Abstract
Defining the chromatin modifications and transcriptional mechanisms that direct the development of different T-cell lineages is a major challenge in immunology. The transcriptional coactivators CREB binding protein (CBP) and the closely related p300, which comprise the KAT3 family of histone/protein lysine acetyltransferases, interact with over 50 T-lymphocyte-essential transcriptional regulators. We show here that CBP, but not p300, modulates the thymic development of conventional adaptive T cells versus those having unconventional innate functions. Conditional inactivation of CBP in the thymus yielded CD8 single-positive (SP) thymocytes with an effector-, memory-, or innate-like T-cell phenotype. In this regard, CD8 SP thymocytes in CBP mutant mice were phenotypically similar to those reported for Itk and Rlk protein tyrosine kinase mutants, including the increased expression of the T-cell master regulatory transcription factor eomesodermin (Eomes) and the interleukin-2 and -15 receptor beta chain (CD122) and an enhanced ability to rapidly produce gamma interferon. CBP was required for the expression of the Itk-dependent genes Egr2, Egr3, and Il2, suggesting that CBP helps mediate Itk-responsive transcription. CBP therefore defines a nuclear component of the signaling pathways that demarcate the development of innate and adaptive naïve CD8+ T cells in the thymus.
The initial event in thymic T-cell development occurs when progenitors derived from hematopoietic stem cells leave the bone marrow and go to the thymus. These progenitors differentiate and expand into a large population of thymocytes, with CD4− CD8− double-negative (DN) thymocytes being the most immature stage. DN cells progress to the CD4+ CD8+ double-positive (DP) stage before commitment to either the CD4+ or CD8+ lineages. CD4+ CD8− and CD4− CD8+ single-positive (SP) thymocytes are the final stage and exit the thymus to the peripheral lymphoid organs as mature T cells.
Conventional adaptive CD8+ T cells undergo two physically separate developmental processes. Naïve adaptive T cells first develop in the thymus and then move to the peripheral lymphoid organs, where after encountering antigen they differentiate to short-lived effector CD8+ T cells with functions that include cytotoxicity and cytokine production (32). Some effector CD8+ T cells become long-lived memory cells that gain effector function rapidly after reexposure to antigen (15). Less abundant unconventional innate T cells also develop in the thymus, but they acquire effector function as part of the maturation process rather than by encountering antigen in the periphery (4). Innate T cells thus are able to respond rapidly to a new foreign antigen such as bacteria (3). The Tec kinases Itk and Rlk (Txk), which modulate T-cell receptor signaling, help demarcate conventional and innate T-cell development in the thymus (2, 4, 5, 8), but the delineating transcriptional events are unclear.
Transcriptional “master regulators” that include Notch, Foxp3, Th-POK/cKrox, T-bet, and Eomes are major determinants of T-cell identity and function (10, 14, 18, 33). Transcription factors like these are probably not truly sufficient to direct cell fate, however, as other regulators act in combination to initiate and maintain a gene expression program (33). Such cooperating regulators may be additional DNA-binding transcription factors, or they may be transcriptional coactivators that possess protein- and chromatin-modifying activities and protein adaptor functions (24, 33, 43). How coactivators influence the expression or function of T-cell master regulatory transcription factors is mostly unknown.
Coactivators typically do not bind DNA but interact with transcription factors to stimulate RNA polymerase II via their associated adaptor or enzymatic functions (e.g., histone acetylation). Often viewed as adding utilitarian functionalities to the transcription factor, in certain tissues “active regulation” of coactivator level or activity appears to control gene expression (35). There are few examples of active coactivator regulation in T cells, however (30). “Passive regulation,” where the unregulated presence of a coactivator helps direct distinct T-cell fates, is also largely unexplored as it is revealed only by mutation of the coactivator gene.
CREB binding protein ([CBP] Crebbp) and p300 (Ep300) are highly conserved and widely expressed histone/protein acetyltransferases that have more than 360 described protein interaction partners (11, 21). Most of the conserved domains of CBP/p300 function to bind a diverse array of proteins, including more than 50 vital T-cell transcriptional regulators (21). Not surprisingly, CBP and p300 are each essential for mouse development (39, 46), and they are collectively expressed in, and required for, T and B cells (21, 44). In vitro, CBP and p300 are often biochemically indistinguishable (20), so there is little reason a priori to contemplate unique roles for them in vivo. Intriguingly, however, conditional inactivation of CBP, but not p300, in thymocytes results in an increased proportion of CD8 SP cells and, under some conditions, increases the tendency for T-cell lymphoma (17, 21). The specialized CBP functions necessary for T-cell development remain unknown.
Using Cre/LoxP conditional knockout mice, we further investigated the role of CBP in T-cell fate decisions. These studies revealed that CBP demarcates adaptive and innate T-cell development in the thymus in a manner reminiscent of Itk and Rlk protein tyrosine kinases, as shown in Itk and Rlk mutant mice.
MATERIALS AND METHODS
Mice.
CBPflox and p300flox mice were described previously and validated to generate null alleles after Cre-mediated recombination (17, 21). Lck-Cre (12) and Cd4-Cre (26) transgenic mice that express Cre specifically in the T-cell lineage were from J. Marth (Howard Hughes Medical Institute—University of California, San Diego, CA) and Taconic (New York), respectively. Gt(Rosa)26Sortm1(EYFP)Cos, version 1 (Jackson Labs), transgenic mice that express yellow fluorescent protein (YFP) in cells where Cre recombinase has been expressed were crossed to the lines above to allow enrichment of CBP-deleted cells for some experiments as indicated (36). Most experiments described in this study were replicated using mice on two genetic backgrounds: a C57BL/6 and 129Sv mixed strain and C57BL/6 × 129Sv F1 hybrid (F1 hybrid) mice that were derived from C57BL/6 and 129Sv congenic parents that had been backcrossed at least nine times. CBPflox/flox; Lck-Cre mice with an increased contribution of C57BL/6 genes tended to have more pronounced thymic hypoplasia and a larger percentage of DN thymocytes, suggestive of an enhanced block at the transition from DN to DP (T. Fukuyama, unpublished data). All mice were used and cared for following protocols approved by the Institutional Animal Care and Use Committee of St. Jude Children's Research Hospital (SJCRH).
Genotyping.
Semiquantitative PCR genotyping for the CBPflox and p300flox alleles was described previously and was used routinely in this study to confirm gene recombination frequency (21).
Statistics.
Data are reported as means ± standard error of the means. Analysis of variance (ANOVA) and t test statistical analyses were performed using GraphPad Prism software. Tukey multiple comparison test was used for ANOVA posttesting of data column pairs to confirm that CBP mutant and control data were significantly different (P values of 0.05 to 0.01, 0.01 to 0.001, or <0.001) when applicable.
Protein extracts and Western blotting.
As a positive control for Eomes protein, the Eomes cDNA (ATCC) was expressed from a murine stem cell virus retroviral plasmid in transiently transfected 293T cells. Nuclear extracts were prepared as previously described (19). To detect Eomes in CD8 SP thymocytes, total thymus cell suspensions were incubated with CD4 (L3T4) MicroBeads (Miltenyi Biotec, Germany). The CD4-negative cell fraction was collected and then incubated with CD8a (Ly-2) MicroBeads (Miltenyi Biotec) to collect CD8 SP thymocytes. One million CD8 SP thymocytes were resuspended in buffer C (20 mM HEPES, pH 7.9, 400 mM NaCl, 1 mM EDTA, 1 mM EGTA) and 2× sodium dodecyl sulfate (SDS) sample buffer, and boiled. Proteins were separated by 8% SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Bio-Rad, CA). Anti-Eomes rabbit polyclonal antibody was from Abcam (Massachusetts), and anti-rabbit immunoglobulin G (IgG), horseradish peroxidase-linked antibody was from GE Healthcare (New Jersey). The signal was detected with ECL Western Blotting Detection Reagents (GE Healthcare). To detect β-actin, the polyvinylidene difluoride membrane was incubated with stripping buffer (100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.7) at 65°C for 30 min, washed with Tris-buffered saline-0.5% Tween 20 (TBS-T) three times, and blocked with 5% nonfat milk-TBS-T at room temperature for 1 h. Anti-β-actin antibody (mouse monoclonal; Abcam), anti-mouse IgG, and horseradish peroxidase-linked antibody (GE Healthcare) were used to detect β-actin.
Flow cytometry and FACS.
Cell acquisition was performed on a FACSCalibur (BD, NJ) using CellQuest software (BD). Antibodies for flow cytometry and fluorescence-activated cell sorting (FACS) were from BD (anti-CD3, -CD4, -CD8, -CD24, -CD122, -CD44, -NK1.1, and -annexin V). Analysis was performed using FlowJo software (Tree Star, OR).
IFN-γ production assays.
Single-cell suspensions of thymocytes or splenocytes were cultured in Dulbecco's modified Eagle's medium (Invitrogen, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) GolgiPlug, and GolgiStop (BD) and with 5 ng/ml of phorbol 12-myristate 13-acetate plus 500 ng/ml of ionomycin (P+I) or with dimethyl sulfoxide vehicle for 5 h. The cells were stained for CD3, CD4, and CD8 and then fixed with BD Cytofix/Cytoperm according to the company's protocol. Cells were stained for intracellular gamma interferon (IFN-γ) (phycoerythrin-Cy7 anti-mouse IFN-γ; eBioscience, CA). IFN-γ induction was checked for SP thymocytes or splenocytes expressing high levels of CD3 (CD3hi) and CD8.
Neonate thymic organ culture.
Thymuses from newborn mixed-background mice were cultured for 6 days in the upper chamber of a Transwell (24-mm diameter with 0.4-μm-pore-size polycarbonate membrane insert; Corning, MA) with the lower chamber filled with Dulbecco's modified Eagle's medium containing 10% heat-inactivated FBS.
Autologous splenic cell transplantation.
Spleens were surgically removed, and splenocyte suspensions were prepared and stained with carboxyfluorescein succinimidyl ester (CFSE; Invitrogen). CFSE-stained splenocytes were injected into the original host mouse via tail vein. Cervical lymph nodes and thymuses were harvested at 1, 4, or 14 days after transplantation, and the CFSE-positive cells were detected by fluorescence microscopy. DAPI (4′,6′-diamidino-2-phenylindole)-stained nuclei served as a normalization control for cell number.
Bone marrow chimeras.
A total of 2 × 106 bone marrow cells from C57BL/6 × 129Sv F1 CBPflox/flox; Lck-Cre or CBPflox/flox control mice were injected via tail vein into lethally irradiated (1,100 rads) 5-week-old C57BL/6 female recipient mice. Eomes mRNA in whole thymus was examined by quantitative reverse transcription-PCR (qRT-PCR), and CBPflox gene recombination was confirmed by PCR.
qRT-PCR.
cDNA was generated from 100 ng (20 ng for anti-CD3-treated splenocytes) of total RNA in a 20-μl reaction mixture using Superscript II reverse transcriptase (Invitrogen). Real-time qRT-PCR was performed as described on an Opticon DNA Engine (MJ Research) using 1 μl of cDNA per 25 μl of PCR mixture with SYBR green dye (Qiagen, CA) (45). PCR primers were designed using the web-based Universal ProbeLibrary Assay Design Center (Roche) and confirmed to yield a single product by melt-curve analysis. Samples were first normalized to Gapdh (glyceraldehyde-3-phosphate dehydrogenase) mRNA, and the expression levels for each test gene were set relative to the lowest value, which was defined as 1. The primer sequences were the following: Eomes/Tbr2, 5′-TTCCGGGACAACTACGATTCA-3′ and 5′-ACGCCGTACCGACCTCC-3′; T-bet/Tbx21, 5′-CCTGTTGTGGTCCAAGTTC-3′ and 5′-CACCAAGACCACATCCAC-3′; Il2rb/CD122, 5′-GTCCATGCCAAGTCGAACCT-3′ and 5′-GGATGCCTGCCTCACAAGAG-3′; Ccr5, 5′-AGGCCATGCAGGCAACAG-3′ and 5′-TCTCTCCAACAAAGGCATAGATGA-3′; Cxcr3, 5′-CAGCCTGAACTTTGACAGAACCT-3′ and 5′-GCAGCCCCAGCAAGAAGAG-3′; Fasl, 5′-AGGAATGTATCAGCTCTTCCACCT-3′ and 5′-GATGATACTTTAAGGCTTTGGTTGG-3′; Prf1, 5′-TCTCCTCCTATGGCACGCAC-3′ and 5′-TGTAAGGACCGAGATGCGG-3′; Egr2, 5′-CGGAGTGGCGGGAGATG-3′ and 5′-GGGAGCGAAGCTACTCGGATA-3′; Egr3, 5′-CAATCTGTACCCCGAGGAGA-3′ and 5′-CCGATGTCCATCACATTCTCT-3′; Il2, 5′-CAGGATGGAGAATTACAGGAACCT-3′ and 5′-TTTCAATTCTGTGGCCTGCTT-3′; Gapdh, 5′-TGCACCACCAACTGCTTAGC-3′ and 5′-TGGATGCAGGGATGATGTTC-3′.
TUNEL assay.
Thymus frozen sections were prepared, and a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay was performed using an In situ Cell Death Detection Kit (TMR red; Roche, IN).
Immunofluorescence.
Single-cell suspensions were made from spleens, and CD8+ splenocytes were isolated by magnetic cell sorting beads as described above. These CD8+ splenocytes were then subjected to FACS analysis as described above for cells expressing low levels of CD44 and CD122 (CD44lo CD122lo), CD44hi CD122hi, and CD44hi and low or intermediated levels of CD122 (CD122lo or int) populations. Cells were fixed for 10 min in 3% paraformaldehyde, spun down and resuspended in phosphate-buffered saline (PBS)-2% FBS, and adhered to 0.1% polyethyleneimine-coated slides for 30 min. Cells were permeabilized with 0.2% Triton in PBS for 10 min and then blocked for 1 h with 3% milk in PBS. Slides were incubated overnight with C-20 and A-22 CBP antibodies (Santa Cruz) diluted 1:1,000 in 3% milk in PBS, followed by a 1-h incubation with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Jackson Immunologicals) and a 5-min DAPI staining.
T-cell receptor (TCR)-responsive transcription in splenic T cells.
Splenocytes were harvested from CBP+/+; Lck-Cre; YFP and CBPflox/flox; Lck-Cre; YFP mice and sorted for YFP+ CD8+ CD44hi and YFP+ CD8+ CD44lo populations (the YFP transgene is expressed when recombined by Cre and was used here to enrich for cells with CBP deletion). YFP+ CD8+ CD44lo splenocytes were seeded in 24-well plates containing plate-bound anti-CD3 (300 μl of PBS and 1.5 μl of 1 mg/ml anti-mouse CD3e [BD Pharmingen] for 90 min at 37°C, followed by two washes with PBS immediately before the cells were added)or PBS-only-treated wells (mock) for 2 h at 37°C and then were harvested for mRNA using an Arcturus PicoPure RNA isolation kit (MDS Analytical Technologies). YFP+ CD8+ CD44hi splenocytes were treated with plate-bound anti-CD3 or were mock treated for 4 h at 37°C, and RNA was harvested as described above. Egr2, Egr3, and Il2 mRNA expression was measured by qRT-PCR analysis, and gene expression was normalized to Gapdh.
RESULTS
Loss of CBP in the T-cell lineage decreases the CD4 SP to CD8 SP thymocyte ratio but not the number of CD8 SP thymocytes.
We previously showed using CBPflox/flox; Lck-Cre and p300flox/flox; Lck-Cre T-cell-specific conditional knockout mice that Cre recombinase-mediated inactivation of a “floxed” CBPflox or p300flox allele starting at the DN stage results in thymocytes that lack CBP or p300 protein (21). CBPflox/flox; Lck-Cre mice have an increased proportion of CD8 SP thymocytes relative to CD4 SP cells, which is not seen in p300flox/flox; Lck-Cre mice (21). This suggested that CBP and p300 have distinct lineage-intrinsic roles in CD8+ T-cell development, possibly related to a unique biochemical property for CBP.
The number of CD8 SP thymocytes in the CBP mutants is similar to that in control mice (21) even though CBPflox/flox; Lck-Cre mice on a mixed-strain background are prone to smaller thymuses (21). Thymic hypoplasia tended to be more severe on a C57BL/6 background (data not shown). To reduce experimental complications due to severe thymic hypoplasia as we continued this study, we used mice with a 129Sv and C57BL/6 mixed background or 129Sv × C57BL/6 F1 hybrids that are heterozygous for all loci except for the introduced mutations. The F1 hybrid background also helps reduce recessive modifier gene effects that arise from using a single strain.
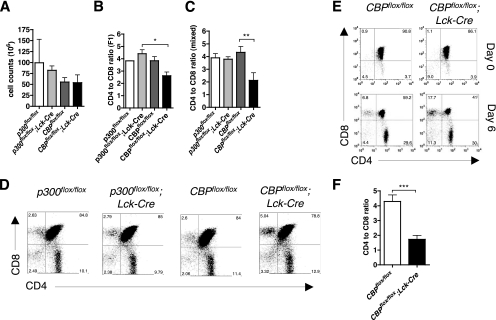
The number of thymocytes in CBPflox/flox; Lck-Cre F1 hybrid mice were not significantly different from the number in controls that included p300flox/flox; Lck-Cre (Fig. 1A) (P = 0.42). F1 hybrid (Fig. 1B and D) and mixed-background (Fig. 1C) strain CBPflox/flox; Lck-Cre mice had significantly reduced (P = 0.019 and 0.0061, respectively) CD4 SP/CD8 SP thymocyte ratios of ∼2, which was lower than the normal ratio of ∼4 seen in p300flox/flox; Lck-Cre, CBPflox/flox, and p300flox/flox controls. This effect on the SP thymocyte ratio was not observed with the Lck-Cre transgene alone (21) (data not shown).
FIG. 1.
Loss of CBP in the T-cell lineage decreases the CD4 SP/CD8 SP thymocyte ratio. (A) Total thymocyte counts of 5-month-old p300flox/flox, p300flox/flox; Lck-Cre, CBPflox/flox, and CBPflox/flox; Lck-Cre F1 hybrid mice are not significantly different (n = 2 to 4; P = 0.42). (B and C) CD4 SP/CD8 SP thymocyte ratio of F1 hybrid (B) (n = 2 to 4; P = 0.019) and mixed background (C) (n = 5 to 7; P = 0.0061) mice. (D) Flow cytometric analysis of CD4 and CD8 expression in p300flox/flox, p300flox/flox; Lck-Cre, CBPflox/flox, and CBPflox/flox; Lck-Cre F1 hybrid mice. (E) Neonatal thymic organ culture (NTOC). Thymuses from mixed-background newborn mice were resected, and one lobe of each thymus was analyzed for CD4 and CD8 expression by flow cytometry on day 0. The other lobe was cultured ex vivo for 6 days before CD4 and CD8 expression was analyzed. (F) CD4-to-CD8 ratio in NTOC from flow cytometric analysis (n = 5 to 8; two-tailed t test, P = 0.0003). Asterisks indicate statistical significance of indicated pairing by Tukey posttest (B and C) or t test (F).
To further test whether the CD8 SP thymocyte requirement for CBP is intrinsic to the T lineage, we used a Cd4-Cre transgene that initiates floxed gene inactivation at the DP stage of T-cell development (which is later than Lck-Cre) (26). Indeed, we observed a ∼10-fold increase in the CD8 SP thymocyte percentage in CBPflox/flox; Cd4-Cre mice on a C57BL/6 background (see Fig. S1 in the supplemental material). Certain aspects of the thymus phenotype (e.g., CD4 SP/CD8 SP ratio) tended to be milder in CBPflox/flox; Cd4-Cre F1 hybrid mice than in CBPflox/flox; Lck-Cre mice on the same genetic background (data not shown). This suggests that CBP is especially important early in T-cell development or that a milder phenotype is caused by residual CBP protein in thymocytes due to the later developmental stage at which the gene is inactivated in CBPflox/flox; Cd4-Cre mice.
The decrease in the CD4 SP/CD8 SP ratio is intrinsic to the thymus.
The larger fraction of CD8 SP thymocytes in CBPflox/flox; Lck-Cre mice could be due to aberrant T-cell development or apoptosis in the thymus or to mature T cells that recirculate from the periphery. We did not see increased apoptosis in CBPflox/flox; Lck-Cre thymuses as measured by TUNEL assay and annexin V staining (see Fig. S2 in the supplemental material). We next tested, by neonate thymic organ culture, whether the skewing of the CD4 SP/CD8 SP ratio was intrinsic to the thymus. This ex vivo assay revealed that the CD4 SP/CD8 SP ratios in CBPflox/flox; Lck-Cre thymuses and CBPflox/flox controls were similar at day 0 but was reduced two- to threefold after 6 days of ex vivo culture (Fig. 1E and F) (P = 0.0003). The thymus-intrinsic effect was further confirmed by taking mutant or control splenic T cells that were stained intracellularly with CFSE and injecting them into the original host via the tail vein to check for recirculating mature lymphocytes in the thymus. No evidence was observed for an increased number of CFSE-stained cells entering the thymuses of CBPflox/flox; Lck-Cre mice 1, 4, and 14 days after injection compared to CBPflox/flox mice (Fig. 2; also data not shown). As expected, comparable numbers of CFSE-stained cells were observed in the cervical lymph nodes of CBPflox/flox and CBPflox/flox; Lck-Cre mice (Fig. 2). As a control for the number of cells on a slide, the number of DAPI-stained nuclei was comparable between control and mutants (data not shown). Thus, the decreased CD4 SP/CD8 SP ratio is an intrinsic property of CBPflox/flox; Lck-Cre thymuses.
FIG. 2.
Decreased CD4 SP/CD8 SP ratio in thymus is not due to returning mature T cells. Autologous transplantation of CBPflox/flox; Lck-Cre or CBPflox/flox control splenocytes. Spleens were surgically resected, and splenocytes were stained with CFSE and injected into the original host via the tail vein. Mice were sacrificed at days 1 (not shown), 4, and 14 after transplantation, and CFSE-stained cells in thymus and cervical lymph nodes (LN) were detected by fluorescence microscopy.
Abnormal expression of maturation and memory cell surface markers in CD8 SP thymocytes of CBPflox/flox; Lck-Cre mice.
The decreased CD4 SP/CD8 SP thymocyte ratio could reflect increased flux through the normal CD8+ T-cell development pathway or the generation of an aberrant CD8+ population. To examine this, we performed flow cytometric analysis for the maturation marker CD24 (heat-stable antigen; mature thymocytes are CD24lo [42]) and found a larger fraction of CD24lo CD8 SP thymocytes from CBPflox/flox; Lck-Cre F1 hybrid mice than in controls (Fig. 3A) (P = 0.03). Mixed-background CBPflox/flox; Lck-Cre mice also showed this CD24lo CD8 SP phenotype (see Fig. S3A in the supplemental material). We confirmed by flow cytometry that CD24lo CD8 SP thymocytes from CBPflox/flox; Lck-Cre F1 hybrid mice express normal levels of the TCR β-chain (see Fig. S3B in the supplemental material). Thus, there is an increased fraction of TCRβ+ CD8 SP thymocytes with a mature CD24lo character in CBPflox/flox; Lck-Cre mice.
FIG. 3.
CD8 SP thymocytes and CD8+ T cells from CBPflox/flox; Lck-Cre F1 hybrid mice have a memory-like phenotype. (A) Flow cytometric analysis of CD24 expression on CD8 SP CD3hi thymocytes (n = 2 to 5; P = 0.03). (B to E) CD122 (B and C) or CD44 (D and E) expression in CD8 SP CD3hi thymocytes (B and D) and CD8+ splenocytes (C and E) (B to E, n = 2 to 4; B to D, P < 0.0001; E, P = 0.0008). Asterisks indicate statistical significance of indicated pairing by Tukey posttest (B to E).
Increased surface expression of the CD44 cell adhesion receptor and the interleukin-2 (IL-2) and IL-15 receptor β-chain (CD122) are hallmarks of adaptive T-cell differentiation in the periphery (i.e., effector or memory CD8+ T cells), whereas mature conventional CD8 SP thymocytes are normally CD44lo CD122lo, like naïve peripheral T cells. Surprisingly, flow cytometry of thymocytes from CBPflox/flox; Lck-Cre F1 hybrid mice (Fig. 3B) and CBPflox/flox; Cd4-Cre C57BL/6 mice (see Fig. S4 in the supplemental material) revealed an unusual CD122hi CD8 SP population. There was also an unusually large percentage of CD44hi CD8 SP thymocytes (Fig. 3D). Thus, many of the CD8 SP cells had a phenotype similar to effector (CD44hi CD122int) or memory (CD44hi CD122hi) peripheral CD8+ T cells (16). Splenic CD8+ T cells from CBPflox/flox; Lck-Cre F1 hybrid mice were also predominantly CD122hi, and many were CD44hi (Fig. 3C and E). The surface staining intensity for CD122 on CBPflox/flox; Lck-Cre CD8 SP thymocytes was at physiological levels as it was as strong as a subpopulation of peripheral T cells from control mice (i.e., memory phenotype cells) (compare Fig. 3B and C; also data not shown).
The CD24lo CD44hi CD122hi TCRβ+ phenotype of CBPflox/flox; Lck-Cre CD8 SP thymocytes is also consistent with innate-phenotype T cells that express the killer cell lectin-like receptor NK1.1 (4). However, flow cytometry revealed that the percentage of CD3+ NK1.1+ CD8 SP thymocytes in CBPflox/flox; Lck-Cre F1 hybrid mice was not significantly different from that in controls (P = 0.112) (see Fig. S5A and B in the supplemental material). In fact, most normal innate NK1.1+ NKT cells do not express CD8 (3), and the fraction of these NK1.1+ CD8− cells in the thymuses of CBPflox/flox; Lck-Cre F1 hybrid mice was lower than in controls (P = 0.0203) (see Fig. S5A and C in the supplemental material). Thus, the typical CD8-negative NK T-cell lineage may require CBP. These results together indicate that some, but not all, facets of innate T cells are present in CD8 SP thymocytes and splenic T cells of CBPflox/flox; Lck-Cre mice.
Several effector, memory, and innate T-cell genes are induced in CBPflox/flox; Lck-Cre CD8 SP thymocytes.
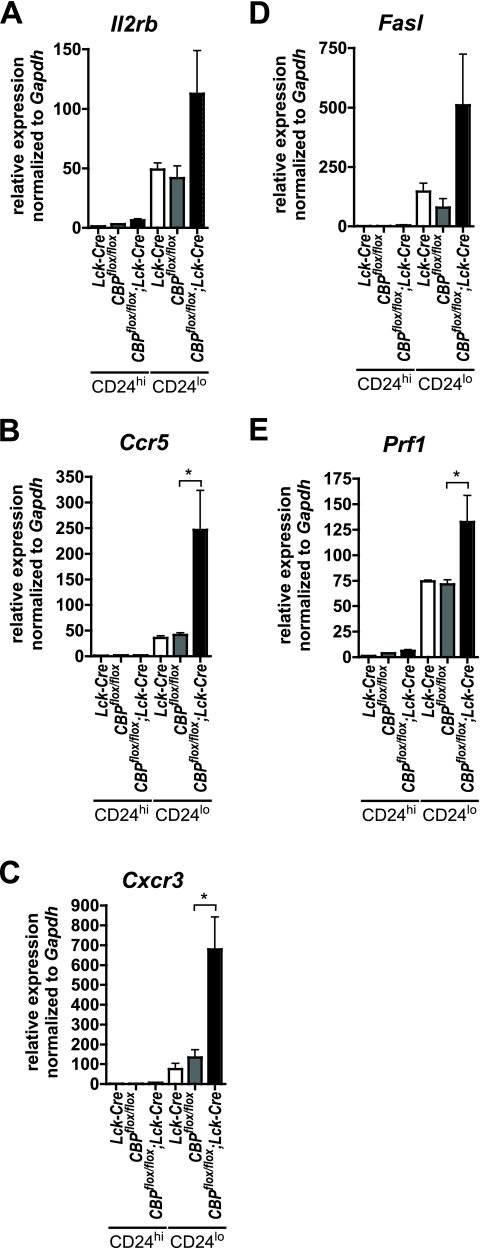
Microarray analysis using total thymocyte RNA from CBPflox/flox; Lck-Cre and CBPflox/flox; MMTV-Cre (where MMTV is mouse mammary tumor virus) mice revealed elevated expression for several effector, memory, and innate T-cell-associated genes (F. Boussouar and J. van Deursen, unpublished data). We used quantitative real-time qRT-PCR to further analyze genes expression in CD24lo and CD24hi CD8 SP thymocytes purified by FACS from CBPflox/flox; Lck-Cre, CBPflox/flox, and Lck-Cre F1 hybrid animals. As expected from flow cytometry results, Il2rb (CD122) mRNA was elevated in CD24lo CD8 SP (mature) thymocytes from CBP mutant mice (Fig. 4A). The chemokine receptor genes Ccr5 and Cxcr3 were also induced more than fivefold compared to controls (Fig. 4B and C) (CCR5 and CXCR3 normally enable activated T-cell migration to inflamed nonlymphoid peripheral tissues). The mRNA levels for the apoptosis-inducer Fas ligand (Fasl) and the cytotoxic pore-forming protein perforin (Prf1) were also elevated (Fig. 4D and E). CD24lo CD8 SP thymocytes from F1 hybrid CBPflox/flox; Cd4-Cre mice also showed enhanced expression of Il2rb, Ccr5, Cxcr3, Fasl, and Prf1 compared to control (see Fig. S6A to E in the supplemental material). These five genes were also strongly expressed in CD8 SP thymocytes from mixed-background CBPflox/flox; Lck-Cre mice (see Fig. S6F to J in the supplemental material). These results together demonstrate that effector genes are inappropriately induced in the CD8 SP thymocytes of multiple mouse strains that inactivate CBP in the T lineage.
FIG. 4.
A subset of effector and memory T-cell genes is induced in CD24lo CD8 SP thymocytes from CBPflox/flox; Lck-Cre F1 hybrid mice. CD8 SP CD24hi and CD24lo cells from Lck-Cre, CBPflox/flox, and CBPflox/flox; Lck-Cre mice were purified by FACS, and mRNAs for Il2rb/Cd122 (A), Ccr5 (B), Cxcr3 (C), Fasl (D), and Prf1 (E) were analyzed by qRT-PCR and normalized to Gapdh (n = 2 to 3) P values were as follows: 0.014 (A), 0.009 (B), 0.003 (C), 0.04 (D), and 0.0006 (E). Asterisks indicate statistical significance of indicated pairing by Tukey posttest (B, C, and E).
CD8 SP thymocytes from CBPflox/flox; Lck-Cre mice can rapidly produce IFN-γ-like peripheral effector, memory, or innate T cells.
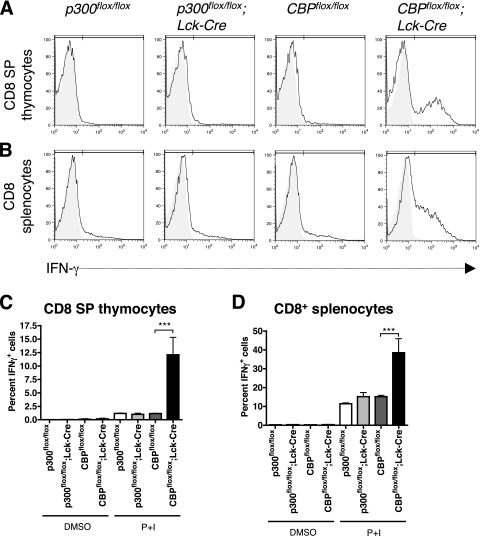
We next determined if CD8 SP thymocytes from CBPflox/flox; Lck-Cre F1 hybrid mice were functionally similar to effector, memory, or innate peripheral CD8+ T cells by measuring their ability to rapidly produce the effector cytokine IFN-γ. Thymocytes or splenic T cells were treated ex vivo for 5 h with P+I to mimic activated TCR signaling. Flow cytometry showed that the fraction of IFN-γ-producing cells was increased about seven- to eightfold in P+I-treated CBPflox/flox; Lck-Cre CD3hi CD8 SP thymocytes compared to controls (Fig. 5A and C). Similar results were seen with CD3hi CD8 SP thymocytes from mixed-background mice (see Fig. S7 in the supplemental material). There was also enhanced IFN-γ production in P+I-treated CD3hi CD8+ T cells from CBPflox/flox; Lck-Cre F1 hybrid mouse spleens (Fig. 5B and D), indicating that CD8+ T cells from both the thymus and periphery have enhanced effector function in these mice.
FIG. 5.
CD8 SP thymocytes and CD8+ T cells from CBPflox/flox; Lck-Cre F1 mice can rapidly produce IFN-γ. Thymocytes (A and C) and splenocytes (B and D) were treated ex vivo with dimethyl sulfoxide (A and B; shaded region) or P+I (A and B; solid line) and GolgiPlug and GolgiStop for 5 h. The cells were stained for CD3, CD4, and CD8; fixed; and then stained for IFN-γ for flow cytometry. IFN-γ was gated on CD3hi CD8 SP cells. (C and D) Percentage of IFN-γ-positive cells (n = 2 to 4; P < 0.0001). Asterisks indicate statistical significance of indicated pairing by Tukey posttest.
Loss of CBP induces the T-cell master regulatory transcription factor Eomes but not its paralog T-bet.
The activation of an effector, memory, or innate T-cell program suggested either that CBP is a repressor of multiple effector genes or that it regulates the expression or activity of a T-cell master transcription factor(s). Affymetrix microarray analysis revealed that Eomes mRNA was elevated in CBPflox/flox; MMTV-Cre and CBPflox/flox; Lck-Cre thymuses (Boussouar and van Deursen, unpublished), as was the Eomes target gene Il2rb (CD122) (14) (Fig. 4A; also data not shown). Eomes is a key transcriptional regulator of effector and memory CD8+ T-cell differentiation (14, 31), and CD122 is a critical component of IL-15 signaling. Notably, IL-15 is essential for normal CD8+ memory T cells (22) and the CD8+ CD44+ CD122+ memory phenotype cells seen in Itk−/− and Itk−/−; Rlk−/− mice (2, 8).
Analysis by qRT-PCR confirmed that the level of Eomes mRNA was strongly increased in more mature CD24lo CD8 SP thymocytes purified from CBPflox/flox; Lck-Cre (Fig. 6A) and CBPflox/flox; Cd4-Cre F1 hybrid mice (see Fig. S8A in the supplemental material). Eomes protein was also abundant in CD8 SP thymocytes from CBPflox/flox; Lck-Cre F1 hybrid mice compared to controls that included p300flox/flox; Lck-Cre mice (Fig. 6C). Thymocytes from mixed-background CBPflox/flox; Lck-Cre mice also showed robust Eomes mRNA and protein expression (see Fig. S8B and E in the supplemental material). Consistent with the idea that Eomes has a functional role in mutant CD8 SP thymocytes, its mRNA was expressed at a level comparable to that found in wild-type CD8+ CD122+ effector and memory phenotype T cells (see Fig. S8C in the supplemental material).
FIG. 6.
Loss of CBP induces Eomes but not T-bet expression. CD8 SP CD24hi and CD24lo thymocytes of Lck-Cre, CBPflox/flox, and CBPflox/flox; Lck-Cre F1 hybrid mice were purified by FACS, and Eomes (A) and T-bet (B) mRNA levels were analyzed by qRT-PCR normalized to Gapdh P values were 0.0004 (A) and 0.0035 (B) (n = 2 to 3). Asterisks indicate statistical significance of indicated pairing by Tukey posttest. (C) Western blot for Eomes protein in CD8 SP thymocytes from multiple CBPflox/flox; Lck-Cre and control F1 hybrid mice. Transfected 293T cell extracts are indicated (control and overexpressing Eomes protein). β-Actin served as a loading control.
In contrast to Eomes, however, mRNA levels for T-bet (Tbx21), a paralog of Eomes that is essential for normal CD8+ and CD4+ T-cell differentiation (13, 37, 38), were not significantly different between control and CBPflox/flox; Lck-Cre CD24lo CD8 SP thymocytes (Fig. 6B). Unlike Eomes mRNA, T-bet mRNA levels were much lower in control and CBPflox/flox; Lck-Cre thymocytes than in wild-type CD8+ CD122+ splenic T cells (see Fig. S8D in the supplemental material). Reiterating a possible connection between CBP and the Tec kinases, the increased expression of Eomes, but not T-bet, in CBPflox/flox; Lck-Cre CD8 SP thymocytes was similar to the phenotype of Itk−/− and Rlk−/−; Itk−/− mice (2). Eomes expression in CBPflox/flox; Lck-Cre CD8 SP thymocytes may occur later in development, however, than in Itk−/− and Rlk−/−; Itk−/− mice, where it is seen in newly arising CD8 SP cells (2). The increased expression of Eomes may be a primary determinant of the effector, memory, or innate phenotype of CP8 SP thymocytes in CBPflox/flox; Lck-Cre mice.
Loss of CBP in the T-cell lineage is not sufficient to generate effector, memory, or innate-phenotype CD8 SP thymocytes.
Shared aspects of the CD8 SP thymocyte phenotype between CBPflox/flox; Lck-Cre, CBPflox/flox; Cd4-Cre, and CBPflox/flox; MMTV-Cre mice strongly support the notion that loss of CBP in the T-cell lineage is causative. Transplantation of CBPflox/flox; Lck-Cre bone marrow into lethally irradiated wild-type C57BL/6 mice failed to recapitulate the effector/memory/ innate phenotype in CD8 SP thymocytes, however (see Table S1 in the supplemental material). This was unexpected because the CD8 SP effector/memory/innate phenotype is observed in lethally irradiated mice transplanted with Itk−/− and Rlk−/−; Itk−/− bone marrow (2, 5). Thus, the in vivo environment is important for T-cell development that is modulated by CBP, and radiation-induced alterations of the bone marrow, thymus, or levels of cytokines may affect this process.
CBP is required for the TCR-responsive expression of Itk-dependent genes in splenic CD8+ T cells.
The similarity in T-cell phenotypes between CBP and Itk mutant mice suggests that CBP and Itk/Rlk act in the same biochemical pathway. Given that Itk and Rlk have established roles in TCR signaling, we tested whether CBP also acts to mediate gene transcription downstream of the TCR. We first tested the Itk-dependent genes Egr2 and Egr3 (early growth response 2 and 3) (29) in naïve YFP+ CD44lo CD8+ T cells isolated from the spleens of CBPflox/flox; Lck-Cre; YFP mice or from CBP+/+; Lck-Cre; YFP controls. (The YFP reporter gene is activated by Cre recombinase and allows for FACS purification of a highly enriched population of CBP null T cells.) In response to a 2-h treatment ex vivo with plate-bound anti-CD3 antibody, the expression of Egr2 and Egr3 was significantly reduced about 50% in CBP null T cells compared to controls (Fig. 7A and B). Egr2 and Egr3 expression was also attenuated in mock-treated CBP null cells. Next we tested the expression of the classic TCR-responsive gene Il2. It has previously been shown that IL-2 production by Itk null T cells is severely impaired after stimulating the TCR pathway with anti-CD3 antibody (34). In addition, chromatin immunoprecipitation has shown that the mouse Il2 promoter is a direct target of CBP (40). Consistent with these studies, effector-memory phenotype YFP+ CD44hi CD8+ T cells that lack CBP had a profound deficit in Il2 gene expression after being treated for 4 h with anti-CD3 (Fig. 7C). Together, these results support the idea that CBP functions in the TCR/Itk/Rlk signaling pathway. Furthermore, it suggests that CBP has a specific role in mediating the expression of certain as yet unidentified Itk/Rlk-dependent genes that control T-cell development.
FIG. 7.
TCR-responsive expression of Itk-dependent genes is decreased in CBP-deficient T cells. (A and B) qRT-PCR analysis of Egr2 and Egr3 mRNAs in YFP+ CD8+ CD44lo splenocytes from CBP+/+; Lck-Cre; YFP and CBPflox/flox; Lck-Cre; YFP mice (the YFP transgene is expressed when recombined by Cre and was used to enrich for cells lacking CBP). Cells were treated ex vivo with plate-bound anti-CD3 or mock treated for 2 h. Gene expression was normalized to Gapdh mRNA. For each experiment, the Lck-Cre anti-CD3-treated value was set to 100 (n = 4; P < 0.001, ANOVA). Asterisks indicate statistical significance of indicated pairing by Tukey posttest. (C) Il2 mRNA expression determined by qRT-PCR of YFP+ CD8+ CD44hi splenocytes treated with plate-bound anti-CD3 or mock treated for 4 h. Gene expression was normalized to Gapdh. Mock-treated samples came up after 40 PCR cycles and were assigned an expression value of zero (for anti-CD3-treated samples, n = 3 and P = 0.033, t test; for mock-treated samples, n = 1 to 2).
DISCUSSION
Our findings show that CBP is critical to delineate the development of the adaptive and innate CD8+ T-cell lineages. It was surprising that p300 was not important to this process even though it is expressed comparably to CBP in thymocytes (21), and it is often functionally indistinguishable from CBP in vitro (20). As CBP and p300 have several genome-unique domains and constitute the two-member KAT3 family of protein lysine acetyltransferases (1), these findings identify a new area of focus in the study of T-cell development.
The phenotype caused by T-cell-lineage-specific inactivation of CBP was remarkably similar to that for the conventional mouse knockouts for the Tec kinase genes Itk and Rlk (2, 5, 8). Like CBP mutant mice, Itk and Rlk mutants have thymocytes with an innate- or conventional memory-like T-cell phenotype (i.e., CD8+ CD44hi CD122hi), which also have high levels of Eomes and the ability to rapidly produce IFN-γ (2, 4, 5, 8). This suggests that CBP and the Tec kinases act in a common pathway that controls T-cell development. Consistent with this idea, we showed that the Itk-dependent genes Egr2, Egr3, and Il2 required CBP for their normal expression in TCR-stimulated T cells. One straightforward explanation is that CBP, Rlk, and Itk function in the same branch of the TCR signaling pathway.
The phenotypes for CBPflox/flox; Lck-Cre and Tec kinase mutant mice differ in several respects, however: the apparent lack of NK1.1 expression in CBP mutant CD8 SP thymocytes and an inability to transplant the CBP mutant phenotype to lethally irradiated wild-type mice. The apparently normal levels of CD8+ NK1.1+ thymocytes we observed in CBPflox/flox; Lck-Cre mice may reflect technical aspects of flow cytometry in our hands although we could easily detect CD8− NK1.1+ T cells (i.e., NKT cells). Tec kinase mutant thymocytes, however, are reported to have low to intermediate NK1.1 surface staining intensity, suggesting that such cells from CBP mutant mice may be hard to identify (2, 5). The inability to transplant the CBP mutant phenotype may reflect differences in the procedure used by us or in the developmental mechanisms that involve CBP and the Tec kinases. It seems unlikely that deletion of CBPflox in non-bone marrow cells causes the thymocyte phenotype in CBPflox/flox; Lck-Cre mice, since we saw a phenotype using two different Cre transgenes that are specific for the T-cell lineage (i.e., Lck-Cre and Cd4-Cre) and a third transgene that is deleted in multiple lineages, including T cells (i.e., MMTV-Cre). The transplant result may provide a clue to the cell-extrinsic factors (e.g., cytokines) that are involved with CBP in T-cell development.
Several possible mechanisms could explain the overlap between the Tec kinase and CBP mutant phenotypes. First, CBP may be required for the Tec kinase-dependent expression of a gene encoding a regulator of CD8+ T-cell development. Our data shown in Fig. 7 are consistent with this model. Second, CBP may be necessary for the expression of a gene(s) that encodes an effector of the Tec kinase pathway, including Itk and Rlk themselves. We did not observe reduced Itk and Rlk mRNA levels in CBP mutant thymocytes, however (data not shown). Third, CBP and the Tec kinases may act independently in CD8+ T-cell development. Fourth, Itk and Rlk may affect the expression or function of CBP.
It is unclear whether the loss of CBP preferentially drives the development of a normal T-cell lineage that is otherwise rare or if it leads to an abnormal T-cell type. It is also uncertain if the induction of Eomes expression in CBP mutants is a primary driving event of atypical T-cell development or secondary to the developmental process. In this regard, loss of CBP is not sufficient to directly induce Eomes expression as we did not observe Eomes mRNA induction in less mature thymocytes (e.g., CD8+ CD24hi) or in the thymuses of CBPflox/flox; Lck-Cre bone marrow transplant-recipient mice (see Table S1 in the supplemental material). In these transplant experiments, a factor such as a cytokine that is needed to induce Eomes expression could be limiting.
Eomes and T-bet are both apparently required for the entire spectrum of cytotoxic T-cell functions (14). Since CBPflox/flox; Lck-Cre CD8 SP thymocytes do not express high levels of T-bet, we also do not expect these cells to have a fully activated effector/memory/innate T-cell program. It is unknown why T-bet is not expressed, but perhaps additional immune response signals are required that are not present in CBPflox/flox; Lck-Cre mice. Alternatively, the CD8 SP thymocytes in these mice may represent a normally rare type of T cell that expresses Eomes but not T-bet.
CBP and p300 have more than 360 described protein interaction partners, including more than 50 T-cell-critical regulators (21). A priori, these extensive roles make it seem unlikely that CBP and p300 would have narrowly defined functions in T cells. Consistent with this view, loss of both CBP and p300 is catastrophic for T-cell development (21), indicating that other histone acetyltransferase coactivators cannot supply redundant activities. Whether the specific T-cell functions of CBP are due to unique protein binding partners or acetylation events is not known.
Mutation studies of other chromatin remodelers and modifiers show a tendency for their broad or very early importance in T-cell development. These include the BAF ATP-dependent chromatin remodeling complex component BRG1, the Mi2β subunit of the nucleosome remodeling deacetylase complex, and the coactivator-associated arginine methyltransferase CARM1 (7, 9) (41) (23). Other coactivators that have more narrowly defined roles in T-cell development, such as KRC and BOB.1/OBF.1, have relatively few interaction partners compared to CBP/p300 (6, 30). None of these coactivators appears to affect the demarcation of conventional and innate T-cell development as CBP does, however.
What could account for the differences in thymocyte phenotype between the CBP and p300 mutant mice? Indirect immunofluorescence analysis of CBP in wild-type naïve CD122− CD8+ and memory-phenotype CD122+ CD8+ peripheral T cells did not indicate that CBP is downregulated as part of the differentiation process (data not shown). Thus, it does not appear that CBP is degraded preferentially over p300 as part of the normal differentiation of naïve T cells into effector or memory cells in the periphery. It remains possible, however, that CBP actively regulates T-cell fate through alterations of its protein level, enzyme activity, or subcellular localization during development in the thymus, whereas p300 does not. Alternatively, CBP may act passively as a facilitator (i.e., its presence is necessary to mediate the activity of another regulated transcription factor). The loss of CBP by mutation could also cause an imbalance between distinct CBP and p300 functions. Arguing against this scenario, T-cell development in p300flox/flox; Lck-Cre mice appears mostly normal apart from decreased numbers of thymocytes in mixed-background mice (21) (it remains possible that a focused examination of p300 mutant CD24lo CD8 SP thymocytes would reveal a moderate phenotype), and the overexpression of p300 does not affect the CD4/CD8 thymocyte profile (47). CBP and p300 share several indistinguishable biochemical properties such as the ability to acetylate histone H3 residues K14 and H4 K5, but H4 K12 is a better substrate for CBP, whereas H4 K8 is better for p300 (28). Other instances where CBP and p300 are reported to have distinct molecular functions are in controlling the expression of the antiapoptotic gene survivin (Birc5) (27) and the mechanism by which sumoylation represses CBP/p300 activity (25). Whether these functions of CBP are relevant to CD8+ T-cell development is unknown.
CBP and p300 are among the most heavily connected “hubs” (i.e., proteins with many different interaction partners) in the mammalian protein-protein interaction network, yet CBP has a specific role in T-cell development. Since p300 lacks this same role, future studies that compare the two coactivators could identify these distinctive activities of CBP. This knowledge would help illuminate the transcriptional mechanisms required for conventional and innate T-cell development.
Supplementary Material
Acknowledgments
We thank J. Opferman for advice; S. Lerach, M. Biesen, and M. Castor for excellent technical assistance; and M. Paktinat for flow cytometry. We also thank the SJCRH Flow Cytometry and Cell Sorting Shared Resource; we thank R. Cross, J. Gatewood, and J. Rogers and the mouse immunophenotyping services in the Department of Immunology and the Animal Resource Center. The Hartwell Center at SJCRH provided oligonucleotides.
This work was supported by NIH grant R01 CA076385 (P.K.B.), a Cancer Center (CORE) support grant P30 CA021765, and the American Lebanese Syrian Associated Charities of SJCRH.
We do not have any conflicts of interest to declare.
Footnotes
Published ahead of print on 11 May 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Allis, C. D., S. L. Berger, J. Cote, S. Dent, T. Jenuwien, T. Kouzarides, L. Pillus, D. Reinberg, Y. Shi, R. Shiekhattar, A. Shilatifard, J. Workman, and Y. Zhang. 2007. New nomenclature for chromatin-modifying enzymes. Cell 131633-636. [DOI] [PubMed] [Google Scholar]
- 2.Atherly, L. O., J. A. Lucas, M. Felices, C. C. Yin, S. L. Reiner, and L. J. Berg. 2006. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity 2579-91. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac, A., P. B. Savage, and L. Teyton. 2007. The biology of NKT cells. Annu. Rev. Immunol. 25297-336. [DOI] [PubMed] [Google Scholar]
- 4.Berg, L. J. 2007. Signalling through TEC kinases regulates conventional versus innate CD8+ T-cell development. Nat. Rev. Immunol. 7479-485. [DOI] [PubMed] [Google Scholar]
- 5.Broussard, C., C. Fleischacker, R. Horai, M. Chetana, A. M. Venegas, L. L. Sharp, S. M. Hedrick, B. J. Fowlkes, and P. L. Schwartzberg. 2006. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity 2593-104. [DOI] [PubMed] [Google Scholar]
- 6.Brunner, C., A. Sindrilaru, I. Girkontaite, K. D. Fischer, C. Sunderkotter, and T. Wirth. 2007. BOB. 1/OBF. 1 controls the balance of TH1 and TH2 immune responses. EMBO J. 263191-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi, T. H., M. Wan, P. P. Lee, K. Akashi, D. Metzger, P. Chambon, C. B. Wilson, and G. R. Crabtree. 2003. Sequential roles of Brg, the ATPase subunit of BAF chromatin remodeling complexes, in thymocyte development. Immunity 19169-182. [DOI] [PubMed] [Google Scholar]
- 8.Dubois, S., T. A. Waldmann, and J. R. Muller. 2006. ITK and IL-15 support two distinct subsets of CD8+ T cells. Proc. Natl. Acad. Sci. USA 10312075-12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebuhr, T. C., G. I. Kovalev, S. Bultman, V. Godfrey, L. Su, and T. Magnuson. 2003. The role of Brg1, a catalytic subunit of mammalian chromatin-remodeling complexes, in T cell development. J. Exp. Med. 1981937-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glimcher, L. H., M. J. Townsend, B. M. Sullivan, and G. M. Lord. 2004. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat. Rev. Immunol. 4900-911. [DOI] [PubMed] [Google Scholar]
- 11.Goodman, R. H., and S. Smolik. 2000. CBP/p300 in cell growth, transformation, and development. Genes Dev. 141553-1577. [PubMed] [Google Scholar]
- 12.Hennet, T., F. K. Hagen, L. A. Tabak, and J. D. Marth. 1995. T-cell-specific deletion of a polypeptide N-acetylgalactosaminyl-transferase gene by site-directed recombination. Proc. Natl. Acad. Sci. USA 9212070-12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Intlekofer, A. M., A. Banerjee, N. Takemoto, S. M. Gordon, C. S. Dejong, H. Shin, C. A. Hunter, E. J. Wherry, T. Lindsten, and S. L. Reiner. 2008. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321408-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Intlekofer, A. M., N. Takemoto, E. J. Wherry, S. A. Longworth, J. T. Northrup, V. R. Palanivel, A. C. Mullen, C. R. Gasink, S. M. Kaech, J. D. Miller, L. Gapin, K. Ryan, A. P. Russ, T. Lindsten, J. S. Orange, A. W. Goldrath, R. Ahmed, and S. L. Reiner. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 61236-1244. [DOI] [PubMed] [Google Scholar]
- 15.Joshi, N. S., and S. M. Kaech. 2008. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J. Immunol. 1801309-1315. [DOI] [PubMed] [Google Scholar]
- 16.Kaech, S. M., S. Hemby, E. Kersh, and R. Ahmed. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111837-851. [DOI] [PubMed] [Google Scholar]
- 17.Kang-Decker, N., C. Tong, F. Boussouar, D. J. Baker, W. Xu, A. A. Leontovich, W. R. Taylor, P. K. Brindle, and J. M. Van Deursen. 2004. Loss of CBP causes T cell lymphomagenesis in synergy with p27(Kip1) insufficiency. Cancer Cell 5177-189. [DOI] [PubMed] [Google Scholar]
- 18.Kappes, D. J., X. He, and X. He. 2005. CD4-CD8 lineage commitment: an inside view. Nat. Immunol. 6761-766. [DOI] [PubMed] [Google Scholar]
- 19.Kasper, L. H., F. Boussouar, P. A. Ney, C. W. Jackson, J. Rehg, J. M. van Deursen, and P. K. Brindle. 2002. A transcription-factor-binding surface of coactivator p300 is required for haematopoiesis. Nature 419738-743. [DOI] [PubMed] [Google Scholar]
- 20.Kasper, L. H., and P. K. Brindle. 2006. Mammalian gene expression program resiliency: the roles of multiple coactivator mechanisms in hypoxia-responsive transcription. Cell Cycle 5142-146. [DOI] [PubMed] [Google Scholar]
- 21.Kasper, L. H., T. Fukuyama, M. A. Biesen, F. Boussouar, C. Tong, A. de Pauw, P. J. Murray, J. M. van Deursen, and P. K. Brindle. 2006. Conditional knockout mice reveal distinct functions for the global transcriptional coactivators CBP and p300 in T-cell development. Mol. Cell. Biol. 26789-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy, M. K., M. Glaccum, S. N. Brown, E. A. Butz, J. L. Viney, M. Embers, N. Matsuki, K. Charrier, L. Sedger, C. R. Willis, K. Brasel, P. J. Morrissey, K. Stocking, J. C. Schuh, S. Joyce, and J. J. Peschon. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191771-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, J., J. Lee, N. Yadav, Q. Wu, C. Carter, S. Richard, E. Richie, and M. T. Bedford. 2004. Loss of CARM1 results in hypomethylation of thymocyte cyclic AMP-regulated phosphoprotein and deregulated early T cell development. J. Biol. Chem. 27925339-25344. [DOI] [PubMed] [Google Scholar]
- 24.Kioussis, D., and K. Georgopoulos. 2007. Epigenetic flexibility underlying lineage choices in the adaptive immune system. Science 317620-622. [DOI] [PubMed] [Google Scholar]
- 25.Kuo, H. Y., C. C. Chang, J. C. Jeng, H. M. Hu, D. Y. Lin, G. G. Maul, R. P. Kwok, and H. M. Shih. 2005. SUMO modification negatively modulates the transcriptional activity of CREB-binding protein via the recruitment of Daxx. Proc. Natl. Acad. Sci. USA 10216973-16978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, P. P., D. R. Fitzpatrick, C. Beard, H. K. Jessup, S. Lehar, K. W. Makar, M. Perez-Melgosa, M. T. Sweetser, M. S. Schlissel, S. Nguyen, S. R. Cherry, J. H. Tsai, S. M. Tucker, W. M. Weaver, A. Kelso, R. Jaenisch, and C. B. Wilson. 2001. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15763-774. [DOI] [PubMed] [Google Scholar]
- 27.Ma, H., C. Nguyen, K. S. Lee, and M. Kahn. 2005. Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene 243619-3631. [DOI] [PubMed] [Google Scholar]
- 28.McManus, K. J., and M. J. Hendzel. 2003. Quantitative analysis of CBP- and P300-induced histone acetylations in vivo using native chromatin. Mol. Cell. Biol. 237611-7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, A. T., and L. J. Berg. 2002. Defective Fas ligand expression and activation-induced cell death in the absence of IL-2-inducible T cell kinase. J. Immunol. 1682163-2172. [DOI] [PubMed] [Google Scholar]
- 30.Oukka, M., M. N. Wein, and L. H. Glimcher. 2004. Schnurri-3 (KRC) interacts with c-Jun to regulate the IL-2 gene in T cells. J. Exp. Med. 19915-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearce, E. L., A. C. Mullen, G. A. Martins, C. M. Krawczyk, A. S. Hutchins, V. P. Zediak, M. Banica, C. B. DiCioccio, D. A. Gross, C. A. Mao, H. Shen, N. Cereb, S. Y. Yang, T. Lindsten, J. Rossant, C. A. Hunter, and S. L. Reiner. 2003. Control of effector CD8+ T cell function by the transcription factor eomesodermin. Science 3021041-1043. [DOI] [PubMed] [Google Scholar]
- 32.Reiner, S. L. 2005. Epigenetic control in the immune response. Hum. Mol. Genet. 14(Spec. no. 1)R41-R46. [DOI] [PubMed] [Google Scholar]
- 33.Rothenberg, E. V. 2007. Cell lineage regulators in B and T cell development. Nature Immunol. 8441-444. [DOI] [PubMed] [Google Scholar]
- 34.Schaeffer, E. M., J. Debnath, G. Yap, D. McVicar, X. C. Liao, D. R. Littman, A. Sher, H. E. Varmus, M. J. Lenardo, and P. L. Schwartzberg. 1999. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science 284638-641. [DOI] [PubMed] [Google Scholar]
- 35.Spiegelman, B. M., and R. Heinrich. 2004. Biological control through regulated transcriptional coactivators. Cell 119157-167. [DOI] [PubMed] [Google Scholar]
- 36.Srinivas, S., T. Watanabe, C. S. Lin, C. M. William, Y. Tanabe, T. M. Jessell, and F. Costantini. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan, B. M., A. Juedes, S. J. Szabo, M. von Herrath, and L. H. Glimcher. 2003. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc. Natl. Acad. Sci. USA 10015818-15823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szabo, S. J., B. M. Sullivan, C. Stemmann, A. R. Satoskar, B. P. Sleckman, and L. H. Glimcher. 2002. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science 295338-342. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka, Y., I. Naruse, T. Hongo, M. Xu, T. Nakahata, T. Maekawa, and S. Ishii. 2000. Extensive brain hemorrhage and embryonic lethality in a mouse null mutant of CREB-binding protein. Mech. Dev. 95133-145. [DOI] [PubMed] [Google Scholar]
- 40.Wang, J., R. A. Barke, and S. Roy. 2007. Transcriptional and epigenetic regulation of interleukin-2 gene in activated T cells by morphine. J. Biol. Chem. 2827164-7171. [DOI] [PubMed] [Google Scholar]
- 41.Williams, C. J., T. Naito, P. G. Arco, J. R. Seavitt, S. M. Cashman, B. De Souza, X. Qi, P. Keables, U. H. Von Andrian, and K. Georgopoulos. 2004. The chromatin remodeler Mi-2β is required for CD4 expression and T cell development. Immunity 20719-733. [DOI] [PubMed] [Google Scholar]
- 42.Wilson, A., L. M. Day, R. Scollay, and K. Shortman. 1988. Subpopulations of mature murine thymocytes: properties of CD4− CD8+ and CD4+ CD8− thymocytes lacking the heat-stable antigen. Cell Immunol. 117312-326. [DOI] [PubMed] [Google Scholar]
- 43.Wilson, C. B., and M. Merkenschlager. 2006. Chromatin structure and gene regulation in T cell development and function. Curr. Opin. Immunol. 18143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu, W., T. Fukuyama, P. A. Ney, D. Wang, J. Rehg, K. Boyd, J. M. van Deursen, and P. K. Brindle. 2006. Global transcriptional coactivators CREB-binding protein and p300 are highly essential collectively but not individually in peripheral B cells. Blood 1074407-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu, W., L. H. Kasper, S. Lerach, T. Jeevan, and P. K. Brindle. 2007. Individual CREB-target genes dictate usage of distinct cAMP-responsive coactivation mechanisms. EMBO J. 262890-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao, T. P., S. P. Oh, M. Fuchs, N. D. Zhou, L. E. Ch'ng, D. Newsome, R. T. Bronson, E. Li, D. M. Livingston, and R. Eckner. 1998. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93361-372. [DOI] [PubMed] [Google Scholar]
- 47.Yu, C. T., M. H. Feng, H. M. Shih, and M. Z. Lai. 2002. Increased p300 expression inhibits glucocorticoid receptor-T-cell receptor antagonism but does not affect thymocyte positive selection. Mol. Cell. Biol. 224556-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.