Abstract
Yeasts used in the production of lagers contain complex allopolyploid genomes, resulting from the fusion of two different yeast species closely related to Saccharomyces cerevisiae and Saccharomyces bayanus. Recombination between the homoeologous chromosomes has generated a number of hybrid chromosomes. These recombination events provide potential for adaptive evolution through the loss or gain of gene function. We have examined the genotypic and phenotypic effects of one of the conserved recombination events that occurred on chromosome XVI in the region of YPR159W and YPR160W. Our analysis shows that the recombination event occurred within the YPR160W gene, which encodes the enzyme glycogen phosphorylase and generates a hybrid gene that does not produce mature mRNA and is nonfunctional due to frameshifts in the coding region. The loss of function of the hybrid gene leads to glycogen levels similar to those found in haploid yeast strains. The implications for the control of glycogen levels in fermentative yeasts are discussed.
Yeasts used in the production of lagers, originally referred to as Saccharomyces carlsbergensis, are now classified as Saccharomyces pastorianus (18). Lager yeasts contain complex polyploid genomes with general tetraploid DNA content and are believed to have arisen from a natural fusion of two different haploid species followed by a genome duplication event or alternatively from the fusion of two diploid yeast species (2, 4, 14, 18). Subsequent genome changes, such as chromosome loss and/or duplication and translocations, have resulted in unequal numbers of chromosomes in the present-day strains, a state referred to as aneuploidy.
A sequence analysis of individual genes indicated that the parental strains contributing to the hybrid strain closely resemble Saccharomyces cerevisiae and Saccharomyces bayanus (6, 15, 16). A whole-genome sequence analysis of the lager yeast strain Weihenstephan (15) identified the presence of three types of chromosomes, referred to as (i) S. cerevisiae-like chromosomes, (ii) S. bayanus-like chromosomes, and (iii) hybrids resulting from recombination events between the homoeologous parental chromosomes.
Competitive genomic hybridization (CGH) analysis of two S. pastorianus strains, named CMBS-33 and 6701, identified as many as 28 specific locations where recombination between homoeologous pairs of chromosomes or chromosomal translocations may have occurred (3). Of the 28 sites identified, 13 occur at unique sites on eight different chromosomes, while the rest are in subtelomeric X elements or within 25 kbp of the telomere. Many of the genes adjacent to the recombination sites encode proteins that play essential roles in fermentation, including ADH2, ADH4, AAD6 and TDH2 (ethanol metabolism), FLO10, and PHD1 (3).
We have recently shown that recombination at these “hot spots” can be induced by the exposure of lager yeasts to environmental stresses such as high temperature and high osmotic stress (13). Furthermore, fermentation under stress conditions leads to the amplification and loss of telomeric regions on a selected set of chromosomes and gene amplification radiating from the rRNA locus on chromosome XII and the DUP locus on chromosome I (13). Since a number of genes, including the MAL (maltose utilization) and the FLO (flocculation) genes, encoding proteins required for the fermentation process reside at the telomeres, such genome dynamics can have important consequences for the immediate quality and outcome of fermentation in addition to severe consequences on strain stability and purity.
One of the recombination events identified by CGH analysis is located on chromosome XVI in the region of YPR159W and YPR160W. DNA to the left of the region hybridizes to S. cerevisiae microarrays, while genes between YPR160W and YPR190C and encompassing approximately 58 kb of DNA displayed a lack of hybridization to these microarrays, suggestive of a hybrid chromosome (3). Whole-genome sequence analysis of the Weihenstephan strain confirmed the existence of hybrid chromosome XVI and indicated the presence a second type of chromosome XVI containing S. bayanus-like sequences to the left of YPR159W (15, 16).
To examine the genotypic and phenotypic outcomes of this recombination event, the right arm of chromosome XVI has been cloned from the yeast strain CMBS-33. Our analysis reveals that the recombination event occurred within the open reading frame (ORF) of YPR160W (GPH1) encoding the enzyme glycogen phosphorylase, which is required for the mobilization of stored glycogen through its conversion into glucose-1-P. The recombination event generates a hybrid gene that does not produce a mature mRNA and is nonfunctional due to frameshifts in the coding region.
MATERIALS AND METHODS
Strains.
The bottom-fermenting lager yeast strain CMBS-33 was from the Centre for Malting and Brewing Science (Leuven, Belgium) lager strain collection (kindly provided by K. Verstrepen). Strain 6701 was from the Guinness yeast collection. The haploid Saccharomyces cerevisiae strain S-150 (MATa leu2-3 leu2-112 his3D1 trp1-289 ura3-52) was obtained from J. Beggs (Edinburgh University). The haploid S. bayanus strain was obtained from the Collection de Levures d'Interet Biotechnologique, Paris, France.
Electrophoretic karyotyping and Southern blotting of lager yeast DNA.
The lager yeast strains CMBS-33 and 6701 and the haploid S. cerevisiae yeast strain S-150 were grown overnight in yeast extract (1%)-peptone (2%) supplemented with maltose (2%) (YEPM) to give a final yield of 1.5 × 107 cells. The total genomic DNA was isolated, and DNA-agar plugs were prepared as previously described (7). Electrophoresis was carried out in 1.2% agarose containing 0.5× Tris-acetate (40 mM), EDTA (1 mM) (TAE) buffer (pH 8.5) at a temperature of 14°C using an initial switching time of 60 s for 15 h and a final switching time of 90 s for 9 h at a 120° pulse angle. After the electrophoresis, the gel was stained with ethidium bromide (10 mg/ml). The gel was then transferred to a nylon membrane (Pall) for hybridization as previously described (8). The membranes were prehybridized for 1 h at 68°C, and the hybridizations were carried out in the same solution with the addition of 10 ng of digoxigenin (DIG)-labeled DNA probes corresponding to either YPR159W or YPR160, which were prepared as previously described (8).
Generation of the genomic DNA library.
High-molecular-weight DNA from the lager strain CMBS-33 was isolated, using the standard phenol-chloroform extraction method as previously described (3). A total of 100 ng of the genomic DNA, partially digested with Sau3A, was incubated with 25 ng of BamHI-digested CopyControl pCC1BAC cloning ready vector (Epicentre) in sterile water and incubated at 55°C for 10 min. The solution cooled to room temperature, and 1× Fast-Link ligation buffer (Epicentre), 10 mM ATP, and 2 μl Fast-Link DNA ligase (Epicentre) were added to the cooled solution. The ligation mixture was incubated at 16°C for 4 h and then heated to 65°C for 15 min. The ligation reaction mixture was desalted using an agarose cone on ice for 1 h and then mixed with TransforMax EPI3000 electrocompetent Escherichia coli cells (Epicentre), and the cells were electroporated. Following the recovery of the cells, the transformants were plated in Luria broth containing chloramphenicol (12.5 μg/ml), X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 40 μg/ml) and IPTG (isopropyl-β-d-thiogalactopyranoside; 0.4 mM) and incubated overnight at 37°C. In total, 7,000 chloramphenicol-resistant colonies were obtained.
Characterization of the bacterial artificial chromosome (BAC) library.
Individual clones were transferred to agar plates containing chloramphenicol-X-Gal-IPTG. The plates were incubated overnight at 37°C and the colonies replica-plated onto a nylon membrane. The membranes were consecutively treated with the following solutions: 10% sodium dodecyl sulfate for 3 min, 0.5 N NaOH, 1.5 M NaCl for 5 min, 0.5 M Tris-Cl, 1.5 M NaCl (pH 7.4) for 5 min, and finally 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 5 min. The membranes were air dried and UV cross-linked. DIG-dUTP-labeled DNA probes were generated by the PCR amplification of CMBS-33 genomic DNA using oligonucleotides based on the S. cerevisiae genome sequence for ORF YPR159 and ORFs to the left of this ORF and the S. bayanus genome sequence for YPR160 and ORFs to the right of this ORF (see Table S1 in the supplemental material). Hybridizations were carried out using the pooled DNA probes as previously described (5). Following hybridization, positive clones were identified, isolated, and grown in 2× YT medium (16 g tryptone, 10 g yeast extract, 5 g NaCl in 1 liter) supplemented with chloramphenicol (12.5 μg/ml) at 37°C overnight. To verify the selection of positive clones, DNA isolated from the picked colonies was hybridized to individual DIG-labeled probes in the region of interest.
Sequencing.
The sequencing of the PCR products and direct sequencing from the BAC clones was performed at GATC, Constance, Germany. The specific primers were designed using the Saccharomyces Genome Database and the Saccharomyces bayanus database (www.yeastgeome.org and www.broad.mit.edu/annotation/fungi/comp_yeasts/, respectively). A list of primers and their genome locations are provided in Table S2 in the supplemental material. Three independent passes in a forward and reverse direction were performed on each fragment.
RNA extraction and Northern blot analysis.
RNA extractions and Northern blot analysis were carried out as previously described (5). The primers used for the preparation of the DIG-labeled hybridization probes are provided in Table 1.
TABLE 1.
Percentage identity of primers
| Oligonucleotide | Sequence (5′-3′) | % Identity to indicated strain
|
||
|---|---|---|---|---|
| S. cerevisiae | S. bayanus | CMBS-33 | ||
| 1 | AATCCCAAGGCTTACAAGGA | 100 | 70 | 95 |
| 2 | CAATGACCAAATTGTC | 100 | 90 | 100 |
| 3 | TTCGTGACAATTTGGTCATTG | 100 | 90 | 100 |
| 4 | TGGCTCTCCCTTTGAATCCT | 60 | 80 | 100 |
| 5 | AGGATTCAAAGGGAGAGCCA | 60 | 100 | 80 |
| 6 | TTCTGGCCTGTCGACGTAACC | 95 | 100 | 95 |
Reverse transcriptase reactions.
The total RNA (30 μg) was incubated with DNase I (Promega, Inc.) at 30°C for 1 h to remove any contaminating DNA. Following digestion, reverse transcription reactions were carried out using 2 μg of DNase-treated RNA as the template, a transcript-specific reverse primer (50 nM), and reverse transcriptase (Applied Biosystems) as specified by the manufacturer in a final volume of 20 μl at 25°C for 10 min and 37°C for 120 min, followed by 85°C for 5 s. PCR amplification was carried out using 2 μl of the cDNA as previously described (3). The primers used for cDNA synthesis are shown in Table 1.
Detection and measurement of the glycogen content of yeast cells.
Yeast cells were patched onto YEP-dextrose or YEPM plates and grown at 30°C overnight. The plates were inverted over iodine crystals. The levels of glycogen were detected by the appearance of a purple coloring of the colony. Additionally, the cell pellets (1 × 108 cells) were washed twice with 5 ml ice-cold sterile distilled water and resuspended in 1 ml of 40 mM sodium acetate (pH 4.8). The cells were boiled for 5 min and cooled on ice. Following vortexing (approximately 12 times with 1-min intervals between on ice), the samples were pelleted by centrifugation at 10,000 rpm. The supernatant was transferred to a fresh tube, and 335 μl of amyloglucosidase (230 U/ml, A1602; Sigma), was added to 125 μl of each sample. The samples were incubated at 57°C for 16 h. The amount of glucose-1-P, generated from the hydrolysis of the glycogen, was determined using a glucose assay kit (GAGO-20; Sigma) according to the supplier's instructions.
Nucleotide sequence accession numbers.
Sequences have been deposited with GenBank (accession numbers bankit1198460 and FJ843080).
RESULTS
Lager yeasts contain hybrid chromosome XVI.
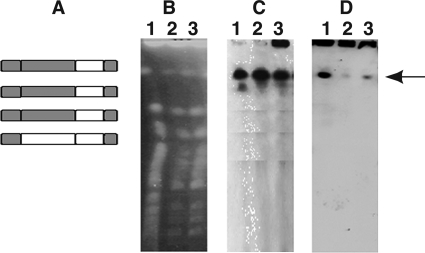
A real-time PCR analysis of genomic DNA from both 6701 and CMBS-33, and correlation with CGH data, predicts the presence of four copies of chromosome XVI in a ratio of three hybrids to one S. bayanus-like chromosome (2, 3, 15, 16). Additionally, in almost all the lager yeast strains tested so far, the telomeric regions of both types of chromosome XVI contain S. cerevisiae-like genes (Fig. 1A), the exceptions being BH-314 and ATCC 24966 (15).
FIG. 1.
(A) Types and numbers of chromosome XVI in CMBS-33. Gray regions are derived from S. cerevisiae, and white regions are derived from S. bayanus. The ends of all four types of chromosome XVI contain S. cerevisiae-like genes. The common white region encompasses genes YPR160W to YPR190C. Genomic DNA from S. cerevisiae (lane 1), lager strain 6701 (lane 2), and lager strain CMBS-33 (lane 3) were separated by pulsed-field electrophoresis. The gels were either stained with ethidium bromide (B) or probed with DIG-labeled DNA to ScYPR159W (C) or ScYPR160W (D). The arrow points to the hybridizing band.
To examine the hybrid nature of chromosome XVI, chromosomal DNA from the lager yeasts CMBS-33 and 6701 and the haploid S. cerevisiae strain S-150 was separated by pulsed-field gel electrophoresis and hybridized to DIG-labeled DNA complementary to either S. cerevisiae YPR159W (ScYPR159W) or ScYPR160. Ethidium bromide staining revealed similar chromosome profiles in all three strains in the high-molecular-weight region of the gel (Fig. 1B). Hybridization to a single chromosome band was observed in the lager DNA samples as well as to the S. cerevisiae DNA with the ScYPR159W probe (Fig. 1C). The intensities of the hybridizing bands in 6701 and CMBS-33 are greater than that observed in the S. cerevisiae sample despite the underloading of the DNA in these samples (Fig. 1C, compare lanes 2 and 3 to lane 1), suggesting a higher copy number for the S. cerevisiae-type YPR159W genes in the two lager strains. A DNA probe in the ScYPR160C ORF also hybridized to a single chromosome band in all three strains; however, the signal was much less intense in the lager strains compared to that observed for the S. cerevisiae laboratory strain (Fig. 1D, compare lanes 2 and 3 to lane 1). Both the ScYPR159W and ScYRP160W probes hybridize to the same band in all three strains, indicating that the ORFs are present on the same chromosome in the lager yeast strains. The reduction in the degree of hybridization with ScYPR160W suggests that this ORF has diverged from the S. cerevisiae sequence or is present at a reduced copy number in the lager yeasts. The reduced hybridization to S. cerevisiae DNA for genes between YPR160W and YPR190C was confirmed by Southern blotting using other S. cerevisiae DNA probes in the region (data not shown).
Chromosome XVI in lager yeasts results from a recombination event between the parental chromosomes.
To examine the region of recombination further, a genomic library of CMBS-33 DNA was generated in a bacterial artificial chromosome vector as described in Materials and Methods. Approximately 7,200 recombinants were recovered, each containing inserts of approximately 100,000 bp. Assuming a minimum tetraploid genome of similar complexity to S. cerevisiae and thus an approximate genome size of 48 Mbp, this represents approximately 15 genome equivalents.
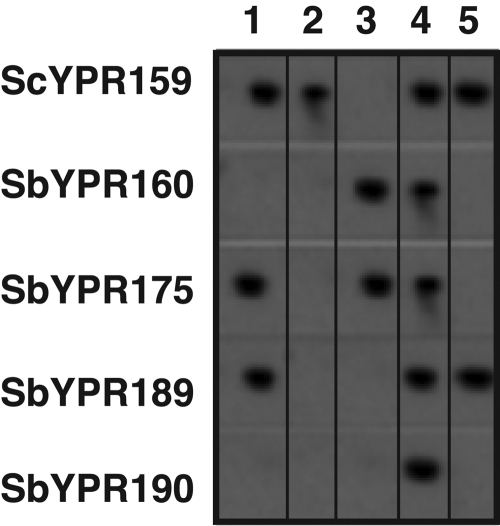
To identify clones encompassing the YPR159W to YPR160W region, the library was screened with a pool of DIG-labeled PCR DNA fragments complementary to ORFs in the region (see Table S1 in the supplemental material). Positive clones were identified, and genomic DNA was isolated from each BAC clone and hybridized to individual S. cerevisiae or S. bayanus probes corresponding to ORFs in the region (Fig. 2). Of the clones screened, only clone 4 hybridized to both the ScYPR159W and S. bayanus YPR160W (SbYPR160W) probes. Interestingly, clone 1 hybridized to the ScYPR159W, SbYPR175C, and SbYPR189W probes and clone 5 to YPR159W and YPR189W, but neither hybridized to the SbYPR160W probe. The differential hybridization of probes to the individual BACs suggests the presence of structurally different forms of chromosome XVI.
FIG. 2.
Bacterial artificial chromosome clones (1 to 5) containing regions of chromosome XVI were hybridized to DIG-labeled DNA probes amplified from either S. cerevisiae (Sc) or S. bayanus (Sb) genomic DNA using the ORF-specific primers.
DNA was isolated from clone 4 and sequenced as described in Materials and Methods. The complete sequence of the region is available in the supplemental material (see Fig. S1). An analysis of the sequence data reveals that the recombination event occurred within the YPR160W ORF (Fig. 3A). The ORF for YPR159W in the lager yeast strain CMBS-33 is 99% identical to the S. cerevisiae YPR159W sequence. The intergenic region between YPR159 and YPR160 is 98% identical to the S. cerevisiae sequence and 77% identical to the S. bayanus sequence and is of the same length as the similar intergenic region in S. cerevisiae, indicating that this region originated from the S. cerevisiae-like chromosome in the lager strain (data not shown).
FIG. 3.
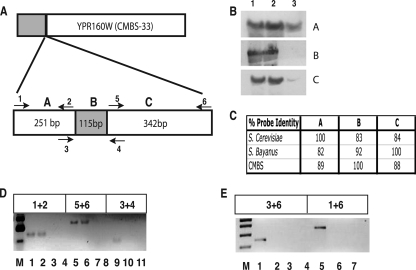
(A) Gene organization of YPR159W and YPR160W in S. cerevisiae (top panels) and the lager strain CMBS-33 (bottom panels). The gray regions are S. cerevisiae-like and the white regions S. bayanus-like. The coordinates for the S. cerevisiae genes on chromosome XVI are shown, and arrows indicate the direction of transcription. (B) Percentage sequence identity of the first 800 nucleotides of the CMBS-33 YPR160W gene to S. cerevisiae (gray) and S. bayanus (black) homologues. (C) Comparison of YPR160W DNA sequences of CMBS-33 (Lager), S. cerevisiae (S.c), and S. bayanus (S.b.) in the region where recombination has occurred. Nucleotides shown in black are common to all three species, those in red are identical in S. cerevisiae and CMBS-33, those in blue are identical in S. bayanus and CMBS-33, and those in pink are identical in S. cerevisiae and S. bayanus. The nine extra nucleotides at positions 450 to 459 present in CMBS and S. bayanus but absent in S. cerevisiae are shown. The in-frame stop codon in the CMBS sequence is underlined. Numbers on the right indicate the nucleotide position relative to the start codon (not shown) for the lager YPR160 gene. The full DNA sequence is shown in Fig. S1 in the supplemental material.
A sequence analysis of the YPR160W gene reveals that the first 360 nucleotides are 97% identical to S. cerevisiae and 83% identical to S. bayanus. After this region, there is a significant reversal in the homologies (Fig. 3B). The DNA sequence from nucleotide 330 to the end of the ORF is 94% identical to the S. bayanus sequence and shares 85% identity with the corresponding S. cerevisiae sequence (see Fig. S1 in the supplemental material). Thus, the recombination event between the parental homoeologous chromosomes appears to have occurred in the 5′ region of YPR160. The DNA sequence encompassing the recombination event is shown in Fig. 3C. An alignment of the lager DNA sequences in this region to the equivalent regions in S. cerevisiae and S. bayanus reveals the presence of an extra three codons in the YPR160W ORF in both the S. bayanus and lager strains, from nucleotides 450 to 459. The translation of the lager YPR160W gene sequence indicates that due to the presence of base insertions, the reading frame is interrupted and that only a truncated form of YPR160W of 15,455 Da could possibly be generated.
The hybrid YPR160 gene is not expressed in lager yeast strains.
Since DNA sequence analysis suggested that the hybrid YPR160W gene, hereafter referred to as Lg (lager-type) YPR160W, cannot encode a functional protein but may produce a truncated form, we set out to determine if this gene is expressed in the lager strain. RNA was extracted from the lager strain CMBS-33 and the parental strains S. cerevisiae and S. bayanus and analyzed by Northern blotting and reverse transcriptase PCR (RT-PCR) using a combination of primers to amplify regions either before, after, or encompassing the recombination site in the Lg YPR160W ORF (Fig. 4A). A DNA probe corresponding to the prerecombination site region (region A) detected a transcript in all three strains, although the level of the transcript in the CMBS-33 strain was much reduced (Fig. 4B). Likewise, a probe corresponding to the postrecombination site region (region C) detected a reduced level of transcript in the CMBS-33 strain compared to the level detected in either the S. cerevisiae or S. bayanus strain. When a probe encompassing the recombination site region (region B) was used, no detectable transcript was observed in the CMBS-33 strain. This lack of hybridization cannot be accounted for by the reduced homology of the recombination site DNA probe to the YPR160 gene in CMBS-33 as indicated by the percentage of homology of the various probes to the DNA sequences in all three strains (Fig. 4C). The lack of a YPR160W transcript containing the recombination site region (region B) was confirmed by RT-PCR analysis (Fig. 4D, lane 10); however, the transcripts corresponding to the pre- and postrecombination sites (regions A and C, respectively) can be detected (Fig. 4D, lanes 2 and 6, respectively). All three regions can be amplified from the CMBS-33 genomic DNA (Fig. 4D, lanes 1, 5, and 9). The quantification of the transcript levels by real-time RT-PCR confirms that the levels of transcripts from all three regions of the gene are much reduced compared to the levels detected in the S. cerevisiae and S. bayanus strains (data not shown).
FIG. 4.
(A) Structure of the YPR160W gene in CMBS-33. The gray region is S. cerevisiae-like and the white region S. bayanus-like. The region where recombination between the homoeologous alleles occurred is shown in the bottom panel; the prerecombination (A), recombination (B), and postrecombination (C) regions are indicated, as are the locations of the primers used for the RT-PCR amplification of the regions. The expected sizes of the RT-PCR products are shown. The location of the primers in YPR160W are shown in Fig. S1 in the supplemental material, and the sequences of the primers are shown in Table 1. (B) Hybridization of RNA from S. cerevisiae (lane 1), S. bayanus (lane 2), and CMBS-33 (lane 3) with DNA probes to regions A, B, and C. Probe A was amplified from S. cerevisiae genomic DNA, probe B from CMBS-33 genomic DNA, and probe C from S. bayanus genomic DNA. (C) Percentage identity of probes A, B, and C to S. cerevisiae, CMBS-33, and S. bayanus YPR160W gene sequences. (D) Amplification of genomic DNA and cDNA corresponding to regions A, B, and C of the CMBS-33 YPR160W gene. Lane M, molecular weight marker hyperladder II (Bioline); lanes 1, 5, and 9, genomic DNA; lanes 2, 6, and 10, cDNA; lanes 3, 7, and 11, cDNA amplification in the absence of reverse transcriptase; lanes 4 and 8, blank. The primers used for amplification are indicated above the lanes. (E) Amplification of genomic DNA and cDNA from the CMBS-33 YPR160W gene. Lane M, molecular weight marker hyperladder I (Bioline); lanes 1 and 5, genomic DNA; lanes 2 and 6, cDNA; lanes 3 and 7, cDNA amplification in the absence of reverse transcriptase; lane 4, blank. The primers used for amplification are shown above the lanes.
To rule out the possibility that sequences within the recombination site region were absent from the final transcript, perhaps due to the introduction of a splice site in the hybrid gene, cDNA synthesis was carried out using reverse primers 4 and 6 in the postrecombination region (Fig. 4A). cDNAs were amplified using the same primers and forward primer 1 (Fig. 4A) in the prerecombination region. The PCR products of the expected sizes (366 and 708 bp, respectively) can be amplified from the CMBS-33 genomic DNA (Fig. 4E, lanes 1 and 5), but no corresponding cDNA transcripts were detected (Fig. 4E, lanes 2 and 6). Transcripts of the expected sizes were detected in both the S. cerevisiae and S. bayanus strains (data not shown). We interpret these data as reflecting the absence of transcription from the hybrid YPR160W gene but the presence of a YPR160W-like RNA, transcribed possibly from the YPR160W gene on the S. bayanus-like chromosome in these strains.
Haploid glycogen levels are observed in the lager yeast strains.
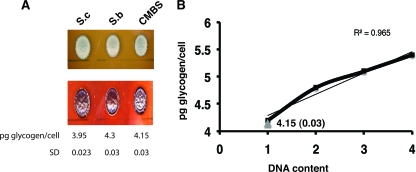
The YPR160W ORF encodes for glycogen phosphorylase (GPH1), which is required for the mobilization of stored glycogen through its conversion into glucose-1-P (12). To determine if the lack of gene expression from the hybrid YPR160W gene affects the glycogen levels in the cells, cellular glycogen levels were determined both qualitatively and quantitatively. First, the S. cerevisiae and S. bayanus parental strains and the lager yeast strain CMBS-33 were grown on glucose- or maltose-supplemented medium and exposed to iodine vapors to visualize the cellular glycogen levels. The glycogen levels were similar for all three strains when grown on either glucose (Fig. 5A) - or maltose (data not shown)-containing medium. The glycogen levels were quantified by measuring the glucose levels following the conversion of glycogen to glucose. Similar levels were detected in all three strains (Fig. 5A). Since the lager yeast strains are considered to be aneuploid but contain a general tetraploid DNA content (J. Usher and U. Bond, unpublished data), we asked if the glycogen content varied with the DNA content by examining the glycogen levels in a series of isogenic S. cerevisiae strains increasing in ploidy from haploid to tetraploid (11). As shown in Fig. 5B, the glycogen content increased as the DNA content increased, displaying a correlation coefficient of 0.965. In all, the glycogen content of CMBS-33 most closely resembles that observed in the 1n isogenic S. cerevisiae strain as well as the levels in haploid S. cerevisiae and S. bayanus strains.
FIG. 5.
(A) Glycogen levels in the S. cerevisiae (S.c), S. bayanus (S.b), and CMBS-33 strains. Cells were plated on YEP-dextrose (top panel) and stained with crystal violet (bottom panel). The levels of glycogen per cell are shown below the panels. Standard deviations (SD) were obtained from the results of three independent experiments. (B) Glycogen levels in isogenic polyploid strains of S. cerevisiae (thick black line). The level of glycogen in CMBS-33 is illustrated by a gray triangle. Thin black line, linear trend line.
DISCUSSION
Polyploidy has the potential to confer evolutionary advantages to cells through the divergent evolution of duplicated loci, increased heterozygosity, increased buffering of deleterious alleles or gene mutations, or increased gene dosage (17). Additionally, polyploid genomes can undergo rapid structural changes and rearrangements which may be seen as a driving mechanism for adaptive evolution (1, 22). The lager yeasts are allopolyploids resulting from the fusion of the S. cerevisiae and S. bayanus genomes and thus have the capacity for adaptive evolution. It can be argued that allopolyploid genomes are in a state of evolutionary flux and can accommodate a higher rate of evolutionary change than their haploid counterparts, allowing for increased opportunities for adaptive evolution.
The allopolyploid genomes have undergone recombination between the homoeologous parental chromosomes at generate hybrid or mosaic chromosomes. Up to 28 putative recombination sites have been identified in the two lager yeasts 6701 and CMBS-33. The majority of these sites are present in all lager yeasts analyzed to date (8), but many strains additionally have a small number of unique recombination sites. There is also evidence for chromosomal translocations between nonhomoeologous chromosomes (8, 16, 20). The recombination events and other chromosomal translocations have the potential to drive adaptive evolution through the loss or gain of functional gene products. To date, only a small number of phenotypic traits resulting from chromosomal rearrangements have been characterized in lager yeasts. A nonreciprocal translocation of approximately 20 kbp from the left telomere region of chromosome IX to the right telomere region of chromosome XIII was identified in a type 1 nonflocculent lager yeast strain (20). The translocation generated a fusion between the 5′ region of the Lg FLO1 gene (FLO5 in S. cerevisiae) and the 3′ region of the YIL169C gene and appears to account for the conversion of the strain from being flocculent to nonflocculent through the loss of function of the Lg FLO1 gene. Flocculation in brewing yeasts is an important phenotypic trait that results in the sedimentation of yeasts to the bottom of the fermentation vat at the end of fermentation.
In this paper, we set out to examine the genotypic and phenotypic outcomes of one of the conserved recombination events between homoeologous chromosomes that occurred between YPR159W and YPR160W on chromosome XVI. A sequence analysis of the region indicates that the recombination site is located in the 5′ region of YPR160W, resulting in the generation of a hybrid gene.
A sequence analysis of the YPR159W gene in CMBS-33, which encodes a protein with glucosidase activity involved in cell wall organization (19), indicates that the gene differs by just two nucleotides from the annotated sequence of S. cerevisiae YPR159W. The nucleotide changes do not affect the protein coding sequence. The intergenic region between YPR159W and YPR160W is also highly conserved and S. cerevisiae-like in origin.
DNA sequence analysis of the hybrid Lg YPR160W gene indicates that the recombination event has resulted in a loss of function in the CMBS-33 lager strain due to specific base insertions that have led to a frameshift in the ORF. Real-time RT-PCR and Northern blot analysis confirm the lack of expression of the gene; however, using specific DNA probes both upstream and downstream of the recombination site, a related transcript that has significantly diverged from the sequences of both S. cerevisiae and S. bayanus is detected in CBMS-33. This divergent transcript most likely emanates from the single S. bayanus-like chromosome XVI that has been identified in lager yeasts. This result may also go some way to explain the clones identified in the screening of the BAC library. While clone 4, which was used in this study, was shown to contain a contiguous copy of the region of interest, both clones 1 and 5 (Fig. 2) showed hybridization to other ORFs in the region but not to an SbYPR160W probe. These clones may contain a divergent copy of YPR160W and may represent S. bayanus-like chromosome XVI or an as-yet-unidentified version of chromosome XVI in which sections between YPR159W and YPR175W have been deleted.
The YPR160W gene (GPH1) encodes the enzyme glycogen phosphorylase (12, 21). Together with Gdb1p, a glycogen-debranching enzyme, GPH1p catalyzes the sequential phosphorolysis of α-1,4-linked glucose units in glycogen to generate glucose-1-phosphate and glucose, thereby mobilizing stored glycogen for energy utilization. In S. cerevisiae, GPH1 expression is regulated by stress-response elements and by the high-osmolarity glycerol mitogen-activated protein kinase pathway and is induced during late exponential growth phase (10, 21). Recent data indicate that the deletion of GPH1 leads to a shortened life span, stress intolerance, and decreased survival in stationary phase (9). Thus, reduced levels of Gph1p in lager yeast may not necessarily be advantageous. However, the lager yeasts still retain one functional GPH1 gene emanating from the S. bayanus-like chromosome. Since cellular glycogen content is determined by the regulation of multiple metabolic pathways and many enzymes, including glycogen synthase, further research will be required to examine the gene dosage effects caused by the loss of three copies of GPH1 in the lager yeasts.
Finally, it is interesting to note that both of the chromosome rearrangements characterized to date in lager yeasts, namely, the YPR160W recombination event described here and the FLO5-YIL169C fusion observed in the nonflocculent type 1 strain (20), result in loss-of-function rather than gain-of-function phenotypes. It will be interesting to examine the other recombination sites in detail to determine if this is a common trend.
Supplementary Material
Acknowledgments
We thank T. C. James for continued expertise and assistance during this project.
This research was supported by a grant to U.B. as part of the IITAC consortium from the Higher Education Authority through the Programme for Research in Third Level Institutions (PRTLI-3).
Footnotes
Published ahead of print on 8 May 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Andalis, A. A., Z. Storchova, C. Styles, T. Galitski, D. Pellman, and G. R. Fink. 2004. Defects arising from whole-genome duplications in Saccharomyces cerevisiae. Genetics 167:1109-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bond, U., and A. Blomberg. 2006. Principles and applications of genomics and proteomics in the analysis of industrial yeast strains, p. 175-213. In A. Querol and G. Fleet (ed.), The yeast handbook. Yeasts in food and beverages. Springer-Verlag, Heidelberg, Germany.
- 3.Bond, U., C. Neal, D. Donnelly, and T. C. James. 2004. Aneuploidy and copy number breakpoints in the genome of lager yeasts mapped by microarray hybridisation. Curr. Genet. 45:360-370. [DOI] [PubMed] [Google Scholar]
- 4.Caesar, R., J. Palmfeldt, J. S. Gustafsson, E. Pettersson, S. H. Hashemi, and A. Blomberg. 2007. Comparative proteomics of industrial lager yeast reveals differential expression of the cerevisiae and non-cerevisiae parts of their genomes. Proteomics 7:4135-4147. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, S. G., M. li del Olmo, P. Beglan, and U. Bond. 2002. A sequence element downstream of the yeast HTB1 gene contributes to mRNA 3′ processing and cell cycle regulation. Mol. Cell. Biol. 22:8415-8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casaregola, S., H. V. Nguyen, G. Lapathitis, A. Kotyk, and C. Gaillardin. 2001. Analysis of the constitution of the beer yeast genome by PCR, sequencing and subtelomeric sequence hybridization. Int. J. Syst. Evol. Microbiol. 51:1607-1618. [DOI] [PubMed] [Google Scholar]
- 7.Casey, G. P. 1986. Molecular and genetic analysis of chromosomes X in Saccharomyces carlsbergensis. Carlsberg Res. Commun. 51:343-362. [Google Scholar]
- 8.Dunn, B., and G. Sherlock. 2008. Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res. 18:1610-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favre, C., P. S. Aguilar, and M. C. Carrillo. 2008. Oxidative stress and chronological aging in glycogen-phosphorylase-deleted yeast. Free Radic. Biol. Med. 45:1446-1456. [DOI] [PubMed] [Google Scholar]
- 10.Francois, J., and J. L. Parrou. 2001. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 25:125-145. [DOI] [PubMed] [Google Scholar]
- 11.Galitski, T., A. J. Saldanha, C. A. Styles, E. S. Lander, and G. R. Fink. 1999. Ploidy regulation of gene expression. Science 285:251-254. [DOI] [PubMed] [Google Scholar]
- 12.Hwang, P. W., S. Tugendreich, and R. J. Fletter. 1989. Molecular analysis of GPHI, the gene encoding glycogen phosphorylase in Saccharomyces cerevisiae. Mol. Cell. Biol. 9:1659-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James, T. C., J. Usher, S. Campbell, and U. Bond. 2008. Lager yeast possess dynamic genomes that undergo rearrangements and amplification in response to stress. Curr. Genet. 3:139-152. [DOI] [PubMed] [Google Scholar]
- 14.Kielland-Brandt, M. C., T. Nillson-Tillgren, C. Gjermansen, S. Holmberg, and M. B. Pedersen. 1995. Genetics of brewing yeasts, p. 223-254. In A. E. Wheals, A. H. Rose, and E. S. Harrison (ed.), The yeasts, vol. 6. Academic Press Ltd., London, United Kingdom. [Google Scholar]
- 15.Kodama, Y., M. C. Kielland-Brandt, and J. Hansen. 2005. Lager brewing yeast, p. 145-164. In P. Sunnerhagen and J. Piskur (ed.), Comparative genomics using fungi as models. Springer-Verlag, Berlin, Germany.
- 16.Nakao, Y., T. Kanamori, T. Itoh, Y. Kodama, S. Rainieri, N. Nakamura, T. Shimonaga, M. Hattori, and T. Ashikari. 2009. Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res. 16:115-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otto, S. P., and J. Whitton. 2000. Polyploid incidence and evolution. Annu. Rev. Genet. 34:401-437. [DOI] [PubMed] [Google Scholar]
- 18.Rainieri, S., Y. Kodama, Y. Kaneko, K. Mikata, Y. Nakao, and T. Ashikari. 2006. Pure and mixed genetic lines of Saccharomyces bayanus and Saccharomyces pastorianus and their contribution to the lager brewing strain genome. Appl. Environ. Microbiol. 72:3968-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roemer, T., and H. Bussey. 1991. Yeast beta-glucan synthesis: KRE6 encodes a predicted type II membrane protein required for glucan synthesis in vivo and for glucan synthase activity in vitro. Proc. Natl. Acad. Sci. USA 88:11295-11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato, M., H. Maeba, J. Watari, and M. Takashio. 2002. Analysis of an inactivated Lg-FLO1 gene present in bottom-fermenting yeast. J. Biosci. Bioeng. 93:395-398. [DOI] [PubMed] [Google Scholar]
- 21.Sunnarborg, S. W., S. P. Miller, I. Unnikrishnan, and D. C. LaPorte. 2001. Expression of the yeast glycogen phosphorylase gene is regulated by stress-response elements and by the HOG MAP kinase pathway. Yeast 18:1505-1514. [DOI] [PubMed] [Google Scholar]
- 22.Torres, E. M., T. Sokolsky, C. M. Tucker, L. Y. Chan, M. Boselli, M. J. Dunham, and A. Amon. 2007. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science 317:916-924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.