Abstract
Crenarchaeol, a membrane-spanning glycerol dialkyl glycerol tetraether (GDGT) containing a cyclohexane moiety in addition to four cyclopentane moieties, was originally hypothesized to be synthesized exclusively by the mesophilic Crenarchaeota. Recent studies reporting the occurrence of crenarchaeol in hot springs and as a membrane constituent of the recently isolated thermophilic crenarchaeote “Candidatus Nitrosocaldus yellowstonii,” however, have raised questions regarding its taxonomic distribution and function. To determine whether crenarchaeol in hot springs is indeed synthesized by members of the Archaea in situ or is of allochthonous origin, we quantified crenarchaeol present in the form of both intact polar lipids (IPLs) and core lipids in sediments of two California hot springs and in nearby soils. IPL-derived crenarchaeol (IPL-crenarchaeol) was found in both hot springs and soils, suggesting in situ production of this GDGT over a wide temperature range (12°C to 89°C). Quantification of archaeal amoA gene abundance by quantitative PCR showed a good correspondence with IPL-crenarchaeol, suggesting that it was indeed derived from living cells and that crenarchaeol-synthesizing members of the Archaea in our samples may also be ammonia oxidizers.
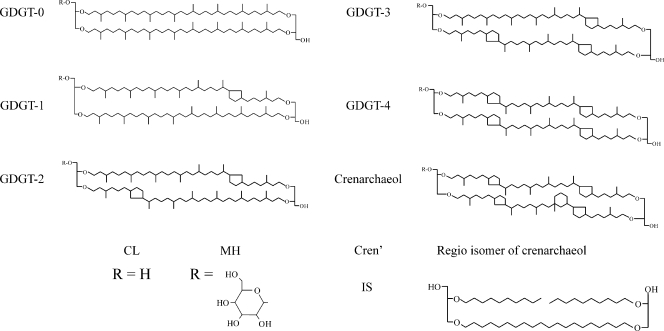
Numerous groups of the Archaea synthesize isoprenoid glycerol dialkyl glycerol tetraethers (GDGTs) as a major component of their core membrane lipids, which can contain up to eight cyclopentane moieties (e.g., see reference 7) (Fig. 1). An increase in the number of cyclopentane moieties results in denser packing of membrane lipids, allowing for the maintenance of both cellular membrane integrity at high temperatures and stable proton gradients under low-pH conditions (8). This biophysical characteristic is hypothesized to be among those traits essential for the survival and persistence of the Archaea in the “extreme” environments in which they are commonly found (42). GDGTs are synthesized by a large number of cultivated members of the Archaea (see overviews in references 20 and 34), and in nature, they are abundant in hot springs (24, 25, 34, 46), for example, where members of the Archaea are known to thrive at high temperatures and over a wide pH range (3, 21).
FIG. 1.
Structures of GDGTs referred to in the text. “IS,” C46 internal standard.
Crenarchaeol is unique among the GDGTs in that it contains a cyclohexane moiety in addition to four cyclopentane moieties (Fig. 1). It was first reported in large abundances from Holocene and ancient sediments collected from various marine settings as supporting evidence for the widespread distribution of low-temperature relatives of the hyperthermophilic Archaea (31). It was later proposed that crenarchaeol was synthesized exclusively by marine group I Crenarchaeota (36), a hypothesis further supported by core lipid analysis of the mesophilic marine group I.1a crenarchaeotes “Cenarchaeum symbiosum” (38) and “Candidatus Nitrosopumilus maritimus” SCM1 (30), which showed that both of these organisms synthesize crenarchaeol at moderate temperatures. In addition to this, the apparent absence of crenarchaeol in cultures of (hyper)thermophilic members of the Archaea (see overviews in references 20 and 34) and molecular modeling (8, 37) led to the hypothesis that crenarchaeol decreases lipid density, effectively allowing archaeal membranes composed of membrane-spanning GDGTs to function at mesophilic temperatures (37). Hence, crenarchaeol synthesis was thought to be instrumental in the evolution and radiation of mesophilic Crenarchaeota from thermophilic habitats (17).
Recent studies, however, have reported the occurrence of crenarchaeol in hot springs with temperatures of up to 86.5°C (24, 25, 34, 46). That work has been debated to some extent, as there exists the potential for the allochtonous input of fossilized lipid material from weathering of nearby soils where mesophilic Crenarchaeota may thrive: Schouten et al. (34) previously found large relative amounts of specific soil bacterium biomarkers in tandem with crenarchaeol in Yellowstone hot springs. In contrast, Reigstad et al. (28) reported the occurrence of crenarchaeol in the absence of soil-specific biomarkers in Icelandic hot springs. Furthermore, the recently isolated thermophilic crenarchaeote “Candidatus Nitrosocaldus yellowstonii” was shown to synthesize crenarchaeol at a growth temperature of 72°C (6).
Core lipids (CLs) that occur in biological membranes generally contain polar head groups such as sugars and phosphates, which are rapidly cleaved upon cell senescence (10, 44). The loss of head groups from intact polar lipids (IPLs) leaves relatively recalcitrant CLs to accumulate in the environment over time as fossil biomarkers. Therefore, depending on the extraction and/or analytical protocols, CLs present in environmental lipid extracts may be derived from both living cells and fossil biomass, including a mixture of both CL-derived GDGTs (CL-GDGTs) and IPL-derived GDGTs (IPL-GDGTs). Most studies of the presence of crenarchaeol in hot springs reported to date have analyzed directly extracted CL-crenarchaeol or CL-crenarchaeol released by the acid hydrolysis of Bligh-Dyer IPL lipid extracts, i.e., without prior separation of CL-GDGTs from IPL-GDGTs (24, 25, 28, 34, 46). In these cases, the reported GDGT distributions represent an integrated signal of both “living” and fossilized material, rendering it impossible to distinguish what proportion (if any) of the observed crenarchaeol was derived from local living archaeal communities. Thus, the in situ production of crenarchaeol in hot springs and its importance relative to that of the in situ production of other archaeal GDGTs remain uncertain.
Here we have used a recently described chromatographic method (22, 26) to separately quantify the potential contributions of both in situ-produced and fossilized crenarchaeol (as well as other archaeal GDGTs) in two Californian hot springs and their surrounding soils. In addition, we have quantified the amounts of archaeal amoA and archaeal 16S rRNA gene copies from one site to make quantitative comparisons between gene abundance and IPL-GDGT concentrations.
MATERIALS AND METHODS
Sampling and study area.
Material from two hot springs, Leonard's hot spring (41°36.086"N, 120°05.135"W) and “Ray's” hot spring (41°31.855"N, 120°04.966"W), both located in Surprise Valley in northwestern California, as well as soils in a perpendicular transect extending from the hot springs were sampled in January 2007. Hot spring material consisted of surface mat and sediment (Leonard's hot spring) and surface sediment material (“Ray's” hot spring) taken with a metal spoon. From the edge of Leonard's and “Ray's” hot springs, soil samples (upper 10 cm) were taken along a perpendicular transect at distances of 10, 30, 60, and 150 cm and 10, 20, 40, 80, and 250 cm, respectively. Sample material to be analyzed for lipids was stored at −20°C until further analysis, and samples from which DNA was to be extracted were stored at −80°C. The in situ soil temperature at a 10-cm depth and the hot spring water temperature of each site were measured with an Ama-Digit Ad 20 Th digital thermometer, and the pH of the soils was determined using freeze-dried samples in distilled water (10:25, wt/vol) after vigorous shaking of the mixture for 1 min and allowing the particles settle for 30 min.
GDGT extraction, column fractionation, and acid hydrolysis.
The mat/sediment and soil samples were freeze-dried and extracted three times using a modified Bligh-Dyer (1) technique to obtain CL- and IPL-GDGTs. A single-phase solvent mixture of methanol-dichloromethane (DCM)-phosphate buffer (2:1:0.8, vol/vol/vol) was added to the sample in a centrifuge tube and placed into an ultrasonic bath for 10 min. DCM and phosphate buffer were added to give a new volume ratio (1:1:0.9, vol/vol/vol). The extract and residue were separated by centrifugation at 2,500 rpm for 5 min. The methanol-phosphate buffer phase was removed, and the DCM phase was collected in a round-bottom flask. We did not substitute with a 5% trichloroacetic acid solution for the aqueous phase in the last two extractions, as previously suggested (39), because we were unsure how this would affect the stability of IPL head groups and potentially artificially create CLs. Thus, IPL concentrations may be potential underestimates based on our extraction procedure. The combined DCM phases were reduced under a rotary vacuum and dried over an Na2SO4 column. The total Bligh-Dyer extract was fractionated over a preactivated silica gel (60 mesh) with 3 column volumes of hexane-ethyl acetate (3:1, vol/vol) to obtain the CL-GDGT fraction and was then fractionated with 3 column volumes of ethyl acetate followed by 3 column volumes of methanol to obtain an IPL-GDGT fraction according to methods described previously by Oba et al. (22) and Pitcher et al. (26).
The IPL-GDGT fraction was subjected to acid hydrolysis to cleave polar head groups by refluxing in 2 ml of 5% HCl in methanol (MeOH) (96%) for 3 h. The pH of the cooled solution was adjusted to pH 5 with 2 N KOH-MeOH (1:1, vol/vol). Bidistilled water was added to a final ratio of H2O-MeOH of 1:1 (vol/vol), and this mixture was washed three times with DCM. The DCM fractions were collected and evaporated to dryness under a rotary vacuum. A known amount of C46 internal GDGT standard (14) was added to both the IPL-GDGT fraction (before acid hydrolysis) and the CL-GDGT fraction, which were subsequently analyzed by high-performance liquid chromatography (HPLC)-mass spectrometry (MS).
GDGT analysis.
Archaeal GDGTs were analyzed using modified procedures described previously by Hopmans et al. (13) and Schouten et al. (33). Analyses were performed using an HP (Palo Alto, CA) 1100 series liquid chromatography-MS device equipped with an autoinjector and Chemstation chromatography manager software. For the first 5 min, elution was isocratic with 99% buffer A (hexane) and 1% buffer B (isopropanol), followed by a gradient to 1.8% buffer B in 45 min. Separation was achieved using a Prevail Cyano column (2.1 by 150 mm, 3 μm; Alltech, Deerfield, IL) maintained at 30°C. The total run time was 40 min, with a flow rate of 0.2 ml min−1. After each analysis, the column was cleaned by back flushing hexane-propanol (9:1, vol/vol) at 0.2 ml min−1 for 10 min. Detection was achieved by positive-ion atmospheric-pressure chemical ionization (APCI) under the following conditions: nebulizer pressure (N2) of 60 lb/in2, vaporizer temperature of 400°C, drying gas (N2) flow of 6 liters min−1, temperature of 200°C, corona current of 5 μA, and capillary voltage of −3 kV. Archaeal GDGTs were detected with single-ion monitoring of their protonated molecules ([M + H]+). Single-ion monitoring parameters were set to detect protonated molecules of six common isoprenoid GDGTs (m/z 1,302, 1,300, 1,298, 1,296, 1,294, and 1,292) as well as the protonated molecule of the C46 GDGT internal standard (m/z 744), with a dwell time of 237 ms per ion.
For the simultaneous analysis of CL- and monohexose (MH)-derived GDGTs (MH-GDGTs) in the total Bligh-Dyer extract, the mobile-phase gradient described above was extended as described previously (26). For the first 5 min, elution was isocratic, with 99% buffer A (hexane) and 1% buffer B (isopropanol), followed by a gradient to 1.8% buffer B in 45 min. This was followed by a gradient to 10% buffer B in 20 min. The total run time for this method was 90 min. Detection was achieved according to the MS method described above.
Quantification of GDGTs was achieved by adding a known amount of C46 GDGT internal standard to each fraction. To check the performance of our column separations of the environmental samples, we quantified the amount of CL-GDGTs present in the IPL-GDGT fraction prior to acid hydrolysis. This showed that, on average, only 8.7% ± 5.4% of IPL-GDGTs were present as CL-GDGTs prior to acid hydrolysis. However, since a proportion of these could have resulted from the degradation of intact GDGTs prior to and during analysis, we did not correct for them in our final concentration measurements. Thus, the IPL-GDGT concentrations presented here may be slight overestimations. Because crenarchaeol and GDGT-4 coelute, the concentrations of each were corrected according to a method described previously by Weijers et al. (43).
DNA extraction and real-time PCR amplification of archaeal genes.
DNA extraction of 0.2 to 0.4 g of sample material from Leonard's hot spring and adjacent soils was carried out using an Ultra Clean soil extraction kit (MoBio Labs) according to the manufacturer's protocol. Archaeal 16S rRNA and archaeal amoA gene copy numbers were determined using primer pairs Parch519f/Arc915r (5) and Arch-amoA-for/Arch-amoA-rev (4). Real-time quantification was performed using an iCycler apparatus (Bio-Rad, Hercules, CA) with all reactions proceeding with an initial denaturing step for 5 min at 94°C, followed by 38 cycles of denaturing for 30 s at 94°C, 40 s of primer annealing at 64°C and 58.5°C for the 16S rRNA and amoA genes, respectively, and 40 s of primer extension at 72°C. The real-time increase in fluorescence determined by use of SYBR green (Molecular Probes) indicated an accumulation of double-stranded amplicons. Calibration of gene copy numbers was performed using real-time standard curves generated with known copy numbers ranging from 102 to 107 copies of an enriched marine group I crenarchaeote from the North Sea (45), which were generated during the same PCRs and with the same primers used to detect the environmental genes.
RESULTS
Distribution and abundance of CL- and IPL-GDGTs.
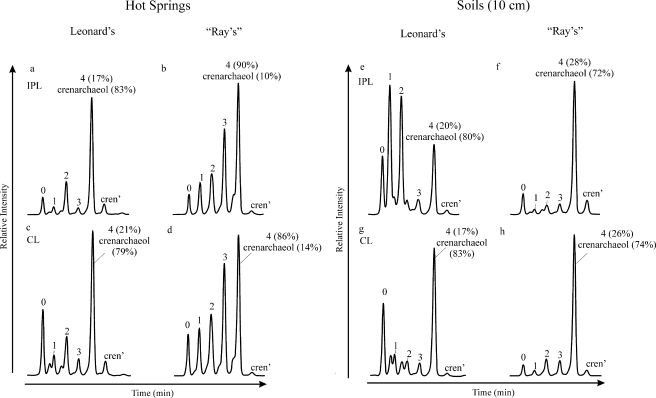
HPLC-MS analysis of the CL- and IPL-GDGT fractions revealed differences in GDGT distributions between both hot springs (Fig. 2a to d). The IPL-GDGT distribution in Leonard's spring was dominated by crenarchaeol, with substantial amounts of GDGTs 0 and 2 and minor amounts of GDGTs 1 and 3 and the crenarchaeol regioisomer (Fig. 2a). In “Ray's” hot spring, IPL-crenarchaeol was also present; however, in contrast to Leonard's hot spring, the relative amounts were small compared to those of the other GDGTs. IPLs from “Ray's” spring were dominated by GDGT-4 but also contained substantial amounts of GDGTs 0 to 3 (Fig. 2b). The CL-GDGT distributions in the hot springs were generally similar to those of the corresponding IPL-GDGTs (Fig. 2c and d). Notable differences include a larger relative contribution of GDGT-0 and a smaller relative contribution of crenarchaeol in both springs. The distributions of GDGTs 1 to 3 were similar in both the IPL and CL fractions. GDGT distributions from the soils differed substantially from those of the corresponding hot spring sediments at each site (Fig. 2e to h). Crenarchaeol was still relatively abundant among the IPL-GDGTs in the soil sampled 10 cm from Leonard's hot spring; however, this soil was dominated by two GDGTs, which appeared to be early-eluting isomers of GDGTs 1 and 2 (Fig. 2e). IPL-GDGTs obtained from soil sampled 10 cm away from the edge of “Ray's” hot spring were dominated by crenarchaeol, with substantially smaller amounts of the other GDGTs (Fig. 2f).
FIG. 2.
HPLC-APCI-MS base peak chromatograms showing the relative abundances of IPL- and CL-GDGTs from Leonard's and “Ray's” hot springs (a to d) and in soils sampled 10 cm from each spring (e to h). Peak numbers and labels correspond to GDGT structures shown in Fig. 1 (e.g., “0” is GDGT-0). Numbers in parentheses next to coeluting GDGT-4 and crenarchaeol indicate their relative contributions to the peak area. cren', regioisomer of crenarchaeol.
The CL-GDGT distribution in the 10-cm soil sampled near Leonard's hot spring was quite different from the corresponding IPL distribution (cf. Fig. 2e and g): GDGTs 1 and 2 were comparatively low in abundance, while crenarchaeol dominated along with a substantial amount of GDGT-0. The early-eluting isomers of GDGTs 1 and 2, which were in high abundance in the IPL fraction, occurred with a much lower relative abundance here, albeit they were still present in near-equal amounts relative to those for GDGTs 1 and 2. The CL-GDGT distribution obtained from the soil near “Ray's” hot spring was similar to that of the corresponding IPLs, with only slightly less GDGT-0 and slightly more GDGT-2 and GDGT-3 (Fig. 2h).
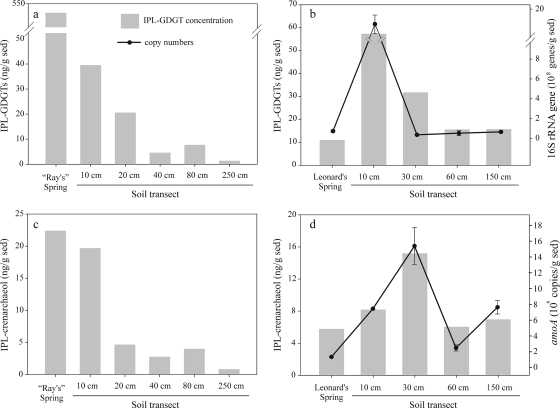
Quantification of the IPL- and CL-GDGT fractions showed that the total IPL-GDGT concentration was always higher than the total CL-GDGT concentration, which ranged from 1.4 to 540 ng g−1 and 0.4 to 44 ng g−1, respectively (Table 1). “Ray's” hot spring contained by far the highest concentration of IPL-GDGTs, with concentrations dropping significantly along the soil transect to ca. 1.4 ng g−1 (Table 1 and Fig. 3a). Much lower total concentrations of IPL-GDGTs were observed in Leonard's hot spring (11 ng g−1), but higher concentrations of IPLs were observed in the surrounding soils (Table 1 and Fig. 3b).
TABLE 1.
Absolute concentrations of IPL- and CL-derived GDGTs for all hot spring and soil samples, IPL-estimated archaeal cell densities, and archaeal 16S rRNA and amoA gene copy numbers (for Leonard's spring only)
| Site | Sample | Distance from spring (cm) | Temp (°C) | pHa | Concn of GDGTs (ng/g dry sample)b
|
Estimated amt of archaeal cells/g | No. of archaeal 16S copies/g sediment | No. of amoA copies/g sediment | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPL
|
CL
|
||||||||||||||||||||||
| 0 | 1 | 2 | 3 | 4 | Cren | Cren′ | Total | 0 | 1 | 2 | 3 | 4 | Cren | Cren′ | Total | ||||||||
| Leonard's | Hot spring | 0 | 58 | 8.4 | 1.1 | 0.4 | 1.6 | 0.4 | 1.1 | 5.8 | 0.6 | 11 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.4 | 1.1E+07 | 7.3E+07 | 5.3E+05 |
| Soil | 10 | 36 | 8.6 | 9.7 | 18* | 17* | 1.9 | 2.1 | 8.2 | 0.6 | 22 | 4.4 | 0.9 | 0.8 | 0.7 | 1.2 | 5.8 | 0.2 | 14 | 5.7E+07 | 1.8E+09 | 7.1E+06 | |
| Soil | 30 | 28 | 8.4 | 1.3 | 6.6 | 4.4 | 0.8 | NA | 15 | 3.4 | 32 | 0.1 | 0.1 | 0.1 | 0.0 | NA | 0.0 | 0.0 | 0.5 | 3.2E+07 | 3.6E+07 | 1.6E+07 | |
| Soil | 60 | 20 | 8.6 | 0.8 | 4.0 | 2.3 | 0.4 | NA | 6.0 | 2.1 | 16 | 0.2 | 0.2 | 0.1 | 0.1 | NA | 2.9 | 0.3 | 3.9 | 1.6E+07 | 5.2E+07 | 1.8E+06 | |
| Soil | 150 | 14 | 8.6 | 2.8 | 1.8 | 1.2 | 0.6 | NA | 7.0 | 2.4 | 16 | 1.4 | 0.1 | 0.1 | 0.1 | NA | 1.3 | 0.3 | 3.2 | 1.6E+07 | 6.5E+07 | 7.3E+06 | |
| “Ray's” | Hot spring | 0 | 89 | 8.2 | 42 | 48 | 75 | 130 | 210 | 22 | 7.5 | 540 | 5.4 | 4.6 | 6.8 | 11 | 13 | 2.1 | 0.4 | 44 | 5.4E+08 | NA | NA |
| Soil | 10 | 42 | 7.0 | 4.8 | 0.9 | 1.8 | 1.9 | 7.6 | 20 | 2.9 | 40 | 2.0 | 0.8 | 2.7 | 2.2 | 5.8 | 16 | 0.9 | 31 | 4.0E+07 | NA | NA | |
| Soil | 20 | 33 | 6.1 | 2.2 | 2.5 | 3.9 | 6.5 | NA | 4.7 | 0.8 | 21 | 0.2 | 0.1 | 0.3 | 0.2 | NA | 3.5 | 0.3 | 4.5 | 2.1E+07 | NA | NA | |
| Soil | 40 | 25 | 6.9 | 0.4 | 0.2 | 0.4 | 0.3 | NA | 2.8 | 0.4 | 4.6 | 0.2 | 0.1 | 0.2 | 0.1 | NA | 0.8 | 0.1 | 1.4 | 4.6E+06 | NA | NA | |
| Soil | 80 | 20 | 7.0 | 1.6 | 1.1 | 0.7 | 0.3 | NA | 4.0 | 0.0 | 7.7 | 0.2 | 0.1 | 0.1 | 0.1 | NA | 1.3 | 0.2 | 1.9 | 7.7E+06 | NA | NA | |
| Soil | 250 | 12 | 7.7 | 0.2 | 0.0 | 0.1 | 0.1 | NA | 0.8 | 0.1 | 1.4 | 0.1 | 0.0 | 0.0 | 0.0 | NA | 0.2 | 0.0 | 0.5 | 1.4E+06 | NA | NA | |
pH was of streamers near source sediment sampled from “Ray's” hot spring.
FIG. 3.
Total archaeal IPL-GDGTs (a and b) and IPL-crenarchaeol (c and d) from “Ray's” hot spring and Leonard's hot spring as well as the corresponding soil transects, shown as bars. Archaeal 16S rRNA (b) and archaeal amoA (d) gene copy numbers measured by quantitative PCR from Leonard's site are shown as marked lines, with error bars (in some cases smaller than the symbol size) representing the standard deviations of copy numbers obtained from triplicate qPCR reactions.
IPL-crenarchaeol concentrations varied from 0.8 to 22 ng g−1 and contributed between 4 and 61% to the total IPL-GDGT concentrations (Table 1). The highest concentration of IPL-crenarchaeol was actually observed in “Ray's” hot spring at 89°C, although it contributed only ca. 4% to the total archaeal IPL-GDGT concentration (Fig. 3c). The IPL-crenarchaeol concentration decreased slightly in the 10-cm soil to 20 ng g−1 and then decreased considerably in soils sampled further away from the hot spring. In contrast, Leonard's hot spring contained smaller absolute amounts of IPL-crenarchaeol (5.8 ng g−1); however, here it represented ca. 63% of the total IPL-GDGTs quantified (Fig. 3d). In soils sampled away from Leonard's spring, the crenarchaeol concentration increased to ca. 15 ng g−1 (at 30 cm) and then decreased again. The concentration of CL-crenarchaeol ranged from 0.0 to 16 ng g−1, and CL-crenarchaeol was always present in smaller amounts than IPL-crenarchaeol in the same sample.
Distribution of MH-GDGTs.
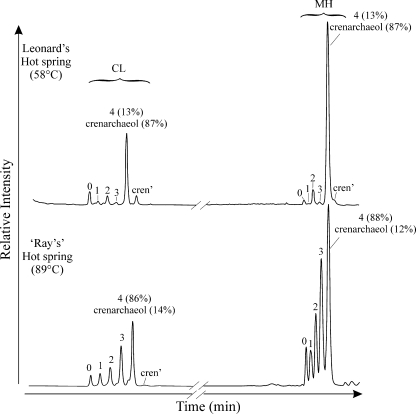
Analysis of the total Bligh-Dyer extracts with HPLC-APCI-MS using a newly described method (26) allowed the detection of both the CL-GDGT and MH-GDGTs (Fig. 1) in a single HPLC-MS run (Fig. 4). The base peak chromatograms of each hot spring showed relatively small amounts of CL-GDGTs compared to those of MH-GDGTs, which is in agreement with the higher concentrations of IPL-GDGTs than of CL-GDGTs quantified after hydrolysis (Table 1). Crenarchaeol dominated both the CL- and MH-GDGTs in Leonard's hot spring, whereas crenarchaeol represented a minor component of the CL- and MH-GDGTs in “Ray's” hot spring. We did not quantify MH-GDGTs directly due to a lack of an authentic MH-GDGT standard, and we therefore cannot conclude what proportion of the IPL-GDGTs in our samples quantified after acid hydrolysis was comprised of IPLs with an MH moiety.
FIG. 4.
HPLC-APCI-MS base peak chromatograms of total Bligh-Dyer extracts showing the relative abundances of CL- and MH-GDGTs in each hot spring. Peak numbers and labels correspond to GDGT structures shown in Fig. 1 (e.g., “0” is GDGT-0). Numbers in parentheses next to GDGT-4 and crenarchaeol indicate the percent composition each in the labeled peak where these GDGTs coelute.
Archaeal 16S rRNA and amoA gene abundance at Leonard's hot spring.
The archaeal 16S rRNA gene abundance in Leonard's hot spring was 7.3 × 107 copies g−1. Similar gene abundances were found in the adjacent soils, with the exception of soil at a 10-cm distance, which had 1.8 × 109 copies g−1 (Table 1 and Fig. 3b). Archaeal amoA gene copy numbers ranged from 5.3 × 105 to 1.6 × 107 copies g−1, with the lowest abundance observed in the hot spring and the highest abundance observed in soil sampled 30 cm from the spring (Fig. 3d). The amoA gene abundance decreased at a 60-cm distance from the hot spring and increased again at 150 cm.
DISCUSSION
Sources of GDGTs in hot springs and soils.
The occurrence of GDGTs that we observed in the hot springs is consistent with the known prevalence of thermophilic Archaea in these environments (3, 21). They are likely sourced by a mixed archaeal community, as GDGTs 0 to 4 are synthesized in various relative abundances by many cultured representatives of (hyper)thermophilic Euryarchaeota and Crenarchaeota (20, 34). For example, most cultured members of the Euryarchaeota, including members of the orders Thermococcales (40), Archaeoglobales (41), Methanosarcinales (12), Methanobacteriales (18), Methanococcales (41), and Methanomicrobiales (38), produce only GDGT-0. Other euryarchaeotes, however, such as the Methanopyrales (9; M. van der Meer et al., unpublished results) and members of the anaerobic methane oxidizer (ANME)-1 (2) and deep-sea hydrothermal-vent euryarchaeotic 2 (DHVE2) lineage (29) clusters, produce various amounts of GDGTs 0 to 4, while some members of the Thermoplasmatales (20) produce GDGTs with up to eight cyclopentane rings. Most cultured (hyper)thermophilic Crenarchaeota synthesize at least GDGTs 0 to 4, and some can also synthesize GDGTs with up to eight cyclopentane moieties (cf. reference 34). In contrast, cultivated representatives of the group I.1a Crenarchaeota, “Candidatus Nitrosopumilus maritimus” SCM (16) and Cenarchaeum symbiosum (27); group I.1b Crenarchaeota, “Nitrosophaera gargensis” (A. Pitcher et al., unpublished results); and the thermophilic ammonia-oxidizing Archaea (ThAOA)/hot-water crenarchaeotic group (HWCG) III cluster of the Crenarchaeota, “Candidatus Nitrosocaldus yellowstonii” (6), all synthesize substantial amounts of GDGT-0 and/or crenarchaeol, with minor amounts of GDGTs 1 to 3 (6, 30, 37). It is therefore likely that GDGTs 0 to 4 measured in this study have multiple sources and that crenarchaeol is derived predominantly from the Crenarchaeota falling in the group I.1a, I.1b, and ThAOA/HWCG III clusters of the Crenarchaeota.
The contrasting IPL-GDGT distributions in Leonard's and “Ray's” hot spring indicate significant differences in the resident archaeal communities. The IPL-GDGT distribution in Leonard's hot spring is similar to those of others observed for isolates of the group I.1a Crenarchaeota, whereas the IPL-GDGT distribution in “Ray's” hot spring is similar to those observed in (hyper)thermophilic Crenarchaeota and some members of the Euryarchaeota (e.g., see references 25 and 34). The high temperature of “Ray's” hot spring (89°C) versus that of Leonard's hot spring (58°C) may be a substantial controlling factor in governing the differences in archaeal community compositions, as Leonard's and “Ray's” hot springs were similar in pH and geographically very close.
For soils, the IPL-GDGT distributions are similar to those commonly observed for isolates of the group I.1a Crenarchaeota, except for the soil closest to Leonard's hot spring, which shows unusually abundant early-eluting isomers of GDGTs 1 and 2. GDGTs 1and 2 are abundant components in ANME-1 Archaea (2, 23, 35). Indeed, in contrast to the other soils, this soil was composed mainly of fine, clay-like material and had a sulfidic smell, indicating anoxic conditions, which could support such a community (Table 1). However, no ANME Archaea in a soil environment have been reported, and the existence of such a community in this soil sample has yet to be identified.
Contributions of fossil and IPL-GDGTs.
The underlying presupposition of our work is that the IPL-GDGTs were derived from living biomass and thus produced in situ. This relies mainly on two assumptions: (i) that GDGTs in the hydrolyzed IPL fraction were not significantly contaminated by CL-GDGTs and (ii) that GDGTs with intact polar head groups are derived only from living biomass. The first assumption is supported by the direct analysis of the MH-GDGTs, which confirmed the presence of GDGTs with polar head groups in our samples and that they are likely more abundant than those of CL-GDGTs (Fig. 4). It should also be noted that MH-GDGTs represent only a part of the total IPL-GDGT pool, as the Archaea are capable of synthesizing a variety of other head groups including dihexoses and phosphohexoses (15, 30, 39).
Support for our second assumption may come by estimating archaeal cell numbers from IPL-GDGT concentrations. If we assume that the average GDGT-synthesizing archaeon in our samples contains ∼1 × 10−3 pg GDGTs cell−1 (8, 36), the IPL-estimated archaeal cell concentrations for Leonard's site (Table 1) fall within an order of magnitude of archaeal 16S rRNA gene copies g−1, with the exception of the 10-cm soil sample (see below). We did not quantify all GDGTs that are known to be produced by the Archaea, e.g., GDGTs with five to eight cyclopentane rings, which could account for some of the observed offset between gene abundance and IPL-GDGT-based cell estimates. Nevertheless, the good correspondence between IPL-GDGT-based cell estimates and gene abundance supports our assumption that the IPL-GDGTs that we quantified were indeed derived mostly from living Archaea.
In the 10-cm soil of Leonard's hot spring, the IPL-GDGT-based cell estimate was ca. 31 times lower than the corresponding number of 16S rRNA gene copies (Table 1). Here, the highest abundances of both archaeal 16S rRNA genes and IPL-GDGTs were measured; however, this maximum represented an increase in archaeal 16S rRNA gene copy numbers from the spring to the 10-cm soil of ca. 25-fold compared to the corresponding increase in the IPL-GDGT concentration of ca. 5-fold only. This discrepancy could be due to the presence of a dominant microbial community consisting primarily, for example, of methanogens, which produce predominantly diglyceride dialkyl ether lipids such as archaeol and hydroxy-archaeol as their main membrane core lipids (15), which we did not measure. Indeed, this anomaly occurred in the soil with an IPL-GDGT distribution typical for ANME-1 Archaea (Fig. 2e) and which appeared to anoxic.
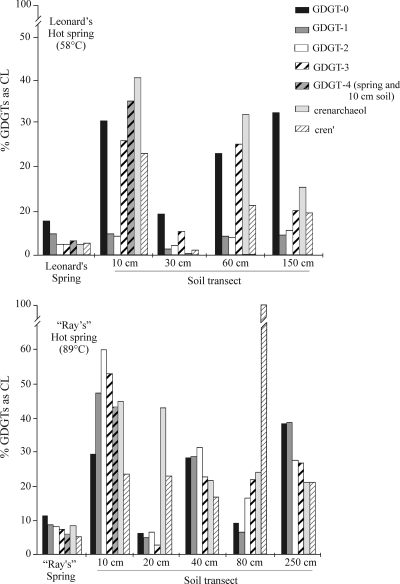
Absolute quantification of GDGTs from both CL- and IPL-GDGT fractions (Table 1) shows that the contribution of fossilized GDGTs to the total GDGT pool (i.e., summed total of IPL- and CL-GDGTs) is as high as 40%. It is also important that this percentage varied widely among individual GDGTs (Fig. 5). For both hot springs, the contribution of fossil GDGTs to the total pool was fairly low (ca. 3 to 8%) and relatively even among individual GDGTs. However, the fossil GDGT contribution in the surrounding soils was substantially higher (up to 44%) and was more variable among individual GDGTs. For example, ca. 2% of the total crenarchaeol measured in Leonard's hot spring was derived from fossil material, whereas crenarchaeol extracted from the soil located 10 cm away from the hot spring contained over 40% fossilized material. Similarly, in “Ray's” hot spring, ca. 9% of crenarchaeol was of fossil origin, whereas fossil crenarchaeol contributed 45% to the total crenarchaeol extracted from the nearest soil. Such a large variation in the fossil contribution to the GDGT distribution in soils and hot springs implies that, in general, analysis of GDGT distributions obtained from directly extracted CL-GDGTs or from CL-GDGTs present in acid-hydrolyzed Bligh-Dyer IPL extracts where background CL-GDGTs have not first been removed may be influenced by a substantial fossil signal and potentially skew the GDGT distribution of the living archaeal community, although the data show that this effect appears to be expressed more strongly in soils than in the hot springs.
FIG. 5.
Contribution of fossilized GDGTs to the total pool in each hot spring and soil transect. Each shaded/patterned bar represents an individual GDGT isomer, as indicated in the key. The amount of GDGT-4 was quantified only for the hot spring and the 10-cm soil samples.
In situ production of crenarchaeol in hot springs and soils.
Separate analyses of CL- and IPL-GDGT fractions allowed us to evaluate whether or not the crenarchaeol found in our hot springs was produced in situ. In both Leonard's and “Ray's” hot springs, we did find IPL-crenarchaeol (Fig. 3c and d) and comparatively minor amounts of fossil crenarchaeol (Table 1), suggesting that crenarchaeol is indeed being synthesized in situ by (hyper)thermophilic Crenarchaeota and that allochtonous input is not important. This is supported by the direct analysis of our total Bligh-Dyer aliquots, which confirmed the presence of MH-crenarchaeol (Fig. 4). An MH moiety is a common head group synthesized by the Archaea and has been identified as being one of the forms of IPL-crenarchaeol in the cell membrane of “Candidatus Nitrosopumilus maritimus” SCM1 (30). There are some differences between the relative distributions of MH-GDGTs and acid-hydrolyzed IPL-GDGTs (Fig. 2), likely indicating the presence of IPL-crenarchaeol with multiple head groups. Indeed, “Candidatus Nitrosopumilus maritimus” SCM1 contains IPL-GDGTs with two hexose moieties and a phosphohexose moiety in addition to MH-GDGTs (30).
The presence of IPL-crenarchaeol shows that crenarchaeol synthesis occurs in hot springs and soils over a wide temperature range (12°C to 89°C). Our findings corroborate those of others who previously found crenarchaeol in hot springs (24, 25, 28, 34, 46) and soils (19, 43) and are supported by work showing that crenarchaeol is synthesized by enrichment cultures of ammonia-oxidizing (hyper)thermophiles (6; Pitcher et al., unpublished). Further confirmation of the in situ production of crenarchaeol comes from analyses of archaeal amoA, the gene coding for a subunit of ammonia monooxygenase, which is the membrane-bound protein that catalyzes the rate-limiting step in the biochemical process of ammonia oxidation. In general, we find that amoA copy numbers and IPL-crenarchaeol concentrations follow similar patterns at Leonard's site (Fig. 3d). This also further corroborates the hypothesis that crenarchaeol is derived predominantly from the Crenarchaeota involved in ammonia oxidation (6).
Interestingly, there is not a significant relationship between the relative abundance of IPL-crenarchaeol and temperature, as might have been expected based on the results of previous studies of hot springs (46) or marine environments (32). This is likely because the other GDGTs are sourced from a variety of members of the Archaea and not only from specific groups of the Crenarchaeota. Shifts in the relative abundances of GDGTs in such terrestrial environments are more likely to reflect changes in community structure than in the marine environment, where archaeal diversity is lower and environmental conditions are comparatively stable. There is, however, some correspondence between IPL-crenarchaeol abundance and pH; i.e., the pH of “Ray's” hot spring is much more alkaline than is the pH of the corresponding soil transect, and there is a substantial decrease in the IPL-crenarchaeol concentration between the spring and soils (Table 1). In contrast, the pH of Leonard's spring is quite similar to that of the surrounding soils, and IPL-crenarchaeol concentrations are quite similar as well. Our data set is too small to derive statistically significant relationships; however, these results compare well with those described previously by Weijers et al. (43) and Pearson et al. (25), who also found a positive relationship between crenarchaeol and pH.
Our results, together with those reported previously (24, 25, 34, 46), raise an intriguing question: why is crenarchaeol specifically synthesized by selected groups of the Crenarchaeota? The fact that all of the crenarchaeol-synthesizing Archaea cultured to date are also ammonia oxidizers suggests that crenarchaeol may be diagnostic for the ammonia-oxidizing Crenarchaeota. This is supported by the correspondence between archaeal amoA gene copy abundances and IPL-crenarchaeol concentrations (Fig. 3d), implying that the crenarchaeol-synthesizing Archaea at our study sites may also be predominantly ammonia oxidizers. Nevertheless, we cannot yet exclude the possibility that crenarchaeol synthesis was an evolutionary trait that allowed the (hyper)thermophilic Archaea to expand into temperate environments (17). It is possible that a group of mesophilic Crenarchaeota subsequently evolved back to hot environments and utilized alternative mechanisms for the maintenance of cellular membrane integrity while still producing crenarchaeol. Further studies of the biophysical properties of crenarchaeol are needed to elucidate its functional utility in cell membranes of the Crenarchaeota.
Conclusions.
IPL-crenarchaeol derived from living Archaea was found in two hot Californian springs, indicating that crenarchaeol is synthesized in situ at high temperatures. The correspondence between IPL-crenarchaeol and archaeal amoA in Leonard's hot spring and surrounding soils further implies that the crenarchaeol-synthesizing Crenarchaeota were predominantly ammonia oxidizers. Quantification of both CL- and IPL-GDGTs showed that the long-term accumulation of fossilized lipids may contribute substantially to the total lipid pool, especially in soils (up to 40%). These fossilized lipids may potentially mask relationships between biomarker distribution and microbial community structure.
Acknowledgments
We thank Francien Peterse and Marcel van der Meer for field assistance in collecting the hot spring and soil samples, Ray Page for permission to sample “Ray's” hot spring, and Ellen Hopmans for analytical assistance.
This study was funded by a grant from the Darwin Institute for Biogeology to J.S.S.D.
Footnotes
Published ahead of print on 1 May 2009.
REFERENCES
- 1.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:912-917. [DOI] [PubMed] [Google Scholar]
- 2.Blumenberg, M., R. Seifert, J. Reitner, T. Pape, and W. Michaelis. 2004. Membrane lipid patterns typify distinct anaerobic methanotrophic consortia. Proc. Natl. Acad. Sci. USA 101:11111-11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaban, B., S. Y. M. Ng, and K. F. Jarrell. 2006. Archaeal habitats—from the extreme to the ordinary. Can. J. Microbiol. 52:73-116. [DOI] [PubMed] [Google Scholar]
- 4.Coolen, M. J. L., B. Abbas, J. van Bleijswijk, E. C. Hopmans, M. M. M. Kuypers, S. G. Wakeham, and J. S. Sinninghe Damsté. 2007. Putative ammonia-oxidizing Crenarchaeota in suboxic waters of the Black Sea: a basin-wide ecological study using 16S ribosomal and functional genes and membrane lipids. Environ. Microbiol. 9:1001-1016. [DOI] [PubMed] [Google Scholar]
- 5.Coolen, M. J. L., E. C. Hopmans, W. I. C. Rijpstra, G. Muyzer, S. Schouten, J. K. Volkman, and J. S. Sinnighe Damsté. 2004. Evolution of the methane cycle in Ace Lake (Antarctica) during the Holocene: response of methanogens and methanotrophs to environmental change. Org. Geochem. 35:1151-1167. [Google Scholar]
- 6.de la Torre, J. R., C. B. Walker, A. E. Ingalls, M. Könneke, and D. A. Stahl. 2008. Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ. Microbiol. 10:810-818. [DOI] [PubMed] [Google Scholar]
- 7.de Rosa, M., and A. Gambacorta. 1988. The lipids of Archaebacteria. Prog. Lipid Res. 27:153-175. [DOI] [PubMed] [Google Scholar]
- 8.Gabriel, J. L., and P. L. G. Chong. 2000. Molecular modeling of archaebacterial bipolar tetraether lipid membranes. Chem. Phys. Lipids 105:193-200. [DOI] [PubMed] [Google Scholar]
- 9.Gattinger, A., M. Scholter, and J. C. Munch. 2002. Phospholipid etherlipid and phospholipid fatty acid fingerprints in selected euryarchaeotal monocultures for taxonomic profiling. FEMS Microbiol. Lett. 213:133-139. [DOI] [PubMed] [Google Scholar]
- 10.Harvey, H. R., R. D. Fallon, and J. S. Patton. 1986. The effect of organic matter and oxygen on the degradation of bacterial membrane lipids in marine sediments. Geochim. Cosmochim. Acta 50:795-804. [Google Scholar]
- 11.Hatzenpichler, R., E. Lebedeva, E. Spieck, K. Stoecker, A. Richter, H. Daims, and M. Wagner. 2008. A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc. Natl. Acad. Sci. USA 105:2134-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoefs, M. E. L., S. Schouten, J. W. De Leeuw, L. L. King, S. G. Wakeham, and J. S. Sinninghe Damsté. 1997. Ether lipids of planktonic archaea in the marine water column. Appl. Environ. Microbiol. 63:3090-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopmans, E. C., S. Schouten, R. D. Pancost, M. T. J. van der Meer, and J. S. Sinninghe Damsté. 2000. Analysis of intact tetraether lipids in archaeal cell material and sediments by high performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 14:585-589. [DOI] [PubMed] [Google Scholar]
- 14.Huguet, C., E. C. Hopmans, W. Febo-Ayala, D. H. Thompson, J. S. Sinninghe Damsté, and S. Schouten. 2006. An improved method to determine the absolute abundance of glycerol dibiphytanyl glycerol tetraether lipids. Org. Geochem. 37:1036-1041. [Google Scholar]
- 15.Koga, Y., and H. Morii. 2005. Recent advances in structural research on ether lipids from Archaea including comparative and physiological aspects. Biosci. Biotechnol. Biochem. 69:2019-2034. [DOI] [PubMed] [Google Scholar]
- 16.Könneke, M., A. E. Bernhard, J. R. de la Torre, C. B. Walker, J. B. Waterbury, and D. A. Stahl. 2005. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543-546. [DOI] [PubMed] [Google Scholar]
- 17.Kuypers, M. M. M., P. Blokker, J. Erbacher, H. Kinkel, R. D. Pancost, S. Schouten, and J. S. Sinninghe Damsté. 2001. Massive expansion of marine Archaea during a mid-Cretaceous oceanic anoxic event. Science 293:92-95. [DOI] [PubMed] [Google Scholar]
- 18.Langworthy, T. A., and J. L. Pond. 1986. Archaebacterial ether lipids and chemotaxonomy. Syst. Appl. Microbiol. 7:253-257. [Google Scholar]
- 19.Leininger, S., T. Urich, M. Schloter, L. Schwark, J. Qi, G. W. Nicol, J. I. Prosser, S. C. Schuster, and C. Schleper. 2006. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806-809. [DOI] [PubMed] [Google Scholar]
- 20.Macalady, J. L., M. M. Vestling, D. Baumler, N. Boekelheide, C. W. Kaspar, and J. F. Banfield. 2004. Tetraether-linked membrane monolayers in Ferroplasma spp: a key to survival in acid. Extremophiles 8:411-419. [DOI] [PubMed] [Google Scholar]
- 21.Madigan, M. T., J. M. Martinko, and J. Parker. 2003. Brock biology of microorganisms, 10th ed., p. 137-166. Prentice Hall, Upper Saddle River, NJ.
- 22.Oba, M., S. Sakata, and U. Tsunogai. 2006. Polar and neutral isopranyl glycerol ether lipids as biomarkers of Archaea in near-surface sediments from the Nankai Trough. Org. Geochem. 37:1643-1654. [Google Scholar]
- 23.Pancost, R. D., E. C. Hopmans, J. S. Sinninghe Damsté, and the MEDINAUT Shipboard Scientific Party. 2001. Archaeal lipids in Mediterranean cold seeps: molecular proxies for anaerobic methane oxidation. Geochim. Cosmochim. Acta 65:1611-1627. [Google Scholar]
- 24.Pearson, A., Z. Huang, A. E. Ingalls, C. S. Romanek, J. Wiegel, K. H. Freeman, R. H. Smittenberg, and C. L. Zhang. 2004. Nonmarine crenarchaeol in Nevada hot springs. Appl. Environ. Microbiol. 70:5229-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson, A., Y. D. Pi, W. D. Zhao, W. J. Li, Y. L. Li, W. Inskeep, A. Perevalova, C. Romanek, S. G. Li, and C. L. Zhang. 2008. Factors controlling the distribution of archaeal tetraethers in terrestrial hot springs. Appl. Environ. Microbiol. 74:3523-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitcher, A., E. C. Hopmans, S. Schouten, and J. S. Sinninghe Damsté. 2009. Separation of core and intact polar archaeal tetraether lipids using silica columns: insights into living and fossil biomass contributions. Org. Geochem. 40:12-19. [Google Scholar]
- 27.Preston, C. M., K. Y. Wu, T. F. Molinski, and E. F. DeLong. 1996. A psychrophilic crenarchaeon inhabits a marine sponge: Cenarchaeum symbiosum gen. nov., sp. nov. Proc. Natl. Acad. Sci. USA 93:6241-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reigstad, L. J., A. Richter, H. Daims, T. Urich, L. Schwark, and C. Schleper. 2008. Nitrification in terrestrial hot springs of Iceland and Kamchatka. FEMS Microbiol. Ecol. 64:167-174. [DOI] [PubMed] [Google Scholar]
- 29.Reysenbach, A. L., Y. Liu, A. B. Banta, T. J. Beveridge, J. D. Kirshtein, S. Schouten, M. K. Tivey, K. Von Damm, and M. A. Voytek. 2006. Isolation of a ubiquitous obligate thermoacidophilic archaeon from deep-sea hydrothermal vents. Nature 442:444-447. [DOI] [PubMed] [Google Scholar]
- 30.Schouten, S., E. C. Hopmans, M. Baas, H. Boumann, S. Standfest, M. Könneke, D. A. Stahl, and J. S. Sinninghe Damsté. 2008. Intact membrane lipids of “Candidatus Nitrosopumilus maritimus,” a cultivated representative of the cosmopolitan mesophilic group I crenarchaeota. Appl. Environ. Microbiol. 74:2433-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schouten, S., E. C. Hopmans, R. D. Pancost, and J. S. Sinninghe Damsté. 2000. Widespread occurrence of structurally diverse tetraether membrane lipids: evidence for the ubiquitous presence of low-temperature relatives of hyperthermophiles. Proc. Natl. Acad. Sci. USA 97:14421-14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schouten, S., E. C. Hopmans, E. Schefuß, and J. S. Sinninghe Damsté. 2002. Distributional variations in marine crenarchaeotal membrane lipids: a new tool for reconstructing ancient sea water temperatures? Earth Planet. Sci. Lett. 204:265-274. [Google Scholar]
- 33.Schouten, S., C. Huguet, E. C. Hopmans, M. V. M. Kienhuis, and J. S. Sinninghe Damsté. 2007. Analytical methodology for TEX86 paleothermometry by high-performance liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry. Anal. Chem. 79:2940-2944. [DOI] [PubMed] [Google Scholar]
- 34.Schouten, S., M. T. J. van der Meer, E. Hopmans, W. I. Rijpstra, A.-L. Reysenbach, D. M. Ward, and J. S. Sinninghe Damsté. 2007. Archaeal and bacterial glycerol dialkyl glycerol tetraether lipids in hot springs of Yellowstone National Park. Appl. Environ. Microbiol. 73:6181-6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schouten, S., S. G. Wakeham, E. C. Hopmans, and J. S. Sinninghe Damsté. 2003. Biogeochemical evidence that thermophilic Archaea mediate the anaerobic oxidation of methane. Appl. Environ. Microbiol. 69:1680-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinninghe Damsté, J. S., W. I. Rijpstra, E. C. Hopmans, F. G. Prahl, S. G. Wakeham, and S. Schouten. 2002. Distribution of membrane lipids of planktonic Crenarchaeota in the Arabian Sea. Appl. Environ. Microbiol. 68:2997-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinninghe Damsté, J. S., S. Schouten, E. C. Hopmans, A. C. T. van Duin, and J. A. J. Geenevasen. 2002. Crenarchaeol: the characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeota. J. Lipid Res. 43:1641-1651. [DOI] [PubMed] [Google Scholar]
- 38.Sprott, G. D., G. Ferrante, and I. Ekiel. 1994. Tetraether lipids of Methanospirillum hungatei with head groups consisting of phospho-N,N-dimethylaminopentanetetrol, phospho-N,N,N-trimethylaminopentanetetrol, and carbohydrates. Biochim. Biophys. Acta 1214:234-242. [DOI] [PubMed] [Google Scholar]
- 39.Sturt, H. F., R. E. Summons, K. Smith, M. Elvert, and K.-U. Hinrichs. 2004. Intact polar membrane lipids in prokaryotes and sediments deciphered by high-performance liquid chromatography/electrospray ionization multistage mass spectrometry—new biomarkers for biogeochemistry and microbial ecology. Rapid Commun. Mass Spectrom. 18:617-628. [DOI] [PubMed] [Google Scholar]
- 40.Sugai, A., I. Uda, Y. Itoh, Y. H. Itoh, and T. Itoh. 2004. The core lipid composition of the 17 strains of hyperthermophilic archaea, Thermococcales. J. Oleo Sci. 53:41-44. [Google Scholar]
- 41.Thurl, S., and W. Schafer. 1988. Lipids from the sulfur-dependent archaeabacterium Thermoproteus tenax. Biochim. Biophys. Acta 961:253-261. [Google Scholar]
- 42.Valentine, D. L. 2007. Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nat. Rev. Microbiol. 5:316-323. [DOI] [PubMed] [Google Scholar]
- 43.Weijers, J. W. H., S. Schouten, O. C. Spaargaren, and J. S. Sinninghe Damsté. 2006. Occurrence and distribution of tetraether membrane lipids in soils: implications for the use of the TEX86 proxy and the BIT index. Org. Geochem. 37:1680-1693. [Google Scholar]
- 44.White, D. C., W. M. Davis, J. S. Nickels, J. D. King, and R. J. Bobbie. 1979. Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40:51-62. [DOI] [PubMed] [Google Scholar]
- 45.Wuchter, C., B. Abbas, M. J. L. Coolen, L. Herfort, J. van Bleijswijk, P. Timmers, M. Strous, E. Teira, G. J. Herndl, J. J. Middelburg, S. Schouten, and J. S. Sinninghe Damsté. 2006. Archaeal nitrification in the ocean. Proc. Natl. Acad. Sci. USA 103:12317-12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, C. L., A. Pearson, Y.-L. Li, G. Mills, and J. Wiegel. 2006. Thermophilic temperature optimum for crenarchaeol synthesis and its implication for archaeal evolution. Appl. Environ. Microbiol. 72:4419-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]