Abstract
In the respiratory tract, different dendritic cell (DC) populations guard a tight balance between tolerance and immunity to infectious or harmless materials to which the airways are continuously exposed. For infectious and noninfectious antigens administered via different routes, different subsets of DC might contribute during the induction of T-cell tolerance and immunity. We studied the impact of primary respiratory syncytial virus (RSV) infection on respiratory DC composition in C57BL/6 mice. We also tracked the migration of respiratory DC to the lymph nodes and studied antigen presentation by lung-derived and lymph node-resident DC to CD4+ and CD8+ T cells. We observed a massive influx of mainly CD103− CD11bhigh CD11c+ conventional DC (cDC) and plasmacytoid DC during the first 7 days of RSV infection, while CD103+ CD11blow CD11c+ cDC disappeared from the lung. The two major subsets of lung tissue DC, CD103+ CD11blow CD11c+ and CD103− CD11bhigh CD11c+ cDC, both transported RSV RNA to the lung-draining lymph node. Furthermore, these lung-derived cDC subsets as well as resident LN DC, which did not contain viral RNA, displayed viral antigen by major histocompatibility complex class I and class II to CD8+ and CD4+ T cells. Taken together, our data indicate that during RSV infections, at least three DC subsets might be involved during the activation of lymph node-homing naïve and memory CD4+ and CD8+ T cells.
Respiratory syncytial virus (RSV) constitutes a major health burden for infants, elderly people, and immunocompromised individuals (16, 19). The virus infects most children in their first year of life and is the main cause of severe lower respiratory tract infections in infants (19). Despite many decades of research, the immune response to RSV is still not completely understood. Infection with RSV leads to poor development of immunity, and recurrent infections are common (23). In mice, it was found that RSV induces virus-specific CD8+ T-cell responses in the lung that are functionally impaired (10). It has been suggested that a functional inactivation of CD8+ T cells by RSV could be a reason for the short-lived immune response. Furthermore, we and others have previously shown that human monocyte-derived dendritic cells (DC) can be infected with RSV, which results in a strong inhibition of their ability to support proliferative responses and induction of effector function in naïve T cells (11, 12). An early vaccine trial with formalin-inactivated RSV in alum administered intramuscularly elicited a memory immune response that caused a strong aberrant secondary immune response in vaccinees upon natural exposure with live virus. This resulted in a high rate of morbidity in the vaccinated children (31). These observations underscore the necessity to understand the components of the immune response that are protective during RSV infections and the need to understand the mechanism by which protective immunity can be elicited for the development of an effective and safe vaccine.
DC play an important role in the initiation of both the innate and adaptive immune responses to pathogens including RSV (3). They are a heterogeneous population of cells represented by two main subsets, the myeloid or “conventional” CD11c+ DC (cDC) and the CD11clow/mPDCA-1+ plasmacytoid DC (pDC) (47, 52). cDC can be further divided based on the expression of surface markers and anatomic location. cDC in the tissue and cDC in lymph nodes (LN) appear to be different subsets arising from different pools of progenitor cells and with specialized functions (13, 17, 30, 33, 46). In the mouse lung, two major cDC populations are derived from blood monocytes. CD11c+ major histocompatibility complex class II (MHC-II)-positive (MHC-II+) CD103− CD11bhigh cDC (CD11bhi cDC) are localized in the parenchyma. These cells are the main producers of chemokines and are important for the recruitment of leukocytes (4). A second cDC population, CD11c+ MHC-II+ CD103+ CD11blow cDC (CD103+ cDC), is located directly underneath the airway epithelium. These CD103+ cDC express the integrin αEβ7; therefore, they are found mainly at the basal lamina of the bronchial epithelia and arterioles, which express E-cadherin, the ligand for αEβ7. Furthermore, CD103+ cDC express the tight-junction proteins ZO-2 and claudin-7, which enables them to sample the airways with their extensions (45). In the lung-draining LN, in addition to pDC, at least two steady-state populations of cDC are present, which are characterized by the expression or absence of CD8α. In contrast to the lung tissue DC, these cells enter the LN from the blood, and they are directly derived from a bone marrow precursor (38, 39, 41). In addition, minor fractions of tissue-derived cDC also access draining LN in the steady state (28). Several studies have addressed the roles of different DC subsets that are present in the tissue and LN draining the infection site. In spleen and skin-draining LN, the role of CD8α+ cDC seems to be important for the initiation of anti-ovalbumin and antiviral CD8+ T-cell responses (6, 26, 35). In mice exposed to innocuous (ovalbumin) or infectious (influenza virus) antigen, functional specialization was described for CD103+ and CD11bhi lung cDC subsets. CD11bhi cDC presented intranasally administered ovalbumin or influenza virus antigen mainly to naïve CD4+ T cells, while CD103+ cDC were important for the induction of CD8+ T-cell responses (14, 32).
The ability of DC to present or cross-present antigens depends on the type of antigenic materials and the uptake mechanism used by antigen-presenting cells. Hence, different pathogens and innocuous antigens might be differently presented by different DC subsets. We studied the kinetics of lung DC migration and repopulation during primary RSV infection in C57BL/6 mice. We found that upon RSV infection, CD103+ cDC disappeared from the lung, while there was a net increase in numbers of CD11bhi cDC, pDC, and macrophages. Within the first 48 h after virus exposure, both CD103+ and CD11bhi cDC rapidly migrated to the lung-draining mediastinal LN (MLN), while this accumulation was absent in the non-lung-draining axillary LN. The migrating cDC showed the highest level of expression of the costimulatory molecules CD40, CD80, and CD86, which are necessary for T-cell stimulation, compared to the MLN-resident cDC. Furthermore, the migrating cDC transported viral RNA to the MLN and were capable of stimulating RSV-specific CD4+ and CD8+ T-cell responses. Resident cDC in the LN were uniformly negative for viral RNA. However, resident cDC in the LN did present viral antigen to CD8+ and CD4+ T cells via MHC-I and MHC-II, respectively.
MATERIALS AND METHODS
Mice.
Pathogen-free 6- to 8-week-old female C57BL/6cjo mice were purchased from Charles River Nederland (Maastricht, The Netherlands). The mouse study protocol was approved by the Animal Ethics Committee of the University Medical Center Utrecht and Utrecht University.
Viruses and infections.
RSV strain A2 was grown on HEp-2 cells and purified by polyethylene glycol 6000 precipitation. Mice were lightly anesthetized with isoflurane and intranasally infected with 106 PFU RSV in a volume of 50 μl diluted in phosphate-buffered saline (PBS) with 10% sucrose.
Isolation of tissue DC.
Mice were sacrificed by intraperitoneal injection of pentobarbital. After performing bronchial alveolar lavage (BAL), the lungs were perfused with 10 ml ice-cold PBS containing 100 U/ml heparin via the right ventricle. The lungs and mediastinal and axillary LN were removed and cut into pieces. The fragments were digested for 25 min at 37°C in 5% CO2 with 3.2 mg collagenase A and 1 mg DNase (Roche Applied Science, Basel, Switzerland). For the last 5 min, 1 mM EDTA was added. Single-cell suspensions were prepared from the pretreated LN by processing the tissue through cell strainers (BD Falcon, Franklin Lakes, NJ).
Flow cytometry.
The DC of the lung and LN were prepared as described above. All cell suspensions were preincubated with 5 μg/ml blocking antibody against CD16/CD32 (2.4G2), obtained from BD biosciences (San Diego, CA), before staining to reduce nonspecific binding. The cells were stained in PBS containing 2% fetal calf serum, 2 mM EDTA, and 0.02% NaN3 with the following monoclonal antibodies: anti-CD4 (L3T4), anti-CD8α (clone 53-6.7), anti-CD11b (clone M1/70), anti-CD11c (clone HL3), anti-CD19 (clone 1D3), anti-CD40 (clone 3/23), anti-CD45R (B220, clone RA3-6B2), anti-CD80 (clone 16-10A1), anti-CD86 (clone GL1), anti-CD103 (clone M290), and anti-MHC-II (I-Ab/I-Eb) (clone M5/114.15.2), obtained from BD Biosciences (San Diego, CA), and anti-mPDCA-1 (clone JF05-1C2.4.1), obtained from Miltenyi Biotec (Germany). Flow cytometry was performed using a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA). Data were analyzed using CellQuest software (BD Biosciences).
CFSE labeling of migrating DC.
To detect migrating DC, mice were lightly anesthetized with isoflurane, and 50 μl 8 mM carboxyfluorescein succinimidyl ester (CFSE) (Fluka, Buchs, Switzerland) diluted in PBS was intranasally administered 6 h before intranasal infection with RSV. This method is based on labeling of intracellular proteins and is stable for at least 8 weeks in nondividing lymphocytes. Cells that undergo cell division can still be distinguished from unlabeled cells after at least five to six cell divisions (36, 51). This labeling procedure has frequently been used to stain cells in the lung after intranasal administration and allowed the tracking of respiratory DC migration. At least 95 to 100% of all respiratory DC labeled in vivo remained CFSE positive up to 6 days after CFSE instillation (7, 8, 21, 27, 29, 34, 37; our unpublished results). At various time points after RSV infection, DC were isolated from the MLN and axillary LN to study the kinetics of migration.
RSV-specific reverse transcription (RT)-PCR.
LN were processed as described above. Subsequently, cDC subsets were sorted on a FACSAria apparatus (BD Biosciences) based on the expression of CD11c and CD103 and the uptake of CFSE. RSV RNA was extracted from 1 × 104 DC using the RNeasy minikit (Qiagen, Valencia, CA) according to the instructions provided by the manufacturer. cDNA was prepared with reverse transcriptase using 2.5 μM random hexamers for 5 min at 25°C, 30 min at 48°C, and 10 min at 95°C. Real-time PCR for the RSV N gene was performed using an ABI Prism 7700 sequence detector (Applied Biosystems, Foster City, CA) using 10 μl of sample in a total volume of 25 μl of master mix under the following run conditions: 1 cycle for 2 min at 50°C and 10 min at 95°C, followed by 45 cycles for 15 s at 95°C and 1 min at 60°C. The following primers and 6-carboxyfluorescein-labeled probe were used: RSV forward primer AGA TCA ACT TCT GTC ATC CAG CAA, RSV reverse primer TTC TGC ACA TCA TAA TTA GGA GTA TCA AT, and RSV probe CAC CAT CCA ACG GAG CAC AGG AGA T. Known concentrations of RSV A2 were used to derive a standard curve. Standards and negative controls were run together with each PCR mixture; the lower limit of detection of the assay was 12 viral copies/ml.
Detection of antigen presentation by LN DC.
At several time points after RSV infection, the MLN cDC populations were isolated as described above and sorted on a FACSAria apparatus (BD Biosciences). MLN cDC (1 × 104 cells) were cocultured with 105 lung lymphocytes harvested 8 days after primary infection and before coculture with cDC depleted of CD19-, NK1.1-, and CD4- or CD8-positive cells. An enzyme-linked immunospot (ELISPOT) assay was performed to detect gamma interferon (IFN-γ) production. The mouse IFN-γ ELISPOT pair (U-cytech, Utrecht, The Netherlands) and Multiscreen-IP filter plates (Millipore, Billerica, MA) were used according to the manufacturers' instructions. Cells were stimulated in 200 μl Iscove's modified Dulbecco's medium (Gibco, Invitrogen) containing 10% fetal calf serum, penicillin-streptomycin, and 50 μM 2-mercaptoethanol with 25 U/ml recombinant human interleukin-2 for 24 h at 37°C in 5% CO2.
RESULTS
Rapid alterations in DC composition in the lung during RSV infection.
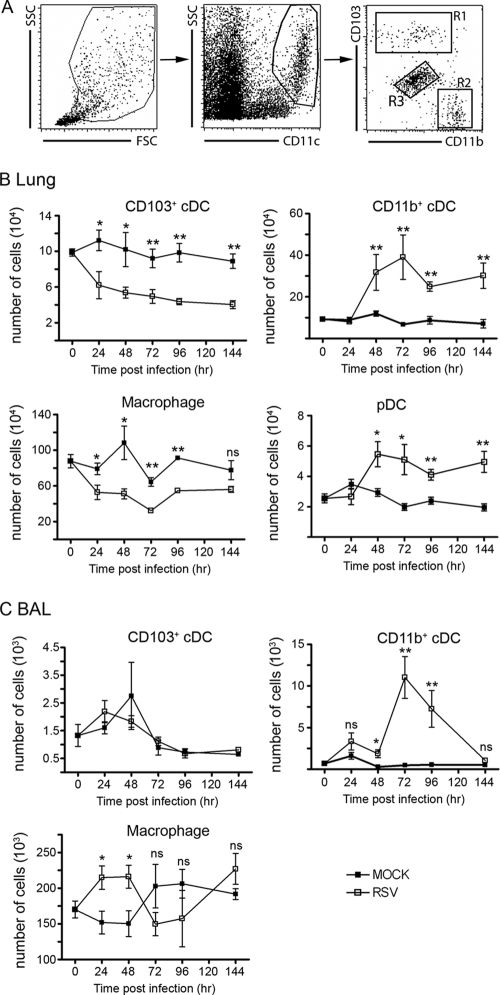
To study the role of RSV infection in lung DC populations, we infected C57BL/6 mice intranasally with RSV or uninfected supernatant of HEp-2 cells as a mock infection. At several time points after infection, we performed BAL to remove most of the alveolar macrophages before analysis of lung DC numbers and phenotypes with flow cytometry. The CD103+ and CD11bhi cDC as well as lung macrophages could easily be detected (Fig. 1A). Within 24 h, we found a rapid decline in absolute numbers of CD103+ cDC and lung macrophages in RSV-infected compared to mock-infected animals (Fig. 1B). Absolute numbers of CD103+ cDC remained significantly smaller throughout the first 6 days of infection. This suggested that the CD103+ cDC had migrated out of the lung or died because of the infection and were not replenished by precursors. In contrast, absolute numbers of CD11bhi cDC and pDC remained constant during the first 48 h of RSV infection and significantly increased during the following 5 days compared to mock-infected mice (Fig. 1B).
FIG. 1.
Migration characteristics of DC subsets in lung and BAL fluid during primary RSV infection. (A) Lungs of naïve mice were used for gating strategies to identify the different cDC populations. R1, CD103+ cDC; R2, CD11bhi cDC; R3, pulmonary macrophages; SSC, side scatter; FSC, forward scatter. (B) At several time points after primary RSV and mock infections, the absolute numbers of CD103+ CD11bhi cDC, macrophages, and pDC (CD11clow, mPDCA-1+, and CD45R/B220+) were determined in the lungs. (C) Similarly, absolute numbers of CD103+ and CD11bhi cDC and alveolar macrophages in the BAL fluid were determined. The experiment was performed twice with five mice per time point. Average values of absolute DC numbers per mouse lung are depicted. Error bars represent the standard errors of the means (SEM) (*, P < 0.05; **, P < 0.01; ns, not significant).
We also enumerated CD103+ and CD11bhi cDC populations in BAL fluid samples to determine whether migration into the airway lumen occurred. We found that the majority of cells in the BAL fluid consisted of alveolar macrophages. There were no significant alterations in absolute numbers of CD103+ cDC in the BAL fluid during an RSV infection. Similar to the lung tissue, we observed an increase in the numbers of CD11bhi cDC in BAL fluid, which was delayed by 24 h compared to that in the lung (Fig. 1C). In conclusion, RSV infection induced a rapid alteration of the DC composition in the lung. We observed a disappearance of CD103+ cDC, while there was an accumulation of CD11bhi cDC and pDC in the lung during RSV infection.
Migration of lung DC to the lung-draining LN.
Naïve T-cell priming occurs in LN draining the site of infection. To study the kinetics of lung DC migration to the lung-draining MLN, we administered the fluorescent dye CFSE intranasally to mice to stably label all the cells in the lung 6 h before intranasal RSV infection (34). This procedure enabled us to track DC migrating from the lung to the MLN during RSV infection and separate migrating from resident DC in the LN with flow cytometry. CFSE treatment alone did not induce significant DC migration, as shown by the minimal numbers of CFSE-labeled cells in the MLN after mock infection (Fig. 2C). After intranasal infection with RSV, predominantly CD11c+ cells were labeled with CFSE in the MLN (Fig. 2A). There was an 8- to 10-fold increase in absolute numbers of cDC during RSV infection in comparison to mice that received a mock infection (Fig. 2B). Absolute numbers of CFSE-labeled cDC in the MLN showed a steep rise in RSV-infected mice, which peaked at 36 h after RSV infection. After 36 h, there was a gradual decline in absolute numbers of CFSE-positive (CFSE+) cDC, while there was still an accumulation of cDC in the MLN (Fig. 2B and C). This might be explained by the migration of CFSE-negative (CSFE−) cDC that originated from cells that entered the lung later after CFSE labeling and hence were not efficiently labeled, or alternatively, these cells were not lung derived but entered the LN from the blood.
FIG. 2.
Migration of cDC to the lung-draining LN (MLN) during primary RSV infections. (A) Mice were given CFSE intranasally 6 h before RSV or mock infection. Using flow cytometry, CFSE+ cDC subsets were identified in the MLN. (B) At several time points after RSV or mock infection, absolute numbers of CD11c+ cDC were determined in MLN and in RSV-infected mice in axillary LN (AxLN). (C) Lung-derived CFSE+/CD11c+ cDC were quantified per lung-draining LN and in axillary LN after RSV exposure or mock infection. (D) During primary RSV infection, absolute numbers of CD103+ cDC and CD11bhi cDC per MLN were determined. (E and F) Percentages (E) and absolute numbers (F) of lung-derived (CFSE+) CD103+ cDC and CD11bhi cDC per MLN during RSV infection were determined. The experiment was performed twice with five mice per time point. Average values of absolute cDC numbers per mouse MLN are depicted. Error bars represent the SEM (*, P < 0.05; **, P < 0.01; ns, not significant).
In the lungs, we found a net accumulation of CD11bhi cDC, while CD103+ cDC numbers declined in the lung during RSV infection. To determine to what extent both populations migrated to the MLN during the RSV infection, we analyzed the fractions of CFSE+ and CSFE− cells within these subpopulations in the MLN. We found equal numbers of total CD103+ and CD11bhi cDC in the MLN during the first 36 to 48 h after RSV infection (Fig. 2D). Both CD103+ and CD11bhi cDC contributed equally to the accumulation of cDC in the MLN during the first 36 to 48 h of RSV infection, as the same percentages of CD103+ and CD11bhi cDC were labeled by CFSE (Fig. 2E). Later during the RSV infection, predominantly unlabeled CD11bhi cDC were responsible for the accumulation of cDC in the MLN (Fig. 2D and F). Thus, we observed a two-step accumulation of cDC in MLN after RSV infection. In the first 48 h, an influx of equal numbers of both subsets of lung cDC was followed in the next 48-h period by the influx of unlabeled cDC, which might not be lung derived.
Migrating cDC have a more mature phenotype than LN-resident cDC.
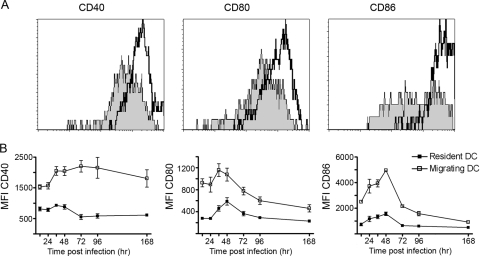
For efficient T-cell stimulation, DC have to be mature; i.e., they should express or upregulate T-cell-costimulatory molecules such as CD40, CD80, and CD86. Therefore, we measured the maturation status of the cDC in the MLN by staining these cells with CD40-, CD80-, and CD86-specific antibodies and compared the expression levels of these surface markers between resident and migrant cDC. We found that at 36 h after RSV infection, the CFSE+ migrating cDC had a higher level of expression of all three costimulatory molecules than did the CFSE− cDC (resident cDC), as judged by the mean fluorescence intensity (Fig. 3A). Over time, the level of expression of CD40 remained significantly higher on migrating cDC than on resident cDC. The levels of expression of CD80 and CD86 also remained significantly higher on migrant cDC than on resident cDC, but in contrast to a constant level of CD40 expression, these molecules showed a clear peak between 36 and 48 h after RSV infection (Fig. 3B).
FIG. 3.
Migrating cDC have a more mature phenotype than do LN-resident cDC. (A) At 48 h after primary RSV infection, MLN cDC were stained with anti-CD40, -CD80, and -CD86. Based on CFSE staining, the cDC were divided into LN-resident cDC (filled histogram) and migrating cDC (open histogram). (B) At several time points after primary RSV infection, the levels of expression of CD40, CD80, and CD86 on LN-resident cDC and migrating cDC were determined. The experiment was performed twice with five mice per time point. Average mean fluorescence intensities (MFI) are depicted. Error bars represent SEM.
Transport of viral RNA from the lung to the lung-draining LN by lung-derived cDC.
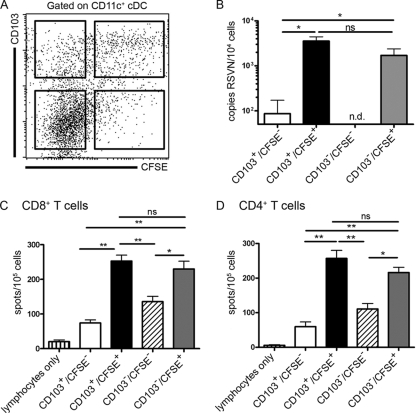
DC start a program of maturation after contact with inflammatory cytokines, pathogens, or tissue damage. The lung-derived cDC that arrived in the MLN had a mature phenotype required for efficient T-cell stimulation. To test whether these cells had acquired RSV via either direct infection or uptake of RSV-containing cell debris, we assayed MLN cDC for the presence of RSV RNA. The MLN were harvested 72 h after RSV infection, and the cDC were sorted based on the expression of CD11c and CD103 and labeling by CFSE (Fig. 4A). Since there was an inverse relationship between the expressions of CD103 and CD11b on lung cDC subsets, we used the absence of expression of CD103 to identify CD11bhi cDC. RNA was isolated from 104 cells per population and assessed for the presence of RSV RNA with quantitative RT-PCR for the RSV N gene. We found most of the viral RNA in the cDC that had been labeled with CFSE and, thus, originated from the lung. There was no significant difference in the presence of RSV RNA in CD103+/CFSE+ or CD103−/CFSE+ cDC, suggesting that they had been infected or had internalized similar amounts of RSV. The CD103+/CFSE− cDC contained very low levels of viral material. The expression of CD103 identified these cells as being lung derived. These cells might have been poorly labeled, or most of these cells might have migrated before CFSE labeling. In unlabeled CD103− cells, the fraction composed of LN-resident DC and possibly inflammatory monocyte-derived DC originating from blood, no viral RNA could be detected (Fig. 4B).
FIG. 4.
Migrating CFSE+ cDC transport viral RNA to the lung-draining LN and present antigens by MHC-I and MHC-II molecules. (A) MLN cDC were harvested 72 h post-RSV infection and sorted into four populations based on CD103 expression and CFSE labeling. (B) Sorted MLN cDC (104 cells/subset) were analyzed for the presence of RSV N RNA by quantitative RT-PCR. (C and D) Antigen presentation was analyzed by culturing RSV-specific CD4+ or CD8+ T-cell populations for 24 h with sorted cDC subsets isolated from the MLN 72 h after RSV infection. To enrich for CD8+ T cells, lung lymphocytes isolated on day 8 after primary RSV infection were depleted of CD4+ T cells, B cells, and NK cells. Similarly, CD4+ T cells were enriched by the depletion of CD8+ T cells, B cells, and NK cells. RSV-specific IFN-γ production was measured by ELISPOT assay. Data shown are the means of data from six individual experiments for the RT-PCR assay. For the ELISPOT assay, the mean values for five individual experiments using cDC purified from five individual mice for each experiment are shown. Error bars represent SEM (*, P < 0.05; **, P < 0.01; ns, not significant; n.d., not detected).
MHC-I and MHC-II restricted antigen presentation by lung-derived DC.
The lung-derived cDC in the MLN contained most of the RSV RNA and were likely candidates to contribute to the induction of RSV-specific T-cell responses. However, several reports have shown that T-cell responses are induced primarily by the LN-resident DC (1) or by both LN-resident and airway-derived cDC (7). Furthermore, it is unclear how the different airway cDC types contribute to MHC-I and MHC-II antigen presentation during RSV infection. To investigate which cDC type was involved in antigen presentation to CD4+ and CD8+ T cells during RSV infection, we again sorted MLN cDC 72 h after RSV infection, based on the expression of CD11c and CD103 and CFSE labeling. These sorted MLN cDC were cocultured with lung lymphocytes isolated 8 days after primary RSV infection that were depleted of B and NK cells and either CD4+ or CD8+ T cells. The enriched CD4+ or CD8+ T-cell populations were cocultured with the different cDC subsets, and the level of IFN-γ production by the virus-specific effector CD8+ or CD4+ T cells was assessed with an IFN-γ ELISPOT assay. We found that the CFSE-labeled cDC subsets were the most effective inducers of IFN-γ production by CD8+ T cells. There was no significant difference in IFN-γ production by CD8+ or CD4+ T cells when these cells where stimulated with CD103+ or CD103− lung-derived (CFSE+) cDC in the T-cell DC ratios used (Fig. 4C and D). CD103+/CFSE− cDC activated a smaller number of CD4+ and CD8+ T cells, which could be explained by the smaller amount of viral RNA detected in this population of lung-derived cells (Fig. 4B to D). Interestingly, a clear response of CD4+ and CD8+ T cells against the CD103−/CFSE− DC population of non-lung-derived DC that did not contain viral RNA was also observed (Fig. 4B to D). In summary, we found that virus-exposed lung cDC subsets migrating to the MLN as well as non-lung-derived cells present in MLN could present viral antigen to CD4+ and CD8+ effector T cells. Moreover, we found no indication that different cDC subsets preferably processed and presented viral materials for presentation to either the CD4+ or CD8+ T-cell subset.
DISCUSSION
In this study, we showed that both major lung cDC subsets, the subepithelial CD103+ CD11blow and the parenchymal CD103− CD11bhigh DC, as well as a population of non-lung-derived MLN cDC display viral antigen in the context of MHC-I and MHC-II molecules in MLN after intranasal RSV infection. Thus, migratory lung DC that might have been directly infected as judged from the observed presence of viral RNA, as well as MLN cDC that did not originate from the upstream infection site in which no viral RNA was detected, triggered T-cell activation in vitro (Fig. 4). In recent studies with influenza virus infection models and intranasal installation of ovalbumin, a functional specialization of cDC subsets in the lung has been described. CD11bhi and CD103+ cDC were found to differ in their efficacies to present antigen to naïve CD8+ and CD4+ T cells (14, 32). It was shown that CD103+ cDC had the unique capacity to cross-present exogenously acquired (noninfectious) antigen in vitro to naïve CD8+ T cells (14). In contrast, CD11bhi lung DC appeared to be more effective in the induction of naïve CD4+ T-cell proliferation. However, the observed functional dichotomy might be less pronounced when antigen is acquired via direct infection. Indeed, there was a clear difference in the abilities of CD11bhi cDC to present live and noninfectious influenza virus particles (32). Upon intranasal infection with live influenza virus, CD11bhi cDC could present antigen by MHC-I molecules to naïve CD8+ T cells in vitro albeit at a somewhat lower efficiency than that of CD103+ cDC. These differences might reflect the more effective uptake of influenza virus by the CD103+ cDC subset that is located directly underneath the infected airway epithelium and/or the fact that these cells are more easily infected by influenza virus in vitro plus the characteristic of these cDC to also be able to capture and present influenza virus-derived antigen via a noninfectious route (24, 32). In our experiments with RSV, we found no substantial difference in the infection levels of both lung cDC subsets as measured by viral RNA content (Fig. 4). Using effector T cells as a readout system to measure antigen display by MHC-I and MHC-II molecules, we found that both migrating lung cDC subsets presented RSV-derived antigens to CD4+ and CD8+ T cells. The presence of viral RNA indicates that a proportion of these DC had been directly infected, but also, material transferred to DC from infected epithelial cells might have contributed to the antigen displayed to T cells. Migrating cDC had upregulated costimulatory molecules CD80, CD86, and CD40. The roles and relative efficacies of the two lung-derived cDC subsets and LN-resident cDC during the initiation of RSV-specific T-cell responses in vivo are unclear. Because T-cell receptor-transgenic mice are not available for RSV, it was not possible for us to study the in vitro stimulation of naïve CD4+ and CD8+ T-cell responses. However, such in vitro studies are inadequate to determine the exact in vivo role of DC in initiating T-cell responses. It was elegantly shown by Allenspach et al. that although migratory DC activated naïve CD4+ T-cell proliferation in vitro, they were unable to initiate T-cell responses in vivo, possibly because they cannot anatomically interact with naïve T cells in the LN (2). Interestingly, the role of migratory cDC was not limited to antigen delivery into the node, as suggested by previous studies, because the full expansion of antigen-specific T cells required, in addition to primary contacts with LN-resident cDC, secondary contacts with migratory cDC (1, 2). A similar cooperation of migrating and resident cDC subsets might be needed for the CD8+ T-cell response because studies in vivo in which CD8α+ LN and splenic cDC were depleted showed the essential role of this DC subset in the initiation of antiviral, antitumor, and ovalbumin-specific CD8+ T-cell responses (6, 7, 26, 35). Because those studies did not look at the role of CD8α+ cDC after the administration of antigen via the intranasal/intratracheal route, it is unclear whether the role of LN-resident cDC in the MLN is obligatory, as it is after antigen exposure via intravenous or intradermal administration (5). The CD103+ lung cDC population has many similarities with the LN-resident CD8α+ cDC subset. They share the ability to cross-present and to react to Toll-like receptor 3 stimulation, and in spleen, CD103 is coexpressed with CD8α. They may therefore function in a similar way in the MLN (14, 15, 45).
For effective in vivo activation of T-cell responses, other cellular interactions might be required. In addition to multiple DC-T-cell contacts, licensing of cDC by CD4+ T cells might contribute to the efficacy of DC to prime CD8+ T-cell responses in vivo, and after primary trigger in the LN, an additional activation of T cells might occur when activated T cells migrate into the infected tissue (37, 44). Thus, the complete picture of how effective T-cell responses are elicited can be learned only from experiments with intact hosts. Because it is currently not possible to delete or functionally inactivate single DC populations in the respiratory tract, the exact in vivo role during T-cell activation awaits tools or genetically engineered mice to perform these experiments.
We found a massive increase in total cDC numbers in the MLN, with a peak influx 96 h after RSV infection. There were five- to eightfold-more cDC in the MLN during RSV infection than in mock-infected mice. The peak influx of CFSE+ cDC occurred 36 h after RSV infection. This is a slower accumulation of cDC in the MLN, as was reported previously for influenza or Sendai virus infection, where the peak influx of respiratory cDC in the MLN is 12 to 24 h after infection (21, 34, 37). This can be explained by differences in virus dose or innate detection of the different viruses by pattern recognition receptors, resulting in a different pattern and, possibly, a different tempo of the inflammatory response. The source of accumulating unlabeled CD11bhi cDC in MLN during RSV infection is not clear. These cells might not be lung derived but enter the LN via blood. Alternatively, they might be derived from cells that enter the lung after CFSE labeling or develop from DC precursors after extensive cell division, which results in the dilution of the CFSE label. It was previously reported that DC precursors that can divide extensively and develop into DC upon exposure to granulocyte-macrophage colony-stimulating factor exist in the lung (50). In contrast, immature DC and mature DC do not extensively divide in the course of 4 days, making these committed DC from the lung an unlikely source of CFSE− cells in the draining LN (53). It has been shown that respiratory cDC migrate only during a short period of time after infection and subsequently become refractory to activation signals, resulting in the inability of subsequent infections to induce DC migration (34). Therefore, local development of DC from lung or monocyte precursors most likely contributes to the enhanced CD11b+ DC numbers in the lung tissue (Fig. 1B), and the late-expanding unlabeled CD11bhi cells in MLN are most likely not lung derived.
We and others have shown that human DC infected with RSV are poor inducers of T-cell proliferation (11, 12, 22). Also, CD4+ T cells that do expand upon exposure to RSV-infected DC do not develop effector function to an extent similar to that of T cells cultured with influenza virus-infected DC or DC matured with Toll-like receptor ligands or cytokines. The exact mechanism of the inhibition effect is still not completely clear. Cell contact mechanisms as well as unidentified soluble components in RSV-DC cultures might contribute to inefficient DC function (11, 12, 20, 22, 40). Despite the fact that RSV infection impairs DC function, we have observed quite robust functional T-cell responses in peripheral blood of infants during primary RSV infections (25). These observations indicate that in vivo cross-presentation could be an important pathway of T-cell priming. Thus, a role for LN-resident DC that are not infected might be important during the activation of RSV-specific T-cell responses.
During respiratory infection with RSV and influenza virus, an increase in DC populations in the lungs of humans and mice has been observed (9, 18) (Fig. 1B). These cells might play a role in the additional activation of LN-primed T cells and/or be important as local antigen-presenting cells that stimulate effector/memory T cells that are directly recruited to inflammatory sites (37, 48, 49). Furthermore, they could also play a role in the production of cytokines and chemokines that are involved in the attraction of different inflammatory cell types (13). Previous studies of RSV-infected mice reported the increase of cDC and pDC populations in the lung, similar to our observations (Fig. 1) (9, 42, 43). However, in those studies, the cDC subsets that accumulated were not further characterized. We show that upon RSV infection, both CD103+ and CD11bhi cDC migrate in similar numbers to the draining LN but that only CD11bhi cDC are replenished and reach higher levels than originally present in the lung tissue. In contrast to LN-resident cDC that develop from precursor cells in the bone marrow, blood monocytes are the precursor cells that give rise to the two major lung tissue cDC subsets (13, 30, 33, 38). Ly6C(Gr1)high CCR2high CX3CR1int monocytes develop into CD103+ cDC, whereas Ly6C(Gr1)low CCR2low CX3CR1high monocytes develop into CD11bhi cDC under homeostatic conditions (30). Landsman et al. previously showed that both monocyte subsets also give rise to cDC under inflammatory (lipopolysaccharide given intratracheally) conditions (33). However, the phenotype of the resulting cDC populations was not determined. It is unclear whether the accumulating CD11bhi cDC subset in the lung after RSV infection is derived from a single monocyte precursor and whether this is different during different viral infections. It is an intriguing idea that the type of inflammatory response induced by the infecting virus might impact the pattern of DC repopulation. During influenza virus infection, CD11bhi cDC numbers were also increased, while it appears that CD103+ cDC numbers stayed stable during the first 3 days after influenza virus infection, suggesting that these cells were replenished but to a lower extent than that for CD11bhi cDC (32).
It was previously described that local ratios of cDC and pDC in the lung might affect the severity of RSV disease, whereby pDC might ameliorate disease by shifting the T-cell response to a Th1 type of response and potentiate more effective viral clearance (42, 43). The exact mechanism behind this more beneficial response by enhanced pDC/cDC ratios needs to be established. Possibly, enhanced IFN-γ-producing CD8+ T-cell numbers could ameliorate Th2 CD4+ T-cell responses. However, it is currently unclear how pDC contribute to enhanced CD8+ T-cell responses; clearly, IFN-α is not crucial for this process (43).
We have shown that at least three subsets of cDC display RSV-derived antigen in the context of MHC-I and MHC-II molecules in the LN draining the infected lung. These subsets might thus be involved in activating RSV-specific T cells circulating through the LN. We further identified the cDC subset that enters the lung after RSV infection as being CD11bhi cells, while CD103+ cells are not replenished in the first 8 days after RSV infection. Future work aimed at studying the contribution of DC subsets during secondary immune responses after natural infection or vaccination and in the presence of preexisting (maternal) antibodies will contribute to our understanding of the exact role that these cells play during protective or pathological immune responses. These studies may therefore contribute to the design of safe vaccine approaches.
Acknowledgments
This work was supported by grant no. OZF-02-004 from the Wilhelmina Research Fund.
Footnotes
Published ahead of print on 6 May 2009.
REFERENCES
- 1.Allan, R. S., J. Waithman, S. Bedoui, C. M. Jones, J. A. Villadangos, Y. Zhan, A. M. Lew, K. Shortman, W. R. Heath, and F. R. Carbone. 2006. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25153-162. [DOI] [PubMed] [Google Scholar]
- 2.Allenspach, E. J., M. P. Lemos, P. M. Porrett, L. A. Turka, and T. M. Laufer. 2008. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity 29795-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. [DOI] [PubMed] [Google Scholar]
- 4.Beaty, S. R., C. E. Rose, Jr., and S. S. Sung. 2007. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J. Immunol. 1781882-1895. [DOI] [PubMed] [Google Scholar]
- 5.Belz, G. T., K. Shortman, M. J. Bevan, and W. R. Heath. 2005. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J. Immunol. 175196-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belz, G. T., C. M. Smith, D. Eichner, K. Shortman, G. Karupiah, F. R. Carbone, and W. R. Heath. 2004. Conventional CD8alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J. Immunol. 1721996-2000. [DOI] [PubMed] [Google Scholar]
- 7.Belz, G. T., C. M. Smith, L. Kleinert, P. Reading, A. Brooks, K. Shortman, F. R. Carbone, and W. R. Heath. 2004. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. USA 1018670-8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belz, G. T., L. Zhang, M. D. Lay, F. Kupresanin, and M. P. Davenport. 2007. Killer T cells regulate antigen presentation for early expansion of memory, but not naive, CD8+ T cells. Proc. Natl. Acad. Sci. USA 1046341-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beyer, M., H. Bartz, K. Horner, S. Doths, C. Koerner-Rettberg, and J. Schwarze. 2004. Sustained increases in numbers of pulmonary dendritic cells after respiratory syncytial virus infection. J. Allergy Clin. Immunol. 113127-133. [DOI] [PubMed] [Google Scholar]
- 10.Chang, J., and T. J. Braciale. 2002. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat. Med. 854-60. [DOI] [PubMed] [Google Scholar]
- 11.Chi, B., H. L. Dickensheets, K. M. Spann, M. A. Alston, C. Luongo, L. Dumoutier, J. Huang, J. C. Renauld, S. V. Kotenko, M. Roederer, J. A. Beeler, R. P. Donnelly, P. L. Collins, and R. L. Rabin. 2006. Alpha and lambda interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. J. Virol. 805032-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Graaff, P. M., E. C. de Jong, T. M. van Capel, M. E. van Dijk, P. J. Roholl, J. Boes, W. Luytjes, J. L. Kimpen, and G. M. van Bleek. 2005. Respiratory syncytial virus infection of monocyte-derived dendritic dells decreases their capacity to activate CD4 T cells. J. Immunol. 1755904-5911. [DOI] [PubMed] [Google Scholar]
- 13.del Rio, M. L., J. I. Rodriguez-Barbosa, J. Bolter, M. Ballmaier, O. Dittrich-Breiholz, M. Kracht, S. Jung, and R. Forster. 2008. CX3CR1+c-kit+ bone marrow cells give rise to CD103+ and CD103− DC with distinct functional properties. J. Immunol. 1816178-6188. [DOI] [PubMed] [Google Scholar]
- 14.del Rio, M. L., J. I. Rodriguez-Barbosa, E. Kremmer, and R. Forster. 2007. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J. Immunol. 1786861-6866. [DOI] [PubMed] [Google Scholar]
- 15.Edwards, A. D., D. Chaussabel, S. Tomlinson, O. Schulz, A. Sher, and C. Reis e Sousa. 2003. Relationships among murine CD11c(high) dendritic cell subsets as revealed by baseline gene expression patterns. J. Immunol. 17147-60. [DOI] [PubMed] [Google Scholar]
- 16.Falsey, A. R., P. A. Hennessey, M. A. Formica, C. Cox, and E. E. Walsh. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 3521749-1759. [DOI] [PubMed] [Google Scholar]
- 17.Geissmann, F., C. Auffray, R. Palframan, C. Wirrig, A. Ciocca, L. Campisi, E. Narni-Mancinelli, and G. Lauvau. 2008. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol. Cell Biol. 86398-408. [DOI] [PubMed] [Google Scholar]
- 18.Gill, M. A., K. Long, T. Kwon, L. Muniz, A. Mejias, J. Connolly, L. Roy, J. Banchereau, and O. Ramilo. 2008. Differential recruitment of dendritic cells and monocytes to respiratory mucosal sites in children with influenza virus or respiratory syncytial virus infection. J. Infect. Dis. 1981667-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glezen, P., and F. W. Denny. 1973. Epidemiology of acute lower respiratory disease in children. N. Engl. J. Med. 288498-505. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez, P. A., C. E. Prado, E. D. Leiva, L. J. Carreno, S. M. Bueno, C. A. Riedel, and A. M. Kalergis. 2008. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc. Natl. Acad. Sci. USA 10514999-15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grayson, M. H., M. S. Ramos, M. M. Rohlfing, R. Kitchens, H. D. Wang, A. Gould, E. Agapov, and M. J. Holtzman. 2007. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J. Immunol. 1791438-1448. [DOI] [PubMed] [Google Scholar]
- 22.Guerrero-Plata, A., A. Casola, G. Suarez, X. Yu, L. Spetch, M. E. Peeples, and R. P. Garofalo. 2006. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 34320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall, C. B., E. E. Walsh, C. E. Long, and K. C. Schnabel. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 163693-698. [DOI] [PubMed] [Google Scholar]
- 24.Hao, X., T. S. Kim, and T. J. Braciale. 2008. Differential response of respiratory dendritic cell subsets to influenza virus infection. J. Virol. 824908-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heidema, J., M. V. Lukens, W. W. van Maren, M. E. van Dijk, H. G. Otten, A. J. van Vught, D. B. van der Werff, S. J. van Gestel, M. G. Semple, R. L. Smyth, J. L. Kimpen, and G. M. van Bleek. 2007. CD8+ T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J. Immunol. 1798410-8417. [DOI] [PubMed] [Google Scholar]
- 26.Hildner, K., B. T. Edelson, W. E. Purtha, M. Diamond, H. Matsushita, M. Kohyama, B. Calderon, B. U. Schraml, E. R. Unanue, M. S. Diamond, R. D. Schreiber, T. L. Murphy, and K. M. Murphy. 2008. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 3221097-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hintzen, G., L. Ohl, M. L. del Rio, J. I. Rodriguez-Barbosa, O. Pabst, J. R. Kocks, J. Krege, S. Hardtke, and R. Forster. 2006. Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J. Immunol. 1777346-7354. [DOI] [PubMed] [Google Scholar]
- 28.Jakubzick, C., M. Bogunovic, A. J. Bonito, E. L. Kuan, M. Merad, and G. J. Randolph. 2008. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J. Exp. Med. 2052839-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakubzick, C., J. Helft, T. J. Kaplan, and G. J. Randolph. 2008. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J. Immunol. Methods 337121-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakubzick, C., F. Tacke, F. Ginhoux, A. J. Wagers, N. van Rooijen, M. Mack, M. Merad, and G. J. Randolph. 2008. Blood monocyte subsets differentially give rise to CD103+ and CD103− pulmonary dendritic cell populations. J. Immunol. 1803019-3027. [DOI] [PubMed] [Google Scholar]
- 31.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89422-434. [DOI] [PubMed] [Google Scholar]
- 32.Kim, T. S., and T. J. Braciale. 2009. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS ONE 4e4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landsman, L., C. Varol, and S. Jung. 2007. Distinct differentiation potential of blood monocyte subsets in the lung. J. Immunol. 1782000-2007. [DOI] [PubMed] [Google Scholar]
- 34.Legge, K. L., and T. J. Braciale. 2003. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity 18265-277. [DOI] [PubMed] [Google Scholar]
- 35.Lin, M. L., Y. Zhan, A. I. Proietto, S. Prato, L. Wu, W. R. Heath, J. A. Villadangos, and A. M. Lew. 2008. Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset. Proc. Natl. Acad. Sci. USA 1053029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171131-137. [DOI] [PubMed] [Google Scholar]
- 37.McGill, J., N. van Rooijen, and K. L. Legge. 2008. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J. Exp. Med. 2051635-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naik, S. H., P. Sathe, H. Y. Park, D. Metcalf, A. I. Proietto, A. Dakic, S. Carotta, M. O'Keeffe, M. Bahlo, A. Papenfuss, J. Y. Kwak, L. Wu, and K. Shortman. 2007. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 81217-1226. [DOI] [PubMed] [Google Scholar]
- 39.Onai, N., A. Obata-Onai, M. A. Schmid, T. Ohteki, D. Jarrossay, and M. G. Manz. 2007. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. Immunol. 81207-1216. [DOI] [PubMed] [Google Scholar]
- 40.Schlender, J., G. Walliser, J. Fricke, and K. K. Conzelmann. 2002. Respiratory syncytial virus fusion protein mediates inhibition of mitogen-induced T-cell proliferation by contact. J. Virol. 761163-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shortman, K., and S. H. Naik. 2007. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 719-30. [DOI] [PubMed] [Google Scholar]
- 42.Smit, J. J., D. M. Lindell, L. Boon, M. Kool, B. N. Lambrecht, and N. W. Lukacs. 2008. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS ONE 3e1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smit, J. J., B. D. Rudd, and N. W. Lukacs. 2006. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J. Exp. Med. 2031153-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith, C. M., N. S. Wilson, J. Waithman, J. A. Villadangos, F. R. Carbone, W. R. Heath, and G. T. Belz. 2004. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nat. Immunol. 51143-1148. [DOI] [PubMed] [Google Scholar]
- 45.Sung, S. S., S. M. Fu, C. E. Rose, Jr., F. Gaskin, S. T. Ju, and S. R. Beaty. 2006. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J. Immunol. 1762161-2172. [DOI] [PubMed] [Google Scholar]
- 46.Varol, C., S. Yona, and S. Jung. 2009. Origins and tissue-context-dependent fates of blood monocytes. Immunol. Cell Biol. 8730-38. [DOI] [PubMed] [Google Scholar]
- 47.von Garnier, C., L. Filgueira, M. Wikstrom, M. Smith, J. A. Thomas, D. H. Strickland, P. G. Holt, and P. A. Stumbles. 2005. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J. Immunol. 1751609-1618. [DOI] [PubMed] [Google Scholar]
- 48.Wakim, L. M., T. Gebhardt, W. R. Heath, and F. R. Carbone. 2008. Local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J. Immunol. 1815837-5841. [DOI] [PubMed] [Google Scholar]
- 49.Wakim, L. M., J. Waithman, N. van Rooijen, W. R. Heath, and F. R. Carbone. 2008. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319198-202. [DOI] [PubMed] [Google Scholar]
- 50.Wang, H., N. Peters, V. Laza-Stanca, N. Nawroly, S. L. Johnston, and J. Schwarze. 2006. Local CD11c+ MHC class II− precursors generate lung dendritic cells during respiratory viral infection, but are depleted in the process. J. Immunol. 1772536-2542. [DOI] [PubMed] [Google Scholar]
- 51.Weston, S. A., and C. R. Parish. 1990. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J. Immunol. Methods 13387-97. [DOI] [PubMed] [Google Scholar]
- 52.Wikstrom, M. E., and P. A. Stumbles. 2007. Mouse respiratory tract dendritic cell subsets and the immunological fate of inhaled antigens. Immunol. Cell Biol. 85182-188. [DOI] [PubMed] [Google Scholar]
- 53.Winzler, C., P. Rovere, M. Rescigno, F. Granucci, G. Penna, L. Adorini, V. S. Zimmerman, J. Davoust, and P. Ricciardi-Castagnoli. 1997. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 185317-328. [DOI] [PMC free article] [PubMed] [Google Scholar]