Abstract
The degradation of nuclear pore components and disruption of nucleocytoplasmic trafficking during rhinovirus infection have been attributed to viral 2A protease. Here we show for the first time that rhinovirus 3C protease may also have a role. Specifically, we show that 3C and its precursor, 3CD, can target green fluorescent protein to the nucleus of living cells, leading to degradation of nuclear pore components, and that incubation with recombinant 3C disrupts active and passive nucleocytoplasmic transport in a semi-intact cell nuclear transport system dependent on 3C protease activity. 3C may thus contribute to host cell shutoff in infected cells by localizing in the nucleus and facilitating nuclear pore breakdown.
Human rhinoviruses (HRVs) are positive-strand RNA viruses belonging to the Picornaviridae family, which includes poliovirus. Although picornavirus replication is completed within the cytoplasm, many nuclear factors have been implicated in the life cycle of both HRVs and polioviruses. Gustin and Sarnow (11) previously showed mislocalization of cellular proteins late in infection in HRV-infected cells that was attributed to inhibited nuclear import due to degradation of several nucleoporins (Nup153 and Nup62) that are critical components of the nuclear pore, the only avenue for transport into and out of the nucleus. Degradation of Nup153 and Nup62 has also been observed in poliovirus infection (10). Recent work (17) indicates that another nucleoporin, Nup98, is also degraded in poliovirus-infected cells and that this precedes cleavage of Nup153 and Nup62. Importantly, results from in vitro cleavage experiments using HRV 2A protease were used to implicate picornavirus 2A protease as the mediator of cleavage of Nup98 (17), but 2A protease cleavage of Nup98 alone was not found to be sufficient to induce alterations in nuclear pore permeability. Importantly, a role in this context was not considered for the other major protease of HRV, 3C. Both 3C protease and its precursor form, 3CD, have been observed in the nucleus of cells infected with HRV or transfected to express 3CD (1), meaning that 3C is an ideal candidate to mediate effects on host cell nuclear transport.
Here we present evidence for the first time that HRV 3C protease has intrinsic nuclear targeting potential, and, dependent on its protease activity, is able to disrupt both active and passive nucleocytoplasmic transport. The results suggest that 3C protease, like 2A (17), is likely to contribute to the disruption of host cell nuclear transport, a key factor in host cell shutdown.
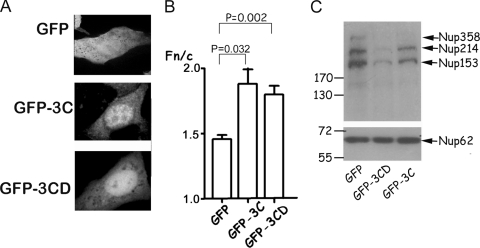
To test 3C's nuclear targeting ability, the coding sequences for HRV16 3CD and 3C were both cloned into the pEPI-DESTC Gateway vector (9) and expressed as C-terminal green fluorescent protein (GFP) fusion derivatives in transfected Vero cells with GFP alone (pEPI-GFP) as a control. Localization of GFP fluorescence was monitored by live-cell confocal laser scanning microscopy (CLSM) at 18 h posttransfection, and the ImageJ1.62 shareware was used to analyze the digital images to determine the relative intensity of fluorescence in the nucleus (Fn) compared to that in the cytoplasm (Fc) (Fn/c ratio) after the subtraction of fluorescence due to background/autofluorescence (Fig. 1A and B). GFP alone was present throughout the transfected cell (Fig. 1A, upper image), while GFP-3C (center image) and GFP-3CD (lower image) showed increased accumulation in the nucleus. The Fn/c ratio increased significantly from about 1.5 for GFP to 1.9 and 1.8 for GFP-3C and GFP-3CD, respectively (Fig. 1B). Clearly, both 3C and 3CD are able to target a heterologous protein (GFP) to the nucleus and hence have intrinsic nuclear localizing ability. The fact that 3C/3CD is nuclear in infected cells, and as shown by Amineva et al. (1), untagged 3C is nuclear in transfected cells, implies that the results are unlikely to stem from sequence anomalies in the GFP fusion constructs.
FIG. 1.
HRV 3C and 3CD can target GFP to the nucleus and degrade nucleoporins. (A) Vero cells were transfected with equal amounts of pEPI-GFP (top image), pEPI-GFP-3C (central image), or pEPI-GFP-3CD (bottom image), and localization of GFP was monitored by CLSM 18 h later. (B) Images such as those shown in panel A were analyzed using the ImageJ1.62 software to determine the Fn/c ratio: Fn/c = (Fn − Fb)/(Fc − Fb). Fn is the nuclear fluorescence, Fc is the cytoplasmic fluorescence, and Fb is the background fluorescence (autofluorescence). Results are means ± standard errors of the mean (n ≥ 50). (C) COS-7 cells were transfected with pEPI-GFP, pEPI-GFP-3C, or pEPI-GFP-3CD; lysed with Laemmli buffer; and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (25 μg total protein per lane) on a 10% gel followed by Western transfer to a polyvinylidene difluoride membrane. After blocking in 5% milk, the membrane was probed with Mab414 (Abcam), followed by species-specific secondary antibodies conjugated to horseradish peroxidase and detection by enhanced chemiluminescence (Perkin Elmer). Molecular mass markers are indicated on the left in kDa, with specific nucleoporins indicated on the right.
Cell lysates were prepared (15) from COS-7 cells transfected to express GFP, GFP-3C, or GFP-3CD and subjected to Western analysis using monoclonal antibody Mab414, which specifically recognizes common motifs within nucleoporins such as Nup358, Nup214, Nup153, and Nup62 and has been used successfully to document degradation of nucleoporins in HRV-infected cells (11). Expression of GFP-3C or GFP-3CD led to degradation of three Mab414-recognized proteins corresponding to Nup153, Nup214, and Nup358 (Fig. 1C) (see also reference 11). There was no evidence for degradation of the band corresponding to Nup62. Clearly, 3C or 3CD alone is sufficient to effect degradation of specific nuclear pore components, independent of other HRV-encoded gene products.
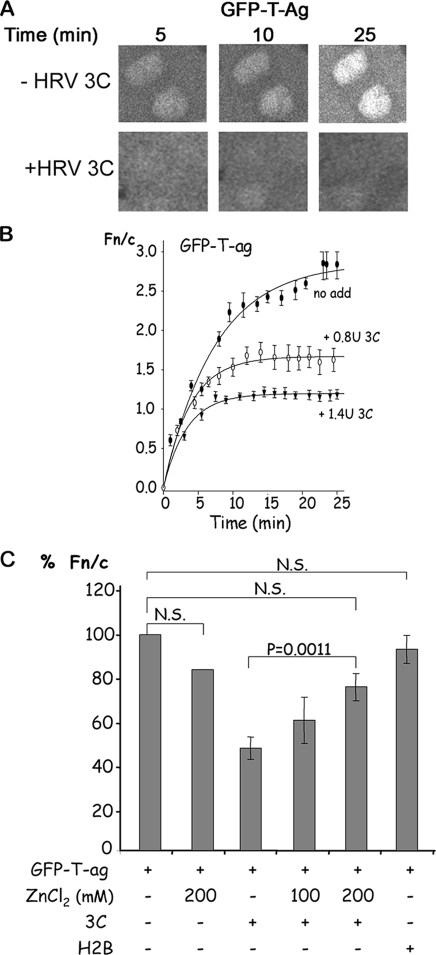
The effect of 3C on active nuclear transport and permeability of the nuclear pore to large molecules was studied at the single-cell level using mechanically perforated HTC rat hepatoma cells in conjunction with CLSM as described previously (8). This system, also applicable to other adherent cell types, is completely comparable to digitonin-permeabilized cells in terms of dependence for transport on importins, Ran, etc., and has been used successfully to study the nuclear transport of various cargos (4, 5, 7, 9, 12, 13, 18). Nuclear localization signal (NLS)-dependent nuclear protein import can be reconstituted in this system through the exogenous addition of cytosolic extract, an ATP-regenerating system (0.125 mg/ml creatine kinase, 30 mM creatine phosphate, 2 mM ATP), and GTP (2 mM). Active nuclear import of GFP-T antigen(111-135) [T-ag(111-135)], which accumulates in the nucleus through recognition of its NLS by the importin-α/β heterodimer (2), was followed by CLSM in the presence or absence of HRV 3C (Novagen) (Fig. 2). CLSM files were analyzed as described above, with the results plotted and curve fitting performed using the SigmaPlot software to determine the kinetics of nuclear transport as described previously (9). Typical CLSM images are shown at various times in Fig. 2A, with quantitative analysis shown in Fig. 2B. In contrast to the absence of 3C, where GFP-T-ag accumulated actively in the nucleus (Fn/c ratio of 3), treatment with 3C led to a marked decrease in nuclear accumulation in a dose-dependent fashion, with the Fn/c value tending to 1 by 25 min in the presence of 1.4 U 3C, indicative of free diffusion of GFP-T-ag through the nuclear pore.
FIG. 2.
HRV 3C inhibits nuclear import of GFP-T-ag in vitro. Active nuclear import across the nuclear membrane in the absence or presence of recombinant HRV 3C was studied in mechanically perforated HTC rat hepatoma cells as described in the text in the presence of cytosolic extract, an ATP-regenerating system, GTP (2 mM), and GFP-T-ag(111-135). (A) Nuclear import/entry of GFP-T-ag alone (−HRV 3C; upper panels) or in the presence of 1.4 U HRV 3C (+HRV 3C; lower panels) was observed for 25 min; images of one field at 5 min, 10 min, and 25 min are presented. (B) Images such as those in panel A were analyzed by ImageJ, the Fn/c ratio was calculated, and the curve was generated with SigmaPlot. Data for GFP-T-ag alone (no add) or in the presence of 0.8 U or 1.4 U of HRV C (+ 3C) are presented. Each data point represents the mean ± standard error of the mean from ≥15 cells. (C) The nuclear import of GFP-T-ag alone or in the presence of 3C or histone H2B in the presence or absence of zinc chloride (ZnCl2) was monitored as in panel B; data are means ± standard errors of the mean from three separate experiments, each of which represents n ≥ 15, where the Fn/c ratio at 25 min is presented as % Fn/c, with 100% being the Fn/c ratio at 25 min for GFP-T-ag in the absence of 3C. N.S., not significant.
The commercial HRV 3C used is a recombinant polyhistidine-tagged protein purified from bacteria; to eliminate the possibility that the observed inhibitory effect on nuclear import in Fig. 2A and B was simply due to the basic polyhistidine tag, 3C was substituted for in the assay by hexahistidine-tagged H2B (19), a histidine-tagged, bacterially expressed protein of comparable size to 3C and able to localize in the nucleus. As shown in Fig. 2C, H2B had no significant effect on nuclear import of GFP-T-ag, the clear implication being that the effect on transport by recombinant 3C was specific. To examine this further, we tested the effect of 3C in the presence or absence of zinc chloride, which inhibits 3C protease activity (6). Preincubation of 3C with various concentrations of zinc chloride before addition to perforated cells resulted in a reduction of the inhibitory effect of 3C to a significant (P = 0.0011) extent in the case of 200 mM zinc chloride. Zinc chloride alone did not have a significant effect on nuclear accumulation of GFP-T-ag. Thus, 3C appears to inhibit nuclear import of GFP-T-ag specifically, dependent on its protease activity.
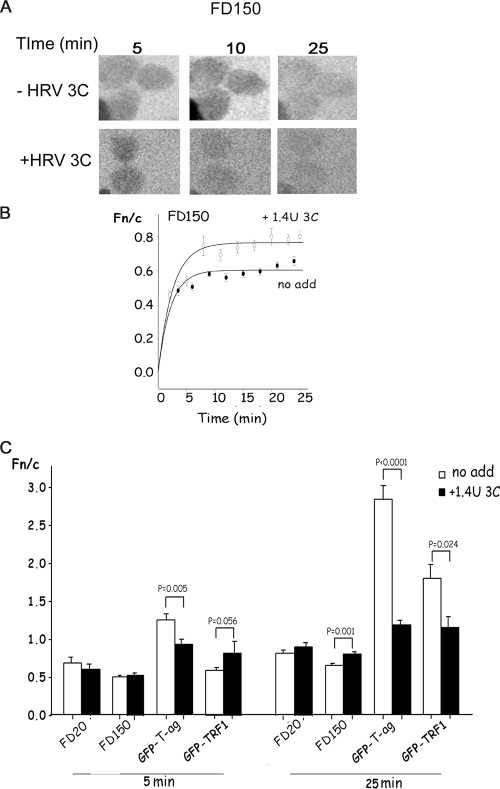
To assess nuclear pore integrity changes effected by 3C activity, 150-kDa fluorescein isothiocyanate-dextran (FD150), which is too large to diffuse through the nuclear pore and is normally excluded from the nucleus, was used (16). CLSM images taken at various times with and without added 1.4 U HRV 3C (Fig. 3A) and quantitative analysis (Fig. 3B) indicate that 3C treatment leads to a lack of nuclear exclusion of FD150. Clearly, 3C activity perturbs the passive nuclear transport properties of the nuclear pore.
FIG. 3.
HRV 3C treatment results in a general disruption of nuclear transport in vitro. Nuclear-cytoplasmic distribution in the absence or presence of recombinant HRV 3C was studied as described in the legend to Fig. 2. (A) Images such as those in Fig. 2A are presented for FD150 imaged by CLSM at the indicated times. (B) Images such as those in panel A were analyzed by ImageJ, the Fn/c ratio was calculated, and the curve was generated with SigmaPlot; data for FD150 alone (no add) or in the presence of 1.4 U of HRV C (+ 3C) are presented. Each data point represents the mean ± standard error of the mean from ≥15 cells. (C) Extent of nuclear accumulation in the absence or presence of 1.4 U 3C at 5 and 25 min for GFP-T-ag, GFP-TRF1, FD20, and FD150. Results are the means ± standard errors of the mean for three separate experiments, for each of which n represents ≥15 measurements.
To test whether 3C can inhibit nuclear import pathways other than that of T-ag, which uses importin α/β (14), we examined the effect of 3C on nuclear import of GFP-TRF1, which is imported actively into the nucleus via binding to importin-β and is retained in the nucleus by binding to nuclear factors (8). Similar to its effect on T-ag nuclear import, 3C inhibited the nuclear import of GFP-TRF1 (Fig. 3C).
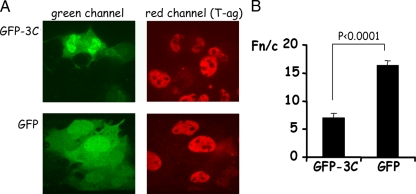
To determine if 3C could induce a similar disruption of nuclear transport pathways in intact cells, we expressed GFP-3C in COS-7 cells and examined the localization of endogenously expressed simian virus 40 T-ag in these cells (Fig. 4). Nuclear accumulation of T-ag in cells expressing GFP-3C was significantly lower than in cells expressing GFP alone. Additionally, expression of 3C appeared to lead to a loss of fluorescent signal for T-ag; whether this is a direct result of proteolytic activity (see reference 1) is unclear.
FIG. 4.
Overexpression of GFP-3C can reduce nuclear accumulation of T-ag in COS-7 cells. COS-7 cells grown on coverslips were transfected to express either GFP alone or GFP-3C and fixed 18 h later with 4% formaldehyde followed by permeabilization of membranes with 0.2% Triton X-100. Cells were probed with rabbit polyclonal antibody to simian virus 40 T-ag (Santa Cruz Biotechnology) followed by detection with Alexa Fluor 568-conjugated antibodies to rabbit immunoglobulin. Coverslips were mounted on glass slides, and CLSM and image analysis were performed as described in the legend to Fig. 1. (A) Single images of cells expressing GFP alone or GFP-3C as indicated. The green channel (GFP and GFP-3C) is on the left, and the red channel (T-ag) is on the right. (B) Images such as those shown in panel A were analyzed as described in the legend to Fig. 1. Results are means ± standard errors of the mean, where n represents ≥15.
Together, our data suggest that 3C treatment results in changes to the nuclear pore in vitro such that large molecules (FD150) are able to freely diffuse across the nuclear pore, while NLS-containing cargoes (GFP-TRF1 and GFP-T-ag) are no longer able to accumulate in the nucleus; 3C had no effect on diffusion of 20-kDa dextran-Texas Red (FD20), which is small enough to diffuse freely across the nuclear pore (Fig. 3C). The functional changes in nuclear pore permeability induced by 3C are presumably due to the degradation of specific nucleoporins such as Nup153, Nup214, and Nup358, as observed in transfected cells (Fig. 1C).
Our study shows, for the first time, that passive as well as active transport across the nuclear pore can be disrupted by 3C, presumably as a direct result of degradation of specific nucleoporins. Previous studies have shown a general inhibition of active nuclear import of NLS-containing cargoes in poliovirus and HRV-infected cells due to degradation of key components of the nuclear pore, with 2A protease being implicated (10, 11, 17). Electron microscopic studies also show that the structure of the nuclear pore may be changed physically in poliovirus-infected cells (3). Our finding that large, normally excluded, molecules are able to diffuse into the nucleus in the presence of HRV 3C is consistent with a breakdown of the nuclear pore in HRV-infected or 3C-expressing transfected cells.
In conclusion, HRV 3C has intrinsic nuclear targeting ability as well as the ability, dependent on its protease activity, to alter the permeability of the nuclear pore. In addition to transcription factor cleavage (1), these properties, together with those of 2A (17), are likely to contribute integrally to host cell shutoff in HRV-infected cells.
Acknowledgments
We acknowledge the support of the National Health and Medical Research Council, Australia (project grant 545844 to R.G. and fellowship 284400 to H.D.).
We thank Belinda Thomas for cell culture of mammalian cells and Alex Fulcher for assistance with in vitro nuclear transport assays.
Footnotes
Published ahead of print on 29 April 2009.
REFERENCES
- 1.Amineva, S. P., A. G. Aminev, A. C. Palmenberg, and J. E. Gern. 2004. Rhinovirus 3C protease precursors 3CD and 3CD′ localize to the nuclei of infected cells. J. Gen. Virol. 852969-2979. [DOI] [PubMed] [Google Scholar]
- 2.Baliga, B. C., P. A. Colussi, S. H. Read, M. M. Dias, D. A. Jans, and S. Kumar. 2003. Role of prodomain in importin-mediated nuclear localization and activation of caspase-2. J. Biol. Chem. 2784899-4905. [DOI] [PubMed] [Google Scholar]
- 3.Belov, G. A., P. V. Lidsky, O. V. Mikitas, D. Egger, K. A. Lukyanov, K. Bienz, and V. I. Agol. 2004. Bidirectional increase in permeability of nuclear envelope upon poliovirus infection and accompanying alterations of nuclear pores. J. Virol. 7810166-10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briggs, L. J., R. W. Johnstone, R. M. Elliot, C. Y. Xiao, M. Dawson, J. A. Trapani, and D. A. Jans. 2001. Novel properties of the protein kinase CK2-site-regulated nuclear-localization sequence of the interferon-induced nuclear factor IFI 16. Biochem. J. 35369-77. [PMC free article] [PubMed] [Google Scholar]
- 5.Briggs, L. J., D. Stein, J. Goltz, V. C. Corrigan, A. Efthymiadis, S. Hubner, and D. A. Jans. 1998. The cAMP-dependent protein kinase site (Ser312) enhances dorsal nuclear import through facilitating nuclear localization sequence/importin interaction. J. Biol. Chem. 27322745-22752. [DOI] [PubMed] [Google Scholar]
- 6.Cordingley, M. G., R. B. Register, P. L. Callahan, V. M. Garsky, and R. J. Colonno. 1989. Cleavage of small peptides in vitro by human rhinovirus 14 3C protease expressed in Escherichia coli. J. Virol. 635037-5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efthymiadis, A., L. J. Briggs, and D. A. Jans. 1998. The HIV-1 Tat nuclear localization sequence confers novel nuclear import properties. J. Biol. Chem. 2731623-1628. [DOI] [PubMed] [Google Scholar]
- 8.Forwood, J. K., V. Harley, and D. A. Jans. 2001. The C-terminal nuclear localization signal of the sex-determining region Y (SRY) high mobility group domain mediates nuclear import through importin beta 1. J. Biol. Chem. 27646575-46582. [DOI] [PubMed] [Google Scholar]
- 9.Ghildyal, R., A. Ho, K. M. Wagstaff, M. M. Dias, C. L. Barton, P. Jans, P. Bardin, and D. A. Jans. 2005. Nuclear import of the respiratory syncytial virus matrix protein is mediated by importin beta1 independent of importin alpha. Biochemistry 4412887-12895. [DOI] [PubMed] [Google Scholar]
- 10.Gustin, K. E., and P. Sarnow. 2001. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. EMBO J. 20240-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustin, K. E., and P. Sarnow. 2002. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J. Virol. 768787-8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hearps, A. C., and D. A. Jans. 2006. HIV-1 integrase is capable of targeting DNA to the nucleus via an importin alpha/beta-dependent mechanism. Biochem. J. 398475-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu, W., and D. A. Jans. 1999. Efficiency of importin alpha/beta-mediated nuclear localization sequence recognition and nuclear import. Differential role of NTF2. J. Biol. Chem. 27415820-15827. [DOI] [PubMed] [Google Scholar]
- 14.Jans, D. A., C. Y. Xiao, and M. H. Lam. 2000. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays 22532-544. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 16.Macara, I. G. 2001. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 65570-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park, N., P. Katikaneni, T. Skern, and K. E. Gustin. 2008. Differential targeting of nuclear pore complex proteins in poliovirus-infected cells. J. Virol. 821647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trapani, J. A., K. A. Browne, M. J. Smyth, and D. A. Jans. 1996. Localization of granzyme B in the nucleus. A putative role in the mechanism of cytotoxic lymphocyte-mediated apoptosis. J. Biol. Chem. 2714127-4133. [DOI] [PubMed] [Google Scholar]
- 19.Wagstaff, K. M., D. J. Glover, D. J. Tremethick, and D. A. Jans. 2007. Histone-mediated transduction as an efficient means for gene delivery. Mol. Ther. 15721-731. [DOI] [PubMed] [Google Scholar]