Abstract
Legionella pneumophila, the causative agent of Legionnaires' disease, is an intracellular parasite of eukaryotic cells. In the environment, it colonizes amoebae. After being inhaled into the human lung, the bacteria infect and damage alveolar cells in a way that is mechanistically similar to the amoeba infection. Several L. pneumophila traits, among those the Dot/Icm type IVB protein secretion machinery, are essential for exploiting host cells. In our search for novel Legionella virulence factors, we developed an agar plate assay, designated the scatter screen, which allowed screening for mutants deficient in infecting Acanthamoeba castellanii amoebae. Likewise, an L. pneumophila clone bank consisting of 23,000 transposon mutants was investigated here, and 19 different established Legionella virulence genes, for example, dot/icm genes, were identified. Importantly, 70 novel virulence-associated genes were found. One of those is L. pneumophila bdhA, coding for a protein with homology to established 3-hydroxybutyrate dehydrogenases involved in poly-3-hydroxybutyrate metabolism. Our study revealed that bdhA is cotranscribed with patD, encoding a patatin-like protein of L. pneumophila showing phospholipase A and lysophospholipase A activities. In addition to strongly reduced lipolytic activities and increased poly-3-hydroxybutyrate levels, the L. pneumophila bdhA-patD mutant showed a severe replication defect in amoebae and U937 macrophages. Our data suggest that the operon is involved in poly-3-hydroxybutyrate utilization and phospholipolysis and show that the bdhA-patD operon is a virulence determinant of L. pneumophila. In summary, the screen for amoeba-sensitive Legionella clones efficiently isolated mutants that do not grow in amoebae and, in the case of the bdhA-patD mutant, also human cells.
Legionella pneumophila is the causative agent of Legionnaires' disease, a potentially fatal pneumonia. Legionellae ubiquitously inhabit aqueous environments and are parasites of amoebae (51). Amoebae share their natural habitat with many kinds of bacteria and usually feed on them (31). Environmental bacteria are therefore constantly challenged by their natural predators. Certain bacteria, among them Legionella pneumophila, resist amoebal predation and digestion (27). Finally, Legionella exploits the amoebal cell for replication and further benefits from the amoeba in several other ways. First, intracellular localization protects the invader from external dangers, like environmental changes or water treatments (56). Second, amoebae are vectors of bacterial spread, and bacteria have frequently been isolated from environmental amoebae (27, 45). Third, after passage through amoebae, Legionella is rendered more invasive and more virulent even for human cells (15). Furthermore, Legionella infections proceed more severely when legionellae enter the human lung with amoebae (9). Amoebal interaction with Legionella is therefore an important step both for the maintenance of the bacterial population in the environment and for facilitating a human infection.
Interestingly, many aspects of amoeba infection show many similarities with human cell infection by Legionella. For example, Legionella replicates within both amoebae and human cells inside a phagosomal compartment, which is surrounded by vesicles and membranes derived from the endoplasmic reticulum (25). At early time points of infection, the phagosome is not acidified, nor does it mature into a phagolysosomal compartment (8).
Not only events within the human or amoebal cell resemble each other; at the same time, certain bacterial determinants for replication within human cells are essential for the exploitation of amoebae. Among others, this is the case for the type IVB secretion system Dot/Icm, because mutations of the dot/icm genes show replication defects in both cell types (54). The Dot/Icm system injects a multitude of effector proteins into the host cell, subverting the functions of both amoebae and human cells (42).
The similarity of human cell and amoeba exploitation mechanisms used by Legionella and other pathogens makes sense from an evolutionary point of view. It is thought that Legionella, like other environmental bacteria, coevolved with amoebal predators, which share many characteristics, such as mechanisms to engulf (phagocytosis) and digest (endosomal degradation) bacteria, with human phagocytic cells (19, 31). This led to the development of bacterial survival strategies, which may also protect them from bacterium-defeating mammalian cells.
Many aspects of bacterial survival and replication in amoebae are still unknown. Previously, Polesky et al. successfully employed an Acanthamoeba castellanii infection model for screening a clone bank of 700 signature-tagged L. pneumophila insertion mutants and isolated six clones showing defects in intracellular replication (49). Due to the similarity in bacterial exploitation mechanisms used for amoebae and human cells as outlined above, the identification of novel amoeba infection strategies might additionally lend insights into mechanisms applied against mammalian macrophages. In a screen to search for L. pneumophila mutants with reduced cytotoxicity against both amoebae and macrophages, Gao and colleagues previously found 89 clones with reduced levels of intracellular replication in Acanthamoeba polyphaga and in U937 macrophages (25).
Here we present a large-scale plate assay for the detection of genes needed for the full virulence of L. pneumophila against A. castellanii. To this end, 23,000 clones of a transposon-mutagenized clone bank were assayed, resulting in the identification of 19 known and 70 novel genes affecting amoeba infection. One of the mutants showed defective expression of the bdhA-patD operon involved in L. pneumophila phospholipolysis, poly-3- hydroxybutyrate (PHB) metabolism, and host cell infection including amoeba and human macrophage cell models.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
L. pneumophila sg1 strain Philadelphia-1 (ATCC 33152) was used for transposon mutagenesis and subsequent screening. L. pneumophila cells were routinely grown on buffered charcoal-yeast extract (BCYE) agar for 2 or 3 days at 37°C and, when appropriate, were subsequently cultured in buffered yeast extract (BYE) broth at 37°C with shaking at 350 rpm. Bacterial growth was checked by determining the optical density at 660 nm (OD660) with a Beckman Coulter (Unterschleißheim, Germany) DU520 spectrophotometer after inoculation to an OD660 of 0.2 to 0.4. Cells of Escherichia coli strain DH5α, the host for new recombinant plasmids, were grown in Luria-Bertani (LB) agar or broth. When appropriate, media were supplemented with antibiotics at final concentrations suitable for L. pneumophila or E. coli as follows: kanamycin at 25 μg/ml for L. pneumophila and 50 μg/ml for E. coli and chloramphenicol at 6 μg/ml for L. pneumophila and 30 μg/ml for E. coli.
Tn5 mutagenesis.
L. pneumophila clone bank construction was performed by using the EZ-Tn5 <KAN-2> transposome (Epicentre, Madison, WI). Transposon-transposase complexes (1 μl per 20 μl bacterial suspension) were introduced into L. pneumophila cells by electroporation by means of a Cell-Porator apparatus (Life Technologies, Paisley, Scotland) used according to the manufacturer's specifications. Subsequently, bacteria from six independent electroporations were recovered in BYE broth with shaking at 350 rpm for 3 h at 37°C and plated onto selective BCYE agar. The number of mutants recovered was determined, and bacteria were washed off the plates by means of BYE broth. The clone bank was stored in BYE-glycerol (1:1) at −80°C.
Screen for infection defects of the L. pneumophila Tn5 clone bank (scatter screen).
Cryostocks of the L. pneumophila Philadelphia-1 clone bank were grown on BCYE agar supplemented with kanamycin. Next, bacteria were subplated onto plain BCYE agar and grown for 3 days. Bacteria were then adjusted to 104 CFU/ml in amoeba infection medium (38) and mixed with 105 A. castellanii amoebae (ATCC 30234) per ml (multiplicity of infection of 0.1). The mixture was plated onto BCYE agar supplemented with kanamycin in appropriate dilutions to acquire approximately 300 Legionella CFU per plate. Subsequently, agar plates were incubated for 3 days at 37°C, followed by 5 days of incubation at 25°C. Finally, plates were analyzed for Legionella colony morphology where colonies seem to scatter and to disappear from the agar plate. The phenomenon was designated the scatter phenotype. Legionella clones showing the scatter phenotype were isolated and subjected to further analysis. In parallel, control clones showing no scatter phenotype at day 5 at 25°C were isolated.
Transposon insertion site analysis.
EZ-Tn5 localization was identified using inverse PCR. In short, HindIII-, SphI-, or PstI (New England Biolabs)-digested chromosomal bacterial DNA was circularized, and the regions flanking the transposon insertion site were amplified and sequenced using primers recommended by Epicentre: KAN-2FP-1 (ACCTACAACAAAGCTCTCATCAACC), KAN-2RP-1 (GCAATGTAACATCAGAGATTTTGAG), Inv-1 (ATGGCTCATAACACCCCTTGTATTA), and Inv-2 (GAACTTTTGCTGAGTTGAAGGATCA). Transposon insertions within all mutants were verified by comparison to the wild-type strain via PCR shift analysis using gene-specific primers.
Further DNA techniques and sequence analysis.
PCR was carried out using a Tgradient thermocycler (Biometra, Göttingen, Germany) and Taq DNA polymerase (New England Biolabs, Frankfurt am Main, Germany) or Pfu DNA polymerase (Fermentas GmbH, St. Leon-Rot, Germany). E. coli DH5α was employed for the propagation of recombinant plasmid DNA. For the expression and complementation of growth-defective mutants, vector pBCKS (backbone in pPA43 [empty vector], pPA49, and pPA58; Stratagene, Heidelberg, Germany) was used and introduced into bacterial strains by electroporation (Cell-Porator; Life Technologies, Paisley, Scotland) according to the manufacturer's specifications. For complementation of the L. pneumophila bdhA-patD mutant, the operon, including its native promoter, was amplified using Pfu DNA polymerase (Fermentas) and primers 2316Sac1_f (ATTACCTCCCTTGAGCTCAACTCTCT) and 2318Xba1_r (AACCCTTCTAGACTTGCTTTACTGAG). Both pBCKS and the bdhA-patD operon were SacI/XbaI digested and ligated using standard protocols, resulting in pPA49. pPA58 is a derivative of pPA49 but contains a stop codon within the bdhA gene. The stop codon was introduced 11 triplets after the start codon of bdhA (by changing GGA [Gly] to TGA [stop]) in pPA49 using the Stratagene QuikChange site-directed mutagenesis kit according to the manufacturer's instructions and mutagenesis primers g34t_sense (ATAAAGTCGCTATTGTTACATGAGCAGCAAGCGGAATTG) and its complementary oligonucleotide g34t_antisense. As an empty vector control, SacI/XbaI-treated pBCKS was religated, resulting in pPA43. Electroporation of E. coli (or L. pneumophila) was carried out using 200 direct current volts (400 direct current volts for L. pneumophila), 4 kΩ (4 kΩ for L. pneumophila), and 330 μF (330 μF for L. pneumophila). DNA was sequenced by using the BigDye 3.1 cycle sequencing mixture (Applied Biosystems, Darmstadt, Germany) and an automated DNA sequencer at the sequencing facility of the Robert Koch Institut. Primers were purchased from Tib Molbiol (Berlin, Germany) or Eurofins MWG (Ebersberg, Germany). Nucleotide and translated protein sequences were analyzed using the DNASTAR package, the pedant website (http://pedant.gsf.de/), the PSORT server (http://www.psort.org), and the L. pneumophila genome project website (http://genome3.cpmc.columbia.edu/_legion). Nucleotide sequences were also analyzed for promoters using the Web-based program BPROM (www.softberry.com) and for secretion signals using the SignalP 3.0 server (http://www.cbs.dtu.dk/services/SignalP). Sequence database searches as well as protein alignments were performed by the BLAST algorithm.
Intracellular infection of Acanthamoeba castellanii amoebae and U937 cells.
A. castellanii amoebae and U937 monocytes (CRL-1593.2; American Type Culture Collection, Manassas, VA), a human cell line that differentiates into macrophage-like cells upon treatment with phorbol esters (80 nM phorbol-12-mystrate-13-acetate [P-8139; Sigma Chemicals, Munich, Germany], with incubation for 36 to 48 h), were used as hosts for in vitro infection by L. pneumophila. Amoebae and monocytes were maintained and infected as previously described (14). To assess the intracellular growth of L. pneumophila, wells containing amoebae or U937 cells at concentrations of 105 amoebae per ml and 106 cells per ml, respectively, were infected with wild-type bacteria or isogenic mutants at multiplicities of infection of 1 (zero time point). Infections proceeded as previously described (5).
Preparations of culture supernatants and cell lysates.
L. pneumophila cell lysates were obtained from bacteria grown on agar for 2 days, which were resuspended to an OD660 of 1.0 in 40 mM Tris-HCl (pH 7.5 at 25°C). For 18-h broth-grown recombinant E. coli cells, bacteria were pelleted by centrifugation for 5 min at 3,400 × g and then adjusted to an OD660 of 1.0 in 40 mM Tris-HCl. One milliliter of bacterial suspensions from either L. pneumophila or E. coli was pelleted as mentioned above, lysed using Triton X-100 and lysozyme as described previously (5), and finally resuspended to the original culture volume with 40 mM Tris-HCl. Tenfold-diluted L. pneumophila lysates or undiluted E. coli lysates were then tested immediately for enzymatic activities.
Enzymatic assay for lipolytic activities.
Lipolytic activities were detected as described previously (for detailed information, see reference 23). In short, a lipid, namely, 1,2-dipalmitoylphosphatidylcholine (DPPC), 1,2-dipalmitoylphosphatidylglycerol (DPPG), 1-monopalmitoyllysophosphatidylcholine (MPLPC), or 1-monopalmitoyllysophosphatidylglycerol (MPLPG), was mixed with the same volume of bacterial cell lysate and incubated at 37°C with continuous agitation at 150 rpm for 3 h (L. pneumophila) and 7 h (E. coli). Amounts of free fatty acids (FFA) were determined by using the NEFA-C kit (Wako Chemicals, Neuss, Germany) according to the manufacturer's instructions.
Light microscopy for visualization of PHB inclusions.
BYE broth-grown bacteria were pelleted and subsequently fixed at room temperature in 3% paraformaldehyde for 30 min. Dry smears were then stained with Nile red (25 mM Nile red in dimethyl sulfoxide, diluted 1/500 in sterile deionized water; Sigma-Aldrich Co. Ltd.) as described elsewhere previously (33, 34). Fluorescence images were obtained using the fluorescein isothiocyanate filter of an inverse microscope (Zeiss Axiovert 200 M) and Axiovision software (V.2.1; Zeiss).
Scanning electron microscopy.
Bacterial agar cultures were first inactivated and fixed in a mixture of formaldehyde and glutaraldehyde in HEPES buffer (pH 7.2) for at least 2 h. Subsequently, specimens were gently washed with phosphate-buffered saline. After stepwise dehydration (15%, 30%, 50%, 70%, 90%, 96%, and 100%) in graded alcohol, samples were critical-point dried in CO2 (CPD 030; Bal Tec, Vaduz, Liechtenstein), mounted onto the sample stubs, sputter coated with 3 nm Au/Pd (Polaron E 5100 sputter coating unit; GaLa Instrumente, Bad Schwalbach, Germany), and examined with a Leo FEG-1530 scanning electron microscope (Carl Zeiss SMT AG, Oberkochen, Germany) at 3 kV.
RESULTS
Amoeba-sensitive L. pneumophila clones show a unique colony morphology.
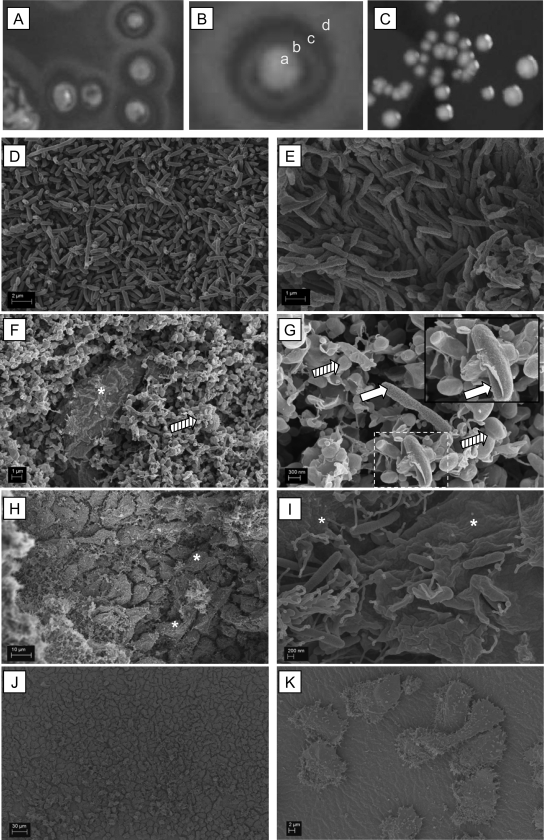
L. pneumophila typically resists predatory amoebae like A. castellanii. The inability to circumvent amoebal digestion, for example, due to the loss of a virulence determinant, renders bacteria sensitive for amoebal feeding. Interestingly, susceptibility to amoebal predation is visible on agar plates simply as a unique colony morphology, designated the scatter phenotype (Fig. 1A to C). L. pneumophila scatter colonies exhibit three defined ring regions, regions b, c, and d, surrounding the core zone a (Fig. 1B). Electron microscopy studies revealed the fine structure of the distinct colony regions. The core region (Fig. 1D and E) and the outermost ring (Fig. 1J and K) contained solely Legionella bacteria or exclusively A. castellanii amoebae, respectively. In contrast, the inner ring area (rings b and c) showed both organisms. Region b was rich in Legionella bacteria and also contained amoebae (Fig. 1F and G). Remarkably, the predominant structure of region b was represented by membranous, vesicular structures (Fig. 1F and G). Some seemed to contain single Legionella bacteria (Fig. 1G, insert). Region c comprised numerous amoebae feeding on bacteria (Fig. 1H and I). Since the ring formation phenomenon was easily detectable and since infection of A. castellanii is considered to be an indicator of bacterial virulence, the method described here has the potential to discriminate amoeba-sensitive from amoeba-resistant clones and to identify clones with reduced infectivity within a population of mutagenized L. pneumophila bacteria.
FIG. 1.
Different morphologies and unique fine structures observed for amoeba-sensitive and -resistant L. pneumophila colonies. (A and B) Amoeba-sensitive clones (A) showed the scatter colony phenotype (B) characterized by the formation of three ring areas (regions b to d) surrounding the original colony (region a). (C) Wild-type L. pneumophila resisted A. castellanii predation and showed the common colony phenotype. (D and E) Central region a of the wild type (D) and a scatter mutant colony comprised solely of Legionella bacteria (E). (F and G) The innermost ring, region b, contained A. castellanii amoebae (asterisks), Legionella bacteria (white arrows), and vesicular structures (hatched arrows). Individual vesicular structures seemed to contain single Legionella bacteria (G, insert, white arrow). (H and I) In region c, clustered A. castellanii amoebae (asterisks) were found phagocytosing bacteria. (J and K) The outermost ring, ring d, was rich in amoebae.
Screening of a transposon-mutagenized L. pneumophila clone bank for amoeba-sensitive clones.
A total of 23,000 clones of a transposon-mutagenized clone bank were screened, and 115 clones showing the scatter morphology were isolated. All of the mutants grew comparably to the wild type on common culture media. Next, the mutants were quantitatively assessed for their abilities for intra-amoebal growth in A. castellanii, and all but three clones showed attenuated replication (data not shown).
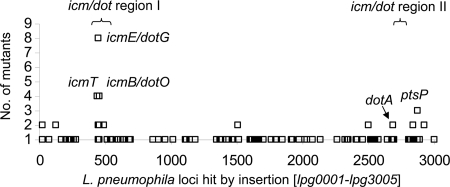
To facilitate the selection of novel virulence genes for further analysis, transposon insertion sites were analyzed, and 89 different genes hit by the transposon in the 112 growth-deficient L. pneumophila clones were identified. EZ-Tn5 insertions in virulence-attenuated clones were distributed relatively uniformly throughout the L. pneumophila genome except that the genes of the type IVB secretion system icm/dot were hit very frequently (22 mutants in icm/dot region I and 4 mutants in region II) (Fig. 2). The genes linked to amoeba infection and virulence were subdivided into two classes: (i) genes that are already known to promote host cell exploitation or virulence and (ii) those that are not. The first group includes 19 different genes affected in 36 scatter mutants (Table 1). The most abundant genes were genes encoding components of the Dot/Icm type IVB protein secretion machinery (nine genes) (54, 59). Other established virulence genes found were, for example, rtxA (16), ptsP (21), and ccmH (49) (Table 1 and Fig. 2). Interestingly, 70 different novel Legionella virulence-related genes were affected in 76 scatter mutants (Table 2). Although many of the identified genes encoded conserved protein domains, giving clues to their function, it remains completely open how they contribute to amoeba infection. For example, lpg1665 encodes a 215-kDa protein possessing an A2M-N_2 alpha-2-macroglobulin domain with characteristic “bait” and “trap” domains of eukaryotic alpha-2-macroglobulins (7). Although Budd et al. previously introduced bacterial alpha-2-macroglobulins as a potential group of host cell colonization factors, no experimental data exist (11). Another example is lpg2527, encoding a hypothetical coiled-coil domain-containing protein lacking any amino acid sequence homology to annotated proteins.
FIG. 2.
Genome-wide distribution of transposon insertion sites in the 112 confirmed L. pneumophila scatter mutants. Clusters of transposon insertion were found within the icm/dot loci with 22 mutants in region I and four mutants in region II (54, 59). The L. pneumophila ptsP gene was identified three times (30).
TABLE 1.
Nineteen known L. pneumophila genes affecting amoeba infection identified by means of the scatter screen
| Locus tag | Gene | Reference(s) |
|---|---|---|
| lpg0025 | rcp | 50 |
| lpg0441 | icmT | 39 |
| lpg0445 | icmP/dotM | 24, 54, 59 |
| lpg0446 | icmO/dotL | 54, 59 |
| lpg0448 | icmM/dotJ | 24 |
| lpg0451 | icmE/dotG | 54, 59 |
| lpg0454 | icmC/dotE | 54, 59 |
| lpg0455 | icmJ/dotN | 54, 59 |
| lpg0456 | icmB/dotO | 21, 54, 59 |
| lpg0693 | ligA | 22 |
| lpg0864 | ccmH | 41, 49 |
| lpg1791 | fliN | 29 |
| lpg2341 | dnaJ | 44 |
| lpg2515 | rtxA | 16 |
| lpg2639 | enhC | 17 |
| lpg2675 | dotC | 54, 58, 59 |
| lpg2686 | dotA | 40, 54, 59 |
| lpg2689 | icmX | 21, 54, 59 |
| lpg2871 | ptsP | 30 |
TABLE 2.
Seventy novel L. pneumophila genes affecting amoeba infection identified by means of the scatter screena
| Locus tag | Closest homolog (organism) | E value |
|---|---|---|
| lpg0026 | Amino acid permease (Cellvibrio japonicus) | 5.00E−132 |
| lpg0072 | Excinuclease ABC subunit B (Pseudomonas mendocina) | 0.0 |
| lpg0129 | Methylmalonate-semialdehyde dehydrogenase (Aeromonas salmonicida) | 1E−164 |
| lpg0166 | Putative membrane protein (Acinetobacter baumannii) | 2E−83 |
| lpg0200 | Cytochrome d ubiquinol oxidase, subunit II (Rickettsiella grylli) | 1E−100 |
| lpg0230 | PleD-like protein (Synechocystis sp.) | 8E−79 |
| lpg0233 | Thiamine pyrophosphate protein binding domain protein (Methylobacterium extorquens) | 2E−71 |
| lpg0237 | Lipolytic enzyme (Nodularia spumigena) | 2E−45 |
| lpg0243 | Probable short-chain dehydrogenase (Pseudomonas aeruginosa) | 6E−68 |
| lpg0265 | Putative multicopper oxidase (Methylobacterium nodulans) | 4E−90 |
| lpg0277 | Hypothetical protein BuboB_27536 (Burkholderia ubonensis) | 1.00E−153 |
| lpg0493 | Amino acid ABC transporter, ATP binding protein (Wolbachia endosymbiont of Drosophila ananassae) | 5E−67 |
| lpg0515 | Hypothetical protein FG10257.1 (Gibberella zeae) | 2E−28 |
| lpg0549 | Gamma-glutamyltranspeptidase (Methylococcus capsulatus) | 4E−150 |
| lpg0599 | Putative poly-beta-hydroxybutyrate synthase (Azoarcus sp. strain EbN1) | 5E−166 |
| lpg0618 | Probable 3-methyladenine-DNA glycosylase I (“Candidatus Protochlamydia amoebophila”) | 2E−66 |
| lpg0635 | Probable melitin resistance protein (Chromobacterium violaceum) | 0.0 |
| lpg0641 | Heat shock protein HslVU, ATPase subunit HslU (Hahella chejuensis) | 0.0 |
| lpg0685 | Hypothetical protein Nmul_A1979 (Nitrosospira multiformis) | 2E−171 |
| lpg1019 | Heavy metal RND efflux membrane fusion protein, CzcB family (Pseudomonas stutzeri) | 2E−71 |
| lpg1110 | None | |
| lpg1121 | None | |
| lpg1146 | Zn-dependent carboxypeptidase (Yersinia frederiksenii) | 5.00E−131 |
| lpg1188 | Potassium uptake protein, Kup system (Geobacter sulfurreducens) | 8E−147 |
| lpg1349 | Apolipoprotein N-acyltransferase (Rickettsiella grylli) | 9.00E−83 |
| lpg1373 | RNase HII (Haemophilus ducreyi) | 2E−59 |
| lpg1376 | Hypothetical protein MED92_07681 (Oceanospirillum sp.) | 2.00E−42 |
| lpg1426 | Hypothetical protein RT0522 (Rickettsia typhi strain Wilmington) | 2.00E−17 |
| lpg1441 | Putative metal-dependent phosphoesterase (marine gammaproteobacterium strain HTCC2207) | 6.00E−58 |
| lpg1466 | Hypothetical protein Rfer_2906 (Rhodoferax ferrireducens) | 1.00E−21 |
| lpg1469 | Peptidase S13 d-Ala-d-Ala carboxypeptidase C (Burkholderia phytofirmans) | 7.00E−154 |
| lpg1507 | Sodium/hydrogen antiporter family protein (Coxiella burnetii) | 1.00E−116 |
| lpg1596 | Putative fatty oxidation complex, alpha subunit (Coxiella burnetii strain Dugway) | 0.0 |
| lpg1610 | Glutamate 5-kinase, ProB related (Prosthecochloris aestuarii) | 5.00E−69 |
| lpg1615 | ABC-type multidrug transport system, ATPase component (Rickettsia bellii) | 6E−142 |
| lpg1634 | Flavin adenine dinucleotide-linked oxidase domain protein (Cyanothece sp. strain PCC 8801) | 0.0 |
| lpg1638 | Integral membrane protein (Streptomyces coelicolor) | 6.00E−78 |
| lpg1653 | d-Xylose-proton symporter (Rickettsiella grylli) | 4.00E−81 |
| lpg1665 | Alpha-2-macroglobulin domain protein (Burkholderia cenocepacia) | 0.0 |
| lpg1702 | Kinectin 1 (kinesin receptor) (Legionella pneumophila Philadelphia-1) | 1E−61 |
| lpg1762 | Two-component response regulator (Oceanospirillum sp. strain MED92) | 5.00E−138 |
| lpg1805 | DNA mismatch repair protein MutS (Coxiella burnetii) | 0.0 |
| lpg1843 | Peptidase S33, proline iminopeptidase 1 (marine gammaproteobacterium strain HTCC2143) | 2.00E−99 |
| lpg1925 | None | |
| lpg1974 | Hypothetical protein Pcar_0519 (Pelobacter carbinolicus) | 2.00E−11 |
| lpg2011 | Hypothetical protein Noc_0956 (Nitrosococcus oceani) | 2.00E−65 |
| lpg2031 | Arginyl-tRNA synthetase (Methylococcus capsulatus strain Bath) | 0.0 |
| lpg2032 | Hypothetical protein SIAM614_20101 (Stappia aggregata) | 3.00E−59 |
| lpg2132 | Diguanylate cyclase/phosphodiesterase with PAS/PAC sensor(s) (Desulfuromonas acetoxidans) | 3E−63 |
| lpg2257 | Hypothetical protein B14911_25090 (Bacillus sp. strain NRRL B-14911) | 4.00E−05 |
| lpg2316 | 3-HB dehydrogenase (Delftia acidovorans) | 1.00E−112 |
| lpg2339 | Conserved hypothetical protein (Mycobacterium marinum) | 5.00E−35 |
| lpg2401 | Beta-lactamase (Flavobacterium johnsoniae) | 3.00E−15 |
| lpg2409 | None | |
| lpg2494 | Kynurenine formamidase (Methylokorus infernorum) | 1.00E−25 |
| lpg2522 | Metal-activated pyridoxal enzyme (Moritella sp. strain PE36) | 1.00E−99 |
| lpg2527 | None | |
| lpg2538 | None | |
| lpg2544 | Transglycosylase, putative (Desulfovibrio desulfuricans) | 7.00E−23 |
| lpg2577 | None | |
| lpg2680 | Cyanophycin synthetase (Clostridium thermocellum ATCC 27405) | 2.00E−37 |
| lpg2806 | None | |
| lpg2814 | Leucine aminopeptidase, putative (Burkholderia thailandensis) | 1.00E−52 |
| lpg2815 | Putative membrane protein (Rickettsiella grylli) | 4.00E−34 |
| lpg2832 | Hypothetical protein Mmc1_1487 (Magnetococcus sp. strain MC-1) | 2.00E−21 |
| lpg2835 | Thiopurine S-methyltransferase (Shewanella baltica) | 3.00E−37 |
| lpg2870 | Hypothetical protein PE36_07941 (Moritella sp. strain PE36) | 1.00E−39 |
| lpg2885 | None | |
| lpg2917 | Conserved hypothetical protein (Mycobacterium abscessus) | 2.00E−91 |
| lpg2996 | Predicted methyltransferase (Hahella chejuensis) | 1.00E−72 |
Proteins with no significant homology showed expected values of ≥0.01.
Numerous Legionella genes possessing homology to virulence factors of other bacteria known to promote host cell interactions were detected. For example, the GGDEF domain-containing gene product of lpg0230 shows partial protein homology to the Ehrlichia chaffeensis PleD response regulator, which is involved in the inhibition of host lysosomal fusion as part of the PleC-PleD two-component system (36). Another mutant with reduced replication in the amoeba infection model carried the transposon insertion in lpg2316 (bdhA), encoding a 260-amino-acid (28-kDa) protein showing full-length protein homology to the 3-hydroxybutyrate (3-HB) dehydrogenase, BdhA, of Sinorhizobium sp. strain NGR234 (E value, 3E−44). PHB is a typical bacterial carbon and energy storage compound, facilitating survival under conditions of nutrient limitation and stress (2, 35). 3-HB dehydrogenases oxidize depolymerized PHB to acetoacetate, allowing metabolization of the energy reserve (20). L. pneumophila BdhA is therefore likely to be an enzyme involved in PHB degradation. Accordingly, Sinorhizobium sp. strain NGR234 bdhA mutants are unable to utilize PHB and interestingly show symbiosis defects with Leucaena host plants (3). L. pneumophila BdhA contains the amino acids S142, Y155, and K159, usually conserved in 3-HB dehydrogenases and likely required for catalysis (47). Furthermore, cytoplasmic localization (http://www.psort.org) and consistently no signal peptide are predicted for L. pneumophila BdhA (http://www.cbs.dtu.dk/services/SignalP/).
Examination of the L. pneumophila Philadelphia-1 bdhA locus revealed that the surrounding open reading frames are organized in the same orientation (see Fig. S1A in the supplemental material). Operon prediction analyses (http://operondb.cbcb.umd.edu/cgi-bin/operondb/operons.cgi) suggested a transcriptional unit of bdhA together with the next downstream open reading frame, lpg2317. Reverse transcriptase PCR revealed that bdhA and lpg2317 are indeed cotranscribed and therefore form an operon (see Fig. S1B in the supplemental material). lpg2317 was recently designated patD (patatin-like protein [PLP] D) and encodes a 386-amino-acid (44-kDa) putative phospholipase A (4). PatD possesses the four conserved blocks of protein homology characteristic for bacterial PLPs in which blocks II and IV harbor the putative active-site serine and aspartic acid residues of the catalytic dyad, respectively (4, 6). PatD is a predicted cytoplasmic membrane protein (http://www.psort.org) and likely does not possess a signal peptide (http://www.cbs.dtu.dk/services/SignalP/). We recently found that the L. pneumophila Philadelphia-1 genome contains the extraordinarily high number of 11 PLP genes. Since a large number of PLP genes has been found, especially in many bacterial pathogens and symbionts, we propose their role in host cell manipulation (4). Following these findings, we investigated the precise growth kinetics of the L. pneumophila bdhA-patD mutant in amoebae and human macrophages and its biochemical properties with respect to PHB content and lipolysis.
The L. pneumophila bdhA-patD mutant possesses an increased number of PHB granula.
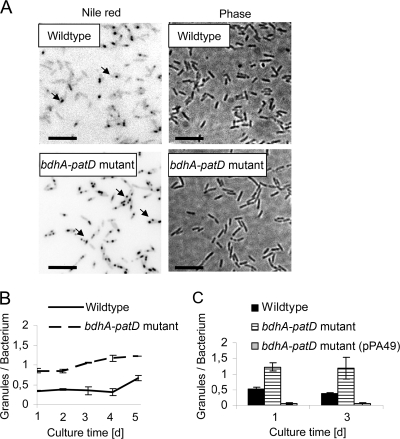
It is known that Legionella accumulates PHB as an endogenous energy reserve and that BdhA is usually an enzyme of PHB metabolism (3, 33). Therefore, we assessed whether the loss of bdhA affects bacterial PHB content. For this, PHB granula of the bdhA-patD mutant were microscopically quantified and compared to the those of the wild type and the complementing strain containing bdhA-patD in trans. Nile red, a dye that becomes fluorescent in hydrophobic environments, was used for detecting PHB inclusions in broth cultures that were incubated up to 5 days. Double amounts of PHB granules per bacterial cell at each time point were detected compared to the wild type (Fig. 3A and B). To be precise, the bdhA-patD mutant possessed about 1 granule per cell, while wild-type bacteria contained ∼0.5 granules per cell. The complementing strain showed PHB granule counts even below wild-type levels (Fig. 3C). In conclusion, we provided evidence that the bdhA-patD operon is involved in L. pneumophila PHB metabolism.
FIG. 3.
The L. pneumophila bdhA-patD mutant contained more intracellular lipid inclusions than did wild-type bacteria. (A) Fluorescence microscopy and phase-contrast images of the L. pneumophila wild type and bdhA-patD mutant stained with Nile red. The L. pneumophila bdhA-patD mutant contained more yellow fluorescent intracellular inclusions than did wild-type bacteria at 3 days postinoculation. (B) The L. pneumophila bdhA-patD mutant possessed twofold-more intracellular fluorescent granules than did wild-type bacteria during 5 days of broth growth. At all days, granule counts in the bdhA-patD mutant were significantly different from wild-type counts (P < 0.01 for days 1, 2, 4, and 5; P < 0.05 for day 3 [determined by a Student's t test]). (C) Granule content of the L. pneumophila wild type, the bdhA-patD mutant, and the complementing bdhA-patD (pPA49) mutant strain at days 1 and 3 of incubation. Whereas bdhA-patD mutant bacteria contained twofold-more intracellular lipid droplets than did the wild-type strain, the complementing strain expressing bdhA and patD in trans showed reduced granule counts. The complementing strain contained significantly less PHB granules than did the wild-type strain and the bdhA-patD mutant at both time points (P < 0.05 by a Student's t test). Results are means and standard deviations of data from two independent cultures and at least 100 counted bacteria per sample.
L. pneumophila bdhA-patD mutants show reduced lipolytic activity.
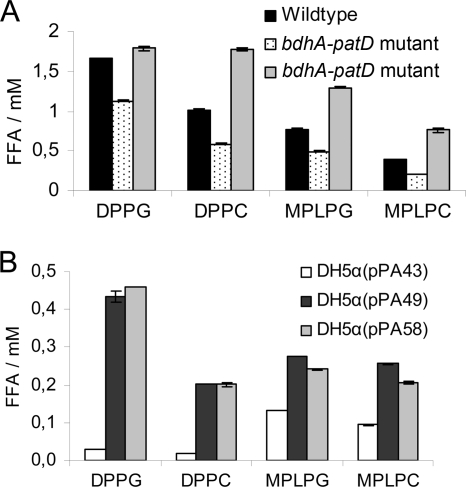
Next, it was of interest to examine whether the loss of the putative phospholipase A PatD affects the lipolytic activity of bdhA-patD mutants. L. pneumophila was previously shown to possess activities directed toward multiple phospholipid substrates (4). We therefore tested bacterial cell lysates for the release of FFA from DPPG, DPPC, MPLPG, and MPLPC. A dramatic decrease in activities hydrolyzing DPPG, DPPC, MPLPG, and MPLPC was found for the mutant (Fig. 4A). These data show that bdhA-patD contributes to the phospholipase A (PLA) and lysophospholipase A (LPLA) activities of L. pneumophila. The ability of the bdhA-patD mutant to fully release FFA from all tested substrates was restored after trans-complementation with bdhA-patD on plasmid pPA49 (Fig. 4A). We then tested cell lysates of recombinant E. coli DH5α clones containing the bdhA and patD genes on plasmid pPA49 or an inactivated bdhA gene (containing a point mutation changing amino acid 12 from Gly to stop) but a functional copy of patD on plasmid pPA58 for lipolytic activity. Both cell lysates of E. coli clones containing either pPA49 or pPA58 liberated comparable amounts of FFA from the employed substrates (Fig. 4B). Our data therefore show that patD contributed to L. pneumophila PLA and LPLA activities and conferred activity to recombinant E. coli clones.
FIG. 4.
The L. pneumophila bdhA-patD mutant possessed reduced PLA/LPLA activities. Lipolytic activities of wild-type, bdhA-patD mutant, and genetically complemented L. pneumophila strains (A) or recombinant E. coli containing either bdhA-patD (pPA49) or inactivated bdhA but functional patD (pPA58). L. pneumophila or recombinant E. coli cell lysates (B) were incubated with DPPG, DPPC, MPLPG, and MPLPC for 3 h (A) or 7 h (B) at 37°C, and the release of FFA was then quantified. L. pneumophila cell lysates were diluted 10-fold prior to incubation. Data are expressed as differences between the amount of FFA released by the cell lysates and the amount released by uninoculated Tris-HCl buffer. Results are means and standard deviations from triplicate values and are representative of two independent experiments. For all substrates, lipolytic activities of the L. pneumophila bdhA-patD mutant strain were significantly reduced compared to those of the wild-type strain, and the activity of the complementing strain was significantly enhanced compared to that of the mutant (P < 0.01 by a Student's t test). E. coli strains containing either pPA49 or pPA58 showed significantly enhanced lipolytic activity against all substrates compared to E. coli harboring the empty vector control pPA43 (P < 0.01 by a Student's t test).
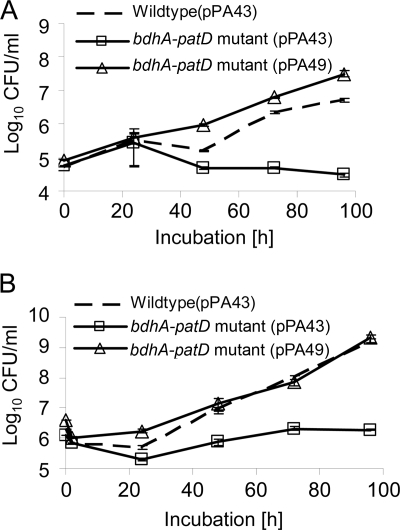
The bdhA-patD operon is essential for infection of amoebae and human macrophages.
After profiling of the L. pneumophila bdhA-patD mutant with regard to lipolysis and PHB content, we investigated the importance of the operon for the intracellular infection of A. castellanii amoebae and U937 macrophages. Whereas the wild type revealed the typical pattern of intracellular growth, in which bacterial numbers increased about 100- to 1,000-fold by 96 h postinoculation, the numbers of the bdhA-patD mutant did not increase (Fig. 5A and B). trans-Complementation with bdhA-patD on plasmid pPA49 fully restored the defect and supported our hypothesis that bdhA-patD is needed for growth in A. castellanii amoebae and U937 macrophages (Fig. 5). Taken together, our data indicate that the bdhA-patD operon is required for the intracellular infection of both A. castellanii amoebae and human U937 macrophages by L. pneumophila and therefore is a novel virulence-associated determinant.
FIG. 5.
Intracellular infection by wild-type and bdhA-patD mutant L. pneumophila strains. The wild-type and bdhA-patD mutant strains, both harboring the empty vector, and the complemented bdhA-patD mutant strain harboring bdhA-patD in trans (pPA49) were used to infect monolayers of A. castellanii amoebae (A) or cultures of U937 macrophages (B) at a multiplicity of infection of 1. At various time points postinoculation, bacteria were quantified by plating aliquots onto BCYE agar. Results are means and standard deviations from triplicate samples and are representative of three independent experiments. CFU counts for the bdhA-patD mutant were significantly lower than those for the wild-type and complementing strains at 72 h and 96 h postinfection in both A. castellanii amoebae and U937 macrophages (P < 0.05 by a Student's t test).
DISCUSSION
Despite increasing research efforts, our understanding of Legionella virulence is still limited. In this study, we present a novel screening procedure for the detection of L. pneumophila host colonization and virulence determinants. The screen, named the scatter screen, is based on the easy recognition of the scatter colony morphology developing from Legionella colonies that are sensitive instead of resistant to amoebal grazing. Using the scatter screen, we examined a clone bank of 23,000 L. pneumophila transposon insertion mutants for amoeba-sensitive clones and identified 89 genes critical for A. castellanii infection (Tables 1 and 2). We thereby found 19 known virulence genes (Table 1), and importantly, 70 novel genes contributing to host cell infection were detected (Table 2), and some will be discussed below. At least two screens for Legionella mutants defective in amoeba infection were reported previously (25, 49). Both screens, like the study presented here, identified novel Legionella virulence genes. In the first one, Gao et al. examined 5,280 clones of a mini-Tn10::Knr-mutagenized L. pneumophila AA100 (derivative of strain 130b) clone bank for replication both in U937 macrophages and in Acanthamoeba polyphaga amoebae. Those researchers found 89 different mutants with defects in intracellular replication in both cells; however, the genes affected were not listed in detail (25). In the second screen, Polesky et al. screened 700 signature-tagged L. pneumophila 130b mutants and isolated six clones that were defective in intracellular replication and invasion (49). In our screen and in the screen reported previously by Polesky et al., genes of similar functional complexes were isolated, including, for example, genes of the cytochrome c biogenesis pathway, ccmH and ccmF, respectively, and motility genes as a homolog of the transcriptional regulator of flagellum synthesis in Vibrio cholerae FlrC (18) and the flagellar motor protein FliN, respectively, underlining conservation between virulence strategies of different pathogenic Legionella isolates. In addition to the overlapping gene functions identified, we here found many genes that were not detected in previous screens (Table 2).
Among the 70 novel L. pneumophila virulence genes that were isolated in the current screen, one, a putative 3-HB dehydrogenase gene, bdhA, was chosen for further characterization. 3-HB dehydrogenases oxidize 3-HB to acetoacetate, which is finally transformed to acetyl coenzyme A and is then introduced into the tricarboxylic acid cycle or the glyoxylate cycle (20, 55). Thus, 3-HB dehydrogenases are involved in PHB degradation and metabolization. L. pneumophila bdhA is therefore likely an enzyme supporting PHB degradation. The closest characterized homolog of L. pneumophila BdhA is Sinorhizobium sp. strain NGR234 BdhA, a 3-HB dehydrogenase essential for PHB utilization by the root bacterium (3). It is known that Legionella accumulates PHB for survival in low-nutrient environments in a vegetative state (33), and several studies previously reported the formation of inclusions resembling PHB granules during intra-amoebal growth of L. pneumophila (1, 57). However, the precise role of the lipid in virulence is still unknown. The identification of a protein that is likely to be involved in PHB degradation is of particular interest, as Brüggemann et al. previously showed that genes putatively involved in Legionella PHB metabolism are transcriptionally induced when intracellular L. pneumophila cells switch from the replicative to the transmissive phase (bdhA, eightfold upregulation) (10). Interestingly, the bdhA gene was found to be cotranscribed with the adjacent PLP gene patD, which is therefore strongly upregulated as well (eightfold) (4).
The second gene of the operon, patD, encodes a protein with homology to patatin, a potato storage glycoprotein with lipid acylhydrolase activity (4, 32). We recently found that bacterial PLPs in addition to ExoU, a potent phospholipase and cytotoxin of Pseudomonas aeruginosa (48, 53), are frequently encoded in many bacterial species (6). Due to the relatedness of PatD to phospholipases, we examined the L. pneumophila bdhA-patD mutant with regard to its lipolytic activity. Indeed, the bdhA-patD mutant possessed strongly diminished cell-associated activity against several PLA and LPLA substrates, and the expression of patD in E. coli further confirmed the enzymatic profile. Additionally, the PHB content was increased in the mutant, and intracellular growth was severely impaired, suggesting that the operon supports PHB usage. Successful complementation of intracellular growth with both bdhA and patD in trans, but not with the single genes (data not shown), demonstrated that both genes are required for growth in the employed host cells. Although its exact role remains open, our data provide evidence that PatD lipolytic activity combined with BdhA 3-HB dehydrogenase activity are essential for intracellular replication and might also be needed for PHB utilization.
It is attractive to speculate whether PatD is directly involved in PHB granule mobilization. PHB depolymerases characteristically contain a catalytic triad, serine (embedded in a G-X-S-X-G motif)/aspartate (or glutamate)/histidine, typical for esterase-lipase superfamily enzymes (NCBI CCD accession number cd00312), and are alpha/beta folded (35, 46). Most of those structural features as a serine/aspartate catalytic dyad and the alpha/beta fold have been found for PLP (52). Thus, PatD is structurally closely related to PHB depolymerases and may fulfill a similar function under PHB-mobilizing conditions. Interestingly, within the four sequenced L. pneumophila (Philadelphia-1, Paris, Lens, and Corby) (12, 13, 26) genomes, we were unable to find genes coding for homologs of known PHB depolymerases. Another scenario of concerted bdhA-patD action is possible. Bacterial and eukaryotic lipid inclusions are surrounded by a phospholipid monolayer with embedded amphiphatic proteins, the phasines. Phasines support the solubilization of granules in hydrophilic environments, as the cytoplasm, and they also avoid an aggregation of granules (28). Phospholipases A partially destruct the granule phospholipid layer, making lipids accessible for degrading enzymes such as depolymerases, thus allowing PHB metabolization (43). Accordingly, May and coworkers previously demonstrated that a plant PLP of Cucumis sativus specifically localized to lipid granules for support of lipid mobilization during seed germination (37). Whether PatD directly facilitates PHB utilization in L. pneumophila requires further work and will be addressed in future studies.
Here we have introduced a novel amoeba-based screening procedure for virulence genes of Legionella, which is likely easily applicable for other amoeba-resistant microorganisms. The procedure identified many known but also new colonization factors of L. pneumophila. Among the latter, the bdhA-patD operon was found to be critical for intracellular replication in amoebae and macrophages, for PHB usage, and for PLA/LPLA activity.
Supplementary Material
Acknowledgments
We thank Kazimierz Madela for valuable advice on microscopy matters.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (grants FL359/3-1 and FL359/3-3).
Footnotes
Published ahead of print on 1 May 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Anand, C. M., A. R. Skinner, A. Malic, and J. B. Kurtz. 1983. Interaction of L. pneumophilia and a free living amoeba (Acanthamoeba palestinensis). J. Hyg. (London) 91:167-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, A. J., and E. A. Dawes. 1990. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Mol. Biol. Rev. 54:450-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aneja, P., and T. C. Charles. 2005. Characterization of bdhA, encoding the enzyme D-3-hydroxybutyrate dehydrogenase, from Sinorhizobium sp. strain NGR234. FEMS Microbiol. Lett. 242:87-94. [DOI] [PubMed] [Google Scholar]
- 4.Banerji, S., P. Aurass, and A. Flieger. 2008. The manifold phospholipases A of Legionella pneumophila—identification, export, regulation, and their link to bacterial virulence. Int. J. Med. Microbiol. 298:169-181. [DOI] [PubMed] [Google Scholar]
- 5.Banerji, S., M. Bewersdorff, B. Hermes, N. P. Cianciotto, and A. Flieger. 2005. Characterization of the major secreted zinc metalloprotease-dependent glycerophospholipid:cholesterol acyltransferase, PlaC, of Legionella pneumophila. Infect. Immun. 73:2899-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerji, S., and A. Flieger. 2004. Patatin-like proteins: a new family of lipolytic enzymes present in bacteria? Microbiology 150:522-525. [DOI] [PubMed] [Google Scholar]
- 7.Barrett, A. J., and P. M. Starkey. 1973. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem. J. 133:709-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozue, J. A., and W. Johnson. 1996. Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion 1. Infect. Immun. 64:668-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brieland, J., M. McClain, M. LeGendre, and C. Engleberg. 1997. Intrapulmonary Hartmannella vermiformis: a potential niche for Legionella pneumophila replication in a murine model of legionellosis. Infect. Immun. 65:4892-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brüggemann, H., A. Hagman, M. Jules, O. Sismeiro, M. A. Dillies, C. Gouyette, F. Kunst, M. Steinert, K. Heuner, J. Y. Coppée, and C. Buchrieser. 2006. Virulence strategies for infecting phagocytes deduced from the in vivo transcriptional program of Legionella pneumophila. Cell. Microbiol. 8:1228-1240. [DOI] [PubMed] [Google Scholar]
- 11.Budd, A., S. Blandin, E. A. Levashina, and T. J. Gibson. 2004. Bacterial alpha2-macroglobulins: colonization factors acquired by horizontal gene transfer from the metazoan genome? Genome Biol. 5:R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cazalet, C., C. Rusniok, H. Bruggemann, N. Zidane, A. Magnier, L. Ma, M. Tichit, S. Jarraud, C. Bouchier, F. Vandenesch, F. Kunst, J. Etienne, P. Glaser, and C. Buchrieser. 2004. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 36:1165-1173. [DOI] [PubMed] [Google Scholar]
- 13.Chien, M., I. Morozova, S. Shi, H. Sheng, J. Chen, S. M. Gomez, G. Asamani, K. Hill, J. Nuara, M. Feder, J. Rineer, J. J. Greenberg, V. Steshenko, S. H. Park, B. Zhao, E. Teplitskaya, J. R. Edwards, S. Pampou, A. Georghiou, I. C. Chou, W. Iannuccilli, M. E. Ulz, D. H. Kim, A. Geringer-Sameth, C. Goldsberry, P. Morozov, S. G. Fischer, G. Segal, X. Qu, A. Rzhetsky, P. Zhang, E. Cayanis, P. J. De Jong, J. Ju, S. Kalachikov, H. A. Shuman, and J. J. Russo. 2004. The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305:1966-1968. [DOI] [PubMed] [Google Scholar]
- 14.Cianciotto, N. P., and B. S. Fields. 1992. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc. Natl. Acad. Sci. USA 89:5188-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cirillo, J. D., S. L. Cirillo, L. Yan, L. E. Bermudez, S. Falkow, and L. S. Tompkins. 1999. Intracellular growth in Acanthamoeba castellanii affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infect. Immun. 67:4427-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirillo, S. L. G., L. E. Bermudez, S. H. El-Etr, G. E. Duhamel, and J. D. Cirillo. 2001. Legionella pneumophila entry gene rtxA is involved in virulence. Infect. Immun. 69:508-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cirillo, S. L. G., J. Lum, and J. D. Cirillo. 2000. Identification of novel loci involved in entry by Legionella pneumophila. Microbiology 146:1345-1359. [DOI] [PubMed] [Google Scholar]
- 18.Correa, N. E., C. M. Lauriano, R. McGee, and K. E. Klose. 2000. Phosphorylation of the flagellar regulatory protein FlrC is necessary for Vibrio cholerae motility and enhanced colonization. Mol. Microbiol. 35:743-755. [DOI] [PubMed] [Google Scholar]
- 19.Cosson, P., and T. Soldati. 2008. Eat, kill or die: when amoeba meets bacteria. Curr. Opin. Microbiol. 11:271-276. [DOI] [PubMed] [Google Scholar]
- 20.Dawes, E. A., and P. J. Senior. 1973. The role and regulation of energy reserve polymers in micro-organisms. Adv. Microb. Physiol. 10:135-266. [DOI] [PubMed] [Google Scholar]
- 21.Edelstein, P. H., M. A. Edelstein, F. Higa, and S. Falkow. 1999. Discovery of virulence genes of Legionella pneumophila by using signature tagged mutagenesis in a guinea pig pneumonia model. Proc. Natl. Acad. Sci. USA 96:8190-8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fettes, P. S., M. Susa, J. Hacker, and R. Marre. 2000. Characterization of the Legionella pneumophila gene ligA. Int. J. Med. Microbiol. 290:239-250. [DOI] [PubMed] [Google Scholar]
- 23.Flieger, A., K. Rydzewski, S. Banerji, M. Broich, and K. Heuner. 2004. Cloning and characterization of the gene encoding the major cell-associated phospholipase A of Legionella pneumophila, plaB, exhibiting hemolytic activity. Infect. Immun. 72:2648-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gal-Mor, O., T. Zusman, and G. Segal. 2002. Analysis of DNA regulatory elements required for expression of the Legionella pneumophila icm and dot virulence genes. J. Bacteriol. 184:3823-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao, L. Y., O. S. Harb, and Y. A. Kwaik. 1997. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect. Immun. 65:4738-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glockner, G., C. Albert-Weissenberger, E. Weinmann, S. Jacobi, E. Schunder, M. Steinert, J. Hacker, and K. Heuner. 2008. Identification and characterization of a new conjugation/type IVA secretion system (trb/tra) of Legionella pneumophila Corby localized on two mobile genomic islands. Int. J. Med. Microbiol. 298:411-428. [DOI] [PubMed] [Google Scholar]
- 27.Greub, G., and D. Raoult. 2004. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17:413-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griebel, R., Z. Smith, and J. M. Merrick. 1968. Metabolism of poly-beta-hydroxybutyrate. I. Purification, composition, and properties of native poly-beta-hydroxybutyrate granules from Bacillus megaterium. Biochemistry 7:3676-3681. [DOI] [PubMed] [Google Scholar]
- 29.Heuner, K., and M. Steinert. 2003. The flagellum of Legionella pneumophila and its link to the expression of the virulent phenotype. Int. J. Med. Microbiol. 293:133-143. [DOI] [PubMed] [Google Scholar]
- 30.Higa, F., and P. H. Edelstein. 2001. Potential virulence role of the Legionella pneumophila ptsP ortholog. Infect. Immun. 69:4782-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilbi, H., S. S. Weber, C. Ragaz, Y. Nyfeler, and S. Urwyler. 2007. Environmental predators as models for bacterial pathogenesis. Environ. Microbiol. 9:563-575. [DOI] [PubMed] [Google Scholar]
- 32.Hirschberg, H. J., J. W. Simons, N. Dekker, and M. R. Egmond. 2001. Cloning, expression, purification and characterization of patatin, a novel phospholipase A. Eur. J. Biochem. 268:5037-5044. [DOI] [PubMed] [Google Scholar]
- 33.James, B. W., W. S. Mauchline, P. J. Dennis, C. W. Keevil, and R. Wait. 1999. Poly-3-hydroxybutyrate in Legionella pneumophila, an energy source for survival in low-nutrient environments. Appl. Environ. Microbiol. 65:822-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jendrossek, D. 2005. Fluorescence microscopical investigation of poly(3-hydroxybutyrate) granule formation in bacteria. Biomacromolecules 6:598-603. [DOI] [PubMed] [Google Scholar]
- 35.Jendrossek, D., and R. Handrick. 2002. Microbial degradation of polyhydroxyalkanoates. Annu. Rev. Microbiol. 56:403-432. [DOI] [PubMed] [Google Scholar]
- 36.Kumagai, Y., Z. Cheng, M. Lin, and Y. Rikihisa. 2006. Biochemical activities of three pairs of Ehrlichia chaffeensis two-component regulatory system proteins involved in inhibition of lysosomal fusion. Infect. Immun. 74:5014-5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.May, C., R. Preisig-Müller, M. Höhne, P. Gnau, and H. Kindl. 1998. A phospholipase A2 is transiently synthesized during seed germination and localized to lipid bodies. Biochim. Biophys. Acta 1393:267-276. [DOI] [PubMed] [Google Scholar]
- 38.Moffat, J. F., and L. S. Tompkins. 1992. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect. Immun. 60:296-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molmeret, M., O. A. Alli, S. Zink, A. Flieger, N. P. Cianciotto, and Y. A. Kwaik. 2002. icmT is essential for pore formation-mediated egress of Legionella pneumophila from mammalian and protozoan cells. Infect. Immun. 70:69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagai, H., and C. R. Roy. 2001. The DotA protein from Legionella pneumophila is secreted by a novel process that requires the Dot/Icm transporter. EMBO J. 20:5962-5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naylor, J., and N. P. Cianciotto. 2004. Cytochrome c maturation proteins are critical for in vivo growth of Legionella pneumophila. FEMS Microbiol. Lett. 241:249-256. [DOI] [PubMed] [Google Scholar]
- 42.Ninio, S., and C. R. Roy. 2007. Effector proteins translocated by Legionella pneumophila: strength in numbers. Trends Microbiol. 15:372-380. [DOI] [PubMed] [Google Scholar]
- 43.Noll, F., C. May, and H. Kindl. 2000. Phospholipid monolayer of plant lipid bodies attacked by phospholipase A2 shows 80 nm holes analyzed by atomic force microscopy. Biophys. Chem. 86:29-35. [DOI] [PubMed] [Google Scholar]
- 44.Ohnishi, H., Y. Mizunoe, A. Takade, Y. Tanaka, H. Miyamoto, M. Harada, and S. Yoshida. 2004. Legionella dumoffii DjlA, a member of the DnaJ family, is required for intracellular growth. Infect. Immun. 72:3592-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pagnier, I., D. Raoult, and S. B. La. 2008. Isolation and identification of amoeba-resisting bacteria from water in human environment by using an Acanthamoeba polyphaga co-culture procedure. Environ. Microbiol. 10:1135-1144. [DOI] [PubMed] [Google Scholar]
- 46.Papageorgiou, A. C., S. Hermawan, C. B. Singh, and D. Jendrossek. 2008. Structural basis of poly(3-hydroxybutyrate) hydrolysis by PhaZ7 depolymerase from Paucimonas lemoignei. J. Mol. Biol. 382:1184-1194. [DOI] [PubMed] [Google Scholar]
- 47.Persson, B., M. Krook, and H. Jornvall. 1991. Characteristics of short-chain alcohol dehydrogenases and related enzymes. Eur. J. Biochem. 200:537-543. [DOI] [PubMed] [Google Scholar]
- 48.Phillips, R. M., D. A. Six, E. A. Dennis, and P. Ghosh. 2003. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J. Biol. Chem. 278:41326-41332. [DOI] [PubMed] [Google Scholar]
- 49.Polesky, A. H., J. T. D. Ross, S. Falkow, and L. S. Tompkins. 2001. Identification of Legionella pneumophila genes important for infection of amoebas by signature-tagged mutagenesis. Infect. Immun. 69:977-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robey, M., W. O'Connell, and N. P. Cianciotto. 2001. Identification of Legionella pneumophila rcp, a pagP-like gene that confers resistance to cationic antimicrobial peptides and promotes intracellular infection. Infect. Immun. 69:4276-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rowbotham, T. J. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rydel, T. J., J. M. Williams, E. Krieger, F. Moshiri, W. C. Stallings, S. M. Brown, J. C. Pershing, J. P. Purcell, and M. F. Alibhai. 2003. The crystal structure, mutagenesis, and activity studies reveal that patatin is a lipid acyl hydrolase with a Ser-Asp catalytic dyad. Biochemistry 42:6696-6708. [DOI] [PubMed] [Google Scholar]
- 53.Sato, H., and D. W. Frank. 2004. ExoU is a potent intracellular phospholipase. Mol. Microbiol. 53:1279-1290. [DOI] [PubMed] [Google Scholar]
- 54.Segal, G., M. Purcell, and H. A. Shuman. 1998. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. USA 95:1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Senior, P. J., and E. A. Dawes. 1973. The regulation of poly-beta-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem. J. 134:225-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas, V., T. Bouchez, V. Nicolas, S. Robert, J. F. Loret, and Y. Levi. 2004. Amoebae in domestic water systems: resistance to disinfection treatments and implication in Legionella persistence. J. Appl. Microbiol. 97:950-963. [DOI] [PubMed] [Google Scholar]
- 57.Vandenesch, F., M. Surgot, N. Bornstein, J. C. Paucod, D. Marmet, P. Isoard, and J. Fleurette. 1990. Relationship between free amoeba and Legionella: studies in vitro and in vivo. Zentralbl. Bakteriol. 272:265-275. [DOI] [PubMed] [Google Scholar]
- 58.Vincent, C. D., J. R. Friedman, K. C. Jeong, E. C. Buford, J. L. Miller, and J. P. Vogel. 2006. Identification of the core transmembrane complex of the Legionella Dot/Icm type IV secretion system. Mol. Microbiol. 62:1278-1291. [DOI] [PubMed] [Google Scholar]
- 59.Vogel, J. P., H. L. Andrews, S. K. Wong, and R. R. Isberg. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279:873-876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.