Abstract
Hantaviruses infect humans following aerosolization from rodent feces and urine, producing hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Due to the high rates of mortality and lack of therapies, vaccines are urgently needed. Nonreplicating adenovirus (Ad) vectors that express Andes hantavirus (ANDV) nucleocapsid protein (AdN) or glycoproteins (AdGN and AdGC) were constructed. Ad vectors were tested for their ability to protect Syrian hamsters from a lethal ANDV infection that mimics the pulmonary disease seen in humans. When administered once, all three Ad vectors, individually or in combination, elicited a robust immune response that protected hamsters. No vaccinated animal died, and there were no obvious clinical signs of disease. Further, hantavirus RNA was not detected by sensitive reverse transcription-PCR in tissues and blood of hamsters immunized with both AdGN and AdGC. Cellular immunity appeared to be important for protection because the AdN vector completely protected animals. All three Ad vectors produced strong cytotoxic T-lymphocyte responses directed to hantavirus proteins in mice. Moreover, hamsters vaccinated with AdN, AdGN, or AdGC produced no detectable neutralizing antibodies yet were protected. These Ad vectors represent the first vaccines that prevent lethal hantavirus disease and, in some instances (AdGN and AdGC), provide sterile immunity. These observations set the stage for a more detailed characterization of the types of immunity required to protect humans from hantavirus infections.
Hantaviruses (genus Hantavirus, family Bunyaviridae) are a closely related group of rodent-borne viruses that are associated with two human diseases involving vascular leakage: hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS). Humans frequently acquire hantaviruses through inhalation of aerosolized infectious material shed by rodents in urine, feces, or saliva (reviewed in references 6, 24, and 38) Throughout Asia and Europe, over 200,000 cases of HFRS requiring hospitalization are documented annually, with lethality rates ranging from 0.1% to 15% (reviewed in references 32 and 45). HPS occurs throughout the Americas and although cases occur less frequently, lethality rates can range as high as 30 to 50% (reviewed in references 22, 24, and 32). Sin Nombre virus (SNV) and Andes virus (ANDV) cause the most frequently recognized and severe cases of human disease in North and South America, respectively (11, 38). SNV, carried primarily by Peromyscus maniculatus (deer mice), was discovered in 1993 in the southwestern United States during an outbreak of acute respiratory distress syndrome (8, 12). ANDV, carried primarily by Oligoryzomys longicaudatus (pygmy rice rat), was identified in 1995 as the agent causing a series of HPS outbreaks in Argentina and Chile (27, 28). With ANDV there is evidence for person-to-person transmission (reviewed in reference 11).
Clinically, HPS is characterized by a febrile, cardiopulmonary, diuretic, and convalescent phase. Following a variable incubation period, humans infected with HPS-causing viruses undergo a rapid disease progression, with symptoms developing from coughing and shortness of breath to severe pulmonary edema and shock requiring intubation and mechanical ventilation (11, 22). This rapid progression limits the utility of specific therapeutic strategies for treating HPS. In contrast to the case for HFRS, it seems that ribavirin is ineffective for HPS (36). The high rates of mortality, capacity to infect via aerosolization, and absence of effective therapies make it imperative that safe, effective hantavirus vaccines are developed and the parameters of protective immunity are established.
Several potential vaccine candidates have been evaluated for efficacy and immunogenicity. These have included inactivated hantaviruses; plasmid DNAs; baculovirus-, yeast-, or Escherichia coli-expressed recombinant proteins; chimeric hepatitis B virus-like particles; transgenic plants; nonreplicating adenovirus (Ad) vectors; vesicular stomatitis virus (VSV) pseudotypes; Sindbis virus replicons; and rodent cytomegalovirus and vaccinia virus (VV) recombinants (reviewed in references 16, 22, 24, and 32). Many of the studies with these vaccines involved HFRS-causing hantaviruses and infection models in which small animals did not exhibit disease similar to that in humans. Certain of these studies have provided evidence for the importance of antibodies in reducing virus load, slowing or reducing disease progression, and mediating virus clearance, while other studies have supported the importance of cellular immune responses (reviewed in references 16, 22, 24, and 32). However, the precise contributions of innate versus adaptive responses and humoral versus cellular immunity and the importance of the immune system in producing pathogenesis are not very clear.
Moreover, the relative roles of individual hantavirus proteins in eliciting a protective immune response are imprecisely defined. The hantavirus genome includes three negative-sense, single-stranded RNAs designated the large (L), medium (M), and small (S) segments (reviewed in reference 44). The L segment encodes the RNA-dependent RNA polymerase, and little is known regarding immune responses to the RNA-dependent RNA polymerase, although it is not a major structural component and is not thought to be a major immunogen (reviewed in references 24, 31, and 44). In contrast, the structural proteins expressed from the M and S segments are known to be important in immune responses (reviewed in references 20, 22, 24, 31, and 32). The M segment encodes a glycoprotein precursor, GPC, which is cotranslationally cleaved into the two envelope proteins GN and GC, which form a heterodimer (reviewed in reference 14). GN/GC is found in the virion envelope and has the capacity to promote virus entry, and GN and GC make up the antigens recognized by neutralizing antibodies (44). The S segment encodes the multifunctional nucleoprotein (N), which has also been shown to elicit cellular and humoral immunity (reviewed in references 20 and 32).
In 2001, a lethal disease model of HPS involving ANDV-infected Syrian hamsters was described (19). This ANDV hamster model remains the only small-animal model for the study of hantavirus disease in which disease progression and outcome mimic those in humans. The few studies using this model have focused on vaccinating hamsters with DNA vaccines that, to date, have not produced immunity sufficient to protect animals against lethal challenge with ANDV (10, 16, 18). Moreover, we do not yet know which types of immunity, whether humoral or cellular, or which viral proteins are necessary for protection against ANDV challenge, although in two studies the transfer of immunoglobulin from vaccinated monkeys or rabbits afforded protection to hamsters (10, 17).
The aim of our studies was to develop new vaccines based on recombinant Ad vectors expressing individual ANDV structural proteins (N, GN, and GC) and to characterize the protective immune responses associated with each of these proteins using the lethal Syrian hamster model of HPS. Surprisingly, a single vaccination of hamsters with Ad vectors expressing any one of the individual hantavirus proteins produced complete protection; no animals died, and no disease was observed. Moreover, there was no detectable ANDV RNA in the majority of hamsters coimmunized with Ad vectors expressing GN and GC. Neutralizing antibodies were detected in some animals but not in others, even though all the vaccinated animals were protected. Robust CD8+ cytotoxic T-lymphocyte (CTL) responses were detected in mice. These data demonstrate that nonreplicating Ad vectors can be used as very effective vaccines for hantaviruses, producing protection from lethal hantavirus infection.
MATERIALS AND METHODS
Cells and viruses.
293 IQ cells, a derivative of 293 cells (which express Ad E1 proteins) that express a Lac repressor protein, were obtained from Microbix (Toronto, Canada), propagated as described previously (33), and used to produce and propagate Ad vectors. U373-MG human glial cells and SVBalb and MC57G mouse cells were all obtained from the American Type Culture Collection (Manassas, VA) and propagated in Dulbecco's modified essential medium (DMEM) containing 7 to 10% fetal bovine serum (FBS). The hantavirus strain ANDV, Chile 9717869, was kindly provided by Connie Schmaljohn, U.S. Army Medical Research Institute of Infectious Diseases, Ft. Detrick, MD, and was propagated in a biosafety level 3 laboratory in Vero E6 (African green monkey kidney) cells maintained in DMEM supplemented with 2% FBS. ANDV focus-forming units (FFU) were calculated by infecting Vero E6 cells, overlaying the cells with 1.4% methylcellulose for 7 to 10 days, fixing the cells with 1:1 acetone-methanol, drying, and staining with rabbit anti-SNV N hyperimmune serum (diluted 1:1,000 in phosphate-buffered saline; a gift from Brian Hjelle) for 1 h. Cells were washed and incubated with peroxidase-conjugated goat anti-rabbit immunoglobulin G antibodies (Dako) for 1 h and then washed, and foci were visualized using NovaRED peroxidase substrate kits (Vector Laboratories).
Antibodies.
The entire coding sequence of the ANDV N protein fused to a polyhistidine (His) sequence was expressed in Escherichia coli and purified using a nickel (Ni) affinity column. A glutathione S-transferase (GST) fusion protein containing ANDV GC sequences (amino acids 898 to 1101) fused at the C terminus of GST was produced using plasmid pGEX-2T in E. coli as described earlier (49). A peptide derived from ANDV GN (RFQELKKSLKKPEVKKGC) was synthesized commercially (Peptido-Genics, Berkeley, CA) and coupled to keyhole limpet hemocyanin (KLH) using maleimidobenzoyl-N-hydroxysuccinimide ester (Sigma, St. Louis, MO) as described previously (41). The KLH-GN peptide, the GST-GC fusion protein, and the His-N fusion protein were used to immunize rabbits as described previously (21).
Construction of nonreplicating Ad vectors.
Nonreplicating (E1− E3−) Ad vectors expressing ANDV proteins were constructed using the AdMax HI-IQ system (Microbix, Toronto, Canada) as described previously (33). The ANDV GN, GC, GPC, and N open reading frames were PCR amplified from plasmids containing the appropriate cDNAs derived from a Chilean ANDV isolate, strain 9717869 (35). The primers listed below amplified the coding regions of specific proteins and introduced a 5′ BglII site and a 3′ AccI site (both underlined below). Primer sequences (5′ to 3′) were as follows: GN forward, TAAAGAAGTGAGAGATCTATGGAAGGGTGGTAT; GN reverse, CCAGCCTGACTCCATGTCGACCTACTATGCACTTGCGGCCCA; GC forward, TATAAAAGTAGAAGATCTGCCACCATGGTATGGTGCCTATTG; GC reverse, GATCAGCGGGTTTAAGTCGACCTACTAGACAGTTTTCTT; N forward, ATATACCCGGGGCCACCATGAGCACCCTCCAAG; and N reverse, TATATGAGCTCTTACAACTTAAGTGGCTC. Amplification of the GPC (containing the coding sequences of both GN and GC) was accomplished using the GN forward and GC reverse primers. PCR fragments were digested with BglII and AccI and inserted into the shuttle plasmid pDC316(io). The authenticity of the ANDV coding regions was then verified by nucleotide sequence analysis in both directions. The ANDV sequences were inserted into plasmid pDC316(io), which contains the Ad type 5 (Ad5) E1 region with a murine cytomegalovirus (MCMV) immediate-early promoter separated from transgenes by a lac repressor binding site which allows downregulation of the transgene in 293 IQ cells, which express the Lac repressor protein. AdEmpty was constructed using plasmid pDC316(io) containing no ANDV sequences. These pDC316(io)-based ANDV plasmids were cotransfected with plasmid pBHGloxΔE1,3Cre, containing the remainder of the Ad5 genomic plasmid, into 293 IQ cells. Supernatants were collected after 6 to 8 days, and the presence of Ad vectors expressing the ANDV proteins (designated AdN, AdGN, AdGC, and AdGPC) was confirmed by immunoprecipitation. Ad vectors were plaque purified, propagated in large-scale infections in 293 IQ cells, and purified using standard CsCl gradient methods (15).
Radiolabeling, immunoprecipitation, and gel electrophoresis of ANDV proteins.
U373-MG cells were infected with Ad vectors (multiplicity of infection of 50 to 100) in DMEM containing 2% FBS for 20 to 24 h. Cells were labeled with [35S]methionine-cysteine for a further 3 h as described previously (51). Cells were lysed in 1% NP-40-0.5% deoxycholate extraction buffer as described previously (51) and stored at −70°C overnight. Lysates were centrifuged at 60,000 × g for 45 min, and then hantavirus proteins were immunoprecipitated and proteins were subjected to electrophoresis on polyacrylamide gels as described previously (51).
CTL assays following Ad vector immunization of mice.
BALB/c mice (Jackson Labs) were vaccinated intraperitoneally (i.p.) with 1 × 108 (293 cell) PFU/animal of AdEmpty, AdN, AdGN, AdGC, or AdGN and AdGC. After 3 to 4 weeks, mice were boosted with an additional injection using the same dose of each Ad vector. Spleens were harvested after 6 to 8 days and splenocyte suspensions prepared as described previously (37). To restimulate CTLs, splenocytes (2 × 107 cells/ml) were cocultured in RPMI medium containing 10% FBS with syngeneic SVBalb cells infected 3 days prior with the Ad vectors expressing the appropriate ANDV proteins and subjected to gamma irradiation (3,000 rads). After 3 days of restimulation, interleukin-2 (1.2 ng/ml; E-Bioscience, San Diego, CA) was added to the cocultures. Following another 4 to 5 days, nonadherent lymphocytes were washed from the plates and tested in CTL assays. The CTL assays involved target cells, SVBalb (syngeneic) or MC57G (major histocompatibility complex [MHC]-mismatched) cells that were infected (multiplicity of infection of 50) with various Ad vectors for 20 to 24 h and loaded with 51Cr (100 μCi per 106 cells) for 1 to 2 h. 51Cr release assays were performed as described previously (14, 53). Spontaneous release of 51Cr from targets (without effectors) never exceeded 20% of the maximal release. The percent specific release [(experimental release − spontaneous release)/(maximum release − spontaneous release) × 100) was calculated as described previously (53).
Immunization of hamsters with Ad vectors followed by ANDV challenge.
Hamster experiments were conducted in accordance with the Canadian Council of Animal Care guidelines and approved by the Canadian Science Centre for Human and Animal Health (CSCHAH) Animal Care Committee. Syrian golden hamsters (Mesocricetus auratus) (4- to 6-week-old males; Charles River, Pointe Claire, Canada) were group housed in microisolator units situated in the biosafety level 4 area of the National Microbiology Laboratory, Public Health Agency of Canada. Prior to vaccine experiments, the 50% lethal dose (LD50) for i.p. injections of ANDV in hamsters was established by inoculating anesthetized animals with 0.8 to 80,000 FFU (using 10-fold dilutions) of ANDV. Hamsters were monitored twice daily for clinical signs of illness according to an approved scoring sheet (ruffled fur, lethargy, inappetence, and labored breathing). For vaccination, hamsters were anesthetized with isoflurane, a preimmunization blood sample was collected, and the animals were immunized with the Ad vectors using 108 (293 cell) PFU of each vector diluted in 100 μl phosphate-buffered saline at two sites in the hind-leg musculature. After 28 days, a second blood sample was collected and hamsters were challenged with ANDV by i.p. injection of 100 LD50s (equivalent to 154 FFU). Hamsters were examined twice daily for signs of illness. Survivors were monitored for 40 to 45 days and then anesthetized and exsanguinated via cardiac puncture.
Viral RNA titration in blood and tissues following Ad vector immunization and ANDV challenge.
At 6 and 9 days after ANDV challenge, hamsters were anesthetized, exsanguinated via cardiac puncture, and necropsied. A 100-mg piece of lung and liver tissue was placed into individual tubes containing 600 μl of RNAlater buffer (Qiagen, Mississauga, Canada) for 12 to 18 h, mechanically homogenized in 600 μl of RLT lysis buffer (Qiagen), clarified by low-speed centrifugation, and then diluted 1:2 with RLT buffer. In addition, 140 μl of cardiac blood was mixed with 560 μl lysis buffer AVL (Qiagen). Samples were extracted for RNA using the RNeasy (solid tissue) or QIAamp (blood) extraction kit (Qiagen). Quantitative real-time one-step reverse transcription-PCR (RT-PCR) was conducted on RNA extracts using a Rotor-Gene RG-3000 instrument (Corbett Life Science, Sydney, Australia) with ANDV S segment-specific primers (ANDV S129f [5′ AAGGCAGTGGAGGTGGAC] and ANDV S291r [5′ CCCTGTTGGATCAACTGGTT]) and a dual-labeled fluorescent probe (ANDV TM [5′ FAM-ACGGGCAGCTGTGTCTACATTGGA-TAMRA]) (all from TIB Molbiol, Adelphia, NJ). A 162-nucleotide fragment was amplified using QuantiTect probe RT-PCR kits (Qiagen) according to the manufacturer's instructions. RT-PCR was carried out in three stages: reverse transcription (50°C for 30 min), Taq activation (95°C for 15 min), and amplification (40 cycles of 94°C for 15 s and 60°C for 60 s). Data acquisition occurred at the end of the annealing/extension stage (60°C for 60 s) of each amplification cycle. Samples were quantified against a standard curve of ANDV RNA extracted from 10-fold serial dilutions of ANDV stocks with predetermined titers.
Humoral immune response in hamsters following Ad vector immunization and ANDV challenge.
Antibodies to the N antigen of ANDV cross-react with SNV N antigen (11). Thus, an indirect enzyme-linked immunosorbent assay (ELISA) using a recombinant E. coli-expressed SNV N was used to test for anti-N antibodies as described previously (12). Assays for ANDV neutralizing antibodies involved the use of vesicular stomatitis virus (VSV) pseudotypes (39). VSV pseudotypes bearing ANDV glycoproteins (designated VSVΔG*AND) that express green fluorescent protein were prepared by using an expression plasmid in which ANDV glycoproteins were expressed using the chicken beta-actin promoter as described previously (39). Diluted serum samples were mixed at 37°C for 1 h with VSVΔG*AND and green fluorescent protein plaques measured using Vero E6 cells after 16 to 24 h. Serum samples were considered neutralizing if there was ≥80% reduction in infectivity.
Statistical analysis.
One-way analysis of variance was conducted on the data with vaccination groups as the treatment factor. Tukey-Kramer multiple-comparison tests were performed to determine the pairwise differences among the vaccine groups. Statistical analysis was carried out on quantitative RT-PCR data from lung, liver, and blood samples collected at 6 and 9 days postinoculation using InStat v3.0 statistical software (GraphPad Software, La Jolla, CA).
RESULTS
Construction of Ad vectors expressing ANDV proteins.
Nonreplicating, E1− Ad vectors can produce large quantities of transgene proteins without expressing substantial amounts of Ad proteins in host cells and can be produced to high titers. One problem associated with the propagation of Ad vectors is that there is high-level expression of transgene proteins in the E1-expressing 293 cells that is associated with toxicity, especially when membrane proteins are involved. The outcome of this toxicity is low-titer stocks of Ad vectors and selection for mutants that do not express the foreign proteins. Ad vectors utilizing conditional promoters have been developed so that vectors can be propagated in 293 cells (13, 33). For our studies, we coupled ANDV cDNA sequences to a modified MCMV immediate-early promoter in the shuttle plasmid pDC316(io) (33). pDC316(io) includes a LacZ repressor-binding site downstream of the MCMV promoter that is bound by a LacZ repressor protein expressed in 293 IQ cells, reducing expression. Also present in the promoter region of this plasmid is an intron that increases expression and nuclear export of mRNAs.
The ANDV S segment containing the N protein-coding sequences and M segment containing the GPC precursor for GN and GC-coding sequences (Fig. 1A) were derived from a Chilean ANDV isolate, amplified by RT-PCR, and inserted into plasmids (35). PCR was used to amplify the N-, GN-, GC-, or GPC (GN plus GC)-coding sequences from these plasmids, and these sequences were inserted adjacent to the modified MCMV promoter in plasmid pDC316(io). These shuttle plasmids were sequenced carefully to ensure that there were no mutations and then cotransfected into 293 IQ cells along with another plasmid, pBHGloxDE1,3Cre (33), which expresses Cre recombinase, which promotes recombination between a loxP site in pDC316(io) and a second loxP site in pBHGloxDE1,3Cre. There is also a deletion in the E3 region of the Ad5 genome in pBHGloxDE1,3Cre. Ad vectors, denoted AdN, AdGN, AdGC, and AdGPC, were obtained after cotransfection of 293 IQ cells (Fig. 1B). A vector without ANDV sequences, denoted AdEmpty, was produced using DC3169(lo).
FIG. 1.
Construction of recombinant Ad vectors expressing ANDV proteins. (A) The ANDV S, M, and L RNA segments encoding the N protein, the GPC protein that gives rise to GN and GC, and polymerase, respectively. (B) N-, GPC-, GN-, and GC-coding regions were amplified by PCR from plasmids and inserted into a shuttle plasmid, pDC316(io), for rescue into E1− E3− Ad vectors. This placed hantavirus coding sequences downstream of an MCMV immediate-early promoter sequence that was separated from the hantavirus genes by a LacZ repressor binding site and an intron, all inserted with Ad5 E1 sequences. These pDC316(io) plasmids were cotransfected with plasmid pBHGloxDE1,3Cre, which contains other Ad sequences and lacks E3 sequences, into 293 IQ cells. Recombination between loxP sites in pDC316(io) and pBHGloxDE1,3Cre produced Ad vectors. 293 IQ cells express a LacZ repressor protein that reduces expression of hantavirus proteins during Ad vector propagation.
Expression of ANDV proteins.
ANDV proteins were detected using rabbit polyclonal antibodies produced against the entire N protein conjugated to a polyhistidine sequence, a synthetic peptide derived from the ANDV GN protein conjugated onto KLH, or the entire ANDV GC protein fused onto the C terminus of GST. Expression of hantavirus proteins was investigated by transducing human U373 cells with AdN, AdGN, AdGC, or AdGPC for 20 to 24 h and then labeling the cells for 3 h with [35S]methionine-cysteine, followed by immunoprecipitation of hantavirus proteins. We observed strong expression of GN, GC, and N proteins by 24 h of Ad vector infection of cells (Fig. 2A). However, expression of the GN and GC proteins in cells infected with AdGPC was much lower in U373 cells (Fig. 2A), as well as in several other cell types (not shown). Moreover, GPC mRNA appeared to be less stable in AdGPC-infected cells than GN mRNA (data not shown), and efforts are under way to characterize this further and construct another GPC-expressing Ad vector. The anti-GN antibodies did not cross-react with GC and vice versa (Fig. 2A). When cells were coinfected with AdGN and AdGC, a complex of GN and GC could be coprecipitated with GN-specific antibodies (Fig. 2B). However, the anti-GC antibodies did not coprecipitate a GN/GC complex from detergent extracts of cells coinfected with AdGN and AdGC. This might be related to the fact that GC-specific antibodies were produced using a synthetic peptide antigen. We concluded that AdN, AdGN, and AdGC vectors produced substantial quantities of GN, GC, or N and that cells coinfected with both AdGN and AdGC produced GN/GC heterodimers observed during viral infection (reviewed in reference 14).
FIG. 2.
Expression of ANDV proteins by Ad vectors. (A) Human U373 cells were infected with Ad vector AdGN, AdGC, AdGPC, or AdN using 50 (293 cell) PFU/cell for 24 h, and then the cells were radiolabeled with [35S]methionine-cysteine for 3 h. (B) U373 cells were infected with AdGN, AdGC, or both AdGN and AdGC using 50 PFU/cell for 22 h, and then cells were radiolabeled with [35S]methionine-cysteine for 3 h. Detergent extracts of cells were mixed with GN-, GC-, or N-specific antibodies, and various proteins were immunoprecipitated and analyzed by gel electrophoresis. Molecular mass markers are at the right, and the positions of GN, GC, and N proteins indicated on the left and right.
CTL responses in mice vaccinated with Ad vectors expressing ANDV proteins.
In order to determine whether cellular immune responses against ANDV proteins were produced, BALB/c mice were vaccinated and then boosted (3 to 4 weeks later) with Ad vectors. Spleens were removed 6 to 8 days after the boost, and splenocytes were restimulated with syngeneic SVBalb cells infected with Ad vectors expressing the relevant ANDV protein(s) (N, GN, GC, or both GN and GC). These lymphocytes were then tested for lysis of syngeneic SVBalb cells in chromium release assays. Lymphocytes from AdN-vaccinated mice efficiently lysed AdN-infected SVBalb cells, but there was only background lysis of cells infected with AdEmpty, AdGN, or AdGC (Fig. 3A). These results suggested strong CD8+ T-cell responses to the N protein and much weaker responses directed toward Ad proteins in these mice. Consistent with this hypothesis, there was little or no lysis of AdN-, AdGN-, and AdGC-infected cells by CTLs from mice vaccinated with AdEmpty (not shown). Together, these results were consistent with relatively low-level expression of Ad proteins produced in these mice. There was also little or no lysis of MHC-mismatched MC57G cells infected with AdN by CTLs from AdN-vaccinated mice (data not shown). Strong CTL responses were also produced in AdGN-vaccinated mice, which efficiently lysed cells infected with AdGN, and there was much lower lysis of cells infected with AdN, AdGC, or AdEmpty (Fig. 3B). Splenocytes from mice vaccinated with AdGC exhibited strong CTL responses specific for AdGC-infected cells (Fig. 3C); however, in these animals the responses to Ad proteins were also relatively higher. Lymphocytes from mice vaccinated with both AdGN and AdGC lysed SVBalb cells infected with AdGN and AdGC but not AdEmpty- or AdN-infected targets (Fig. 3D). In this specific experiment, the CTL responses produced in mice vaccinated with a combination of AdGN and AdGC were lower (20% specific lysis) than CTL responses observed with either AdGN- or AdGC-vaccinated animals (35%). Note that mice were separately vaccinated with AdN, AdGC, AdGN, or AdGC plus AdGN in some cases in different experiments, but in every case the CTL experiments were internally controlled so that any preparation of lymphocytes was tested for lysis of AdEmpty-infected targets as well as target cells infected with Ad vectors, and lysis of these controls was always substantially lower. It was not clear whether the lower lysis of AdGN-plus-AdGC-infected targets (compared with targets infected with AdGN alone) might reflect the requirement to cotransduce mouse cells during vaccination (so that both viruses were present in the same cell). Alternatively, there might be competition between Ad vectors for protein expression (i.e., so that restimulation with SVBalb cells infected with both viruses was weaker). However, overall we concluded that AdN, AdGN, AdGC, or both AdGN and AdGC all produced robust and specific CD8+ CTL responses in mice.
FIG. 3.
Mouse CTL lysis of mouse cells transduced with hantavirus proteins. Groups of five mice were vaccinated with 1 × 108 (293 cell) PFU per animal of AdN (A), AdGN (B), AdGC (C), or both AdGN and AdGC (D). Splenocytes were removed after 6 to 8 days, pooled for all five animals, and restimulated with interleukin-2 and gamma-irradiated syngeneic SVBalb cells infected with the relevant Ad vectors for 7 to 8 days. The splenocytes (effectors) were mixed with 51Cr-labeled target cells consisting of SVBalb cells that were left uninfected (UI) or infected with Ad vectors (including AdEmpty, which expresses no hantavirus proteins) using various effector-to-target cell ratios in triplicate wells. After 4 to 5 h the release of 51Cr into cell culture supernatants was measured, as well as spontaneous release and maximum release. The percent specific release was calculated as described in the Materials and Methods.
Vaccination with Ad vectors expressing ANDV N, GN, or GC protects hamsters from lethal challenge.
For protection studies using the Ad vectors, it was first necessary to establish the ANDV hamster model (19) in our biosafety level 4 laboratory and determine an ANDV challenge dose for Syrian hamsters. Groups of six hamsters were injected via the i.p. route with 10-fold dilutions of ANDV ranging from 0.8 to 80,000 FFU/animal, where FFU were as defined in Materials and Methods. Hamsters that succumbed to ANDV infection exhibited symptoms including breathing distress, beginning with rapid, shallow breaths, which rapidly advanced to severe dyspnea. At the onset of symptoms, hamsters appeared active, but as symptoms progressed, they quickly became moribund. Death occurred within approximately 12 to 36 h of symptom onset, between 7 and 14 days postinfection (p.i.), in a dose-dependent manner (data not shown). Postmortem observations included cyanosis in the lips of the majority of hamsters as well as occasional observations of dried blood around the nares and mouth. Hamsters that survived challenge were monitored for an additional 2 weeks after the final death, and none of the animals that survived displayed any obvious clinical signs of disease. Based on the data, the LD50 for ANDV inoculated i.p. was calculated to be 1.54 FFU, and in subsequent experiments we used a challenge dose of 100 LD50s (equivalent to 154 FFU ANDV).
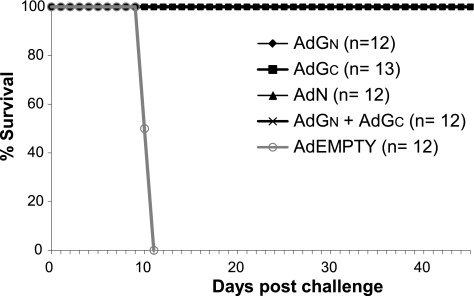
Hamsters (five groups of 12 or 13 animals) were vaccinated using a single inoculation via the intramuscular route with 108 PFU (determined using 293 cells) of AdEmpty, AdN, AdGC, AdGN, or a combination of AdGN and AdGC and were challenged 28 days later with 100 LD50 of ANDV. AdEmpty-immunized hamsters showed clinical signs (i.e., respiratory symptoms) of ANDV infection at 9 days after challenge, and they all died by day 11. In contrast, none of the animals receiving any of the single Ad vectors or the combination of AdGN and AdGC displayed any obvious signs of ANDV disease postchallenge, and all survived the lethal challenge (Fig. 4).
FIG. 4.
Protection of hamsters from lethal ANDV disease following vaccination with Ad vectors. Groups of 12 or 13 hamsters were immunized once using 108 PFU/animal with various Ad vectors by the intramuscular route. Twenty-eight days later, the animals were challenged using 100 LD50s of ANDV and monitored for 45 days for signs of disease and survival.
ANDV replication in hamsters vaccinated with different Ad vectors was investigated by obtaining blood, lung, and liver samples from three immunized animals of each group at 6 and 9 days after ANDV challenge and analyzing for the presence of viral RNA using real-time quantitative RT-PCR. AdEmpty-immunized hamsters had considerable ANDV RNA in blood and tissues at both 6 and 9 days p.i. (Fig. 5). Samples collected from hamsters immunized with single vectors (AdN, AdGN, or AdGC) had measurably lower quantities of ANDV RNA in all tissues at both time points. ANDV RNA was not detected in the blood of hamsters immunized with AdN, AdGN, or AdGC at 6 days postchallenge. At day 9 postchallenge there were low levels of RNA in the blood of AdN- and AdGC-vaccinated animals. ANDV RNA was detected in all of the livers and most of the lungs of AdN- and AdGC-vaccinated animals at both times, although the quantities of RNA were lower than those in AdEmpty-vaccinated animals (Fig. 5). In five of the six hamsters vaccinated with AdGN, there was no detectable ANDV RNA in both lung and liver, and the single hamster with detectable ANDV RNA had a very low level. Animals vaccinated with the combination AdGN and AdGC exhibited no detectable ANDV RNA in any of the tissues tested either at day 6 or 9 postchallenge (Fig. 5).
FIG. 5.
Detection of ANDV RNA in hamsters vaccinated with Ad vectors and then challenged with ANDV. Three hamsters from each group (AdEmpty, AdN, AdGN, AdGC, or both AdGN and AdGC) were euthanized at 6 (upper panel) and 9 (lower panel) days postchallenge with ANDV. Lung, liver, and blood samples were collected and RNA extracted and analyzed for the presence of ANDV RNA using real-time quantitative RT-PCR. The data represents the average values for triplicate analyses of tissues from three hamsters. Numbers above bars indicate the number of hamsters that were positive for RNA. Asterisks indicate statistically significant differences compared with AdEmpty-immunized hamsters (*, P < 0.0001; **, P < 0.0096). Error bars represent 2 times the standard error of the mean.
Humoral immune responses in hamsters following immunization and challenge.
We tested humoral immune responses in hamsters to determine whether antibodies were produced in response to vaccination and whether ANDV challenge boosted existing antibodies. N-specific antibodies were characterized using ELISA and ANDV-neutralizing antibodies responses tested using ANDV glycoprotein/VSV pseudotypes. Sera were collected from hamsters before vaccination, 28 days after vaccination (before challenge with ANDV), and 40 to 45 days after ANDV challenge. Prevaccination sera were uniformly negative for the presence of hantavirus N-specific and neutralizing antibodies (data not shown). Moderate levels of N-specific antibodies were detected in all animals vaccinated with one dose of AdN, and N-specific antibodies were boosted to high titers following ANDV challenge (Fig. 6). There were no detectable neutralizing antibodies in AdN-vaccinated hamsters prior to challenge, but there were good neutralizing titers following challenge. Both responses were consistent with ANDV replication in these animals. Animals vaccinated with AdGC exhibited no neutralizing antibodies following vaccination and good neutralizing titers following challenge, and there were robust anti-N responses following in all animals (Fig. 6). Again, this supported ANDV replication in AdGC-vaccinated animals. All hamsters vaccinated with AdGN displayed no neutralizing antibodies before challenge and either no neutralizing antibodies or modest responses following challenge (Fig. 6). Moreover, AdGN-vaccinated hamsters displayed no or low levels of anti-N antibodies after challenge, consistent with much lower levels of ANDV replication. The majority (10 of 12) of animals vaccinated with both AdGN and AdGC exhibited no anti-N specific antibodies following challenge, and those detected in two animals were weak. Approximately two-thirds of the AdGN/AdGC-vaccinated animals had no neutralizing antibodies before ANDV challenge, and there was modest elevation of titers after challenge so that all displayed low levels of neutralizing antibodies (Fig. 6). Together, these data show that neutralizing antibodies were produced in response to AdGN/AdGC vaccination but none were detected with AdGN or AdGC alone, yet these animals were completed protected. These data also supported relatively limited or no ANDV replication in most of the AdGN/AdGC- and some of the AdGN-vaccinated animals.
FIG. 6.
N protein-specific and neutralizing antibody responses in hamsters vaccinated with Ad vectors before and after ANDV challenge. Sera from vaccinated hamsters were obtained 28 days after vaccination with Ad vectors and prior to ANDV challenge (pre) or 45 days after ANDV challenge (post). Sera were tested for N-specific antibodies using recombinant SNV N protein in ELISA (upper panel). Neutralizing antibodies were detected using VSV pseudotypes bearing the ANDV glycoproteins (lower panel). Each symbol represents the titer for a single hamster. †, hamster died and serum was not collected.
DISCUSSION
New World hantaviruses produce HPS, involving rapid progression from mild influenza-like symptoms to severe respiratory distress, and have been frequently associated with mortality in otherwise healthy individuals. Currently there are no licensed therapeutic interventions for HPS treatment or prevention (22, 32), and vaccines are urgently required. Inactivated vaccines have been tested for safety and efficacy in human volunteers, and these vaccines produced neutralizing antibodies, although these responses were often relatively short-lived and these vaccines are likely less effective in producing strong cellular responses (reviewed in references 24 and 32). A recombinant VV expressing Hantaan virus glycoproteins and N protein was tested in phase I and II clinical trials in North America and produced good immunity in immunologically naive volunteers (34, 43). However, the widespread use of VV-based vaccines is not likely to be approved in the near term because of rare cases of VV-induced disease. DNA vaccines produce neutralizing antibodies in experimental animals (10, 16-18, 20, 23). However, DNA vaccines generally give much lower expression than virus vectors; it is frequently necessary to vaccinate multiple times, and for humans this involves relatively large amounts of DNA. Perhaps reflecting relatively poor expression, vaccination of hamsters with ANDV M segment cDNA (expressing GPC) failed to produce antibodies and was not protective in the lethal HPS model (10).
Nonreplicating Ad vectors offer advantages over other vaccine approaches, as they replicate to high titers, express large quantities of transgenes and low levels of Ad proteins, and have a proven safety record (reviewed in references 1 and 48). Importantly, Ad vectors can produce potent and long-lasting cellular immunity, as well as humoral responses. Comparisons of nonreplicating Ad and VV vectors and DNA vaccinations of monkeys with human immunodeficiency virus or simian immunodeficiency virus Gag proteins suggested more robust immunity with Ad vectors (7, 46). However, preexisting immunity to Ad5 and other common human Ad serotypes is a major problem (48). It appears likely that this problem can be overcome by using rare human Ad serotypes (2, 26) or by using simian or other animal Ad vectors (25, 47). Alternatively, evidence is accumulating that other routes of immunization, e.g., nasal delivery, may provide partial or complete resistance to neutralizing antibodies, which are the principal problem with preexisting immunity to Ad5 vaccines (9). Delivery of Ad vectors expressing ANDV proteins by the nasal or oral route may be especially useful because HPS-causing hantaviruses replicate in the lung.
In this study, we constructed nonreplicating Ad vectors that expressed ANDV N, GN, or GC proteins by coupling hantavirus cDNAs to a promoter that was negatively regulated in the helper 293 cells used to propagate the vectors. We observed high-level expression of all three proteins expressed individually, and cells coinfected with AdGN and AdGC produced the GN/GC heterodimer. Hamsters immunized with individual vectors (AdGN, AdGC, or AdN) or with a combination of AdGN and AdGC were completely protected from 100 times the LD50 of ANDV. Importantly, no single animal vaccinated with any of these vectors displayed any obvious clinical signs of disease. In contrast, all hamsters vaccinated with AdEmpty (a control vector that does not express ANDV proteins) demonstrated breathing distress within 8 to 10 days postchallenge, and all these animals died by day 11. These Ad vectors represent the first vaccines that produced immunity sufficient to protect animals from a highly lethal hantavirus challenge.
All of the hamsters vaccinated with both AdGN and AdGC displayed no detectable ANDV RNA in the blood or in liver and lung tissues after 6 and 9 days p.i. Furthermore, the majority (10 of 12) of the AdGN/AdGC-vaccinated hamsters exhibited no detectable N-specific antibodies following ANDV challenge, although there was modest boosting of neutralizing antibody titers in some animals. These data are consistent with very low or no ANDV replication in these animals. Much of the strong protective immunity produced in animals vaccinated with AdGN and AdGC appears to relate to expression of GN, because ANDV RNA was not detected or was low in AdGN-vaccinated hamsters and was higher in AdGC-vaccinated hamsters. Moreover, about half of the AdGN-vaccinated animals did not produce detectable N-specific antibodies following ANDV challenge, whereas there was a much more robust N-specific antibody response in AdGC-immunized animals. There was also evidence of ANDV replication in hamsters vaccinated with AdN, in the form of viral RNA detected in tissues, boosting of anti-N antibodies, and neutralizing antibodies produced after ANDV challenge. Even though ANDV clearly replicated in AdN- and AdGC-vaccinated hamsters, there were reduced levels of ANDV RNA and delays in the appearance of RNA compared with AdEmpty-vaccinated animals. Moreover, all the AdGC- and AdN-vaccinated animals survived with no obvious disease symptoms. Our observations with AdN were consistent with previous observations that hantavirus N protein vaccines produce good immunity (29, 42). The observation that ANDV RNA was undetected in AdGN/GC-vaccinated hamsters was quite remarkable, given the highly pathogenic nature of ANDV in these hamsters.
The superior immunity produced by vaccination with the combined AdGN and AdGC, compared with GN or GC alone, might reflect more accurate folding and assembly of GN/GC heterodimers or posttranslational modification. Any of these effects might increase protective antibody responses. However, there might also be additive effects of expressing both GN and GC, thereby producing more CD8+ T-cell peptide epitopes. The notion that the GN/GC heterodimers are formed in AdGN/AdGC-vaccinated animals depends upon coexpression of both proteins in a population of cells (cis expression) in vivo. At present, we have no information on the frequency of cis expression in animal tissues, and it is also possible that GN and GC produce additive immunity by being expressed in trans. Further experimentation with AdGN and AdGC, in comparison with a vector expressing GPC, is required to characterize this further.
It is well established that the hantavirus glycoproteins are the principal, or sole, targets of neutralizing antibodies. The induction of strong neutralizing antibody responses correlates with protection against hantaviruses in small-animal infection models (18, 40, 42, 52) and with clinically milder courses of HPS in patients (5). Additionally, passive transfer of immune sera is sufficient to protect hamsters from lethal HPS disease (10, 17). No antibodies capable of neutralizing ANDV without complement were produced in AdN-, AdGN-, and AdGC-vaccinated animals. Relatively low levels of neutralizing antibodies were produced in about one-third of hamsters vaccinated with both AdGN and AdGC. These observations are consistent with the notion that high titers of neutralizing antibodies were not necessary for protection, because AdN-, AdGN-, and AdGC-immunized animals were completely protected. However, it is also very possible that nonneutralizing antibodies played an important role in protection, e.g., acting in conjunction with complement or by antibody-dependent cell-mediated cytotoxic mechanisms. It is likely that antibodies work in conjunction with cellular immunity to provide protection, but the precise role of antibodies in such protective mechanisms requires further testing in antibody transfer experiments.
Our results also have important implications for the understanding of how cellular immunity affects the outcome of hantavirus infections. AdN-vaccinated hamsters survived a lethal challenge with ANDV without obvious symptoms. These observations are consistent with the hypothesis that N-specific cellular immunity, and specifically CD8+ T lymphocytes, is sufficient for protection from lethal hantavirus disease. N-specific antibodies are not likely to be neutralizing, and given that the N protein is not presented on the surfaces of ANDV-infected cells, it is much more likely that the N protein is recognized by MHC class I-mediated presentation of peptide antigens. Obviously, this result does not prove that cellular immunity is sufficient for protection from hantavirus disease in hamsters. CTL experiments and T-cell transfer experiments (which are much more difficult in hamsters) will be needed for further proof of this hypothesis. However, consistent with the hypothesis, all three Ad vectors, AdN, AdGN, and AdGC, produced strong CD8+ CTL responses in mice. Previous studies involving an Ad vector vaccine expressing the SNV N protein demonstrated strong, long-lived T-cell responses in mice (30). There are numerous other reports that hantavirus N proteins produce cellular immunity with protective effects (29, 42, 50, 52), although protection from disease was not measured.
It is also highly likely that peptide epitopes derived from the glycoproteins GN and GC were recognized by CD8+ T cells and that these also contributed substantially to the protective immunity observed in AdGN-, AdGC-, and AdGN/AdGC-vaccinated hamsters. As noted above, many of these animals did not exhibit neutralizing antibodies yet resisted ANDV infection well. Coupled with the observations of protection of AdN-vaccinated hamsters, it is very likely that T-cell recognition of GN and GC contributed substantially to protection and rapid clearance, especially with AdGN/AdGC-vaccinated animals, which exhibited little ANDV replication. Supporting this, studies by Bharadwaj and colleagues (3, 4) described protective T-cell epitopes in both the SNV GN and N proteins using DNA-based vaccines in mice. Similar to the findings here, deer mice were protected from SNV infection following vaccination with plasmids expressing specific fragments of GN or N but not GC. Neutralizing antibodies were not detected in immunized mice, but rather protection correlated with increased splenocyte proliferation following stimulation with cognate antigen (3, 4).
In summary, these are the first studies demonstrating protection of experimental animals from a lethal hantavirus infection. These Ad vectors produced immune responses able to prevent disease, and with some hamsters, little or no ANDV replication was detected. These nonreplicating Ad vectors have substantial benefits over inactivated vaccines because they produce robust cellular immunity and over DNA vaccines that, to date, have not protected hamsters from ANDV. More studies with these Ad vectors are required to understand the relative contributions of humoral versus cellular immunity and to obtain a more exact characterization of protective antigens and mechanisms. These studies provide proof that Ad vectors can produce robust and fully protective immunity to hantaviruses. However, the problem of preexisting immunity to Ad5 remains substantial, and we expect that other types of Ad vectors with less prevalent human immunity will be necessary for clinical trials of hantavirus vaccines. Toward this end, we are designing other vectors based on less common Ad serotypes (Ad6 and Ad35) or based on porcine Ad3.
Acknowledgments
We are most grateful to Frank Graham (now retired to a farm in Umbria) for advice in the early stages of this work and to Jason Gren and Shane Jones (National Microbiology Laboratory, Public Health Agency of Canada) for assistance with animal work.
This work was supported by grants from NIH/NIAID (R21 AI059245) to D.C.J., a grant from the Canadian Institutes of Health Research (CIHR MOP 7471) to H.F., and funding through the Public Health Agency of Canada. S.S.J. received grant support from the Pacific Southwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases (AI065359). D.S. was supported in part by a fellowship from a training program in infectious diseases sponsored by CIHR, the International Centre for Infectious Diseases (ICID), and the University of Manitoba (UM) (CIHR/ICID, UM).
Footnotes
Published ahead of print on 29 April 2009.
REFERENCES
- 1.Bangari, D. S., and S. K. Mittal. 2006. Current strategies and future directions for eluding adenoviral vector immunity. Curr. Gene Ther. 6215-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 1726290-6297. [DOI] [PubMed] [Google Scholar]
- 3.Bharadwaj, M., C. R. Lyons, I. A. Wortman, and B. Hjelle. 1999. Intramuscular inoculation of Sin Nombre hantavirus cDNAs induces cellular and humoral immune responses in BALB/c mice. Vaccine 172836-2843. [DOI] [PubMed] [Google Scholar]
- 4.Bharadwaj, M., K. Mirowsky, C. Ye, J. Botten, B. Masten, J. Yee, C. R. Lyons, and B. Hjelle. 2002. Genetic vaccines protect against Sin Nombre hantavirus challenge in the deer mouse (Peromyscus maniculatus). J. Gen. Virol. 831745-1751. [DOI] [PubMed] [Google Scholar]
- 5.Bharadwaj, M., R. Nofchissey, D. Goade, F. Koster, and B. Hjelle. 2000. Humoral immune responses in the hantavirus cardiopulmonary syndrome. J. Infect. Dis. 18243-48. [DOI] [PubMed] [Google Scholar]
- 6.Carroll, D. S., J. N. Mills, J. M. Montgomery, D. G. Bausch, P. J. Blair, J. P. Burans, V. Felices, A. Gianella, N. Iihoshi, S. T. Nichol, J. G. Olson, D. S. Rogers, M. Salazar, and T. G. Ksiazek. 2005. Hantavirus pulmonary syndrome in Central Bolivia: relationships between reservoir hosts, habitats, and viral genotypes. Am. J. Trop. Med. Hyg. 7242-46. [PubMed] [Google Scholar]
- 7.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 776305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Childs, J. E., T. G. Ksiazek, C. F. Spiropoulou, J. W. Krebs, S. Morzunov, G. O. Maupin, K. L. Gage, P. E. Rollin, J. Sarisky, R. E. Enscore, et al. 1994. Serologic and genetic identification of Peromyscus maniculatus as the primary rodent reservoir for a new hantavirus in the southwestern United States. J. Infect. Dis. 1691271-1280. [DOI] [PubMed] [Google Scholar]
- 9.Croyle, M. A., A. Patel, K. N. Tran, M. Gray, Y. Zhang, J. E. Strong, H. Feldmann, and G. P. Kobinger. 2008. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS One 3e3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Custer, D. M., E. Thompson, C. S. Schmaljohn, T. G. Ksiazek, and J. W. Hooper. 2003. Active and passive vaccination against hantavirus pulmonary syndrome with Andes virus M genome segment-based DNA vaccine. J. Virol. 779894-9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enria, D. A., A. M. Briggiler, N. Pini, and S. Levis. 2001. Clinical manifestations of New World hantaviruses. Curr. Top. Microbiol. Immunol. 256117-134. [DOI] [PubMed] [Google Scholar]
- 12.Feldmann, H., A. Sanchez, S. Morzunov, C. F. Spiropoulou, P. E. Rollin, T. G. Ksiazek, C. J. Peters, and S. T. Nichol. 1993. Utilization of autopsy RNA for the synthesis of the nucleocapsid antigen of a newly recognized virus associated with hantavirus pulmonary syndrome. Virus Res. 30351-367. [DOI] [PubMed] [Google Scholar]
- 13.Hardy, S., M. Kitamura, T. Harris-Stansil, Y. Dai, and M. L. Phipps. 1997. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 711842-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegde, N. R., C. Dunn, D. M. Lewinsohn, M. A. Jarvis, J. A. Nelson, and D. C. Johnson. 2005. Endogenous human cytomegalovirus gB is presented efficiently by MHC class II molecules to CD4+ CTL. J. Exp. Med. 2021109-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hitt, M., A. J. Bett, C. L. Addison, L. Prevec, and F. L. Graham. 1995. Techniques for human adenovirus vector construction and characterization. Methods Mol. Genet. 7813-30. [Google Scholar]
- 16.Hooper, J. W., D. M. Custer, J. Smith, and V. Wahl-Jensen. 2006. Hantaan/Andes virus DNA vaccine elicits a broadly cross-reactive neutralizing antibody response in nonhuman primates. Virology 347208-216. [DOI] [PubMed] [Google Scholar]
- 17.Hooper, J. W., A. M. Ferro, and V. Wahl-Jensen. 2008. Immune serum produced by DNA vaccination protects hamsters against lethal respiratory challenge with Andes virus. J. Virol. 821332-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooper, J. W., K. I. Kamrud, F. Elgh, D. Custer, and C. S. Schmaljohn. 1999. DNA vaccination with hantavirus M segment elicits neutralizing antibodies and protects against Seoul virus infection. Virology 255269-278. [DOI] [PubMed] [Google Scholar]
- 19.Hooper, J. W., T. Larsen, D. M. Custer, and C. S. Schmaljohn. 2001. A lethal disease model for hantavirus pulmonary syndrome. Virology 2896-14. [DOI] [PubMed] [Google Scholar]
- 20.Hooper, J. W., and D. Li. 2001. Vaccines against hantaviruses. Curr. Top. Microbiol. Immunol. 256171-191. [DOI] [PubMed] [Google Scholar]
- 21.Hutchinson, L., H. Browne, V. Wargent, N. Davis-Poynter, S. Primorac, K. Goldsmith, A. C. Minson, and D. C. Johnson. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J. Virol. 662240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonsson, C. B., J. Hooper, and G. Mertz. 2008. Treatment of hantavirus pulmonary syndrome. Antiviral Res. 78162-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamrud, K. I., J. W. Hooper, F. Elgh, and C. S. Schmaljohn. 1999. Comparison of the protective efficacy of naked DNA, DNA-based Sindbis replicon, and packaged Sindbis replicon vectors expressing hantavirus structural genes in hamsters. Virology 263209-219. [DOI] [PubMed] [Google Scholar]
- 24.Khaiboullina, S. F., S. P. Morzunov, and S. C. St. Jeor. 2005. Hantaviruses: molecular biology, evolution and pathogenesis. Curr. Mol. Med. 5773-790. [DOI] [PubMed] [Google Scholar]
- 25.Kobinger, G. P., H. Feldmann, Y. Zhi, G. Schumer, G. Gao, F. Feldmann, S. Jones, and J. M. Wilson. 2006. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology 346394-401. [DOI] [PubMed] [Google Scholar]
- 26.Lemckert, A. A., S. M. Sumida, L. Holterman, R. Vogels, D. M. Truitt, D. M. Lynch, A. Nanda, B. A. Ewald, D. A. Gorgone, M. A. Lifton, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2005. Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-Ad5 immunity. J. Virol. 799694-9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levis, S., J. E. Rowe, S. Morzunov, D. A. Enria, and S. St. Jeor. 1997. New hantaviruses causing hantavirus pulmonary syndrome in central Argentina. Lancet 349998-999. [DOI] [PubMed] [Google Scholar]
- 28.Lopez, N., P. Padula, C. Rossi, M. E. Lazaro, and M. T. Franze-Fernandez. 1996. Genetic identification of a new hantavirus causing severe pulmonary syndrome in Argentina. Virology 220223-226. [DOI] [PubMed] [Google Scholar]
- 29.Lundkvist, A., H. Kallio-Kokko, K. B. Sjolander, H. Lankinen, B. Niklasson, A. Vaheri, and O. Vapalahti. 1996. Characterization of Puumala virus nucleocapsid protein: identification of B-cell epitopes and domains involved in protective immunity. Virology 216397-406. [DOI] [PubMed] [Google Scholar]
- 30.Maeda, K., K. West, D. Hayasaka, F. A. Ennis, and M. Terajima. 2005. Recombinant adenovirus vector vaccine induces stronger cytotoxic T-cell responses than recombinant vaccinia virus vector, plasmid DNA, or a combination of these. Viral Immunol. 18657-667. [DOI] [PubMed] [Google Scholar]
- 31.Maes, P., J. Clement, I. Gavrilovskaya, and M. Van Ranst. 2004. Hantaviruses: immunology, treatment, and prevention. Viral Immunol. 17481-497. [DOI] [PubMed] [Google Scholar]
- 32.Maes, P., J. Clement, and M. Van Ranst. 2009. Recent approaches in hantavirus vaccine development. Expert Rev. Vaccines 867-76. [DOI] [PubMed] [Google Scholar]
- 33.Matthews, D. A., D. Cummings, C. Evelegh, F. L. Graham, and L. Prevec. 1999. Development and use of a 293 cell line expressing lac repressor for the rescue of recombinant adenoviruses expressing high levels of rabies virus glycoprotein. J. Gen. Virol. 80345-353. [DOI] [PubMed] [Google Scholar]
- 34.McClain, D. J., P. L. Summers, S. A. Harrison, A. L. Schmaljohn, and C. S. Schmaljohn. 2000. Clinical evaluation of a vaccinia-vectored Hantaan virus vaccine. J. Med. Virol. 6077-85. [DOI] [PubMed] [Google Scholar]
- 35.Meissner, J. D., J. E. Rowe, M. K. Borucki, and S. C. St. Jeor. 2002. Complete nucleotide sequence of a Chilean hantavirus. Virus Res. 89131-143. [DOI] [PubMed] [Google Scholar]
- 36.Mertz, G. J., L. Miedzinski, D. Goade, A. T. Pavia, B. Hjelle, C. O. Hansbarger, H. Levy, F. T. Koster, K. Baum, A. Lindemulder, W. Wang, L. Riser, H. Fernandez, and R. J. Whitley. 2004. Placebo-controlled, double-blind trial of intravenous ribavirin for the treatment of hantavirus cardiopulmonary syndrome in North America. Clin. Infect. Dis. 391307-1313. [DOI] [PubMed] [Google Scholar]
- 37.Mishell, R. I., J. M. Shiigi, B. B. Mishell, K. H. Grabstein, and S. M. Shiigi. 1980. Prevention of the immunosuppressive effects of glucocorticosteroids by cell-free factors from adjuvant-activated accessory cells. Immunopharmacology 2233-245. [DOI] [PubMed] [Google Scholar]
- 38.Nichol, S. T., R. M. Elliot, A. Goldbach, C. S. Plyusnin, C. S. Schmaljohn, and R. B. Tesh. 2005. Bunyaviridae, p. 695-716. In C. M. Fauquet, M. A. Mayo, J. Manilfoff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy: VIIth report of the International Committtee on Taxonomy of Viruses. Academic Press, San Diego, CA.
- 39.Ogino, M., H. Ebihara, B. H. Lee, K. Araki, A. Lundkvist, Y. Kawaoka, K. Yoshimatsu, and J. Arikawa. 2003. Use of vesicular stomatitis virus pseudotypes bearing Hantaan or Seoul virus envelope proteins in a rapid and safe neutralization test. Clin. Diagn. Lab Immunol. 10154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pensiero, M. N., G. B. Jennings, C. S. Schmaljohn, and J. Hay. 1988. Expression of the hantaan virus M genome segment by using a vaccinia virus recombinant. J. Virol. 62696-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryckman, B. J., B. L. Rainish, M. C. Chase, J. A. Borton, J. A. Nelson, M. A. Jarvis, and D. C. Johnson. 2008. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J. Virol. 8260-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmaljohn, C. S., Y. K. Chu, A. L. Schmaljohn, and J. M. Dalrymple. 1990. Antigenic subunits of Hantaan virus expressed by baculovirus and vaccinia virus recombinants. J. Virol. 643162-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmaljohn, C. S., S. E. Hasty, and J. M. Dalrymple. 1992. Preparation of candidate vaccinia-vectored vaccines for haemorrhagic fever with renal syndrome. Vaccine 1010-13. [DOI] [PubMed] [Google Scholar]
- 44.Schmaljohn, C. S., and S. T. Nichol. 2007. Bunyaviridae, p. 1742-1789. In D. M. Knipe and P. A. Howley (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 45.Schonrich, G., A. Rang, N. Lutteke, M. J. Raftery, N. Charbonnel, and R. G. Ulrich. 2008. Hantavirus-induced immunity in rodent reservoirs and humans. Immunol. Rev. 225163-189. [DOI] [PubMed] [Google Scholar]
- 46.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415331-335. [DOI] [PubMed] [Google Scholar]
- 47.Sridhar, S., A. Reyes-Sandoval, S. J. Draper, A. C. Moore, S. C. Gilbert, G. P. Gao, J. M. Wilson, and A. V. Hill. 2008. Single-dose protection against Plasmodium berghei by a simian adenovirus vector using a human cytomegalovirus promoter containing intron A. J. Virol. 823822-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tatsis, N., and H. C. Ertl. 2004. Adenoviruses as vaccine vectors. Mol. Ther. 10616-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomazin, R., A. B. Hill, P. Jugovic, I. York, P. van Endert, H. L. Ploegh, D. W. Andrews, and D. C. Johnson. 1996. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 153256-3266. [PMC free article] [PubMed] [Google Scholar]
- 50.Ulrich, R., A. Lundkvist, H. Meisel, D. Koletzki, K. B. Sjolander, H. R. Gelderblom, G. Borisova, P. Schnitzler, G. Darai, and D. H. Kruger. 1998. Chimaeric HBV core particles carrying a defined segment of Puumala hantavirus nucleocapsid protein evoke protective immunity in an animal model. Vaccine 16272-280. [DOI] [PubMed] [Google Scholar]
- 51.Wisner, T., C. Brunetti, K. Dingwell, and D. C. Johnson. 2000. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J. Virol. 742278-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu, X., S. L. Ruo, J. B. McCormick, and S. P. Fisher-Hoch. 1992. Immunity to Hantavirus challenge in Meriones unguiculatus induced by vaccinia-vectored viral proteins. Am. J. Trop. Med. Hyg. 47397-404. [DOI] [PubMed] [Google Scholar]
- 53.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77525-535. [DOI] [PubMed] [Google Scholar]