Abstract
Effective vaccines for human immunodeficiency virus type 1 (HIV-1) will likely need to stimulate protective immunity in the intestinal mucosa, where HIV-1 infection causes severe CD4+ T-cell depletion. While replication-competent recombinant adenovirus (rAd) vectors can stimulate adenovirus-specific mucosal immunity after replication, oral delivery of replication-defective rAd vectors encoding specific immunogens has proven challenging. In this study, we have systematically identified barriers to effective gut delivery of rAd vectors and identified sites and strategies to induce potent cellular and humoral immunity. Vector-mediated gene transfer by rAd5 was susceptible to low-pH buffer, gastric and pancreatic proteases, and extracellular mucins. Using ex vivo organ explants, we found that transduction with rAd5 was highest in the ileum and colon among all intestinal segments. Transgene expression was 100-fold higher after direct surgical introduction into the ileum than after oral gavage, with rAd5 showing greater potency than the rAd35 or the rAd41 vector. A single immunization of rAd5 encoding HIV-1 gp140B to the ileum stimulated potent CD8+ T-cell responses in the intestinal and systemic compartments, and these responses were further enhanced by intramuscular rAd5 boosting. These studies suggest that induction of primary immune responses by rAd5 gut immunization and subsequent systemic boosting elicits potent antigen-specific gut mucosal responses.
Human immunodeficiency virus type 1 (HIV-1) infection is characterized by uncontrolled virus replication and cytopathicity in the intestinal mucosa, the site of major T-cell depletion after primary infection. The gastrointestinal (GI) tract is the predominant site of a pronounced CD4+ T-cell loss in the early stages of HIV infection and simian immunodeficiency virus (SIV) infection in the nonhuman primate model (3, 23, 26, 43). It has been suggested that a mucosal vaccine which generates HIV-specific CD8+ T cells in the gut could prevent the loss of CD4+ cells in gut-associated lymphoid tissue, establishment of infection, or spread of virus (13, 34). Therefore, targeted delivery of vaccines to the GI tract to stimulate mucosal responses has the potential to improve the efficacy of immune protection against HIV-1; however, the site of gene-based transduction and the barriers to vaccine delivery have not been well defined.
Adenoviruses (Ads) have been used extensively as vectors for both gene transfer and vaccine development. They offer several advantages as tools for vaccine delivery, such as the ability to transduce both dividing and nondividing cells, relative safety and stability in vivo, ease of production in high titers, and lack of integration (2, 35). These vectors are promising because parenteral administration in both animals and humans has been shown to generate strong and long-lasting humoral and cellular immune responses. The immune responses surpass those achieved with other types of gene vectors and genetic vaccines (5, 38, 46). As a result, recombinant Ad (rAd) vectors have been developed and tested as vaccine vehicles to immunize against a number of pathogens (4, 10, 15, 18, 41).
Orally (p.o.) delivered vaccines are attractive in theory because of their ease of administration and potential to deliver antigen to gut-associated lymphoid tissue, permitting induction of immune responses in both mucosal and systemic compartments. At the same time, p.o. delivery of replication-defective rAd vectors has posed a challenge and has met with variable levels of success. Immunization with rAd5 encoding rabies virus antigens, influenza virus antigens, or other antigens has generated some protection against infection in animal models (9, 27, 31, 39, 41), but p.o. immunization has elicited much lower CD8+ T-cell responses than systemic delivery (33), and a much higher dose is required to induce immune responses (37). We have recently shown in an HIV vaccine model that rAd41, a human enteric Ad-based vector, induced potent CD8+ T-cell responses in both systemic and mucosal compartments when primed p.o. or in the ileum (17). The previous study showed that rAd41 vectors delivered through direct ileal injection elicited mucosal cell immunity, but whether other rAd vectors could stimulate these responses and which factors affected delivery and immunogenicity were unknown. In this report, we have investigated the mechanisms associated with the low immunogenicity of rAd5 dosed through the p.o. route in mice. The purpose was to identify barriers to effective delivery of rAd vectors to gut tissues and to ascertain sites and strategies for induction of potent cellular and humoral immunity. To investigate the mechanism of the low immunogenicity of rAd vectors through the p.o. route and develop effective delivery of rAd5 and rare serotype rAd35 vectors as gut mucosal HIV vaccines, we have analyzed the obstacles to p.o. immunization, characterized vector transgene expression, and systematically compared immune responses induced by p.o. and local immunization strategies. These studies demonstrated that the higher immune responses were strongly associated with higher gene expression in the intestine and support further study of gut mucosal immunization in SIV challenge models as a potential HIV vaccine strategy.
MATERIALS AND METHODS
Animals and viruses.
Six- to 8-week-old female BALB/c mice were purchased from Jackson Laboratories and housed in the experimental animal facility of the Vaccine Research Center, NIAID, NIH (Bethesda, MD). All animal experiments were reviewed and approved by the Animal Care and Use Committee, VRC, NIAID, NIH, and performed in accordance with all relevant federal and NIH guidelines and regulations.
rAd5 vectors are replication-defective E1-, E3-, and E4-deleted human Ads, and rAd35 vectors are E1- and E3-deleted replication-defective vectors. rAd35/41L and rAd35/41S vectors are rAd35-based vectors with chimeric Ad41 long fiber or short fiber, respectively. rAd5-luc, rAd35-luc, rAd35/41L-luc, and rAd35/41S-luc encode luciferase reporter genes under the control of the cytomegalovirus promoter/enhancer. rAd5-gp140B, rAd35-gp140B, and rAd35/41L- or rAd35/41S-gp140B (rAd35 with Ad41 long or short fiber, respectively) encode gp140ΔCFIΔV1V2 of HIV-1 clade B (20). Ad vectors were propagated in 293-ORF6 cells and purified by cesium chloride gradients (6).
Mouse intestinal explants.
Explant culture medium consisted of Dulbecco's modified Eagle's medium-F-12 medium supplemented with 5% fetal bovine serum, l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), gentamicin (50 μg/ml), and epidermal growth factor (10 U/ml). All media and supplements were purchased from Invitrogen, Inc. (Carlsbad, CA). Mouse intestines were isolated and cut open longitudinally. After being washed thoroughly, the tissues were cut into 5- by 5-mm pieces and placed on presoaked Gelfoam (Pfizer, New York, NY), with the epithelium uppermost, in 24-well plates containing 0.3 ml of culture medium. Four microliters of virus solution containing 1 × 108 to 5 × 108 virus particles (VP) of treated or untreated rAd vectors encoding luciferase was applied directly to the upper surface of each explant. After overnight incubation, the tissues were rinsed in phosphate-buffered saline (PBS) and placed in 0.1% Triton X-100. Following a 1-min ultrasound treatment and three freeze-thaw cycles, the lysates were centrifuged at 2,000 rpm for 10 min. The supernatants were used for a luciferase assay. The luminescence was measured using a microplate scintillation and luminescence counter (PerkinElmer, Shelton, CT). The protein concentration of the cleared supernatant was determined using a DC protein microplate assay kit (Bio-Rad, Hercules, CA). The results are shown as relative luminescence units per μg protein.
For the acid and protease sensitivity study, rAd5-luc was (i) treated with 0.1 M HCl and 2.5 mg/ml pepsin from porcine gastric mucosa (Sigma, St. Louis, MO) for 5 min at 37°C and neutralized with 10× THB (200 mM Tris, 500 mM HEPES, 1.5 M NaCl), (ii) treated with 25 μg/ml trypsin from bovine pancreas (Sigma, St. Louis, MO) or 2.5 U/ml chymotrypsin (Sigma, St. Louis, MO) for 30 min at 37°C, or (iii) exposed to the combination of treatments with HCl-pepsin and trypsin and/or chymotrypsin and then applied to the tissue explants.
To test the susceptibility of intestinal segments to Ad vectors, mouse duodenum (1 cm downstream of the stomach), jejunum (median part of the small intestine), ileum (from the junction of the ileum and cecum to 2 cm upstream of the cecum), and colon (2 cm downstream of the cecum) were isolated and either washed thoroughly (native) or gently stripped to remove the mucus for culture. Ten pieces from three mice for each segment were cultured with 4 μl virus solution containing 1 × 108 VP of rAd5-luc and harvested as described above.
Treatment of intestinal explants with DTT, hyaluronidase, and exoglycosidases.
The mouse intestinal explants were either not treated or treated with dithiothreitol (DTT) (20 mM, catalogue number 43816; Sigma, St. Louis, MO) or hyaluronidase (1 or 10 mg/ml, catalogue number H 3506; Sigma, St. Louis, MO) for 30 min or exoglycosidases from Trimeresurus cornutus (1, 5, or 10 mg/ml; Seikagaku Biobusiness, Tokyo, Japan) for 2 min at 37°C. The explants were then rinsed in the prewarmed medium and cultured with 4 μl virus solution containing 1 × 108 VP of rAd5-luc. The stripped jejunum and the untreated ileum were used as the positive controls, while the medium only was used for the negative controls. The tissue explants were harvested as described above.
Gene expression in mouse intestine transduced with rAd5-luc in vivo.
Three BALB/c mice per group were either administered p.o. 1010 VP of rAd5-luc in 500 μl PBS or given an intraileal injection with 1010 VP of rAd5-luc in 100 μl PBS. Twenty-four hours after injection, the mice were sacrificed, and intestinal segments, including duodenum, jejunum, ileum, colon, and mesenteric lymph nodes (MLN), were lysed and assayed for luciferase activity. The group injected with 100 μl rAd5 empty vector via the ileum lumen was used as the control. The intraileal-injection procedure was described previously (17).
Immunization.
For the immunogenicity study, immune responses induced by a single injection of rAd5 encoding HIV-1 gp140B into the ileum lumen were tested. Five mice from each group were immunized with 1010 VP of rAd5-gp140B vector in 500 μl PBS p.o. or with 1010 VP of rAd5-gp140B vector in 100 μl PBS via ileal injection as described above. Mice that received rAd5 empty vector p.o. or via ileal injections were used as the negative controls. Three weeks after inoculation, peripheral blood mononuclear cells (PBMC), spleens, and small intestines were collected for H-2Dd/PA-9 tetramer staining. The spleens were also used for detecting HIV-1 peptide-specific cytokine-producing CD4+ or CD8+ T lymphocytes. The serum immunoglobulin G (IgG) antibodies were determined by enzyme-linked immunosorbent assay (ELISA).
In the prime-boost experiments, five mice per group were primed with 1010 VP of rAd5-gp140B via p.o. dosing or with rAd5, rAd35, Ad35/41L, and rAd35/41S, all encoding gp140B, via intraileal injections. Three weeks after immunization, blood was collected and PBMC were isolated and stained for H-2Dd/PA-9 tetramer. These mice were then boosted intramuscularly (i.m.) with rAd5-gp140B at 109 VP. Two weeks after the boost, PBMC, spleens, MLN, and small intestines were harvested for H-2Dd/PA-9 tetramer staining, and the spleens were also used for detecting HIV-1 peptide-specific cytokine-producing CD4+ or CD8+ T lymphocytes. The IgG and IgA antibodies in the sera and vaginal washes were determined by ELISA.
Lymphocyte isolation.
The isolation of lymphocytes from blood, spleen, and MLN was described previously (17). For lymphocyte isolation from the gut, the small intestines from individual mice were collected in cold medium. The intestine was opened and washed thoroughly following excision of Peyer's patches. Intestinal sections were then minced and digested with 0.5 mg/ml collagenase II (C-6885; Sigma) for 30 min at 37°C. The resulting supernatants were filtered through a 40-μm cell strainer. After centrifugation, the cell pellets were suspended in 6 ml of 40% Percoll (Amersham Biosciences, Piscataway, NJ) and overlaid on 4 ml of 75% Percoll. The samples were then subjected to centrifugation at 800 × g for 20 min at 22°C. The cells from the interface of 75% and 40% Percoll were collected, washed with a large volume of medium, and counted.
Tetramer staining and ICS.
Lymphocytes isolated from blood, spleen, MLN, and the small intestine were used for tetramer staining. The splenic lymphocytes from individual mice were used for intracellular cytokine staining (ICS), while the combined intestinal cells from the group of five mice were also used for ICS in the rAd35/41 chimeric fiber experiment, using ViViD dye (Invitrogen Life Sciences, Carlsbad, CA) to exclude nonviable cells. The detailed methods for tetramer staining and ICS were described previously (M. Honda, R. Wang, W.-P. Kong, M. Kanekiyo, W. Akahata, L. Xu, K. Matsuo, K. Natarajan, H. Robinson, T. E. Asher, D. A. Price, D. C. Douek, D. H. Margulies, and G. J. Nabel, submitted for publication; 17).
ELISA for IgG and IgA antibodies to HIV Env.
Levels of HIV gp140B-specific IgG and IgA in the sera or vaginal washes of vaccinated mice were assessed using ELISA (17). The sera were diluted at 1:1,000 for IgG and 1:50 for IgA, while the vaginal washes were diluted at 1:3 for the detection of both IgG and IgA. Absorbance at 450 nm was determined by a Spectra Max instrument (Molecular Devices).
Data and statistical analysis.
All results are presented as means with standard errors. Statistical analyses were performed based upon comparisons between the control groups and the treated groups or between differently treated groups by using the two-tailed Student t test. P values of less than 0.05 were considered statistically significant.
RESULTS
Determination of barriers to effective delivery of rAd5 vectors to the GI tract.
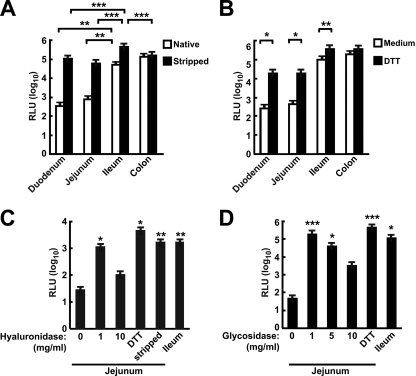
To identify potential mechanisms that inhibit transduction of the GI tract after rAd gene transfer after p.o. delivery, we first examined the transduction of mouse intestinal explants by an rAd5 vector expressing luciferase. Intestinal explants of the duodenum, jejunum, ileum, and colon were cultured ex vivo, and the relative rAd transduction efficiencies were determined. The ileum and the colon were transduced at significantly higher levels than were the duodenum and jejunum (Fig. 1A, open bars). To determine whether the superficial layer played a role in the inhibition of transduction of intestinal cells by rAd, mucus was removed by mechanical and biochemical means. Mechanical removal of the mucus significantly enhanced the transduction activity in all segments (P < 0.05 for the duodenum and jejunum, P < 0.001 for the ileum) except for the colon (Fig. 1A, filled versus open bars). The differences between transductions of native and mucus-stripped explants were 100-fold in the duodenum and jejunum and 10-fold in the ileum. Previous in vitro studies have shown that intestinal mucus can also be dissolved by a variety of agents, including enzymes, detergents, and sulfhydryl compounds (1, 28). The effect of DTT, hyaluronidase, and glycosidases on the transduction of intestinal explants by rAd5 was investigated using explants treated and exposed to vector ex vivo. Transduction of explants treated with DTT was significantly higher than transduction of those that were untreated, specifically in the upper intestinal segments, duodenum and jejunum (Fig. 1B, filled versus open bars). When mouse jejunum was treated with hyaluronidase or exoglycosidases (see Materials and Methods), transduction was significantly higher than that of the jejunum treated with medium (Fig. 1C and D, 1 mg/ml). However, high concentrations of these enzymes did not enhance transduction, possibly due to nonspecific cell damage.
FIG. 1.
The distal regions of the intestine were efficiently transduced by rAd5, and removal of luminal substances increased transduction of the proximal intestine. (A) Efficient transduction of intestinal segments. Segments of intestine were left untreated (native), or the mucus was gently removed (stripped) and then transduced with rAd5-luc. (B) Treatment of intestinal segments with DTT increased the transduction efficiency of rAd5-Luc. (C and D) Effect of removal of the glycocalyx and hyaluronic acid on transduction. Mouse jejuna were treated with hyaluronidase (C) or mixed glycosidases (D) prior to transduction with rAd5-luc. *, P < 0.05; **, P < 0.01; ***, P < 0.001. RLU, relative luminescence units.
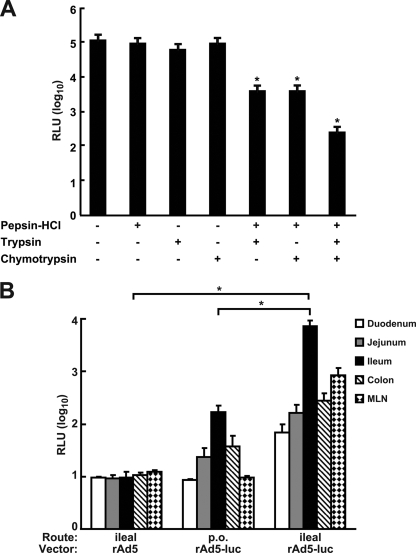
We next examined the effect of acid and selected proteases on rAd5 transduction of mouse intestinal explant cultures. Gastric acid and pepsin are the major components secreted into the stomach, and trypsin and chymotrypsin are the major proteases produced by the pancreas. To mimic the passage of rAd through the gastric environment and into the proximal section of the small intestine, rAd5-luciferase vector was treated with combinations of acid and protease and then applied to the tissue explants. Although we did not observe a significant decrease in luciferase activity after acid-pepsin, trypsin, or chymotrypsin treatment alone, the combination of acid-pepsin treatment with either trypsin or chymotrypsin treatment significantly reduced transgene expression (Fig. 2A, lanes 5 and 6). The combination of acid-pepsin with both trypsin and chymotrypsin further reduced luciferase activity, to approximately 100-fold lower than transduction with untreated rAd5-luc (Fig. 2A, lane 7). This result suggested that rAd5 vectors were susceptible to degradation by gastric proteases. Taken together, these ex vivo experiments indicated that multiple barriers reduce transduction of intestinal epithelia by rAd5 vectors and demonstrated differential transduction of specific intestinal segments.
FIG. 2.
Transduction of intestinal explant cultures by rAd5-luc was reduced by exposure to low pH and proteases and enhanced gene expression by intraileal delivery of rAd5 in vivo. (A) The rAd vector encoding luciferase was treated as indicated (+, treatment; −, no treatment) and applied to explants of mouse ileum. *, P < 0.05. (B) Mice were administered rAd5-luc via p.o. or intraileal injection, and the levels of luciferase activity in intestinal segments and MLN were determined 24 h later. *, P < 0.05. RLU, relative luminescence units.
Development of intraileal delivery to increase gene delivery to the small intestine.
Since the untreated ileum was transduced most effectively in ex vivo organ culture, we developed an in vivo route of administration that targeted the ileum and bypassed the significant barriers encountered by rAd in the GI tract. It has been reported that receptor binding and uptake of rAd5 occur relatively quickly, with the majority (80 to 85%) of the bound virions being taken up by permissive cells within 5 to 10 min (12). We found that a 10-min exposure of ileum explants to rAd5 resulted in levels of transgene expression similar to those obtained with longer time exposures (see Fig. S1 in the supplemental material). Therefore, for in vivo studies, the ileum loop was formed to hold the virus solution for 20 min to ensure efficient binding of viruses within the intestine and the separation of virus from undigested food in the ileum.
To determine the relative efficiency of rAd5-mediated transduction, mice were administered rAd5 (1010 VP) encoding luciferase either p.o. or via the intraileal-injection method. Twenty-four hours later, mice were sacrificed, and the intestinal segments were assayed for luciferase activity. A low level of luciferase activity was detected in the ileum after p.o. administration, but the luciferase activity was not significantly different from that for the empty rAd5 control group (Fig. 2B) (P > 0.05). Following intraileal injection, there was approximately a 10- to 100-fold-higher luciferase activity in the ileum and MLN than for the control group (Fig. 2B) (P < 0.05). Importantly, the luciferase activity in the ilea from injected mice was significantly higher than that for the p.o. dosed mice (Fig. 2B) (P < 0.05).
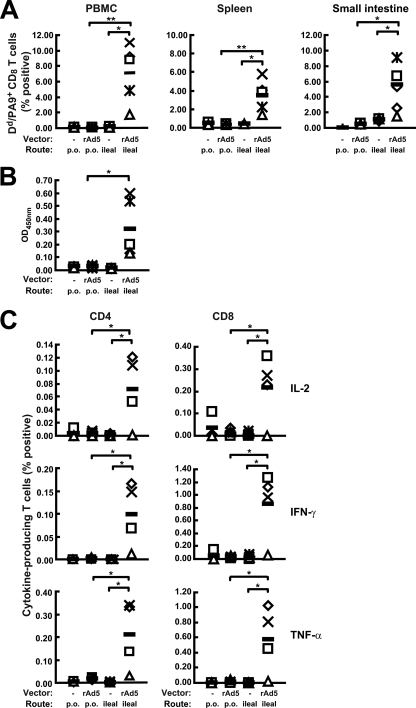
Immunization via intraileal injection generated humoral and cellular responses in both mucosal and systemic compartments.
The higher efficiency of intestinal cell transduction observed with ileal injection than with p.o. dosing suggested the possibility that intraileal administration of rAd vectors would induce a more potent immune response to the encoded transgene. Mice received a single administration of rAd5-gp140B (1010 VP) p.o. or via intraileal injection. Three weeks later, the mice immunized with rAd5-gp140B via intraileal injection showed significantly higher levels of H-2Dd/PA-9+ CD8+ T cells in both the small intestine and the systemic compartments than did the mice immunized p.o. (Fig. 3A). Oral administration did not result in detectable responses higher than background (Fig. 3A) (P > 0.05). The average levels of H-2Dd/PA-9+ CD8+ T cells in the mice given an intraileal injection were 7.11% (ranging from 2 to 11%) in PBMC, 3.47% (ranging from 1.44 to 5.82%) in the spleen, and 5.53% (ranging from 2.13 to 9.65%) in the small intestine. HIV Env-specific IgG responses were also detected in sera from mice immunized via the intraileal injection but not in sera from the p.o. dosed mice (Fig. 3B). ICS analysis of splenocytes was consistent with the tetramer staining results; numbers of HIV-1-specific CD4+ and CD8+ T cells producing interleukin-2 (IL-2), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) were significantly higher in the mice that received rAd5-gp140B via intraileal injection than in the mice that received equal amounts of rAd5-gp140B p.o. (Fig. 3C). In this analysis, the magnitudes of CD8+ T-cell response differed between tetramer staining and ICS, suggesting that there was a disproportionate representation of the dominant tetramer response or that not all of the tetramer-positive CD8+ T cells were secreting the measurable cytokines, a dichotomy described to occur previously when using these techniques (42). Tetramer staining measures CD8+ T cells responding to an immunodominant HIV-Env peptide regardless of function, including inactivated cells and activated cells, while ICS detects only activated cells or functional cells. Oral immunization generated only a background level of cytokine-producing CD4+ or CD8+ T cells. These data demonstrate that the rAd5 vector induced substantial antigen-specific cellular and humoral immune responses after a single intraileal immunization, while p.o. immunization of the same amount of rAd5 did not induce any detectable immune responses.
FIG. 3.
Mucosal and systemic immune responses induced by a single intraileal injection of rAd5-gp140B. Mice were immunized with rAd5gp-140B (rAd5) or rAd5 empty vector (−) via the p.o. or the intraileal route as indicated. (A) Percentages of HIV-specific H-2Dd/PA-9+ CD8+ T cells from PBMC, spleens, and small intestines. (B) IgG antibody in the serum. OD450nm, absorbance at 450 nm. (C) Percentages of HIV-specific IL-2-, IFN-γ-, and TNF-α-secreting CD4+ or CD8+ T cells from the spleens. The data from each individual mouse and the mean values from the five mice (black bars) are shown. *, P < 0.05; **, P < 0.01.
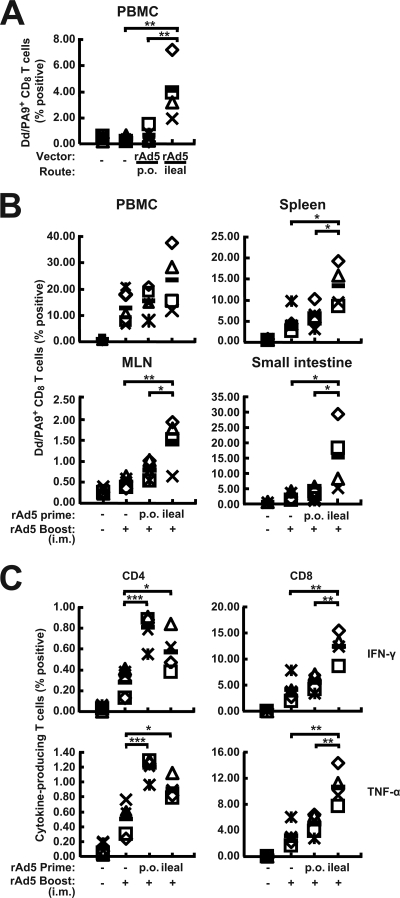
A mucosal prime-systemic boost regimen elicited potent cellular immunity in the small intestine and the systemic compartments.
The relative potencies of p.o. and intraileal routes of administration as primes for systemic boosting were next examined. Mice were primed on day 0 with empty rAd5 or rAd5-gp140B p.o. or via intraileal injection and were boosted i.m. on day 21 with rAd5-gp140B. Before the boosting step, PBMC were harvested for tetramer staining, and consistent with the results described above, immunization with rAd5-gp140B via intraileal injections induced significantly higher responses than p.o. administration (Fig. 4A). After the mice were boosted with rAd5-gp140B i.m., the mice primed via the intraileal route showed a dramatic increase in antigen-specific H-2Dd/PA-9+ CD8+ T-cell responses in both systemic and mucosal compartments compared to the responses of the group primed p.o. or i.m. (Fig. 4B). HIV-1 peptide-specific cytokine-producing CD8+ T cells and CD4+ T cells were also boosted effectively in the mice given an ileal prime and an i.m. boost. On average, 12.44% of CD8+ splenic T cells produced IFN-γ, and 10.65% of CD8+ splenic T cells were TNF-α positive (Fig. 4C). The mice primed p.o. or via ileal injection and boosted i.m. generated higher levels of HIV-1 peptide-specific IFN-γ- and TNF-α-producing CD4+ T cells than the mice immunized by i.m. injection only (Fig. 4C). There were no significant differences in CD4 frequencies between the prime-boost groups. The only differences were relative to the single-immunization group, indicating that prime-boost regimens induce higher frequencies of immune cells than a single immunization. HIV-1-specific IgG and IgA antibodies were also detected in sera and vaginal washes, but the differences between the mice primed p.o. and the mice primed by ileal injection were not significant (see Fig. S2 in the supplemental material). These results demonstrate that priming directly in the ileum and boosting i.m. induced the most potent CD8+ T-cell immunity in the small intestine as well as in the systemic compartments.
FIG. 4.
Immune responses induced by ileum prime and i.m. boost. Mice were administered rAd5gp-140B (rAd5) or empty vector (−) via the p.o. or intraileal route and boosted i.m. (+) 3 weeks later. (A) Percentages of HIV-specific H-2Dd/PA-9+ CD8+ T cells from the PBMC immediately prior to the i.m. injection of rAd5-gp140B. (B) Percentages of HIV-specific H-2Dd/PA-9+ CD8+ T cells from the PBMC, spleens, MLN, and small intestines at 2 weeks after the i.m. injection of rAd5-gp140B. (C) HIV-1-specific IFN-γ- and TNF-α-secreting CD4+ or CD8+ T lymphocytes from the spleens 2 weeks after i.m. injection of rAd5-gp140B. The data from each individual mouse and the mean values from the five mice (black bars) are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The fiber proteins of Ad41 did not increase the mucosal immunogenicity of a rare serotype vector, rAd35.
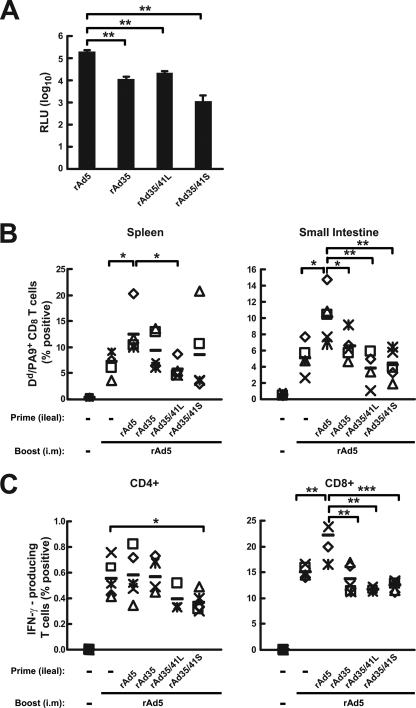
The swapping of Ad5 fiber proteins into Ad35 has previously been shown to increase immunogenicity (29). Since we have seen intestinal and systemic immune responses induced by rAd41 vectors of a magnitude similar to that of responses induced by rAd5 (17), we determined whether the swap of Ad41 proteins into rAd35 would affect rAd35-mediated transduction and immune responses. Explant cultures of murine intestine were transduced with rAd35, rAd35/41L, and rAd35/41S, encoding luciferase at a 1- to 2-log-lower efficiency than that for rAd5 (Fig. 5A). We also compared the relative potencies of the rAd35 and rAd5 vectors in priming for an i.m. rAd5 boost. While an intraileal prime-i.m. boost with rAd5-gp140B elicited significantly higher levels of CD8+ T-cell responses in the spleen and in the small intestine than did rAd5 i.m. alone, intraileal administration of rAd35, rAd35/41L, and rAd35/41S failed to increase the rAd5 i.m boost (Fig. 5B and C, right, lane 3 versus lane 2, 4, 5, or 6). However, CD4+ T-cell responses did not show a difference after rAd5 boost, possibly due to the lower magnitude of responders than CD8+ cells typically seen with rAd5 immunization. In addition, an ileal prime and i.m. boost with rAd5-gp140B induced a twofold-greater IFN-γ+ CD8+ T-cell response in the small intestine than did rAd5 i.m. alone. In contrast, an ileal prime with rAd35, rAd35/41L, and rAd35/41S did not show a boost response in the small intestine higher than that with rAd5 i.m. alone (see Fig. S3 in the supplemental material). These results demonstrate that the direct intraileal injection of rAd5, but not that of rAd35, rAd35/41L, and rAd35/41S, could prime for an i.m. rAd5 boost to generate potent immune responses in the systemic and mucosal compartments.
FIG. 5.
Transduction efficiencies and immune responses induced by rAd35 and rAd35/41 chimeras. (A) Transduction of ileum explants by rAd vectors. RLU, relative luminescence units. (B and C) Immune responses induced by rAd vectors in mice. Mice were administered the indicated rAd vectors or empty rAd5 vector (−) via the intraileal route and boosted i.m. 3 weeks later. (B) Percentages of HIV-specific H-2Dd/PA-9+ CD8+ T cells from spleens and small intestines 2 weeks after the i.m. rAd5-gp140B boost. (C) HIV-1-specific IFN-γ-secreting CD4+ or CD8+ T lymphocytes from spleens 2 weeks after i.m. rAd5-gp140B boost. The data from each individual mouse and the mean values from the five mice (black bars) are shown. -, empty vector. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
The intestinal mucosa represents a primary site of HIV replication during the peak of infection (3, 23, 26, 43). Vaccination through the GI mucosa could potentially induce mucosal immunity to prevent virus entry and/or limit the spread of infection. Oral immunization is the most convenient way to deliver mucosal vaccines. Although several studies have reported that the use of this route of immunization of Ad vectors encoding various virus antigens generated antibody responses in the serum and mucosal secretions (33, 37), the administration of Ads encoding Gag or Env from HIV-1 or SIV elicited weak or undetectable intestinal mucosa responses (24, 30). The major obstacles to successful induction of mucosal immunity and/or systemic immunity via GI delivery of vaccine have not been defined previously. In this study utilizing murine intestinal explant cultures and in vivo gene expression assessments, we demonstrate that the combination of a low-pH environment and the presence of proteases as well as the mucus/glycocalyx in the GI tract prevents rAd5 from effectively transducing the intestinal cells when delivered p.o. Therefore, p.o. immunization of vaccine vectors must overcome or bypass these barriers in the GI tract in order to be effective. We also show that the ileum is the most receptive site for rAd transduction and that intraileal injection of rAd5 results in a significantly higher transgene expression in the ileum than does p.o. delivery. In addition, intraileal immunization with rAd5 did not induce significant systemic antivector neutralizing activity, since the rAd5 i.m. administration was effective in the homologous rAd5 prime-rAd5 boost regimen. This is in agreement with our previous work demonstrating the absence of circulating neutralizing antibodies following p.o. administration of rAd5 and rAd41 (17). It has also been shown that preexisting immunity to the vaccine carrier did not impair p.o. vaccination with Ad vectors in mice (45). Thus, the ileum represents a valid target for delivery of rAd vectors.
Utilization of a route of administration that bypassed the barriers to ileal transduction revealed the potency of rAd5 as a mucosal immunogen. A single immunization of mice with rAd5 encoding HIV-1 gp140B via intraileal injection generated significantly higher numbers of H-2Dd/PA-9+ CD8+ T cells in both mucosal and systemic compartments and antigen-specific IFN-γ-, TNF-α-, and IL-2-producing CD4+ and CD8+ T cells in the spleen than did p.o. administration (Fig. 3A and C). In fact, p.o. immunization did not generate any detectable immune response after a single dose in our study. These results indicate that the higher transduction from intraileal injections than from p.o. delivery (Fig. 2B) is associated with the stronger immune responses to HIV-1 gp140B. Systemic boosting with rAd5-gp140B dramatically increased CD8+ T-cell responses in the mice primed via the intraileal route in both systemic and mucosal compartments compared to responses in the group primed p.o. (Fig. 4B and C, right) as well as splenic CD4+ T-cell responses compared to those in the mice primed p.o. or via ileal injection (Fig. 4C, left). Whether the activation of CD4+ T cells would provide more target cells for HIV acquisition will be addressed in nonhuman primate (NHP) studies, though a previous study in which such mucosal responses were detected showed that these responses led to protection (21, 25). Oral dosing with replication-competent Ad vectors generates systemic immune responses in nonhuman primates, but multiple primes and protein boosting are required for protective efficacy against SIVmac251 challenge (32, 47). Oral vaccination with replication-competent Ad4 and Ad7 has been shown to generate protective Ad neutralizing antibodies in humans (11), indicating that replication-competent vectors elicit antivector neutralizing antibodies, which may compete with transgene-specific responses or may prevent multiple p.o. administrations of the same vector and affect efficacy. In addition, the magnitude and contribution of the transgene-specific response in mediating protection by replication-competent rAd remain unknown in this model because the gut mucosal immune responses were not measured and compared to systemic responses. Thus, there are potential efficacy and regulatory concerns with replication-competent rAds, and further studies are needed to assess the potential efficacy of gut mucosal immunization against lentivirus infection in NHP. Direct mucosal introduction of replication-defective vectors offers an alternative approach to address questions of immunogenicity and efficacy in this model. Although cellular responses were robust after intraileal injection, we did not detect a significant level of IgG or IgA antibody from fecal extracts (data not shown). Some animals showed IgA responses from the vaginal washes, but the responses were low and inconsistent. Though collection methods for mucosal secretions can be optimized (19), it is likely that these responses are low in magnitude in the GI tract.
While the results reported here describe the immunogenicity of rAd5- and rAd35-based vectors, we have previously analyzed rAd41, an enteric tropic Ad, for its ability to generate immune responses through direct ileum lumenal administration (17). When rAd41 was administered directly to the lumen of the ileum, it efficiently primed antigen-specific cellular immunity. Ad41, a member of the Ad species F, has been reported to bind/uptake more efficiently than Ad5 in the isolated intestinal loops of rats. Furthermore, gene delivery with Ad5 to differentiated enterocytes in vitro and to the rat jejunum in vivo is extremely inefficient (7, 8, 44). However, the immune responses induced by Ad41 and Ad5 through intraileal injection priming and i.m. boosting were not significantly different, which highlighted that vectors derived from mucosal pathogens, both enteric and respiratory, can be used in these regimens. However, due to the prevalence of neutralizing antibodies to Ad5 and Ad41 in humans, the development and use of alternative serotypes of Ad vectors for virus vaccines remain a priority. Ad35 is one relatively well-characterized rare serotype vector and has been associated with infections of the kidney and urinary tract. Therefore, the fiber swap study was done for two reasons: (i) to define the contribution of fiber versus hexon to transduction of mucosal epithelium and (ii) to generate vectors resistant to anti-Ad41 hexon neutralizing antibodies, should they be mediated by fiber. Here, we found that rAd35 did not transduce the organ cultures of intestinal segments as efficiently as rAd5 and that rAd35 was less immunogenic in the ileal priming regimen, thus allowing us to address these questions. Unexpectedly, grafting the long fiber or the short fiber of Ad41 to the rAd35 vector did not improve the transduction efficiency of intestinal explants or immunogenicity, suggesting that the Ad41 fiber is not the only determinant of enteric tropism. However, these results suggest that the immunogenicity of rAd vectors in vivo correlates with the efficiency of transduction of intestinal explants ex vivo. We conclude that the lower small intestine represents a target for antigen delivery with certain rAd vectors. Further development of special formulations to deliver rAd to the ileum should be emphasized for practical vaccination.
Our results suggest that it is important to pursue a mucosal route of administration of rAd vaccine vectors for HIV. The strong cellular immune responses induced by intraileal injection, further strengthened by i.m. boost, suggest a strategy for protection of gut-associated lymphoid tissue lymphocytes from HIV-1 infection. Possible mechanisms of immune stimulation include persistent antigen expression from lamina propria cells transduced by the rAd vector (7, 40). Further characterization of the immune responses induced by the vaccination regimens employed in this work, such as the balance of CD8+ and CD4+ T-cell responses, the polyfunctionality of the T cells, and the duration of the immune response, could provide a better prediction as to which is the most important type of immune response for protection against HIV-1 (22). Regarding the applicability of these data to other species, as well as the uncertain applicability to humans, this study represents one of a stepwise series of studies to determine whether mucosal immunity can be enhanced by different rAd vectors with alternative routes of administration. Having been demonstrated with rodents, this will next be evaluated with nonhuman primate challenge models to determine its contribution to immune protection. Should these results support the concept of a mucosal vaccine, an effort will be made to formulate an Ad vector for delivery to the ileum in humans, since the site-specific delivery technology to deliver drugs and vaccines to the ileum has been developed with partial success so far (14, 16, 27, 36).
The use of rAd5 vaccines in populations with moderate to high neutralizing antibodies to Ad5 based on the STEP trial (3a) results is a concern. It will be difficult to define mechanisms responsible for this effect or even to document it definitively. At the same time, rAd5 vectors are well characterized and induce strong immune responses. There are numerous differences in vector design, insert combination, and immunization regimen that may result in different performances in future clinical studies. Regardless of its future clinical utility, rAd5 remains a useful model vector for inducing mucosal immunity and for determining how mucosal immune responses contribute to protection. Once these key questions are addressed, rAd5 vectors can be used if justified by efficacy/safety considerations, or, if concerns remain, other vectors can be substituted.
Supplementary Material
Acknowledgments
We thank Ati Tislerics for help with manuscript preparation, Brenda Hartman and Morteza Loghmani for assistance with figures, Srinivas Rao and Saran Bao for help with surgical techniques, D. Margulies and the NIAID Tetramer Core Facility for production of tetramers, and members of the Nabel laboratory for helpful discussions.
This work was supported by the Intramural Research Program of the National Institutes of Health, Vaccine Research Center, NIAID, and by the Bill and Melinda Gates Foundation.
Footnotes
Published ahead of print on 6 May 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Aoki, Y., M. Morishita, and K. Takayama. 2005. Role of the mucous/glycocalyx layers in insulin permeation across the rat ileal membrane. Int. J. Pharm. 29798-109. [DOI] [PubMed] [Google Scholar]
- 2.Benihoud, K., P. Yeh, and M. Perricaudet. 1999. Adenovirus vectors for gene delivery. Curr. Opin. Biotechnol. 10440-447. [DOI] [PubMed] [Google Scholar]
- 3.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, M. N. Robertson, and the Step Study Protocol Team. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 3721881-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casimiro, D. R., A. J. Bett, T. M. Fu, M. E. Davies, A. Tang, K. A. Wilson, M. Chen, R. Long, T. McKelvey, M. Chastain, S. Gurunathan, J. Tartaglia, E. A. Emini, and J. Shiver. 2004. Heterologous human immunodeficiency virus type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J. Virol. 7811434-11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 776305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, C., J. G. Gall, W. P. Kong, R. L. Sheets, P. L. Gomez, C. R. King, and G. J. Nabel. 2007. Mechanism of ad5 vaccine immunity and toxicity: fiber shaft targeting of dendritic cells. PLoS Pathog. 3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croyle, M. A., M. Stone, G. L. Amidon, and B. J. Roessler. 1998. In vitro and in vivo assessment of adenovirus 41 as a vector for gene delivery to the intestine. Gene Ther. 5645-654. [DOI] [PubMed] [Google Scholar]
- 8.Croyle, M. A., E. Walter, S. Janich, B. J. Roessler, and G. L. Amidon. 1998. Role of integrin expression in adenovirus-mediated gene delivery to the intestinal epithelium. Hum. Gene Ther. 9561-573. [DOI] [PubMed] [Google Scholar]
- 9.Ertl, H. C. 2005. Immunological insights from genetic vaccines. Virus Res. 11189-92. [DOI] [PubMed] [Google Scholar]
- 10.Gao, W., A. C. Soloff, X. Lu, A. Montecalvo, D. C. Nguyen, Y. Matsuoka, P. D. Robbins, D. E. Swayne, R. O. Donis, J. M. Katz, S. M. Barratt-Boyes, and A. Gambotto. 2006. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 801959-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaydos, C. A., and J. C. Gaydos. 1995. Adenovirus vaccines in the U.S. military. Mil. Med. 160300-304. [PubMed] [Google Scholar]
- 12.Greber, U. F. 2002. Signalling in viral entry. Cell. Mol. Life Sci. 59608-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haase, A. T. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5783-792. [DOI] [PubMed] [Google Scholar]
- 14.Hamman, J. H., G. M. Enslin, and A. F. Kotze. 2005. Oral delivery of peptide drugs: barriers and developments. BioDrugs 19165-177. [DOI] [PubMed] [Google Scholar]
- 15.Jaiswal, S., N. Khanna, and S. Swaminathan. 2003. Replication-defective adenoviral vaccine vector for the induction of immune responses to dengue virus type 2. J. Virol. 7712907-12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein, S., J. Stein, and J. Dressman. 2005. Site-specific delivery of anti-inflammatory drugs in the gastrointestinal tract: an in-vitro release model. J. Pharm. Pharmacol. 57709-719. [DOI] [PubMed] [Google Scholar]
- 17.Ko, S. Y., C. Cheng, W. P. Kong, L. Wang, M. Kanekiyo, D. Einfeld, C. R. King, J. G. Gall, and G. J. Nabel. 2009. Enhanced induction of intestinal cellular immunity by oral priming with enteric adenovirus 41 vectors. J. Virol. 83748-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobinger, G. P., H. Feldmann, Y. Zhi, G. Schumer, G. Gao, F. Feldmann, S. Jones, and J. M. Wilson. 2006. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology 346394-401. [DOI] [PubMed] [Google Scholar]
- 19.Kozlowski, P. A., R. M. Lynch, R. R. Patterson, S. Cu-Uvin, T. P. Flanigan, and M. R. Neutra. 2000. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J. Acquir. Immune Defic. Syndr. 24297-309. [DOI] [PubMed] [Google Scholar]
- 20.Lemiale, F., H. Haddada, G. J. Nabel, D. E. Brough, C. R. King, and J. G. Gall. 2007. Novel adenovirus vaccine vectors based on the enteric-tropic serotype 41. Vaccine 252074-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 3121530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Letvin, N. L., S. S. Rao, V. Dang, A. P. Buzby, B. Korioth-Schmitz, D. Dombagoda, J. G. Parvani, R. H. Clarke, L. Bar, K. R. Carlson, P. A. Kozlowski, V. M. Hirsch, J. R. Mascola, and G. J. Nabel. 2007. No evidence for consistent virus-specific immunity in simian immunodeficiency virus-exposed, uninfected rhesus monkeys. J. Virol. 8112368-12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 4341148-1152. [DOI] [PubMed] [Google Scholar]
- 24.Lin, S. W., A. S. Cun, K. Harris-McCoy, and H. C. Ertl. 2007. Intramuscular rather than oral administration of replication-defective adenoviral vaccine vector induces specific CD8+ T cell responses in the gut. Vaccine 252187-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattapallil, J. J., D. C. Douek, A. Buckler-White, D. Montefiori, N. L. Letvin, G. J. Nabel, and M. Roederer. 2006. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J. Exp. Med. 2031533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 4341093-1097. [DOI] [PubMed] [Google Scholar]
- 27.Mercier, G. T., P. N. Nehete, M. F. Passeri, B. N. Nehete, E. A. Weaver, N. S. Templeton, K. Schluns, S. S. Buchl, K. J. Sastry, and M. A. Barry. 2007. Oral immunization of rhesus macaques with adenoviral HIV vaccines using enteric-coated capsules. Vaccine 258687-8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monteiro-Riviere, N. A., and J. A. Popp. 1986. Ultrastructural evaluation of acute nasal toxicity in the rat respiratory epithelium in response to formaldehyde gas. Fundam. Appl. Toxicol. 6251-262. [PubMed] [Google Scholar]
- 29.Nanda, A., D. M. Lynch, J. Goudsmit, A. A. Lemckert, B. A. Ewald, S. M. Sumida, D. M. Truitt, P. Abbink, M. G. Kishko, D. A. Gorgone, M. A. Lifton, L. Shen, A. Carville, K. G. Mansfield, M. J. Havenga, and D. H. Barouch. 2005. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J. Virol. 7914161-14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oomura, K., K. Q. Xin, M. Takakura, K. Shinoda, N. Jounai, and K. Okuda. 2006. Oral administration of the adenovirus vector induces systemic immunity rather than intestinal mucosal immunity. Vaccine 241045-1046. [DOI] [PubMed] [Google Scholar]
- 31.Patel, A., Y. Zhang, M. Croyle, K. Tran, M. Gray, J. Strong, H. Feldmann, J. M. Wilson, and G. P. Kobinger. 2007. Mucosal delivery of adenovirus-based vaccine protects against Ebola virus infection in mice. J. Infect. Dis. 196(Suppl. 2)S413-S420. [DOI] [PubMed] [Google Scholar]
- 32.Patterson, L. J., N. Malkevitch, D. Venzon, J. Pinczewski, V. R. Gomez-Roman, L. Wang, V. S. Kalyanaraman, P. D. Markham, F. A. Robey, and M. Robert-Guroff. 2004. Protection against mucosal simian immunodeficiency virus SIVmac251 challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J. Virol. 782212-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinto, A. R., J. C. Fitzgerald, G. P. Gao, J. M. Wilson, and H. C. Ertl. 2004. Induction of CD8+ T cells to an HIV-1 antigen upon oral immunization of mice with a simian E1-deleted adenoviral vector. Vaccine 22697-703. [DOI] [PubMed] [Google Scholar]
- 34.Pope, M., and A. T. Haase. 2003. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat. Med. 9847-852. [DOI] [PubMed] [Google Scholar]
- 35.Robbins, P. D., and S. C. Ghivizzani. 1998. Viral vectors for gene therapy. Pharmacol. Ther. 8035-47. [PubMed] [Google Scholar]
- 36.Schellekens, R. C., F. Stellaard, D. Mitrovic, F. E. Stuurman, J. G. Kosterink, and H. W. Frijlink. 2008. Pulsatile drug delivery to ileo-colonic segments by structured incorporation of disintegrants in pH-responsive polymer coatings. J. Control. Release 13291-98. [DOI] [PubMed] [Google Scholar]
- 37.Sharpe, S., A. Fooks, J. Lee, K. Hayes, C. Clegg, and M. Cranage. 2002. Single oral immunization with replication deficient recombinant adenovirus elicits long-lived transgene-specific cellular and humoral immune responses. Virology 293210-216. [DOI] [PubMed] [Google Scholar]
- 38.Shiver, J. W., and E. A. Emini. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55355-372. [DOI] [PubMed] [Google Scholar]
- 39.Stahl-Hennig, C., S. Kuate, M. Franz, Y. S. Suh, H. Stoiber, U. Sauermann, K. Tenner-Racz, S. Norley, K. S. Park, Y. C. Sung, R. Steinman, P. Racz, and K. Uberla. 2007. Atraumatic oral spray immunization with replication-deficient viral vector vaccines. J. Virol. 8113180-13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatsis, N., J. C. Fitzgerald, A. Reyes-Sandoval, K. C. Harris-McCoy, S. E. Hensley, D. Zhou, S. W. Lin, A. Bian, Z. Q. Xiang, A. Iparraguirre, C. Lopez-Camacho, E. J. Wherry, and H. C. Ertl. 2007. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood 1101916-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorner, A. R., and D. H. Barouch. 2007. HIV-1 vaccine development: progress and prospects. Curr. Infect. Dis. Rep. 971-75. [DOI] [PubMed] [Google Scholar]
- 42.Tobery, T. W., S. A. Dubey, K. Anderson, D. C. Freed, K. S. Cox, J. Lin, M. T. Prokop, K. J. Sykes, R. Mogg, D. V. Mehrotra, T. M. Fu, D. R. Casimiro, and J. W. Shiver. 2006. A comparison of standard immunogenicity assays for monitoring HIV type 1 gag-specific T cell responses in Ad5 HIV type 1 gag vaccinated human subjects. AIDS Res. Hum. Retrovir. 221081-1090. [DOI] [PubMed] [Google Scholar]
- 43.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280427-431. [DOI] [PubMed] [Google Scholar]
- 44.Walter, E., M. A. Croyle, B. J. Roessler, and G. L. Amidon. 1997. The absence of accessible vitronectin receptors in differentiated tissue hinders adenoviral-mediated gene transfer to the intestinal epithelium in vitro. Pharm. Res. 141216-1222. [DOI] [PubMed] [Google Scholar]
- 45.Xiang, Z. Q., G. P. Gao, A. Reyes-Sandoval, Y. Li, J. M. Wilson, and H. C. Ertl. 2003. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J. Virol. 7710780-10789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiang, Z. Q., Y. Yang, J. M. Wilson, and H. C. Ertl. 1996. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 219220-227. [DOI] [PubMed] [Google Scholar]
- 47.Zhou, Q., R. Hidajat, B. Peng, D. Venzon, M. K. Aldrich, E. Richardson, E. M. Lee, V. S. Kalyanaraman, G. Grimes, V. R. Gomez-Roman, L. E. Summers, N. Malkevich, and M. Robert-Guroff. 2007. Comparative evaluation of oral and intranasal priming with replication-competent adenovirus 5 host range mutant (Ad5hr)-simian immunodeficiency virus (SIV) recombinant vaccines on immunogenicity and protective efficacy against SIV(mac251). Vaccine 258021-8035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.