Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV), like other herpesviruses, has two stages to its life cycle: latency and lytic replication. KSHV is required for development of Kaposi's sarcoma, a tumor of endothelial origin, and is associated with the B-cell tumor primary effusion lymphoma (PEL) and the plasmablastic variant of multicentric Castleman's disease, all of which are characterized by predominantly latent KSHV infection. Recently, we and others have shown that the activated form of transcription factor X-box binding protein 1 (XBP-1) is a physiological trigger of KSHV lytic reactivation in PEL. Here, we show that XBP-1s transactivates the ORF50/RTA promoter though an ACGT core containing the XBP-1 response element, an element previously identified as a weakly active hypoxia response element (HRE). Hypoxia induces the KSHV lytic cycle, and active HREs that respond to hypoxia-inducible factor 1α are present in the ORF50/RTA promoter. Hypoxia also induces active XBP-1s, and here, we show that both transcription factors contribute to the induction of RTA expression, leading to the production of infectious KSHV under hypoxic conditions.
Kaposi's sarcoma-associated herpesvirus (KSHV) (human herpesvirus 8) is the etiological agent of Kaposi's sarcoma (KS) (12), a malignancy that accounts for 10% of all cancers in areas of sub-Saharan Africa (18, 40). Additionally, the B-cell tumor primary effusion lymphoma (PEL), also associated with KSHV infection (11), represents 4% of all AIDS-related non-Hodgkin lymphomas (8). In these tumors, KSHV expresses only a subset of its genes, thereby defining the pattern of viral gene expression in latency. Like other herpesviruses, KSHV can enter a lytic replication cycle, resulting in the production of new viral particles (20), a property that is essential for viral transmission. Low-level viral lytic replication is also detectable in KS, PEL, and multicentric Castleman's disease, suggesting a role in the pathogenesis of these diseases (45, 53). The viral protein of replication and transactivation (RTA), encoded by open reading frame 50 (ORF50), is both necessary (29) and sufficient (15) for inducing KSHV lytic replication. Therefore, determining the physiological triggers that induce RTA expression is critical for understanding the control of the KSHV lytic cycle in asymptomatic infection and disease.
Recently, we and others identified the transcription factor X-box binding protein 1 (XBP-1) as being capable of inducing the KSHV lytic cycle through transactivation of the RTA promoter (50, 52). XBP-1, a basic leucine zipper transcription factor, is essential for the induction of the unfolded protein response (UPR) that is triggered by a variety of different cellular stresses (39). XBP-1 is also a necessary transcription factor for terminal differentiation of B cells to plasma cells (35). PEL cells predominantly express the inactive form of XBP-1, namely, XBP-1u, but when the active form, XBP-1s, is induced, the KSHV lytic cycle is activated (50). This raises the possibility that in KSHV infected B cells, latency is maintained until plasma cell differentiation, which through XBP-1s induces the KSHV lytic cycle. If this occurs in the reticulated epithelium of the oral cavity and the lymphoid tissue of the Walydeyers ring, KSHV could be shed from infected plasma cells along with antibodies into the saliva (10, 25).
Hypoxia, acting through the transcription factor hypoxia-inducible factor 1 alpha (HIF-1α), is also a cellular stress that induces the KSHV lytic cycle (13). Under normoxic conditions, the HIF-α subunits are constitutively expressed but rapidly degraded (19, 38). Under hypoxic conditions, the HIF-1α subunits are stabilized, accumulate, and dimerize with the HIF-1β subunit (22). This basic helix-loop-helix transcription factor acts on promoters via hypoxic response elements (HREs) to upregulate genes involved in angiogenesis and anaerobic glycolysis (49); the hypoxia response is therefore crucial for solid-tumor-cell survival (17). ORF50 is hypoxia inducible, with its promoter containing seven putative HREs (16), of which three respond to HIF-1α (4). Hypoxia-induced HIF-1α is therefore a relevant biological activator of KSHV lytic replication. These observations outline a potential role for hypoxia in the pathology of KS where HIF-1α is abundantly expressed (9); however, why only 1 to 5% (45) of cells in KS display the lytic cycle, despite HIF-1α expression, is not known.
KSHV infection of activated B cells has recently been demonstrated in vitro (33). HIF-1α is also stabilized and sequestered to the nucleus in PEL, although again, no large-scale lytic replication is induced (4). HIF-1α is important in normal B-cell physiology, as HIF-1α knockout RAG2−/− chimeric mice display an increase in appearance of abnormal B-1-like peritoneal lymphocytes, with an associated increase in the level of autoimmunity and a disturbed maturation of the B-2 cell subset in the bone marrow (26). Indicative of hypoxia, HIF-1α is stabilized in the microenvironments of the germinal centers, other secondary lymphoid tissues, and bone marrow, raising the possibility that KSHV-infected B cells encountering these environments may induce viral lytic replication (31). Concomitantly, XBP-1, alongside its function in normal B-cell development, has also been shown to be necessary for tumor growth (37) and is activated under hypoxic conditions (47). Together, therefore, XBP-1s and HIF-1α are both lytic cycle triggers for KSHV, with these two pathways intersecting at normal B-cell development, response to cellular stress, and promotion of tumor survival.
Here, we identify a novel and active binding site for XBP-1 in the ORF50 promoter containing the core sequence ACGT; intriguingly, this sequence is identical to the core sequence of HIF-1α binding HREs (41). Because of the presence of both stress-inducible factors under hypoxic conditions and their involvement in normal B-cell biology, we further investigate their roles in KSHV reactivation in response to hypoxia. Here, we show that both XBP-1s and HIF-1α are induced in PEL exposed to acute hypoxia and that both contribute to induction of the lytic cycle of KSHV. Interestingly, HIF-1α overexpression is not sufficient to induce the KSHV lytic cycle in PEL whereas XBP-1s is sufficient under conditions of normoxia (50).
MATERIALS AND METHODS
Cell culture.
The PEL cell lines JSC-1 and HBL-6 were grown in RPMI 1640 medium (Invitrogen) with 10% fetal calf serum (BioSera) and 100 units/ml penicillin-streptomycin (Invitrogen) at 37°C in 5% CO2-21% oxygen or in a hypoxic chamber gassed regularly with a 5% CO2-95% nitrogen mix and placed within the standard incubator. Oxygen levels of media were measured with a FOXY-R stainless steel 1/16-inch OD fiber optic probe (Ocean Optics) (kindly supported by Tim Arnett, University College London). HEK 293T cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) with 10% fetal calf serum and 100 units/ml penicillin-streptomycin. Recombinant KSHV (rKSHV.219; Jeff Vieira, University of Washington [48])-infected cells were maintained with 2 μg of puromycin/ml (Calbiochem). To induce KSHV reactivation, 4 × 105 JSC-1 PEL cell lines were cultured in medium containing 12-O-tetradecanoyl-phorbol 13-acetate (20 ng/μl; Sigma).
p50Redi reporter assays.
Plasmid p50Redi was constructed as previously described (50), and mutation of this promoter was achieved using the QuikChange mutagenesis system (Stratagene) and forward (5′-GCT TTT CAG GAG AGT TAG GTC GAC ACT GAG GAT GTG GAC AAG CTT CTG C-3′) and reverse (5′-GCA GAA GCT TGT CCA CAT CCT CAG TGT CGA CCT AAC TCT CCT GAA AAG C −3′) primers, in accordance with the manufacturer's instructions. HEK 293T cells were transfected with 1 μg of p50Redi or the mutant pMUTRedi and 2.8 μg of pXBPsIG or the molar equivalent of the other plasmids. At 48 h posttransfection, cells were harvested and emerald green fluorescent protein and DsRed-express expression levels quantified using flow cytometry. In experiments delivering two transcription factors, 1.4 μg of pXBPsIG or the molar equivalent of the other plasmids were used in combination.
Immunoblotting.
Samples were lysed in sample buffer (0.2 M Tris HCl, pH 6.8, 5.2% sodium dodecyl sulfate, 20% glycerol, and bromophenol blue). Protein concentration was normalized by cell number and by blotting for a housekeeping protein. cells (8 × 105) were lysed directly with 100 μl sample buffer and 20 μl of the sample was resolved by 10% sodium dodecyl sulfate-polyacrylamide gels and transferred to a polyvinylidene fluoride membrane. After being blocked overnight with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (Sigma) (TBST), the membrane was probed with primary antibodies against HIF-1α (monoclonal; BD Transduction Laboratories, San Jose, CA) or RTA (polyclonal; Don Ganem, University of California—San Francisco [30]) and appropriate horseradish peroxidase-conjugated secondary antibodies (GE Healthcare) in 1% nonfat dry milk in TBST at room temperature. The anti-hemagglutinin (anti-HA) (horseradish peroxidase conjugated) and β-actin (ABcam) primary antibodies as well as corresponding secondary antibody as required were incubated for 1 h at room temperature in TBST. Blots were washed five times with TBST at intervals of 5 min each before developing. Positive controls for HIF-1α (pCDNA3-HIF-1α) were kindly provided by Peter Ratcliffe, University of Oxford (34).
RNA extraction and reverse transcriptase PCR (RT-PCR).
Total RNA was purified from 8 × 105 to 10 × 105 cells resuspended in 1 ml of Trizol (Invitrogen). After an initial chloroform extraction step, RNA was isolated using an RNA extraction kit (Qiagen) with on-column DNase (Promega) digestion. Oligonucleotide dT (Promega)-primed cDNA was used for PCR amplification across the XBP-1 atypical splice junction as previously described (50). KSHV ORF29a/b was amplified using forward (5′-GCA CGT AGC CAA CTC CGT G-3′) and reverse (5′-GCA GGA AAC TCG TGG AGC G-3′) primers. The PCR mixture contained 1.6 mM MgCl2, and a PCR cycle consisting of 95°C for 30 s, 65°C for 30 s, and 72°C for 1 min and 30s was repeated 30 times. β-Actin forward (5′-CTG TGG CAT CCA CGA AAC TA-3′) and reverse (5′-ACA TCT GCT GGA AGG TGG AC-3′) primers were used under the same PCR cycle as that described above, except with an annealing temperature of 60°C.
Virion DNA extraction and TaqMan PCR.
One hundred eighty microliters from 1 ml of supernatant containing approximately 1 × 105 to 2 × 105 PEL cells in culture under normoxic or hypoxic conditions was treated with DNase (Promega), followed by the addition of 10 μg of salmon sperm DNA as a carrier. Virion DNA was extracted using a QIAamp DNA extraction kit (Qiagen). The amount of recovered DNA was used to normalize the DNA inputs of different samples for calculation of viral load. KSHV ORF37 TaqMan PCR was performed as described previously, with modification (44). Each 25-μl PCR mixture contained 1× Platinum quantitative PCR (qPCR) SuperMix-uracil DNA glycosylase with ROX (Invitrogen), 300 nM each ORF37 primer,150 nM ORF37 probe (Sigma), and 5 μl sample DNA. Following 2 min incubation at 50°C for activation of the uracil DNA glycosylase, the sample was denatured for 3 min at 95°C. Fifty cycles of standard qPCR consisting of 95°C for 15s and 60°C for 50s were performed. Amplification was carried out with an ABI Prism 7000 sequence detection system (Applied Biosystems). Each PCR run also contained a standard dilution curve of ORF37 and no template-negative controls. All samples were run in duplicate reactions.
Luciferase promoter assays.
HEK 293T cells were plated at a density of 2 × 104 cells in 96-well plates; on the next day, cells were transfected using 20 ng of the appropriate ORF50 reporter plasmids (a kind gift from Erle Robertson, University of Pennsylvania [4]) in combination with a molar equivalent transcription factor expressing plasmid DNA. After 48 h, quantification of relative light units was performed using the luciferase Bright Glo reagent in accordance with the manufacturer's instructions (Promega) and a GloMax 96-microplate luminometer with a single injector (Promega). All assays were performed in triplicate. Transfection efficiency was monitored by parallel transfection with a fluorescent protein and flow cytometry.
Lentiviral vector construction and RNA interference.
Lentiviral vector-genome expressing HIF-1α was generated by amplifying HIF-1α with forward (5′-GGA TCC GAG GGC GCC GGC GGC GCG AAC-3′) and reverse (5′-GCG GCC GCG TTA ACT TGA TCC AAA GCT C-3′) primers from pCDNA HIF-1α. The resulting 2.4-kb amplicon was cloned into pGEM-T-Easy vector (Promega) and sequence verified. The BamHI/NotI HIF-1α insert was then subcloned into a modified version of the lentivirus vector pCSGW (50), allowing the expression of HA-tagged HIF-1α. The lentiviral vector expressing short hairpin RNA (shRNA) targeting XBP-1 was described previously (50). The lentiviral vector expressing shRNA targeting HIF-1α was generated using same methodology. Briefly, the reverse primer (5′-CGA AAA AGA TGA CCA GCA ACT TGA GGA AGT CGA AAC CTC CCC AAG CTA CTG GTC ATC GGT GTT TCG TCC TTT CCA CAA GAT ATA TAA AG-3′) and the forward primer (5′-GGG GCT GCA GAA GGT CGG GCA GGA AGA GGG CCT ATT TCC C-3′) were used to PCR amplify an shRNA expression cassette. This amplicon was cloned into the pGEM-T-Easy vector, sequence verified, and subcloned into antibiotic resistance-modified versions of pCSGW, and lentiviral vectors were produced as described previously (2).
Lentiviral vector transduction.
PEL cells (4 × 105) were transduced with lentiviral vectors at an input equivalent to a multiplicity of infection of 5 on HEK 293T cells. At 48 h posttransduction, samples were taken or the cells were put under respective selection and stable lines were maintained under selection.
RESULTS
The KSHV ORF50 promoter contains a novel XBP-1 responsive element.
Previously, we demonstrated that the highly active plasma cell transcription factor XBP-1 is a lytic switch trigger for KSHV. Only the expression of the spliced active isoform of XBP-1 (XBP-1s) in PEL cell lines robustly increased the expression of RTA, and this expression was sufficient to initiate the full lytic cycle. The direct action of XBP-1s on the RTA promoter was mapped to the 200 bp preceding the RTA start codon (50). Examination of the targets for XBP-1 binding previously identified by chromatin immunoprecipitation (1) revealed a potential XBP-1 response element (XRE) in the ORF50 promoter with an ACGT core-containing motif (Fig. 1A). To assess the relevance of this, we determined the effect of XBP-1s expression on a red fluorescent protein (DsRed-express) reporter gene cloned downstream of the wild-type ORF50 promoter and an ORF50 promoter sequence containing a mutant putative XRE in transient-transfection assays. Both the wild-type and the mutant promoters responded to overexpressed RTA with an 18-fold increase in activity with respect to the level for the empty vector (pIG) (Fig. 1B). Overexpression of XBP-1s was able to activate transcription from the wild-type promoter to a level similar to that for RTA (17-fold) but failed to transactivate the mutant ORF50 promoter (Fig. 1B). These data identify a functional XRE 76 bp upstream of the starting methionine in the ORF50 promoter. This element was previously identified as a weak HIF-1α response element (HRE4) in the ORF50 promoter (4), and we propose that this element should be renamed XRE.
FIG. 1.
The KSHV ORF50 promoter contains a functional XRE. (A) Schematic representation of the ORF50 promoter showing the location of the dominant HRE (HRE2) as well as the newly identified XRE, previously identified as the putative HRE4 element (4). Numbers in brackets indicate the response element start sites relative to the KSHV genome sequence (NC_003409). The wild-type sequences of both HRE2 and XRE are shown, with the core ACGT sequence element highlighted in gray. The mutated sequence of the XRE is indicated underneath, with mutated nucleotides indicated by •. (B) HEK 293T cells were transfected with the DsRed reporter plasmid with the wild-type (p50redi; black bars) or the mutant (pMUT50redi; white bars) ORF50 promoter sequence, together with pIG (control), RTA-expressing (pCMV-RTA), or XBP-1s-expressing (pXBP-sIG) plasmids. The percentages of DsRed-positive cells were quantified by flow cytometry at 48 h posttransfection. Percentages of DsRed-positive cells are expressed relative to the level for empty-vector control pIG.
Acute hypoxia stabilizes HIF-1α and activates XBP-1 in PEL.
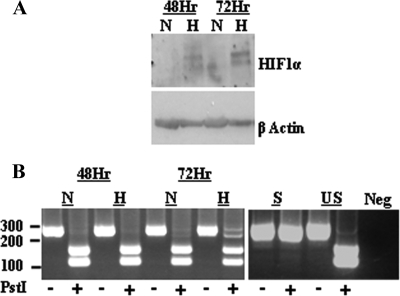
Because hypoxia is able to induce both XBP-1s and HIF-1α and both transcription factors can transactivate the ORF50 promoter through ACGT cores containing XREs and HREs, respectively, we examined the ability of hypoxia to induce both transcription factors in PEL. HIF-1α is stabilized and activated at oxygen concentrations below 3% in a variety of cell lines (28). Under conditions of acute hypoxia (<3% O2), HIF-1α is stabilized and readily detected in the PEL cell line rKSHV.219 JSC-1 (Fig. 2). Levels of HIF-1α are increased by 48 h of hypoxia, compared to levels for cells cultured in normoxia, and robustly expressed by 72 h (Fig. 2A). We have previously shown that XBP-1 is present in PEL as the unspliced, inactive transcript which can be activated to the spliced bZIP-containing transcript by the reducing agent dithiothreitol (50). XBP-1 has also been shown to be activated by hypoxia in cardiac myocytes (47). Consistent with these data, exposure of rKSHV.219 JSC-1 to hypoxia leads to significant conversion of XBP-1 to the spliced, active form (XBP-1s) (Fig. 2B). Therefore, hypoxia induces both HIF-1α and XBP-1s transcription factors in PEL.
FIG. 2.
Acute hypoxia stabilizes HIF-1α and activates XBP-1 in PEL. (A) Western blot analysis of whole-cell lysates of the PEL cell line rKSHV.219 JSC-1 cultured in normoxia (N; 21%O2) or exposed to hypoxia (H; 3% O2) 48 or 72 h with anti-HIF-1α antibody (BD transduction laboratories). HIF-1α is stabilized in rKSHV.219 JSC-1 in response to hypoxia at 48 and 72 h, compared to the level for normoxia, and β-actin acts as a loading control. (B) RT-PCR amplification across the XBP-1 intron produces a 249-bp amplicon from XBP-1u mRNA and a 223-base amplicon from XBP-s mRNA. PstI digests only the XBP-1u amplicon, resulting in two bands, whereas XBP-1s results in a single band (50). RT-PCR amplification from the total mRNA of the rKSHV.219 JSC-1 cell line cultured under normoxic conditions (N; 21%O2) or exposed to hypoxia (H; <3% O2) for 48 or 72 h shows that XBP-1s is produced after 72 h of hypoxia but is absent in cells under normoxic conditions. In the 72-h-hypoxia sample, a slower-migrating, non-PstI-digestible PCR hybrid between the XBP-1s and XBP-1u products is visible, similar to what was previously described (50).
Acute hypoxia leads to KSHV reactivation in PEL.
The effects of hypoxia and hypoxia mimics, such as cobalt chloride, on KSHV lytic cycle induction have been demonstrated (4, 13, 16); therefore, we wanted to test if the low-oxygen conditions that led to HIF-1α and XBP-1s expression (Fig. 2) also induced KSHV RTA in PEL. Under normoxic conditions, RTA is not significantly expressed (Fig. 3A). Hypoxia readily induces RTA expression in rKSHV.219 JSC-1 cells (Fig. 3A). RT-PCR for the spliced KSHV lytic cycle transcript ORF29a/b shows that KSHV enters the full lytic cycle (Fig. 3B), and ORF37 qPCR for the extracellular KSHV viral genome confirms production of virus in response to hypoxia in PEL cell lines JSC-1 and HBL-6 (Fig. 3C). Therefore, RTA induced by low-oxygen conditions is able to induce the full lytic cycle of KSHV in PEL.
FIG. 3.
Acute hypoxia leads to accumulation of KSHV RTA, induces ORF29a/b lytic gene expression, and produces progeny virions, as measured by ORF37. (A) Western blot analysis of whole-cell lysates of the PEL cell line rKSHV.219 JSC-1 cultured in normoxia (N; 21%O2) or exposed to hypoxia (H; <3% O2) for 48 or 72 h with rabbit anti-RTA. RTA is induced by hypoxia at 48 h and further increased at 72 h of exposure, compared to cells under normoxic conditions. β-Actin acts as a loading control. (B) Total cellular RNA was isolated from rKSHV.219 JSC-1 cultured under normoxic or hypoxic conditions for 48 and 72 h. With RT-PCR, cDNA was amplified with gene-specific primers for the spliced lytic transcript, ORF29a/b. The 300-bp product is seen only in samples exposed to hypoxia. (C) qPCR for ORF37 from KSHV virion DNA extracted from the supernatants of JSC-1 and HBL-6 cells cultured for 72 h in normoxia (N; 21%O2; black bars) or hypoxia (H; <3% O2; white bars). Copy numbers were determined using an ORF37 standard curve and normalized for total DNA input. Hypoxia leads to a significant increase in virus copies present in the supernatant. Western blots for HIF-1α and RTA of cells corresponding to the supernatants at 48 h show that samples are HIF-1α positive at 48 h and that RTA is induced.
The RTA promoter has the potential to respond to HIF-1α and XBP-1s.
The RTA promoter can be induced by the transcription factors XBP-1s and HIF-1α (Fig. 1) (4). Because both response elements contain essentially the same core ACGT sequence, we determined if these elements respond to both transcription factors. A series of truncated constructs of the RTA promoter fused to a luciferase reporter were used to isolate HRE2 from XRE (HRE4) (Fig. 4A). Expression of RTA efficiently induced luciferase activity from the full-length and truncated ORF50 promoters (Fig. 4B). HIF-1α was also able to drive luciferase expression from the full-length promoter as well as the HRE-containing truncated promoters, as previously seen (4). However, XBP-1s is able to activate expression of luciferase from the full-length ORF50 reporter and the XRE (HRE4)-containing promoter only. Therefore, the RTA promoter of KSHV can be transactivated by both HIF-1α and XBP-1s, and both factors act through ACGT binding sites.
FIG. 4.
The KSHV RTA promoter contains response elements for XBP-1 and HIF-1α and can respond to both. (A) Schematic of the predicted HREs in the KSHV RTA promoter region (adapted from reference 4). (B) Schematic of reporter plasmids pRpluc1-3087+s containing a 3-kb sequence upstream of the RTA transcriptional start site and a 1-kb splicing sequence of RTA that drives the expression of firefly luciferase (FL) (4) as well as truncated promoters named pRpluc1115-1327 (HRE2 only) and pRpluc1-550 XRE (HRE4 only). (B) A fixed amount of the reporter plasmids was transfected into HEK 293T cells, together with pIG (empty vector control; ↓), RTA-expressing (pCMV-RTA; black bars), XBP-1s-expressing (pXBP-sIG; striped bars), or HIF-1α-expressing (pHIF-1αIG; white bars) plasmids, normalized by plasmid copy number. Promoter activity levels are expressed as log relative light units (logRLU) relative to the levels for the reporter plus the pIG control plasmid. Means and standard errors are from three independent transfections. HIF-1α acts on all reporters containing HRE2 and HRE4 while XBP-1s acts on the full-length and HRE4-only promoter but not HRE2 only.
XBP-1 can induce RTA under hypoxic conditions.
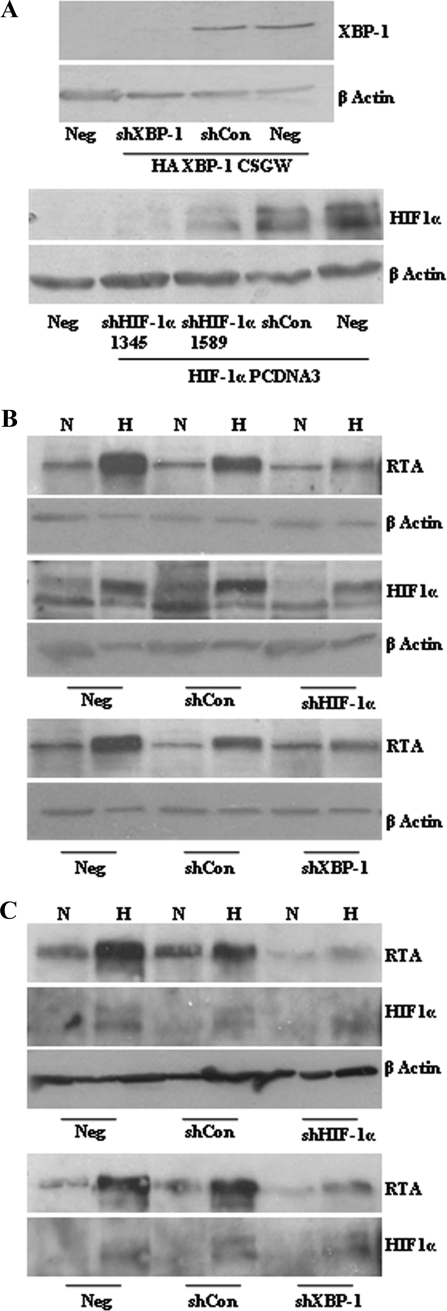
As both HIF-1α and XBP-1s are activated by hypoxia at times similar to those of RTA induction, we wished to determine if one transcription factor was dominant for inducing KSHV lytic reactivation under hypoxic conditions. We produced interfering shRNAs targeting XBP-1 and HIF-1α. These hairpins led to efficient reduction in HIF-1α and XBP-1s protein levels when each protein was overexpressed in HEK 293Ts cells (Fig. 5A). Transduction of the rKSHV.219 HEK 293T cells (Fig. 5B) and the PEL cell line JSC-1 (Fig. 5C) with each lentivirus vector expressing these hairpins shows that decreasing the levels of HIF-1α under hypoxic conditions clearly results in attenuated RTA induction (Fig. 5B and C). Identical results were achieved for the PEL cell line HBL-6 (data not shown). The shRNA targeting XBP-1s also led to a reduction in RTA induction under the same hypoxic conditions (Fig. 5B and C). The presence of shRNA does not interfere with the processing of XBP-1 (data not shown). These data suggest that both HIF-1α and XBP-1s contribute to the lytic cycle induction of KSHV under hypoxic conditions.
FIG. 5.
XBP-1s contributes to RTA induction by hypoxia. (A) The ability of shRNA to knock down target protein levels was demonstrated by cotransfection of HEK 293Tcells with 1 μg of target-expressing plasmid and 3 μg of relevant/irrelevant control hairpins in a pGEMT backbone. Western blots for target protein using anti-HA for HA-tagged XBP-1 detection (upper panel) and anti-HIF-1α for HIF-1α (lower panel) are shown (see Materials and Methods for plasmid descriptions). The shRNA targeting XBP-1 and the two shRNAs targeting HIF-1α are able to reduce target protein levels substantially, compared to the irrelevant control shRNA and untransduced cells (Neg). shRNA 1345 targeting HIF-1α was used for further experiments. rKSHV.219 HEK 293T cells (B) and JSC-1 cells (C) untransduced (Neg) or stably transduced with lentiviruses containing shRNA targeting XBP-1, HIF-1α, and an irrelevant control independently were cultured in normoxia (N; 21% O2) or hypoxia (H; 3% O2) for 72 h. Western blot analysis of whole-cell lysates was performed for HIF-1α, which was upregulated in all hypoxic samples except those expressing the shRNA targeting HIF-1α. The same samples probed with anti-RTA show induction of RTA in response to hypoxia, which is reduced in the presence of shRNA targeting HIF-1α or XBP-1s.
HIF-1α overexpression under normoxic conditions does not lead to RTA induction.
Because of the stabilization of HIF-1α in PEL and KS lesions (6, 9), we wanted to determine the contribution of HIF-1α to KSHV lytic cycle induction under normoxic conditions. We transduced the PEL cell lines JSC-1 and HBL-6 with lentivirus expressing HIF-1α, XBP-1s, or empty vector. Overexpression of HIF-1α from the lentivirus vector led to HIF-1α protein expression in both PEL cell lines (Fig. 6A) and resulted in transactivation of the ORF50 promoter in HEK293T cells (Fig. 4B and data not shown). However RTA, was induced only in cells overexpressing XBP-1s under normoxic conditions (Fig. 6B). Therefore, only XBP-1s is able to reactivate KSHV under conditions of normoxia and hypoxia.
FIG. 6.
Overexpression of HIF-1α under normoxic conditions does not induce RTA. (A) Anti-HIF-1α Western blot of whole-cell lysates of JSC-1 and HBL-6 cells transduced with lentivirus expressing HIF-1α. (B) RTA Western blot of JSC-1 and HBL-6 cells untransduced (Neg) or transduced with empty vector (pIG), HIF-1α-expressing (pHIF-1αIG), and XBP-1s-expressing (pXBP-sIG) plasmids. RTA is induced only in the presence of XBP-1s expression (pXBP-sIG) under normoxic conditions.
DISCUSSION
KSHV lytic replication can be induced by cellular stress, and we previously reported the ability of the basic leucine zipper transcription factor XBP-1s to cause KSHV reactivation (50). XBP-1s is an important mediator of the UPR and is necessary for B-cell differentiation to antibody-producing plasma cells (35, 42). Therefore, plasma cell differentiation and XBP-1s expression can act as a cellular cue for induction of the KSHV lytic cycle. Here, we demonstrate that the mechanism for this transactivation is the utilization of an XBP-1-responsive ACGT-containing element in the ORF50 promoter (Fig. 1). This response element was previously identified as HRE4 (4). XBP-1 can be activated by nonphysiological triggers that result in unfolded proteins, such as dithiothreitol, which induces KSHV reactivation (50), by B-cell receptor ligation (43, 51), and, importantly, by hypoxia (14, 28, 47). Here, we show that hypoxia induces RTA expression through XBP-1s and HIF-1α, both of which recognize common core ACGT sequence elements.
Hypoxia was previously identified as an inducer of KSHV lytic replication (13), with HREs being present within the promoter regions of KSHV viral genes, including ORF50/RTA. Studies of RTA promoter activity in response to HIF-1α overexpression (16, 23) or hypoxia mimics (9, 16) show minimal or cell-type-specific activation by HIF-1α under otherwise normoxic culture conditions. Here, we have shown that under authentic hypoxic conditions, both HIF-1α stabilization and XBP-1 activation concordantly occur (Fig. 2), the RTA protein is robustly upregulated, the viral lytic transcript ORF29a/b is induced (Fig. 3A and B), and infectious virions are produced (Fig. 3C). Through RNA interference knockdowns, we demonstrate that both HIF-1α and XBP-1s are required for this lytic induction of KSHV under hypoxic conditions (Fig. 5).
The ability of HIF-1α to activate transcription from ORF50 promoter-reporter vectors also appears to be cell type specific, as HIF-1α overexpression under normoxic conditions in HEK 293 cells increased RTA promoter activity more than overexpression in BJAB cells (4). In contrast, overexpression of HIF-1α in Hep3B cells weakly induced the ORF50 promoter and RTA expression in the absence of hypoxia (16), a situation more akin to that found in KSHV-infected endothelial cells (9). Indeed, overexpression of HIF-1α under normoxic conditions in PEL cell lines failed to induce RTA expression, whereas XBP1s overexpression initiates the full KSHV lytic cycle (Fig. 6) (50). Together, this suggests a role for KSHV in maintaining latency through the control or suppression of HIF-1α activity on the ORF50 promoter under normoxic conditions.
Under normoxic culture conditions, KSHV LANA mediates degradation of the Von Hippel-Lindau factor, the master regulator of HIF stability, thereby stabilizing HIF-1α upon KSHV infection (6). LANA, via its N-terminal domain, mediates HIF-1α nuclear accumulation in PEL cell lines (5). HIF-1α mRNA is also induced in KSHV-infected endothelial cells, and HIF-1α protein is abundantly expressed in KS lesions, where it is nuclearly localized (9). Despite the nuclear accumulation and stabilization of HIF-1α in PEL, infected endothelial cells, and KS lesions, very little KSHV lytic replication is observed, typical of the infected cells being predominantly latent. The nuclear accumulations of HIF-1α in response to KSHV latent infection and hypoxia are spatially distinct, suggesting that accumulation in nuclear compartments under normoxic conditions does not result in induction of RTA and that redistribution under hypoxic conditions is required for induction of the lytic cycle (5). Alternatively, KSHV-associated HIF-1α accumulation could prime infected cells so that nuclear, stabilized HIF-1α, when exposed to hypoxia, is further activated (for example, by phosphorylation) (36), resulting in lytic reactivation (9). This “superinduction” of HIF-1α in response to hypoxia has been observed through the increased activity of HIF-1α induced by v-SRC (21). However, the VEGF gene, a HIF-1α-responsive gene, is upregulated in a KSHV LANA-induced, HIF-1α-dependent way under normoxic conditions (5, 9), indicating that HIF-1α can be transcriptionally active under latent normoxic infection. It is possible that an additional factor, activated by hypoxia and absent during latent KSHV infection, is necessary for HIF-1α-mediated lytic induction, and we suggest that XBP-1s can fulfill this role.
PEL cell lines have minimal KSHV lytic induction and contain inactive XBP-1u and nuclear HIF-1α, both of which can be rapidly activated by relevant stresses leading to lytic replication (6, 50). It is not known why XBP-1 is inactive or why nuclear accumulation of HIF-1α fails to induce the KSHV lytic cycle in PELs. However, both transcription factors play important and varied roles at different stages of normal B-cell differentiation. XBP-1s, as part of the physiological UPR, is critical for plasma cell differentiation but is also transiently induced after B-cell receptor stimulation (35, 43). Analogously, B cells also experience hypoxic microenvironments and HIF-1α stabilization in normal physiology (7). Germinal centers are exclusively HIF-1α positive in secondary lymphoid organs (31), and HIF-1α-deficient chimeric mice display a distortion in the number of B-2 lymphocytes in the bone marrow as well as an accumulation of peritoneal B-1 cells and autoimmunity (26). This indicates that HIF-1α and XBP-1s have roles in normal B-cell biology and are usurped in reactivation of KSHV. It is possible that antigen-stimulated cells of the HIF-1α-positive germinal center provide the cues for KSHV lytic cycle induction in primary infection, whereas terminal differentiation of B cells to plasma cells provides the lytic cues during lifelong persistent oral shedding.
The coinvolvement of HIF-1α and XBP-1s may also be of significance to the life cycles of other herpesviruses. Epstein-Barr virus (EBV), the human herpesvirus most closely related to KSHV, is also induced into its lytic cycle by hypoxic treatment (24), and XBP-1s has been implicated as an EBV lytic cycle inducer, although most likely in the presence of other coactivators, such as protein kinase D (3, 46). For murine herpesvirus 4, a related rhadinovirus, ORF50 is also upregulated in response to hypoxia (32), although a response to XBP-1s has not been reported. Similarly to KSHV LANA, the EBV latent membrane protein (LMP-1) has been reported to upregulate HIF-1α (27). Together, these data suggest that gammaherpesviruses have evolved the ability to respond to cellular stress transcription factors and that these same factors are crucial for normal B-cell development, where their expression during differentiation may facilitate controlled KSHV lytic cycle activation.
Acknowledgments
We thank Peter Ratcliffe for the gifts of HIF-1α expression vectors; Erle Robertson for the luciferase constructs; Don Ganem for the gift of the RTA polyclonal antibodies; Jeff Vieira for the recombinant KSHV, rKSHV.219-infected Vero, and JSC-1 cell lines; and Tim Arnett for advice with hypoxic culture conditions and access to instrumentation for determining oxygen levels. We also acknowledge David Bibby and Duncan Clark of Barts and the London NHS trust for the gift of the pORF37 plasmid as standards for the KSHV qPCR. Finally, we thank everyone from the VGB group at Windeyer, especially Edward Tsao, for technical help and advice.
L.D.-G. and S.J.W. were supported by the MRC, and P.K. was supported by grants from the Wellcome Trust and Cancer Research UK.
Footnotes
Published ahead of print on 29 April 2009.
REFERENCES
- 1.Acosta-Alvear, D., Y. Zhou, A. Blais, M. Tsikitis, N. H. Lents, C. Arias, C. J. Lennon, Y. Kluger, and B. D. Dynlacht. 2007. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell 2753-66. [DOI] [PubMed] [Google Scholar]
- 2.Besnier, C., Y. Takeuchi, and G. Towers. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 9911920-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhende, P. M., S. J. Dickerson, X. Sun, W. H. Feng, and S. C. Kenney. 2007. X-box-binding protein 1 activates lytic Epstein-Barr virus gene expression in combination with protein kinase D. J. Virol. 817363-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai, Q., K. Lan, S. C. Verma, H. Si, D. Lin, and E. S. Robertson. 2006. Kaposi's sarcoma-associated herpesvirus latent protein LANA interacts with HIF-1alpha to upregulate RTA expression during hypoxia: latency control under low oxygen conditions. J. Virol. 807965-7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai, Q., M. Murakami, H. Si, and E. S. Robertson. 2007. A potential alpha-helix motif in the amino terminus of LANA encoded by Kaposi's sarcoma-associated herpesvirus is critical for nuclear accumulation of HIF-1 alpha in normoxia. J. Virol. 8110413-10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai, Q. L., J. S. Knight, S. C. Verma, P. Zald, and E. S. Robertson. 2006. EC5S ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and p53 tumor suppressors. PLoS Pathog. 2e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell, C. C., H. Kojima, D. Lukashev, J. Armstrong, M. Farber, S. G. Apasov, and M. V. Sitkovsky. 2001. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J. Immunol. 1676140-6149. [DOI] [PubMed] [Google Scholar]
- 8.Carbone, A., and A. Gloghini. 2008. KSHV/HHV8-associated lymphomas. Br J. Haematol. 14013-24. [DOI] [PubMed] [Google Scholar]
- 9.Carroll, P. A., H. L. Kenerson, R. S. Yeung, and M. Lagunoff. 2006. Latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors. J. Virol. 8010802-10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattani, P., M. Capuano, F. Cerimele, I. L. La Parola, R. Santangelo, C. Masini, D. Cerimele, and G. Fadda. 1999. Human herpesvirus 8 seroprevalence and evaluation of nonsexual transmission routes by detection of DNA in clinical specimens from human immunodeficiency virus-seronegative patients from central and southern Italy, with and without Kaposi's sarcoma. J. Clin. Microbiol. 371150-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS- related body-cavity-based lymphomas. N. Engl. J. Med. 3321186-1191. [DOI] [PubMed] [Google Scholar]
- 12.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 2661865-1869. [DOI] [PubMed] [Google Scholar]
- 13.Davis, D. A., A. S. Rinderknecht, J. P. Zoeteweij, Y. Aoki, E. L. Read-Connole, G. Tosato, A. Blauvelt, and R. Yarchoan. 2001. Hypoxia induces lytic replication of Kaposi sarcoma-associated herpesvirus. Blood 973244-3250. [DOI] [PubMed] [Google Scholar]
- 14.Feldman, D. E., V. Chauhan, and A. C. Koong. 2005. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol. Cancer Res. 3597-605. [DOI] [PubMed] [Google Scholar]
- 15.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 746207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haque, M., D. A. Davis, V. Wang, I. Widmer, and R. Yarchoan. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) contains hypoxia response elements: relevance to lytic induction by hypoxia. J. Virol. 776761-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, A. L. 2002. Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer 238-47. [DOI] [PubMed] [Google Scholar]
- 18.Hengge, U. R., T. Ruzicka, S. K. Tyring, M. Stuschke, M. Roggendorf, R. A. Schwartz, and S. Seeber. 2002. Update on Kaposi's sarcoma and other HHV8 associated diseases. Part 1: epidemiology, environmental predispositions, clinical manifestations, and therapy. Lancet Infect. Dis. 2281-292. [DOI] [PubMed] [Google Scholar]
- 19.Huang, L. E., J. Gu, M. Schau, and H. F. Bunn. 1998. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA 957987-7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenner, R. G., M. M. Alba, C. Boshoff, and P. Kellam. 2001. Kaposi's sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 75891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang, B. H., F. Agani, A. Passaniti, and G. L. Semenza. 1997. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res. 575328-5335. [PubMed] [Google Scholar]
- 22.Jiang, B. H., E. Rue, G. L. Wang, R. Roe, and G. L. Semenza. 1996. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J. Biol. Chem. 27117771-17778. [DOI] [PubMed] [Google Scholar]
- 23.Jiang, B. H., J. Z. Zheng, S. W. Leung, R. Roe, and G. L. Semenza. 1997. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J. Biol. Chem. 27219253-19260. [DOI] [PubMed] [Google Scholar]
- 24.Jiang, J. H., N. Wang, A. Li, W. T. Liao, Z. G. Pan, S. J. Mai, D. J. Li, M. S. Zeng, J. M. Wen, and Y. X. Zeng. 2006. Hypoxia can contribute to the induction of the Epstein-Barr virus (EBV) lytic cycle. J. Clin. Virol. 3798-103. [DOI] [PubMed] [Google Scholar]
- 25.Koelle, D. M., M. L. Huang, B. Chandran, J. Vieira, M. Piepkorn, and L. Corey. 1997. Frequent detection of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in saliva of human immunodeficiency virus-infected men: clinical and immunologic correlates. J. Infect. Dis. 17694-102. [DOI] [PubMed] [Google Scholar]
- 26.Kojima, H., H. Gu, S. Nomura, C. C. Caldwell, T. Kobata, P. Carmeliet, G. L. Semenza, and M. V. Sitkovsky. 2002. Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1alpha-deficient chimeric mice. Proc. Natl. Acad. Sci. USA 992170-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondo, S., S. Y. Seo, T. Yoshizaki, N. Wakisaka, M. Furukawa, I. Joab, K. L. Jang, and J. S. Pagano. 2006. EBV latent membrane protein 1 up-regulates hypoxia-inducible factor 1alpha through Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Cancer Res. 669870-9877. [DOI] [PubMed] [Google Scholar]
- 28.Koumenis, C., and B. G. Wouters. 2006. “Translating” tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways. Mol. Cancer Res. 4423-436. [DOI] [PubMed] [Google Scholar]
- 29.Lukac, D. M., J. R. Kirshner, and D. Ganem. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 739348-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukac, D. M., R. Renne, J. R. Kirshner, and D. Ganem. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252304-312. [DOI] [PubMed] [Google Scholar]
- 31.Piovan, E., V. Tosello, S. Indraccolo, M. Masiero, L. Persano, G. Esposito, R. Zamarchi, M. Ponzoni, L. Chieco-Bianchi, R. Dalla-Favera, and A. Amadori. 2007. Differential regulation of hypoxia-induced CXCR4 triggering during B-cell development and lymphomagenesis. Cancer Res. 678605-8614. [DOI] [PubMed] [Google Scholar]
- 32.Polcicova, K., Z. Hrabovska, J. Mistrikova, J. Tomaskova, J. Pastorek, S. Pastorekova, and J. Kopacek. 2008. Up-regulation of Murid herpesvirus 4 ORF50 by hypoxia: possible implication for virus reactivation from latency. Virus Res. 132257-262. [DOI] [PubMed] [Google Scholar]
- 33.Rappocciolo, G., H. R. Hensler, M. Jais, T. A. Reinhart, A. Pegu, F. J. Jenkins, and C. R. Rinaldo. 2008. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J. Virol. 824793-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raval, R. R., K. W. Lau, M. G. Tran, H. M. Sowter, S. J. Mandriota, J. L. Li, C. W. Pugh, P. H. Maxwell, A. L. Harris, and P. J. Ratcliffe. 2005. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol. Cell. Biol. 255675-5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reimold, A. M., P. D. Ponath, Y. S. Li, R. R. Hardy, C. S. David, J. L. Strominger, and L. H. Glimcher. 1996. Transcription factor B cell lineage-specific activator protein regulates the gene for human X-box binding protein 1. J. Exp. Med. 183393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richard, D. E., E. Berra, E. Gothie, D. Roux, and J. Pouyssegur. 1999. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 27432631-32637. [DOI] [PubMed] [Google Scholar]
- 37.Romero-Ramirez, L., H. Cao, D. Nelson, E. Hammond, A. H. Lee, H. Yoshida, K. Mori, L. H. Glimcher, N. C. Denko, A. J. Giaccia, Q. T. Le, and A. C. Koong. 2004. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 645943-5947. [DOI] [PubMed] [Google Scholar]
- 38.Salceda, S., and J. Caro. 1997. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 27222642-22647. [DOI] [PubMed] [Google Scholar]
- 39.Schroder, M., and R. J. Kaufman. 2005. The mammalian unfolded protein response. Annu. Rev. Biochem. 74739-789. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz, R. A., G. Micali, M. R. Nasca, and L. Scuderi. 2008. Kaposi sarcoma: a continuing conundrum. J. Am. Acad. Dermatol. 59179-208. [DOI] [PubMed] [Google Scholar]
- 41.Semenza, G. L., B. H. Jiang, S. W. Leung, R. Passantino, J. P. Concordet, P. Maire, and A. Giallongo. 1996. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 27132529-32537. [DOI] [PubMed] [Google Scholar]
- 42.Shen, X., K. Zhang, and R. J. Kaufman. 2004. The unfolded protein response—a stress signaling pathway of the endoplasmic reticulum. J. Chem. Neuroanat. 2879-92. [DOI] [PubMed] [Google Scholar]
- 43.Skalet, A. H., J. A. Isler, L. B. King, H. P. Harding, D. Ron, and J. G. Monroe. 2005. Rapid B cell receptor-induced unfolded protein response in nonsecretory B cells correlates with pro- versus antiapoptotic cell fate. J. Biol. Chem. 28039762-39771. [DOI] [PubMed] [Google Scholar]
- 44.Stamey, F. R., M. M. Patel, B. P. Holloway, and P. E. Pellett. 2001. Quantitative, fluorogenic probe PCR assay for detection of human herpesvirus 8 DNA in clinical specimens. J. Clin. Microbiol. 393537-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun, C. C., and D. A. Thorley-Lawson. 2007. Plasma cell-specific transcription factor XBP-1s binds to and transactivates the Epstein-Barr virus BZLF1 promoter. J. Virol. 8113566-13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thuerauf, D. J., M. Marcinko, N. Gude, M. Rubio, M. A. Sussman, and C. C. Glembotski. 2006. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ. Res. 99275-282. [DOI] [PubMed] [Google Scholar]
- 48.Vieira, J., and P. M. O'Hearn. 2004. Use of the red fluorescent protein as a marker of Kaposi's sarcoma-associated herpesvirus lytic gene expression. Virology 325225-240. [DOI] [PubMed] [Google Scholar]
- 49.Wang, G. L., B. H. Jiang, E. A. Rue, and G. L. Semenza. 1995. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 925510-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson, S. J., E. H. Tsao, B. L. Webb, H. Ye, L. Dalton-Griffin, C. Tsantoulas, C. V. Gale, M. Q. Du, A. Whitehouse, and P. Kellam. 2007. X box binding protein XBP-1s transactivates the Kaposi's sarcoma-associated herpesvirus (KSHV) ORF50 promoter, linking plasma cell differentiation to KSHV reactivation from latency. J. Virol. 8113578-13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan, B. C., T. Adachi, and T. Tsubata. 2008. ER stress is involved in B cell antigen receptor ligation-induced apoptosis. Biochem. Biophys. Res. Commun. 365143-148. [DOI] [PubMed] [Google Scholar]
- 52.Yu, F., J. Feng, J. N. Harada, S. K. Chanda, S. C. Kenney, and R. Sun. 2007. B cell terminal differentiation factor XBP-1 induces reactivation of Kaposi's sarcoma-associated herpesvirus. FEBS Lett. 5813485-3488. [DOI] [PubMed] [Google Scholar]
- 53.Zhong, W., H. Wang, B. Herndier, and D. Ganem. 1996. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 936641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]