrhrlit/lit), which are homozygous for a missense mutation in the GH-releasing hormone receptor (Ghrhr) gene (13) and have substantially reduced levels of circulating GH (1% of the normal level) (12). We have used two age groups of animals: 5- and 24-month-old mice. We found that the GSK3β levels are reduced, while levels of cyclin D3 are increased, in 5-month-old Little mice compared with levels in WT mice of the same age. In 24-month-old WT mice, cyclin D3 is elevated, while GSK3β is reduced (Fig. 7A). These patterns of expression are in agreement with the hypothesis that reduction of GH in old mice causes the reduction of GSK3β and stabilization of cyclin D3.
FIG. 7.
The GH-GSK3β-cyclin D3 pathway is altered in young GH-deficient Little mice. (A) Examination of protein levels of GSK3β, cyclin D3, total C/EBPα, the phosphorylated form of C/EBPα (ph-S193-C/EBPα), and CUGBP1 in young and old Little mice. Nuclear and cytoplasmic (for CUGBP1) extracts from livers of 5- and 24-month-old WT control and Little mice were examined by Western blotting with antibodies to cyclin D3, GSK3β, C/EBPα, and CUGBP1. C/EBPα is expressed in the liver as two isoforms with molecular masses of 42 and 30 kDa. The S193-ph 42-kDa isoform of C/EBPα was determined in C/EBPα-IPs from the nuclear extracts. The membranes were reprobed with antibodies to β-actin to verify protein loading. CRM, cross-reactive molecule observed on the CUGBP1 membrane. (B) Hyperphosphorylated isoforms of CUGBP1 are abundant in young Little mice. Cytoplasmic extracts from livers of young WT and Little mice were separated by 2D gel electrophoresis and probed with antibodies to CUGBP1. Positions of hyperphosphorylated isoforms of CUGBP1 are shown by red arrows. (C) Injection of GH into young Little mice rescues the GSK3β-cyclin D3-C/EBPα pathway. GH was injected into 5-month-old Little mice for 7 days. Proteins were isolated and examined for expression of GSK3β, cyclin D3, C/EBPα, and CUGBP1. Phosphorylation of C/EBPα at S193 was examined by IP of C/EBPα and Western blotting with antibodies to the ph-S193 isoform of C/EBPα. Each filter was reprobed with β-actin. (D) Examination of CUGBP1 isoforms in young control and GH-treated Little mice. The 2D Western blotting was performed as described for panel B. Positions of hyperphosphorylated isoforms of CUGBP1 are shown by red arrows.
Previous studies have shown that cyclin D3 is accumulated in both the nuclei and cytoplasm of old livers and hyperphosphorylates C/EBPα and RNA binding protein CUGBP1 (29, 33). To further examine the consequences of the elevation of cyclin D3 in young Little mice, we examined C/EBPα and CUGBP1 in livers of Little mice. Western blotting of nuclear extracts showed that while protein levels of C/EBPα are not changed, phosphorylation of C/EBPα at Ser193 is significantly increased in livers of young Little mice (Fig. 7A). This phosphorylation of C/EBPα mimics alterations observed in WT old mice, which have also reduced expression of GH (Fig. 7A, lanes 5 and 6). A different pattern of expression was observed for CUGBP1. Protein levels of total CUGBP1 are increased in livers of young Little mice, which mimics the pattern of expression of CUGBP1 in livers of WT old mice (lanes 7 and 8). Since phosphorylation of CUGBP1 is difficult to detect by regular Western blotting, we applied a 2D Western approach. As one can see in Fig. 7B, Little mice contain several additional phosphorylated isoforms of CUGBP1. Thus, the investigations with GH-deficient mice revealed that young Little mice have alterations of GSK3β, cyclin D3, C/EBPα, and CUGBP1 similar to those observed in livers of old mice which have reduced concentrations of GH.
Since Little mice are deficient in GH, we asked whether injections of GH into Little mice might rescue the GSK3β-cyclin D3-C/EBPα pathway. We first examined expression of GSK3β and cyclin D3 by Western blotting. These studies showed that treatment of Little mice with GH increases expression of GSK3β and reduces levels of cyclin D3. We next examined phosphorylation of C/EBPα and CUGBP1 in livers of GH-treated Little mice. An IP-Western approach showed that the phosphorylation of C/EBPα at S193 is inhibited in GH-treated Little mice (Fig. 7C). Examination of another target of cyclin D3, CUGBP1, by Western blotting and a 2D Western approach showed that GH reduces protein levels of CUGBP1 to the normal level and that phosphorylation of CUGBP1 is significantly reduced in GH-treated mice (Fig. 7C and D). Thus, these studies demonstrated that the reduction of GH in Little mice is the major cause of the alteration in GSK3β-cyclin D3-C/EBPα/CUGBP1 pathways and that injection of GH into Little mice corrects these pathways.
GSK3β negatively regulates growth-inhibitory activity of C/EBPα.
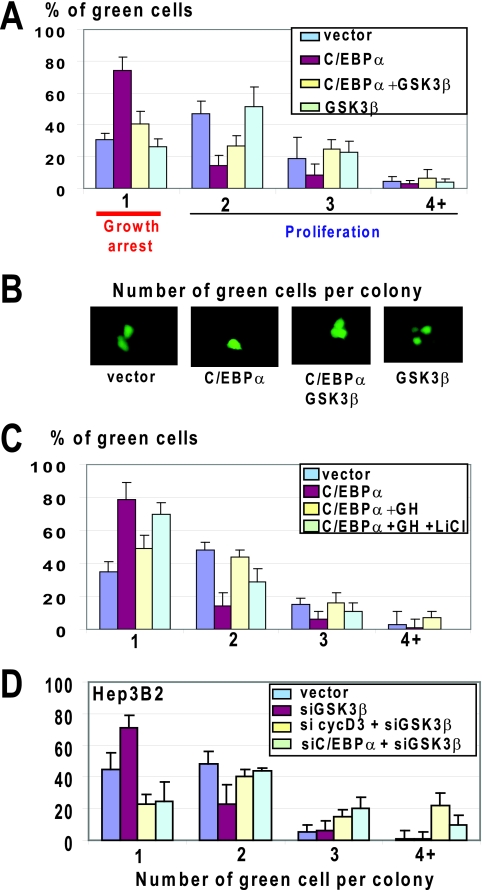
C/EBPα is a key protein which is involved in the age-associated loss of regenerative capacities of the liver (7, 16). Given a strong correlation between downregulation of GSK3β and phosphorylation of C/EBPα at S193 (Fig. 7A), we asked whether GSK3β might control growth-inhibitory activity of C/EBPα. We first examined whether the ectopic expression of GSK3β might block the ability of C/EBPα to arrest cell proliferation. For this, we used HEK293 cells, which express high levels of cyclin D3 supporting growth-inhibitory activity of C/EBPα (35). Figure 8A and B show that C/EBPα alone inhibits proliferation of HEK293 cells; however, ectopic expression of GSK3β abolishes growth-inhibitory activity of C/EBPα. Since GH operates upstream of GSK3β, we asked whether the inhibition of GSK3β might block GH-mediated neutralization of C/EBPα activity in HEK293 cells. Untreated and GH-treated HEK293 cells were transfected with pAdTrack C/EBPα, and cell proliferation was examined. These studies showed that GH eliminates growth-inhibitory activity of C/EBPα through GSK3β, since the inhibition of GSK3β by LiCl blocks the ability of GH to eliminate C/EBPα activities (Fig. 8C).
FIG. 8.
GSK3β negatively regulates growth-inhibitory activity of C/EBPα. (A) Overexpression of GSK3β blocks the ability of C/EBPα to inhibit cell proliferation. C/EBPα was cotransfected with GSK3β into HEK293 cells, and cell proliferation was examined by colony formation assay. Bar graphs show a summary of three independent experiments. Error bars indicate standard deviations. (B) Typical pictures of cells on the experimental plates. (C) GH eliminates growth-inhibitory activity of C/EBPα through GSK3β. C/EBPα was transfected into HEK293 cells treated with GH or with GH plus LiCl. Cell proliferation was examined as described for panel A. The bar graph shows a summary of three independent experiments. (D) Downregulation of GSK3β in Hep3B2 cells leads to growth arrest through the activation of the cyclin D3-C/EBPα pathway. The expression of GSK3β was inhibited in Hep3B2 cells by siRNA. The expression of C/EBPα and cyclin D3 was inhibited with specific siRNAs by simultaneous transfections of siRNAs to these proteins and to GSK3β. A summary of three experiments is shown.
The studies with HEK293 cells were performed with overexpression of C/EBPα and GSK3β; therefore, we asked whether GSK3β might block activities of C/EBPα when both proteins are expressed from the endogenous promoters in appropriate biological environments. For these studies, we have chosen hepatoma cell line Hep3B2, which expresses endogenous C/EBPα but proliferates due to low levels of cyclin D3 and lack of phosphorylation of C/EBPα (30, 35). To reduce expression of GSK3β, we cotransfected siRNA to GSK3β and GFP vector (to visualize transfected cells). Figure 8D shows that expression of siRNA to GSK3β inhibits proliferation of Hep3B2 cells. Downregulation of cyclin D3 or C/EBPα by siRNAs abolished this inhibition, clearly demonstrating that siRNA to GSK3β inhibits proliferation through activation of the cyclin D3-C/EBPα pathway. Thus, these studies showed that GSK3β is a negative regulator of growth-inhibitory activity of C/EBPα.
Downregulation of GSK3β in young mice inhibits liver proliferation via activation of the cyclin D3-C/EBPα pathway.
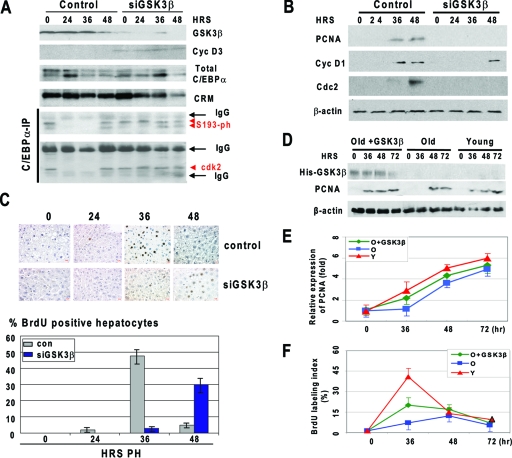
Given the reduction of GSK3β in livers of old mice which have lost the proper proliferative response, we asked whether the inhibition of GSK3β in the livers of young mice might inhibit liver proliferation after PH. siRNA to GSK3β was injected in mice a day before PH, and liver proliferation was examined at different time points after PH by several approaches. Western blotting showed that siRNA to GSK3β significantly reduced levels of GSK3β at all time points examined (Fig. 9A). This inhibition resulted in the elevation of cyclin D3 and hyperphosphorylation of C/EBPα at S193 at all stages of liver proliferation examined, while phosphorylation of S193 was not detectable in control animals at 24 and 36 h after PH. We next determined whether the ph-S193 isoform of C/EBPα forms complexes with cdk2. In control mice, C/EBPα was associated with cdk2 at time zero and 48 h after PH, while no interaction was observed at 24 and 36 h after PH. In contrast, the S193-ph isoform of C/EBPα is associated with cdk2 in siGSK3β-inhibited livers at all stages of liver regeneration examined. Given the association of C/EBPα with cdk2, we next determined whether this association inhibits liver proliferation. We found that the livers with inhibited GSK3β failed to activate PCNA and cdc2 and that the elevation of cyclin D1 was delayed (Fig. 9B). We next examined DNA synthesis by measuring BrdU uptake. In control mice, around 45% of hepatocytes incorporate BrdU at 36 h after PH, while in livers with inhibited GSK3β, only 5% of hepatocytes are BrdU positive at that time point after PH. We found that the peak of DNA synthesis is reduced and is shifted in these animals to 48 h (Fig. 9C). These data showed that, similar to the case for old animals, the reduction of GSK3β in young mice leads to the inhibition of liver proliferation via activation of the cyclin D3-C/EBPα pathway.
FIG. 9.
Downregulation of GSK3β in young mice inhibits liver proliferation, while ectopic expression of GSK3β in old liver accelerates proliferation of the liver. (A) Downregulation of GSK3β in the liver activates the cyclin D3-C/EBPα pathway of inhibition of proliferation. Protein levels of GSK3β, cyclin D3, and C/EBPα were examined by Western blotting with corresponding antibodies. The 42- and 40-kDa isoforms of C/EBPα are shown. CRM, cross-reactive molecule observed on the C/EBPα membrane. C/EBPα-IP, C/EBPα was immunoprecipitated from nuclear extracts and probed with antibodies to ph-S193 and to cdk2. IgG, heavy and light chains of immunoglobulin G. (B) Expression of cell cycle proteins in livers with inhibited levels of GSK3β. Western blotting was performed with nuclear extracts isolated from control and siGSK3β-treated mice at different time points after PH. (C) Inhibition of GSK3β in the liver leads to delayed and reduced DNA synthesis after PH. The upper panel shows BrdU staining; the lower panel shows a summary of three independent experiments. Error bars indicate standard deviations. (D) Expression of His-GSK3β and PCNA after PH in livers of young mice, old mice, and old mice injected with GSK3β. Western blotting was performed with nuclear extracts isolated at different time points after PH (shown on the top) using antibodies to the His tag and to PCNA. The filter was reprobed with β-actin. (E) Ectopic expression of GSK3β corrects expression of PCNA in old livers. Levels of PCNA were calculated as ratios to β-actin. (F) Ectopic expression of GSK3β accelerates proliferation of old livers after PH. BrdU uptake was examined in livers of young mice (Y), old mice (O), and old mice injected with GSK3β. A summary of three independent experiments is shown.
Ectopic expression of GSK3β in old mice accelerates liver proliferation after PH.
To demonstrate a causal role of the reduction of GSK3β in the inhibition of liver proliferation in old mice, we delivered a vector expressing GSK3β into old mice and examined liver proliferation after PH. To distinguish the injected GSK3β and endogenous protein, GSK3β was fused to a His tag. GSK3β was injected in animals 24 h before surgery. Figure 9D shows that His-GSK3β is stably expressed in the liver within 72 h after PH. To examine liver proliferation, we used two approaches: examination of the S-phase-specific protein PCNA and examination of BrdU uptake. Proliferation of young livers after PH was examined in parallel studies. As one can see in Fig. 9D and E, elevation of PCNA is delayed in livers of old mice compared to expression in young mice. The ectopic expression of GSK3β partially corrects the expression of PCNA. The partial correction seems to reflect the fact that the efficiency of plasmid delivery to hepatocytes was around 50%. Consistent with previous publications, examination of BrdU uptake showed that DNA synthesis is reduced and delayed in livers of old mice; however, the ectopic expression of GSK3β accelerates DNA synthesis in livers of old mice (Fig. 9F). Similar to correction of PCNA expression, the correction of DNA synthesis is also partial because 50% or fewer hepatocytes received GSK3β plasmid. Taken together, these studies revealed that ectopic expression of GSK3β in livers of old mice accelerates liver proliferation after PH.
DISCUSSION
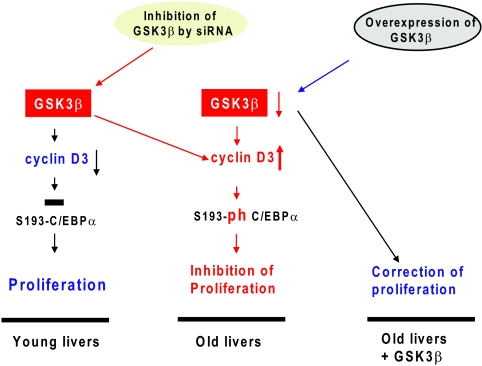
The reduction of the regenerative capacities of tissues is one of the major characteristics of aging. The inhibited proliferation of livers in old mice is mediated by alterations in expression and activities of several proteins, including transcription factors C/EBPα (7, 16) and FoxM1B (37) as well as RNA binding protein CUGBP1 (29, 31). The data in this paper show that GSK3β is a kinase which operates upstream of C/EBPα and CUGBP1 and that the age-associated reduction of GSK3β and the following stabilization of cyclin D3 play a key role in the inhibition of liver proliferation (Fig. 10). We and other groups have previously found that cyclin D3 is expressed in differentiated tissues and supports quiescence of the liver and skeletal muscle (2, 5, 10, 33). In this paper, we have focused our studies on tissues which express high levels of C/EBPα, the target of cyclin D3. These tissues included liver, lung, brain, and adipose tissues. The expression of cyclin D3 is elevated in all of these tissues, and this elevation is associated with the reduction of GH. Examination of tissues from young, old, and old GH-treated mice clearly showed that both GSK3β and cyclin D3 are controlled by GH. Searching for mechanisms by which GH upregulates GSK3β, we have found that the GSK3β promoter contains several binding sites for C/EBP proteins and that the GSK3β promoter is repressed in livers of old mice by C/EBPβ-HDAC1 complexes. Treatment of old mice with GH leads to the activation of the GSK3β promoter via removal of the C/EBPβ-HDAC1 complexes.
FIG. 10.
A hypothetical model for the role of GSK3β in control of liver proliferation.
Our data demonstrate that cyclin D3 levels are tightly controlled by GH in the liver and in cultured hepatocytes. The reduction of GH in old mice and in young Little mice causes the downregulation of GSK3β, which leads to a change of the GSK3β/PP1 balance in favor of PP1 and to increased association of PP1 with cyclin D3. Our data also show that Thr283 is a key residue of cyclin D3 which is required for the GH-mediated degradation of cyclin D3 in the livers of old mice and that the cyclin D3 Thr283A mutant cannot be degraded by a GH-dependent mechanism in the liver (Fig. 6). It is interesting to note that Lahne et al. have previously demonstrated that the degradation of cyclin D3 can be achieved using a cyclin D3 T283A mutant, independently of GSK3β activity (21). We think that these differences are associated with the existence of tissue-specific pathways of degradation of cyclin D3. While our data were obtained with cultured hepatocytes and with liver, the experiments in the study by Lahne et al. (21) were performed with the cultured B-lymphoid precursor cell line Reh and with the leukemic T-cell line Jurkat. We suggest that, although cyclin D3 might be degraded by several pathways, the GH-GSK3β pathway of degradation of cyclin D3 is the major pathway in the liver.
The key role of GSK3β in the inhibition of liver proliferation is supported by our studies of liver proliferation in young mice with inhibited levels of GSK3β and in old mice with ectopic expression of GSK3β. Investigations with young animals clearly demonstrated that the downregulation of GSK3β leads to the inhibition of liver proliferation and that this inhibition is associated with an abundance of ph-S193 isoforms of C/EBPα and with the elevation of C/EBPα-cdk2 complexes (Fig. 9A). Most important, we found that ectopic expression of GSK3β in old mice accelerates liver proliferation after PH. These studies demonstrate that the reduction of GSK3β is a key event in the reduced proliferative capacities of the old liver (Fig. 10). In support of this hypothesis, experiments in different tissue culture systems showed that GSK3β can regulate cell proliferation by controlling growth-inhibitory activity of C/EBPα (Fig. 8). Although the majority of the data in this paper were obtained with the liver, we suggest that similar mechanisms might be involved in the age-associated reduction of regenerative capacities of other tissues. Since the reduction of GSK3β and elevation of cyclin D3 in other tissues are also GH dependent, we suggest that the known targets of cyclin D3 (C/EBPα and CUGBP1) might be also changed in lung, brain, and adipose tissues. We also suggest that cyclin D3-cdk4 might have additional targets in other tissues and that phosphorylation of these targets might be involved in the age-associated reduction of the regenerative capacities of tissues. Taken together, our data provide a molecular basis for the age-associated loss of the regenerative capacity of the liver.
Acknowledgments
This work is supported by NIH grants GM55188, CA100070, and AG20752 (N.A.T.) and AG028865 and DK56338 (G.J.D.).
We thank Jim Woodgett for GSK3β KO MEFs and Daniel Amador-Noguez and Adam Dean for help with the work with Little mice.
Footnotes
Published ahead of print on 27 April 2009.
REFERENCES
- 1.Aktas, H., H. Cai, and G. M. Cooper. 1997. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol. Cell. Biol. 173850-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartkova, J., J. Lukas, M. Strauss, and J. Bartek. 1998. Cyclin D3: requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene 171027-1037. [DOI] [PubMed] [Google Scholar]
- 3.Bollen, M. 2001. Combinatorial control of protein phosphatase-1. Trends Biochem. Sci. 26426-431. [DOI] [PubMed] [Google Scholar]
- 4.Casanovas, O., M. Jaumot, A. B. Paules, N. Agell, and O. Bachs. 2004. P38SAPK2 phosphorylates cyclin D3 at Thr-283 and targets it for proteasomal degradation. Oncogene 237537-7544. [DOI] [PubMed] [Google Scholar]
- 5.Cenciarelli, C., F. De Santa, P. L. Puri, E. Mattei, L. Ricci, F. Bucci, A. Felsani, and M. Caruso. 1999. Critical role played by cyclin D3 in the MyoD-mediated arrest of cell cycle during myoblast differentiation. Mol. Cell. Biol. 195203-5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, P. T. 2002. Protein phosphatase 1—targeted in many directions. J. Cell Sci. 115241-256. [DOI] [PubMed] [Google Scholar]
- 7.Conboy, I. M., M. J. Conboy, A. J. Wagers, E. R. Girma, I. L. Weissman, and T. A. Rando. 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433760-764. [DOI] [PubMed] [Google Scholar]
- 8.de la Serna, I. L., K. Roy, K. A. Carlson, and A. N. Imbalzano. 2001. MyoD can induce cell cycle arrest but not muscle differentiation in the presence of dominant negative SWI/SNF chromatin remodeling enzymes. J. Biol. Chem. 27641486-41491. [DOI] [PubMed] [Google Scholar]
- 9.Doble, B. W., and J. R. Woodgett. 2003. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 1161175-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doglioni, C., C. Chiarelli, E. Macri, A. P. Dei Tos, E. Meggiolaro, P. Dalla Palma, and M. Barbareschi. 1998. Cyclin D3 expression in normal, reactive and neoplastic tissues. J. Pathol. 185159-166. [DOI] [PubMed] [Google Scholar]
- 11.Everitt, A., and J. Meites. 1989. Aging and anti-aging effects of hormones. J. Gerontol. 44B139-B147. [DOI] [PubMed] [Google Scholar]
- 12.Flurkey, K., J. Papaconstantinou, R. A. Miller, and D. E. Harrison. 2001. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. USA 986736-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godfrey, P., J. O. Rahal, W. G. Beamer, N. G. Copeland, N. A. Jenkins, and K. E. Mayo. 1993. GHRH receptor of Little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat. Genet. 4227-232. [DOI] [PubMed] [Google Scholar]
- 14.Goff, B. L., J. A. Roth, L. H. Arp, and G. S. Incefy. 1987. Growth hormone treatment stimulates thymulin production in aged dogs. Clin. Exp. Immunol. 68580-587. [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeflich, K. P., J. Luo, E. A. Rubie, M. S. Tsao, O. Jin, and J. R. Woodgett. 2000. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 40686-90. [DOI] [PubMed] [Google Scholar]
- 16.Iakova, P., S. S. Awad, and N. A. Timchenko. 2003. Aging reduces proliferative capacities of liver by switching pathways of C/EBPalpha growth arrest. Cell 113495-506. [DOI] [PubMed] [Google Scholar]
- 17.Inaba, T., H. Matsushime, M. Valentine, M. F. Roussel, C. J. Sherr, and A. T. Look. 1992. Genomic organization, chromosomal localization, and independent expression of human cyclin D genes. Genomics 13565-574. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, P. F. 2005. Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J. Cell Sci. 1182545-2555. [DOI] [PubMed] [Google Scholar]
- 19.Krishnamurthy, J., M. R. Ramsey, K. L. Ligon, C. Torrice, A. Koh, S. Bonner-Weir, and N. E. Sharpless. 2006. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443453-457. [DOI] [PubMed] [Google Scholar]
- 20.Krupczak-Hollis, K., X. Wang, M. B. Dennewitz, and R. H. Costa. 2003. Growth hormone stimulates proliferation of old-aged regenerating liver through forkhead box m1b. Hepatology 381552-1562. [DOI] [PubMed] [Google Scholar]
- 21.Lahne, H. U., M. M. Kloster, S. Lefdal, H. K. Blomhoff, and S. Naderi. 2006. Degradation of cyclin D3 independent of Thr-283 phosphorylation. Oncogene 252468-2476. [DOI] [PubMed] [Google Scholar]
- 22.Muise-Helmericks, R. C., H. L. Grimes, A. Bellacosa, S. E. Malstrom, P. N. Tsichlis, and N. Rosen. 1998. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 27329864-29872. [DOI] [PubMed] [Google Scholar]
- 23.Naderi, S., K. B. Gutzkow, H. U. Lahne, S. Lefdal, W. J. Ryves, A. J. Harwood, and H. K. Blomhoff. 2004. cAMP-induced degradation of cyclin D3 through association with GSK-3beta. J. Cell Sci. 1173769-3783. [DOI] [PubMed] [Google Scholar]
- 24.Nelsen, C. J., D. G. Rickheim, N. A. Timchenko, M. W. Stanley, and J. H. Albrecht. 2001. Transient expression of cyclin D1 is sufficient to promote hepatocyte replication and liver growth in vivo. Cancer Res. 618564-8568. [PubMed] [Google Scholar]
- 25.Nerlov, C. 2007. The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 17318-324. [DOI] [PubMed] [Google Scholar]
- 26.Reichert, M., and D. Eick. 1999. Analysis of cell cycle arrest in adipocyte differentiation. Oncogene 18459-466. [DOI] [PubMed] [Google Scholar]
- 27.Rickheim, D. G., C. J. Nelsen, J. T. Fassett, N. A. Timchenko, L. K. Hansen, and J. H. Albrecht. 2002. Differential regulation of cyclins D1 and D3 in hepatocyte proliferation. Hepatology 3630-38. [DOI] [PubMed] [Google Scholar]
- 28.Sarruf, D. A., I. Iankova, A. Abella, S. Assou, S. Miard, and L. Fajas. 2005. Cyclin D3 promotes adipogenesis through activation of peroxisome proliferator-activated receptor gamma. Mol. Cell. Biol. 259985-9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timchenko, L. T., E. Salisbury, G. L. Wang, H. Nguyen, J. H. Albrecht, J. W. Hershey, and N. A. Timchenko. 2006. Age-specific CUGBP1-eIF2 complex increases translation of CCAAT/enhancer-binding protein beta in old liver. J. Biol. Chem. 28132806-32819. [DOI] [PubMed] [Google Scholar]
- 30.Wang, G. L., P. Iakova, M. Wilde, S. Awad, and N. A. Timchenko. 2004. Liver tumors escape negative control of proliferation via PI3K/Akt-mediated block of C/EBP alpha growth inhibitory activity. Genes Dev. 18912-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, G. L., E. Salisbury, X. Shi, L. Timchenko, E. E. Medrano, and N. A. Timchenko. 2008. HDAC1 cooperates with C/EBPalpha in the inhibition of liver proliferation in old mice. J. Biol. Chem. 28326196-26198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, G. L., E. Salisbury, X. Shi, L. Timchenko, E. E. Medrano, and N. A. Timchenko. 2008. HDAC1 promotes liver proliferation in young mice via interactions with C/EBP beta. J. Biol. Chem. 28326179-26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, G. L., X. Shi, E. Salisbury, Y. Sun, J. H. Albrecht, R. G. Smith, and N. A. Timchenko. 2006. Cyclin D3 maintains growth-inhibitory activity of C/EBPα by stabilizing C/EBPα-cdk2 and C/EBPα-Brm complexes. Mol. Cell. Biol. 262570-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, G. L., X. Shi, E. Salisbury, Y. Sun, J. H. Albrecht, R. G. Smith, and N. A. Timchenko. 2007. Growth hormone corrects proliferation and transcription of phosphoenolpyruvate carboxykinase in livers of old mice via elimination of CCAAT/enhancer-binding protein alpha-Brm complex. J. Biol. Chem. 2821468-1478. [DOI] [PubMed] [Google Scholar]
- 35.Wang, G. L., X. Shi, E. Salisbury, and N. A. Timchenko. 2008. Regulation of apoptotic and growth inhibitory activities of C/EBPalpha in different cell lines. Exp. Cell Res. 3141626-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, G. L., and N. A. Timchenko. 2005. Dephosphorylated C/EBPalpha accelerates cell proliferation through sequestering retinoblastoma protein. Mol. Cell. Biol. 251325-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, X., K. Krupczak-Hollis, Y. Tan, M. B. Dennewitz, G. R. Adami, and R. H. Costa. 2002. Increased hepatic Forkhead Box M1B (FoxM1B) levels in old-aged mice stimulated liver regeneration through diminished p27Kip1 protein levels and increased Cdc25B expression. J. Biol. Chem. 27744310-44316. [DOI] [PubMed] [Google Scholar]
- 38.Woodgett, J. R. 1990. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 92431-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]