Abstract
Mersacidin binds to lipid II and thus blocks the transglycosylation step of the cell wall biosynthesis. Binding of lipid II involves a special motif, the so-called mersacidin-lipid II binding motif, which is conserved in a major subgroup of lantibiotics. We analyzed the role of Ca2+ ions in the mode of action of mersacidin and some related peptides containing a mersacidin-like lipid II binding motif. We found that the stimulating effect of Ca2+ ions on the antimicrobial activity known for mersacidin also applies to plantaricin C and lacticin 3147. Ca2+ ions appear to facilitate the interaction of the lantibiotics with the bacterial membrane and with lipid II rather than being an essential part of a peptide-lipid II complex. In the case of lacticin 481, both the interaction with lipid II and the antimicrobial activity were Ca2+ independent.
Bacteriocins are a heterogeneous group of ribosomally synthesized antibiotic peptides and proteins which were proposed to fall into three classes, the lanthionine-containing bacteriocins (class I), the non-lanthionine-containing bacteriocins (class II), and the bacteriolysins, respectively (for a review, see reference 12).
The lanthionine-containing bacteriocins (lantibiotics) are produced by and are effective against a broad spectrum of gram-positive bacteria. They are small, posttranslationally modified antimicrobial peptides containing characteristic thioether ring structures (lanthionine and 3-methyllanthionine) and other unusual amino acids, e.g., d-Ala (3, 49).
Mersacidin was the first lantibiotic shown to interact with a defined target molecule, the ultimate cell wall precursor lipid II (6) (Fig. 1). Further studies revealed that this molecule is also the target of nisin and many other lantibiotics (19). Lipid II is synthesized on the cytoplasmic side of the membrane and translocated to the outside of the bacterial cell membrane, where the disaccharide pentapeptide part of lipid II is incorporated into the growing peptidoglycan network by the cell wall biosynthesis machinery (for reviews, see references 5 and 45).
FIG. 1.
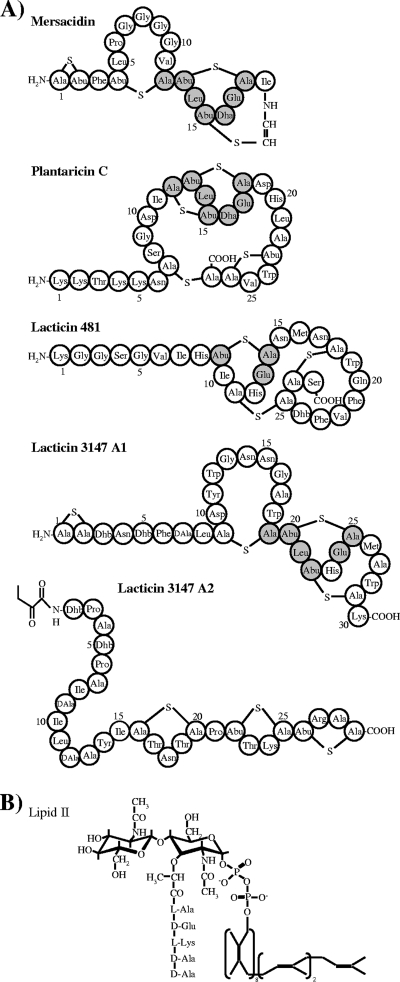
Primary structure of lantibiotics containing the mersacidin-lipid II binding motif (A) and the structure of the cell wall precursor lipid II (B). The binding motif of mersacidin and identical amino acids in the mersacidin-like lantibiotics are highlighted in gray. Dha, dehydroalanine; Dhb, dehydrobutyrine; Ala-S-Ala, lanthionine; Abu-S-Ala, methyllanthionine; DAla, d-alanine.
To date, two different lipid II binding motifs in lantibiotics have been identified, referred to as the nisin-lipid II and mersacidin-lipid II binding motifs, and a classification regarding their interaction with the cell wall precursor was recently proposed by Bierbaum and Sahl (3).
The nisin-lipid II binding motif is also found in related lantibiotics, e.g., gallidermin, epidermin (4), mutacin 1140 (40), and subtilin (30). Nisin displays a dual mode of action by binding to lipid II. It prevents lipid II incorporation into the growing murein layer, thereby blocking cell wall biosynthesis (8), and it uses lipid II as an anchor molecule for subsequent pore formation (48). The nisin/lipid II interaction was analyzed by nuclear magnetic resonance spectroscopy and it was shown that the N-terminal part of the peptide forms a cage-like structure encompassing the pyrophosphate group of the lipid II molecule, leading to the formation of five intermolecular hydrogen bonds between the backbone amids of the lantibiotic and pyrophosphate groups (22).
The second binding motif occurs in mersacidin and related lantibiotics (Fig. 1). The interaction of mersacidin with lipid II leads to inhibition of the peptidoglycan biosynthesis at the level of transglycosylation (7). In contrast to nisin, the activity of mersacidin is influenced by Ca2+ ions, since its antimicrobial activity increased twofold in Ca2+-containing medium (2). When a Ca2+ binding pocket was identified in the mersacidin-like lantibiotic actagardine by crystal structure determination, it was suggested that the deprotonated Glu17 in the mersacidin-lipid II binding motif (Fig. 1) is involved in Ca2+ binding (24). Furthermore, nuclear magnetic resonance studies revealed that, upon binding of lipid II, mersacidin effectively alters its overall backbone geometry with Ala-12 and Abu-13, acting as a hinge region. The conformational change exposes the amino group of Lys1 and the carboxyl group of Glu17 to the lipid II molecule (21). It was speculated that Ca2+ is needed to bridge the mersacidin Glu17 side chain to the negatively charged groups of lipid II; alternatively, a direct salt bridge with the positively charged side chain of Lys3 in lipid II is formed (21). This hypothesis is in good agreement with the observation that replacement of Glu17 by Ala abolished the antimicrobial activity of mersacidin (43).
To analyze the impact of Ca2+ on the activity of mersacidin-like lantibiotics, we selected four peptides which possess the respective lipid II-binding motif, yet show significant differences in primary structures (Fig. 1). Like mersacidin, plantaricin C and the two-component lantibiotic lacticin 3147 have been shown to inhibit cell wall biosynthesis at the level of transglycosylation (46, 47). Additionally, lacticin 3147 shows a dual mode of action and is able to form lipid II-dependent pores (28, 47). The mode of action of lacticin 481 so far has not been characterized in sufficient detail.
We found that Ca2+ increases the antimicrobial activity of all peptides containing the mersacidin-lipid II binding motif, except for lacticin 481, however, which was also found to bind to lipid II.
MATERIALS AND METHODS
Chemicals and materials.
All chemicals were of analytical grade or better. Radiolabeled uridine diphospho-N-acetyl-d-[U-14C]glucosamine (9.69 GBq mmol−1) was purchased from Amersham (GE Healthcare, Buckinghamshire, United Kingdom). The phospholipids 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL), used without further purification, and stored at −20°C in chloroform. Carboxyfluorescein (CF) was purchased from Sigma (Deisenhofen, Germany).
Bacterial strains and culture conditions.
The lacticin 3147-overproducing strain Lactococcus lactis subsp. cremoris MG1363 (pMRC01, pOM02) (11), Lactococcus lactis IPLA C270 (lacticin 481 producer) (34), and the indicator strain Lactococcus lactis subsp. cremoris HP were grown in M17 medium (Oxoid, Basingstoke, United Kingdom) supplemented with 0.5% glucose (GM17) at 30°C without aeration. Micrococcus luteus DSM 1790 was grown at 37°C in Trypticase soy broth (Merck, Darmstadt, Germany) with aeration.
Antimicrobial peptides.
All bacteriocins were purified to homogeneity and were stored as stock solutions (1 mg/ml) at −20°C. Nisin Z was purified from culture supernatants of Lactococcus lactis NIZO22186 (25) by chloroform extraction (4). Mersacidin was obtained from Hoechst (Frankfurt am Main, Germany). Plantaricin C purification was performed as previously described (46). The lacticin 3147 A1 and A2 peptides were isolated as reported previously (47).
Lacticin 481 was purified as described previously for other lantibiotics (4) with slight modifications. The lacticin 481-producing strain L. lactis IPLA C270 was grown in nisin production medium (26) for 8 h at 30°C. Purification was achieved by high-performance liquid chromatography using a linear gradient (buffer A [H2O, 0.1% (vol/vol) trifluoroacetic acid]) from 0 to 25% with buffer B (isopropanol, 0.1% [vol/vol] trifluoroacetic acid) for 3 min, from 25 to 42% for 22 min, from 42 to 50% for 15 min, and from 50 to 100% for 2 min at a flow rate of 8 ml/min. The peptide eluted at 40 to 42% with buffer B.
Lipid II preparation.
Lipid II was synthesized in vitro using membrane preparations of M. luteus DSM 1790 and purified as described previously (39).
Preparation of radiolabeled lipid II.
Radiolabeled lipid II was synthesized and purified as described above with some modifications. The lipid II assay was performed in a total volume of 1.95 ml containing 5.2 to 10.4 mg of protein from a membrane preparation, 130 nmol undecaprenylphosphate, 1.3 mmol UDP-N-acetylmuramyl pentapeptide, and 1.3 μmol of a 50:1 mixture of UDP-N-acetylglucosamine, and UDP-N-acetyl-d-[U-14C]glucosamine (9.69 GBq mmol−1) in 60 mM Tris-HCl, 5 mM MgCl2, pH 8, and 0.5% (vol/vol) Triton X-100. After incubation for 1 h at 30°C, lipids were extracted with the same volume of n-butanol-6 M pyridine acetate (2:1, vol/vol), pH 4.2. Purification of radiolabeled lipid II was performed on a DEAE-cellulose column (1 ml; HiTrap DEAE FF; GE Healthcare, Buckinghamshire, United Kingdom) and eluted in a stepwise gradient from chloroform-methanol-water (2:3:1, vol/vol/vol) to chloroform-methanol-300 mM ammonium bicarbonate (2:3:1, vol/vol/vol). Identification of the radiolabeled lipid II-containing fractions was performed by using thin-layer chromatography (TLC) (silica plates, 60 F254; Merck, Darmstadt, Germany) using chloroform-methanol-water-ammonia (88:48:10:1, vol/vol/vol/vol) as solvent (33). Lipid spots were visualized by using iodine vapor, scraped off, and used for phosphate determination (35).
MIC determinations.
MIC determinations were carried out in microtiter plates. M. luteus DSM 1790 was grown in half-concentrated Mueller-Hinton broth (contains less than 0.05 mM Ca2+) (Oxoid). When indicated, 1.25 mM CaCl2 was added. MICs with serial twofold dilution steps were performed with additional intermediate steps (1:2) starting at three-quarters of the highest concentration used. Bacteria were added to give a final inoculum of 105 CFU/ml in a volume of 0.2 ml. After incubation for 24 h at 37°C for M. luteus DSM 1790, the MIC was read as the lowest peptide concentration causing inhibition of visible growth.
Potassium release from whole cells.
Potassium release experiments were performed as described previously (48) with some modifications. L. lactis subsp. cremoris HP cells were harvested at an optical density at 600 nm of 1.0 to 1.5 (3,300 × g at 4°C for 3 min), washed with 50 ml of cold choline buffer (300 mM choline chloride, 30 mM morpholineethanesulfonic acid [MES], and 20 mM Tris, pH 6.5), and resuspended in the same buffer to an optical density at 600 nm of 30. Peptide-induced potassium efflux was monitored by using the pH meter pH 213 (Hanna Instruments, Kehl am Rhein, Germany) with an MI-442 potassium electrode and an MI-409F reference electrode (Microelectrodes, Inc., Bedford, MA). Measurements were performed in buffer and Ca2+-free buffer (treated with 10 mM EGTA), since curves obtained from experiments in the presence of additional 1.25 mM CaCl2 were identical to the ones in buffer (data not shown). K+ leakage was expressed relative to the total amount of potassium release induced by the addition of 1 μM nisin (data not shown). The amount of K+ released by nisin correlated to an intracellular K+ concentration of approximately 300 mM. The experiments were repeated three times.
Preparation of unilamellar vesicles.
Large unilamellar vesicles of DOPC were prepared for CF and tryptophan fluorescence experiments by the extrusion technique (27). When indicated, vesicles (400 nm) were supplemented with 0.1 mol% or 1 mol% lipid II (referring to the total amount of phospholipids).
CF efflux experiments.
CF-loaded vesicles were prepared with 50 mM CF and then diluted in 1.5-ml K+ buffer (50 mM MES-KOH, pH 6.0, 100 mM K2SO4) to a final concentration of 25 μM phospholipid on a phosphorous base. When indicated, the buffer was supplemented with 1.25 mM CaCl2 or 10 mM EGTA. After the addition of peptides, the increase of fluorescence intensity was measured at 520 nm (excitation at 492 nm) at room temperature (RT). Peptide-induced leakage was documented relative to the total amount of marker release after solubilization of the vesicles by the addition of 10 μl of 20% Triton X-100. All measurements were performed at least twice, and one representative experiment is shown.
Tryptophan fluorescence measurements.
Tryptophan fluorescence measurements were performed in 1.5 ml of K+ buffer at RT. The peptides were added at a concentration of 0.25 μM (lacticin 3147), 0.5 μM (plantaricin C), and 1 μM (lacticin 481), respectively, in the absence and presence of different liposomes at a concentration of 100 μM lipid Pi. Emission spectra were recorded from 300 to 400 nm with excitation at 280 nm and corrected for the vesicle blank. All fluorescence measurements were recorded using an RF-5301 spectrophotometer (Shimadzu, Duisburg, Germany).
Inhibition of the in vitro peptidoglycan synthesis.
The penicillin binding protein 2 (PBP2) assay was performed as described by Schneider et al. (38) with some modifications. In short, the assay was performed in a total volume of 50 μl containing 1 nmol of radiolabeled lipid II, 9 μg His-tagged PBP2 from Staphylococcus aureus NCTC 8325 in 0.1 M MES, pH 5.5, 10 mM MgCl2, 0.1% Triton X-100, and 1.25 mM CaCl2 when noted. The bifunctional high-molecular-weight PBP2 from S. aureus ATCT 8325 catalyzes the transpeptidation and transglycosylation reaction in peptidoglycan biosynthesis, using the ultimate cell wall precursor lipid II as the substrate (37). In competition experiments, radiolabeled lipid II was preincubated with 2 nmol peptide (molar ratio of lipid II/peptide, 1:2) for 30 min at RT before addition of the PBP2 enzyme to the reaction mixture. After incubation for 2 h at 30°C, the reaction assays were analyzed by TLC using solvent B (butanol-acetic acid-water-pyridine [75:60:15:50, vol/vol/vol/vol]) (32). Radiolabeled spots or lanes were visualized and quantified using a phosphor storage screen and a Storm 820 optical scanner (GE Healthcare, Munich, Germany). One hundred percent PBP2 activity corresponds to the reduction of free radiolabeled lipid II, which was converted to polymeric peptidoglycan in the absence of antibiotic peptides (positive control).
RESULTS
Antimicrobial activity of lantibiotics in the presence of Ca2+ ions.
The antimicrobial activity of mersacidin, plantaricin C, and lacticin 3147 was enhanced by Ca2+ ions. In contrast, the MIC of lacticin 481 remained unaltered (Table 1). The individual lacticin 3147 peptides, when tested on their own, did not show antimicrobial activity against the indicator strain even when Ca2+ was present. The presence of MgCl2 (1.25 mM) showed no stimulating effect on the antimicrobial activity of mersacidin (a MIC of 2.5 μg/ml instead of 1.25 μg/ml).
TABLE 1.
MICs of lantibiotics against M. luteus DSM 1790 in the absence and presence of 1.25 mM CaCl2a
| Lantibiotic | MIC (μg/ml)
|
|
|---|---|---|
| Without CaCl2 | With 1.25 mM CaCl2 | |
| Mersacidin | 1.25 | 0.31 |
| Plantaricin C | 2.5 | 1.25 |
| Lacticin 481 | 1.25 | 1.25 |
| Lacticin 3147 | 1.25 | 0.63 |
| LtnA1 | >10 | >10 |
| LtnA2 | >10 | >10 |
LtnA1 and LtnA2 were used at a 1:1 molar ratio; calculations of the MIC are based on the molecular weight of the A1 peptide.
Inhibition of the in vitro peptidoglycan synthesis in the absence and presence of Ca2+ ions.
We used the in vitro PBP2 assay to analyze the influence of Ca2+ ions on the interaction of mersacidin and the related lantibiotics with lipid II. In such an assay, the substrate lipid II is converted into polymeric peptidoglycan.
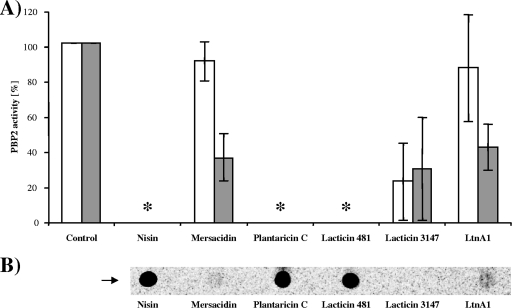
Nisin, plantaricin C, and lacticin 481 are potent inhibitors of the reaction and abolished the PBP2 activity completely (Fig. 2A). Under the conditions applied, these peptides formed complexes with the substrate lipid II, which did not dissociate during TLC and remained at the application point (Fig. 2B). In contrast, mersacidin and the individual lacticin 3147-lipid II binding component LtnA1 interfered only marginally with the PBP2 reaction and were not capable of complex formation during TLC (Fig. 2B).
FIG. 2.
(A) Inhibition of the in vitro peptidoglycan synthesis by lantibiotics in the presence and absence of Ca2+. Reaction mixtures contained radiolabeled lipid II and PBP2 in the absence (white bars) and presence (gray bars) of 1.25 mM CaCl2. Peptides (40 μM) are added at a 2:1 molar ratio (peptide/lipid II). Mean values of three independent experiments are shown. *, inhibition was complete, and all lipid II was found at the application spot. (B) Complex formation of lantibiotics with radiolabeled lipid II. Reaction mixtures (described above) were analyzed by TLC. Since complex formation was Ca2+ independent, one representative experiment is shown. The application spot is indicated by an arrow.
When 1.25 mM CaCl2 was added to the reaction mixture, we observed a pronounced increase in the lipid II binding capacity of mersacidin and the LtnA1 reduction of the PBP2 activity to 36% and 42%, respectively.
When both lacticin 3147 peptides, i.e., LtnA1 and LtnA2, were tested simultaneously, the Ca2+ dependence of the A1 peptide disappeared.
Tryptophan fluorescence spectroscopy.
The primary structures of the mersacidin-like lantibiotics used in this study, except for mersacidin, contain at least one Trp residue, which enables fluorescence spectroscopy to study the interaction of the peptides with different types of liposomes in the absence and presence of Ca2+ and of lipid II.
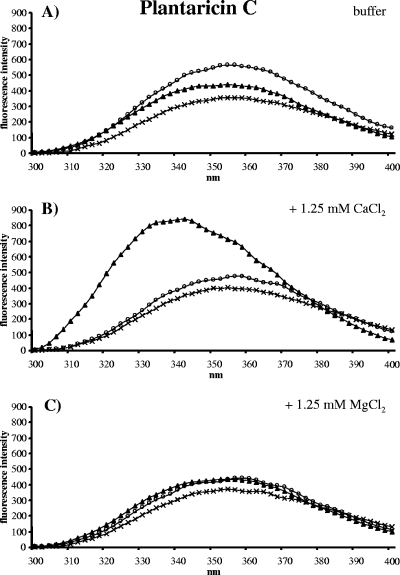
Plantaricin C contains one Trp at position 24 (Fig. 1), and the fluorescence emission spectrum showed a typical maximal emission at 354 nm (42) (Fig. 3A), which was essentially unaltered in the presence of pure DOPC vesicles or DOPC vesicles supplemented with 1 mol% lipid II. In contrast, with lipid II-doped DOPC vesicles and 1.25 mM CaCl2, the emission spectrum of plantaricin C changed drastically. A blue shift from 354 nm to 342 nm and a strong increase in the tryptophan fluorescence was observed (Fig. 3B), which is indicative of the Trp residue moving into an environment with decreased polarity (14), e.g., insertion into a lipid bilayer (42). The interaction of plantaricin C with lipid II-supplemented vesicles was Ca2+ specific, since Mg2+ ions did not change the emission spectrum (Fig. 3C).
FIG. 3.
Fluorescence emission spectra of plantaricin C (0.5 μM) (crosses) in the presence of vesicles (100 μM lipid Pi) made of DOPC (open circles) and vesicles made of DOPC supplemented with 1 mol% lipid II (triangles) in buffer (A) and buffer containing 1.25 mM Ca2+ (B) or 1.25 mM Mg2+ (C).
The tryptophan of lacticin 481 at position 19 (Fig. 1) is apparently not involved in the interaction with the cell wall precursor and the vesicle membranes, respectively. We observed no changes in the Trp fluorescence spectra (data not shown).
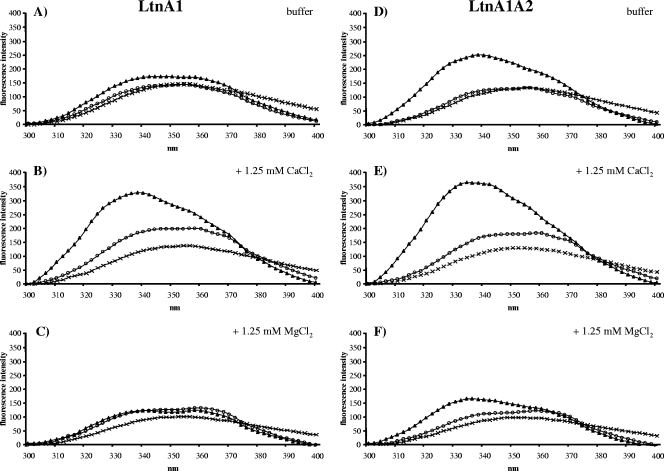
The lacticin 3147 A1 peptide contains three Trp residues (Fig. 1), and the emission spectra (Fig. 4A) are similar to the spectra of plantaricin C. The presence of 1.25 mM CaCl2 and DOPC vesicles supplemented with lipid II resulted in a strong increase in the fluorescence intensity and a blue shift to 341 nm of the fluorescence maximum (Fig. 4B), which was not induced by Mg2+ ions (Fig. 4C). Such a Trp fluorescence spectrum indicates a strong interaction of the A1 peptide with the vesicle membrane and lipid II, which involves the immersion of at least one of the three Trp residues of the peptide into the lipid membrane.
FIG. 4.
Fluorescence emission spectra of LtnA1 (0.25 μM) (A to C) and LtnA1/A2 (0.25 μM each) (D to F) (crosses) in the presence of vesicles (100 μM lipid Pi) made of DOPC (open circles) and DOPC with 1 mol% lipid II (triangles) in buffer (A and B) containing 1.25 mM Ca2+ (C and D) and 1.25 mM Mg2+ (E and F), respectively.
When LtnA1 and its partner peptide LtnA2 were present in the experiments (Fig. 4D to F), a blue shift and an increase in the fluorescence intensity of the emission spectrum in the presence of lipid II-doped vesicles was already observed in the buffer (Fig. 4D). The addition of Ca2+ further enhanced both the Trp fluorescence and the blue shift (Fig. 4E), whereas Mg2+ ions showed an opposite effect (Fig. 4F). These results indicate that Ca2+ ions and the LtnA2 peptide may have a similar effect on the conformation of the LtnA1 peptide, which is favorable for both LtnA1-lipid II interaction and immersion of LtnA1 into the membrane bilayer.
Influence of Ca2+ ions on LtnA1 binding to whole cells.
The two-component lantibiotic lacticin 3147 is unusual in that the lipid II binding and pore-forming functions can be attributed to two distinct peptides, LtnA1 and LtnA2, respectively. We analyzed the role of Ca2+ ions in the interaction of the A1 peptide with the bacterial cell surface and in the LtnA1A2-mediated pore formation process monitoring the potassium release from whole cells.
In the presence of Ca2+, the simultaneous addition of both lacticin 3147 peptides to lactococcal cells resulted in a rapid efflux of potassium ions (100%) (Fig. 5A). When bacterial cells were preincubated with the lipid II binding component LtnA1 and washed with buffer, the subsequent addition of LtnA2 also induced a complete K+ efflux. Since LtnA2 possesses no pore-forming activity, the A1 peptide must have remained bound to the cell surface during the washing step before the addition of LtnA2. The reduced intracellular content of K+ in such an experiment was due to the washing process, as observed with appropriate controls.
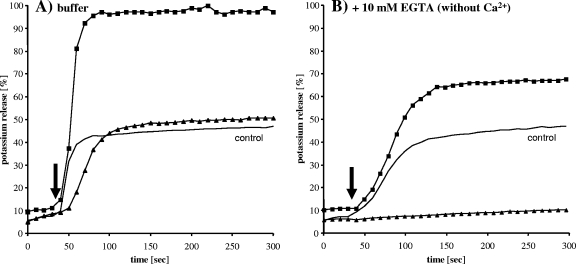
FIG. 5.
Potassium release from Lactococcus lactis HP whole cells induced by lacticin 3147 in the absence (A) and presence (B) of 10 mM EGTA. Lacticin 3147 A1/A2 (1:1 molar ratio) was added simultaneously at a concentration of 1 μM each to whole cells (squares). Cells were preincubated with the lacticin A1 peptide for 3 min prior to a washing step, followed by the addition (black arrow) of lacticin A2 (triangles). In the control experiment, both lacticin 3147 peptides were added directly to washed cells (black line). One representative experiment is shown.
After the addition of EGTA to remove Ca2+ ions from the system, a different picture emerged. The fast K+ release induced by lacticin 3147 that was observed in buffer (Fig. 5A) was delayed in the absence of Ca2+ ions (Fig. 5B); i.e., the maximum release was reached after 50 s and 100 s after the peptide addition, respectively, and the maximum release level was reduced to about 70% compared to that shown in Fig. 5A.
Furthermore, LtnA1-preincubated and washed cells remained stable after the subsequent addition of LtnA2; i.e., the A1 peptide was obviously removed by the washing step, demonstrating that Ca2+ ions are important for attachment of the A1 peptide to the cell surface.
Influence of Ca2+ ions on the pore-forming activity of lacticin 3147 in liposomes.
CF release experiments were performed to analyze the influence of Ca2+ ions on the pore-forming activity of lacticin 3147 in more detail.
As depicted in Fig. 6, pore formation induced by lacticin 3147 was not influenced by the addition of 1.25 mM CaCl2 to the buffer, nontargeted unspecific permeabilization of pure DOPC vesicles, nor targeted poration of lipid II-containing vesicles.
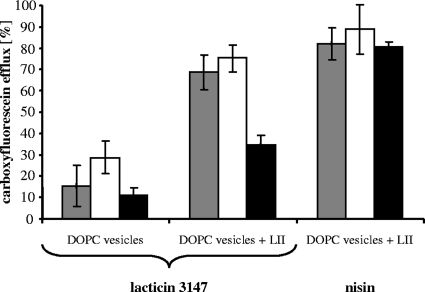
FIG. 6.
Lacticin 3147 and nisin-induced CF-release from liposomes made of DOPC and DOPC supplemented with 0.1 mol% lipid II (LII). Peptides were added at a concentration of 0.25 μM to liposomes (25 μM phospholipid on a phosphorous basis) in buffer (gray bars), buffer with 1.25 mM CaCl2 (white bars) and buffer with 10 mM EGTA (black bars). Marker release was determined 2.5 min after peptide addition. Mean values of three independent experiments are shown.
In contrast, deprivation of Ca2+ ions (by the addition of EGTA) resulted in a significant reduction of the pore-forming activities in both DOPC vesicles to an extent of about 50%. These data indicate that Ca2+ ions are not only important for the LtnA1-lipid II interaction, as shown by the potassium release assay, but that they are also involved in the interaction of the two lacticin 3147 components. Nisin, which was used as a control, exhibited a Ca2+-independent pore-forming activity throughout the experiments.
DISCUSSION
Divalent cations are naturally present in the cell envelope of all bacteria, and especially Ca2+ ions impact the mode of action of a number of antibiotics, such as lipopeptides, e.g., daptomycin and friulimicin (13, 16, 17).
In this study, we focused on lantibiotics, which possess a mersacidin-like lipid II binding motif with a conserved Glu residue, which had been proposed to play a role in the Ca2+-dependent interaction with lipid II.
We found Ca2+ ions to improve the activities of some peptides but not all, and the ways in which the divalent ions contribute to activity differ for the individual peptides. However, Ca2+ ions may (i) confer to peptides with no net charge a positive overall charge which improves the interaction with the bacterial membrane and/or (ii) increase the affinity of the lantibiotic for lipid II, resulting in a deeper immersion of the peptides into the lipid bilayer.
Two types of mersacidin-like lantibiotics may be distinguished among the peptides studied here, based on their characteristics in a TLC system. Mersacidin and lacticin 3147, as well as the LtnA1 peptide of lacticin 3147, form complexes with lipid II, which dissociate in the TLC, while the plantaricin C-lipid II and lacticin 481-lipid II complexes remained stable and were visible at the application spot (Fig. 2B).
In line with this observation, mersacidin and the LtnA1 peptide share interesting similar overall physicochemical properties. The peptides possess no net charge and an N-terminal thioether ring (Ala1-Ala2). The lipid II binding motif differs only at one position (His instead of Dha at position 23), and the potential hinge region (Ala19-Abu20) and a C-terminal thioether bridging pattern are conserved (Fig. 1).
Concordantly, we observed a clearly improved activity of mersacidin and LtnA1 in the presence of Ca2+ ions in our test systems. Both peptides required Ca2+ ions for full antimicrobial activity (Table 1) and potent inhibition of the in vitro peptidoglycan synthesis, i.e., inhibition of the PBP2 reaction (Fig. 2A). Moreover, immersion of the LtnA1 peptide into the lipid bilayer of lipid II-containing vesicles as detected by Trp fluorescence spectroscopy was greatly enhanced (Fig. 4B).
Remarkably, the Glu residues in the lipid II binding motif of both peptides, i.e., Glu17 in mersacidin and Glu24 in LtnA1, are essential for antimicrobial activity as shown by using site-directed mutagenesis (10, 43) and might be involved in the observed Ca2+ stimulation of the activity.
Recently, a mersacidin-like lipid II binding motif with an essential Glu was also identified in the two-peptide lantibiotic haloduracin (29). Interestingly, the conserved ring structure of the binding motif (ring B; Abu18-Ala23) in the haloduracin A1 peptide is not necessary for the antibacterial activity. Rather, the neighboring thioether ring structure (ring C) was essential (9), indicating that in particular the correct conformation of the amino acids Abu-X-Glu-Ala is a critical determinant for activity. This may also hold true for mersacidin and the LtnA1 peptide.
In addition to the interaction of Ca2+ ions with the conserved Glu residue in the lipid II binding motif, it is conceivable that Ca2+ ions are necessary to give mersacidin and LtnA1 an overall positive charge to facilitate the interaction with the negatively charged surface of the bacterial membrane. A mechanism like this has been recently described for daptomycin. It was suggested that the Ca2+ binding of daptomycin occurs in a 1:1 molar ratio in solution, serving to form micelles with an overall positive charge, which may deliver high concentrations of the antibiotic to the bacterial membrane. In close proximity to the membrane, such micelles may dissociate and daptomycin may insert into the lipid bilayer, resulting in positive membrane curvature and ion leakage (20, 23, 38, 41).
In contrast to mersacidin and LtnA1, plantaricin C and lacticin 481 were found to form stable complexes with lipid II in our TLC system (Fig. 2B). Both peptides possess a positive net charge.
The N terminus seems to play an important role in the activity of both peptides, since its cleavage in plantaricin C by trypsin treatment abolished the activity (18) and a truncated form of lacticin 481 (T-lacticin 481) lacking the amino acids 1 to 5 featured a 10-fold-reduced specific activity (44). A proteolytic fragment of lacticin 481 missing the N-terminal lysine showed reduced antibacterial activity (50).
The positively charged amino acids in plantaricin C and lacticin 481 may facilitate the interaction of both peptides with the bacterial membrane as shown for nukacin ISK-1 (36), a lantibiotic of the lacticin 481 group (15). Nukacin ISK-1 contains three N-terminal lysine residues which were recently shown to be responsible for its strong affinity to anionic lipid membranes (1). Mutants containing alanine replacements or absent lysine residues showed an impaired binding to membranes and a 32-fold-reduced antibacterial activity, indicating that electrostatic binding of nukacin ISK-1 to the bacterial membrane is an important step in its mode of action. Mutants with additional lysine residues at the N terminus exhibited an improved binding to anionic membranes but no further increased antibacterial activity (1).
Plantaricin C combines Ca2+-dependent and Ca2+-independent characteristics. It forms a complex with the target structure lipid II even in the absence of Ca2+ ions (Fig. 2) and, as indicated by the Trp fluorescence experiments, appears to locate on the membrane surface only when it binds to lipid II. Immersion of the Trp-containing part of plantaricin C into the lipid bilayer occurred only in the presence of Ca2+ ions (Fig. 3B). Possibly, Ca2+ stabilizes a conformation of the lantibiotic that is more compatible with membrane insertion: we propose that immersion of the peptide into the bacterial membrane is an important Ca2+-dependent step that leads to an improved in vivo activity. However, it is not known to which extent the presence of the conserved Glu in the lipid II binding motif in plantaricin C might contribute to the activity, since site-directed mutagenesis of this peptide has not been reported to clarify if the Glu residue is essential.
Interestingly, the activity of lacticin 481 is completely Ca2+ independent. Neither the antimicrobial activity nor the activity of the peptide in the PBP2 assay was enhanced by the presence of Ca2+ ions. Lacticin 481 binds to lipid II as stably as plantaricin C (Fig. 2); however, although the Trp residues in lacticin 481 and plantaricin C are located at similar positions in the molecule, the residue does not appear to insert into the vesicle membrane (data not shown).
Obviously, lacticin 481 activity does not involve Ca2+ ions. This conclusion is further supported by the finding of Patton et al., who showed that the Glu13 residue in the mersacidin-lipid II binding motif of lacticin 481 is not essential for the antibacterial activity (31).
Information about the influence of Ca2+ ions on the pore formation process can only be obtained with the two-peptide lantibiotic lacticin 3147. The mode of action of lacticin 3147 had been recently analyzed, and it was shown that after interaction of LtnA1 with lipid II, the LtnA2 peptide binds to the LtnA1-lipid II complex for subsequent pore formation (47). Ca2+ ions seem to have an overall stimulating effect on the pore-forming process by lacticin 3147, as seen in the in vitro CF release assay (Fig. 6).
However, Ca2+ ions are indispensable for immersion of the LtnA1 peptide into lipid II-containing vesicles (Fig. 4B). Interestingly, the spectral shift indicative for peptide penetration into the membrane was also observed in the absence of Ca2+ ions when the LtnA2 peptide was present (Fig. 4D). This observation is of special interest since the C-terminal segment of LtnA2 peptide contains two positively charged residues (Fig. 1), emphasizing the important role of positive charges in the activity of lacticin 3147. This also holds true for the activity in vivo. In potassium release experiments, we observed that attachment of LtnA1 to whole cells, necessary for subsequent pore formation, occurred only in the presence of Ca2+ ions or the LtnA2 peptide (Fig. 5).
In summary, based on our results, Ca2+ ions are not essential for the interaction of mersacidin-like lantibiotics with lipid II. However, we found that, overall, especially neutral peptides require Ca2+ ions to obtain full activity; e.g., mersacidin and the A1 peptide of lacticin 3147 may achieve an overall positive net charge in the presence of Ca2+ that facilitates the interaction of the peptides with the bacterial membrane. In contrast, plantaricin C and lacticin 481 already possess positively charged amino acids. In addition, the Ca2+-dependent immersion of lantibiotics into the bacterial membrane is a process, which is obviously important for the activity of all lantibiotics harboring an essential Glu residue in the lipid II binding motif.
Acknowledgments
This work has been funded by the German Research Foundation (DFG, Sa 292/91-1 to 9-4) and the BONFOR program of the Medical Faculty of Bonn.
We are grateful to P. Cotter, C. Hill, and P. Ross for providing the lacticin 3147-overproducing strain.
Footnotes
Published ahead of print on 8 May 2009.
REFERENCES
- 1.Asaduzzaman, S. M., J. Nagao, Y. Aso, J. Nakayama, and K. Sonomoto. 2006. Lysine-oriented charges trigger the membrane binding and activity of nukacin ISK-1. Appl. Environ. Microbiol. 72:6012-6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett, M. S., R. P. Wenzel, and R. N. Jones. 1992. In vitro activity of mersacidin (M87-1551), an investigational peptide antibiotic tested against gram-positive bloodstream isolates. Diagn. Microbiol. Infect. Dis. 15:641-644. [DOI] [PubMed] [Google Scholar]
- 3.Bierbaum, G., and H. G. Sahl. 2009. Lantibiotics: mode of action, biosynthesis and bioengineering. Curr. Pharm. Biotechnol. 10:2-18. [DOI] [PubMed] [Google Scholar]
- 4.Bonelli, R. R., T. Schneider, H. G. Sahl, and I. Wiedemann. 2006. Insights into in vivo activities of lantibiotics from gallidermin and epidermin mode-of-action studies. Antimicrob. Agents Chemother. 50:1449-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouhss, A., A. E. Trunkfield, T. D. Bugg, and D. Mengin-Lecreulx. 2008. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol. Rev. 32:208-233. [DOI] [PubMed] [Google Scholar]
- 6.Brötz, H., G. Bierbaum, K. Leopold, P. E. Reynolds, and H. G. Sahl. 1998. The lantibiotic mersacidin inhibits peptidoglycan synthesis by targeting lipid II. Antimicrob. Agents Chemother. 42:154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brötz, H., G. Bierbaum, P. E. Reynolds, and H. G. Sahl. 1997. The lantibiotic mersacidin inhibits peptidoglycan biosynthesis at the level of transglycosylation. Eur. J. Biochem. 246:193-199. [DOI] [PubMed] [Google Scholar]
- 8.Brötz, H., M. Josten, I. Wiedemann, U. Schneider, F. Götz, G. Bierbaum, and H. G. Sahl. 1998. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol. Microbiol. 30:317-327. [DOI] [PubMed] [Google Scholar]
- 9.Cooper, L. E., A. L. McClerren, A. Chary, and W. A. van der Donk. 2008. Structure-activity relationship studies of the two-component lantibiotic haloduracin. Chem. Biol. 15:1035-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotter, P. D., L. H. Deegan, E. M. Lawton, L. A. Draper, P. M. O'Connor, C. Hill, and R. P. Ross. 2006. Complete alanine scanning of the two-component lantibiotic lacticin 3147: generating a blueprint for rational drug design. Mol. Microbiol. 62:735-747. [DOI] [PubMed] [Google Scholar]
- 11.Cotter, P. D., L. A. Draper, E. M. Lawton, O. McAuliffe, C. Hill, and R. P. Ross. 2006. Overproduction of wild-type and bioengineered derivatives of the lantibiotic lacticin 3147. Appl. Environ. Microbiol. 72:4492-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 13.Counter, F. T., N. E. Allen, D. S. Fukuda, J. N. Hobbs, J. Ott, P. W. Ensminger, J. S. Mynderse, D. A. Preston, and C. Y. Wu. 1990. A54145 a new lipopeptide antibiotic complex: microbiological evaluation. J. Antibiot. (Tokyo) 43:616-622. [DOI] [PubMed] [Google Scholar]
- 14.Cowgill, R. W. 1967. Fluorescence and protein structure. X. Reappraisal of solvent and structural effects. Biochim. Biophys. Acta 133:6-18. [DOI] [PubMed] [Google Scholar]
- 15.Dufour, A., T. Hindré, D. Haras, and J. P. Le Pennec. 2007. The biology of lantibiotics from the lacticin 481 group is coming of age. FEMS Microbiol. Rev. 31:134-167. [DOI] [PubMed] [Google Scholar]
- 16.Eliopoulos, G. M., C. Thauvin, B. Gerson, and R. C. Moellering, Jr. 1985. In vitro activity and mechanism of action of A21978C1, a novel cyclic lipopeptide antibiotic. Antimicrob. Agents Chemother. 27:357-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eliopoulos, G. M., S. Willey, E. Reiszner, P. G. Spitzer, G. Caputo, and R. C. Moellering, Jr. 1986. In vitro and in vivo activity of LY 146032, a new cyclic lipopeptide antibiotic. Antimicrob. Agents Chemother. 30:532-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González, B., P. Arca, B. Mayo, and J. E. Suárez. 1994. Detection, purification, and partial characterization of plantaricin C, a bacteriocin produced by a Lactobacillus plantarum strain of dairy origin. Appl. Environ. Microbiol. 60:2158-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Héchard, Y., and H. G. Sahl. 2002. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 84:545-557. [DOI] [PubMed] [Google Scholar]
- 20.Ho, S. W., D. Jung, J. R. Calhoun, J. D. Lear, M. Okon, W. R. Scott, R. E. Hancock, and S. K. Straus. 2008. Effect of divalent cations on the structure of the antibiotic daptomycin. Eur. Biophys. J. 37:421-433. [DOI] [PubMed] [Google Scholar]
- 21.Hsu, S. T., E. Breukink, G. Bierbaum, H. G. Sahl, B. de Kruijff, R. Kaptein, N. A. van Nuland, and A. M. Bonvin. 2003. NMR study of mersacidin and lipid II interaction in dodecylphosphocholine micelles. Conformational changes are a key to antimicrobial activity. J. Biol. Chem. 278:13110-13117. [DOI] [PubMed] [Google Scholar]
- 22.Hsu, S. T., E. Breukink, E. Tischenko, M. A. Lutters, B. de Kruijff, R. Kaptein, A. M. Bonvin, and N. A. van Nuland. 2004. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat. Struct. Mol. Biol. 11:963-967. [DOI] [PubMed] [Google Scholar]
- 23.Jung, D., A. Rozek, M. Okon, and R. E. Hancock. 2004. Structural transitions as determinants of the action of the calcium-dependent antibiotic daptomycin. Chem. Biol. 11:949-957. [DOI] [PubMed] [Google Scholar]
- 24.Kärcher, J. 2000. Mersacidin analoge Typ-B-Lantibiotika: Kristallisation, Datensammlung und Strukturaufklärung. Universität Göttingen, Göttingen, Germany.
- 25.Kuipers, O. P., H. S. Rollema, W. M. Yap, H. J. Boot, R. J. Siezen, and W. M. de Vos. 1992. Engineering dehydrated amino acid residues in the antimicrobial peptide nisin. J. Biol. Chem. 267:24340-24346. [PubMed] [Google Scholar]
- 26.Kuipers, O. P., W. M. G. J. Yap, H. S. Rollema, M. M. Beerthuyzen, R. J. Siezen, and W. M. de Vos. 1991. In G. Jung and H.-G. Sahl (ed.), Nisin and novel lantibiotics. ESCOM Science Publishers, Leiden, The Netherlands.
- 27.Mayer, L. D., M. J. Hope, and P. R. Cullis. 1986. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim. Biophys. Acta 858:161-168. [DOI] [PubMed] [Google Scholar]
- 28.McAuliffe, O., M. P. Ryan, R. P. Ross, C. Hill, P. Breeuwer, and T. Abee. 1998. Lacticin 3147, a broad-spectrum bacteriocin which selectively dissipates the membrane potential. Appl. Environ. Microbiol. 64:439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClerren, A. L., L. E. Cooper, C. Quan, P. M. Thomas, N. L. Kelleher, and W. A. van der Donk. 2006. Discovery and in vitro biosynthesis of haloduracin, a two-component lantibiotic. Proc. Natl. Acad. Sci. USA 103:17243-17248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parisot, J., S. Carey, E. Breukink, W. C. Chan, A. Narbad, and B. Bonev. 2008. Molecular mechanism of target recognition by subtilin, a class I lanthionine antibiotic. Antimicrob. Agents Chemother. 52:612-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patton, G. C., P. Moushumi, L. E. Cooper, C. Chatterjee, and W. A. van der Donk. 2008. The importance of the leader sequence for directing lanthionine formation in lacticin 481. Biochemistry 47:7342-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reisinger, P., H. Seidel, H. Tschesche, and W. P. Hammes. 1980. The effect of nisin on murein synthesis. Arch. Microbiol. 127:187-193. [DOI] [PubMed] [Google Scholar]
- 33.Rick, P. D., G. L. Hubbard, M. Kitaoka, H. Nagaki, T. Kinoshita, S. Dowd, V. Simplaceanu, and C. Ho. 1998. Characterization of the lipid-carrier involved in the synthesis of enterobacterial common antigen (ECA) and identification of a novel phosphoglyceride in a mutant of Salmonella typhimurium defective in ECA synthesis. Glycobiology 8:557-567. [DOI] [PubMed] [Google Scholar]
- 34.Rilla-Villar, N. 2003. Aplicación de bacterias lácticas productoras de bacteriocinas en bioconservación. Ph.D. thesis. Universidad de Santiago de Compostela, Santiago de Compostela, Spain.
- 35.Rouser, G., S. Fkeischer, and A. Yamamoto. 1970. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5:494-496. [DOI] [PubMed] [Google Scholar]
- 36.Sashihara, T., H. Kimura, T. Higuchi, A. Adachi, H. Matsusaki, K. Sonomoto, and A. Ishizaki. 2000. A novel lantibiotic, nukacin ISK-1, of Staphylococcus warneri ISK-1: cloning of the structural gene and identification of the structure. Biosci. Biotechnol. Biochem. 64:2420-2428. [DOI] [PubMed] [Google Scholar]
- 37.Sauvage, E., F. Kerff, M. Terrak, J. A. Ayala, and P. Charlier. 2008. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 32:234-258. [DOI] [PubMed] [Google Scholar]
- 38.Schneider, T., K. Gries, M. Josten, I. Wiedemann, S. Pelzer, H. Labischinski, and H. G. Sahl. 2009. The lipopeptide antibiotic friulimicin B inhibits cell wall biosynthesis through complex formation with bactoprenol-phosphate. Antimicrob. Agents Chemother. 53:1610-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider, T., M. M. Senn, B. Berger-Bächi, A. Tossi, H. G. Sahl, and I. Wiedemann. 2004. In vitro assembly of a complete, pentaglycine interpeptide bridge containing cell wall precursor (lipid II-Gly5) of Staphylococcus aureus. Mol. Microbiol. 53:675-685. [DOI] [PubMed] [Google Scholar]
- 40.Smith, L., H. Hasper, E. Breukink, J. Novak, J. Čerkasov, J. D. Hillman, S. Wilson-Stanford, and R. S. Orugunty. 2008. Elucidation of the antimicrobial mechanism of mutacin 1140. Biochemistry 47:3308-3314. [DOI] [PubMed] [Google Scholar]
- 41.Straus, S. K., and R. E. Hancock. 2006. Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: comparison with cationic antimicrobial peptides and lipopeptides. Biochim. Biophys. Acta 1758:1215-1223. [DOI] [PubMed] [Google Scholar]
- 42.Surewicz, W. K., and R. M. Epand. 1984. Role of peptide structure in lipid-peptide interactions: a fluorescence study of the binding of pentagastrin-related pentapeptides to phospholipid vesicles. Biochemistry 23:6072-6077. [DOI] [PubMed] [Google Scholar]
- 43.Szekat, C., R. W. Jack, D. Skutlarek, H. Farber, and G. Bierbaum. 2003. Construction of an expression system for site-directed mutagenesis of the lantibiotic mersacidin. Appl. Environ. Microbiol. 69:3777-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uguen, P., T. Hindré, S. Didelot, C. Marty, D. Haras, J. P. Le Pennec, K. Vallée-Réhel, and A. Dufour. 2005. Maturation by LctT is required for biosynthesis of full-length lantibiotic lacticin 481. Appl. Environ. Microbiol. 71:562-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Heijenoort, J. 2007. Lipid intermediates in the biosynthesis of bacterial peptidoglycan. Microbiol. Mol. Biol. Rev. 71:620-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiedemann, I., T. Böttiger, R. R. Bonelli, T. Schneider, H. G. Sahl, and B. Martínez. 2006. Lipid II-based antimicrobial activity of the lantibiotic plantaricin C. Appl. Environ. Microbiol. 72:2809-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiedemann, I., T. Böttiger, R. R. Bonelli, A. Wiese, S. O. Hagge, T. Gutsmann, U. Seydel, L. Deegan, C. Hill, P. Ross, and H. G. Sahl. 2006. The mode of action of the lantibiotic lacticin 3147: a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol. Microbiol. 61:285-296. [DOI] [PubMed] [Google Scholar]
- 48.Wiedemann, I., E. Breukink, C. van Kraaij, O. P. Kuipers, G. Bierbaum, B. de Kruijff, and H. G. Sahl. 2001. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 276:1772-1779. [DOI] [PubMed] [Google Scholar]
- 49.Willey, J. M., and W. A. van der Donk. 2007. Lantibiotics: peptides of diverse structure and function. Annu. Rev. Microbiol. 61:477-501. [DOI] [PubMed] [Google Scholar]
- 50.Xie, L., L. M. Miller, C. Chatterjee, O. Averin, N. L. Kelleher, and W. A. van der Donk. 2004. Lacticin 481: in vitro reconstitution of lantibiotic synthetase activity. Science 303:679-681. [DOI] [PubMed] [Google Scholar]