Abstract
Low concentrations of furfural are formed as a side product during the dilute acid hydrolysis of hemicellulose. Growth is inhibited by exposure to furfural but resumes after the complete reduction of furfural to the less toxic furfuryl alcohol. Growth-based selection was used to isolate a furfural-resistant mutant of ethanologenic Escherichia coli LY180, designated strain EMFR9. Based on mRNA expression levels in the parent and mutant in response to furfural challenge, genes encoding 12 oxidoreductases were found to vary by more than twofold (eight were higher in EMFR9; four were higher in the parent). All 12 genes were cloned. When expressed from plasmids, none of the eight genes in the first group increased furfural tolerance in the parent (LY180). Expression of three of the silenced genes (yqhD, dkgA, and yqfA) in EMFR9 was found to decrease furfural tolerance compared to that in the parent. Purified enzymes encoded by yqhD and dkgA were shown to have NADPH-dependent furfural reductase activity. Both exhibited low Km values for NADPH (8 μM and 23 μM, respectively), similar to those of biosynthetic reactions. Furfural reductase activity was not associated with yqfA. Deleting yqhD and dkgA in the parent (LY180) increased furfural tolerance, but not to the same extent observed in the mutant EMFR9. Together, these results suggest that the process of reducing furfural by using an enzyme with a low Km for NADPH rather than a direct inhibitory action is the primary cause for growth inhibition by low concentrations of furfural.
A wide variety of fermentation products can be made using sugars from lignocellulosic biomass as a substrate (9, 13, 16, 39). Prior to fermentation, however, the carbohydrate polymers cellulose and hemicellulose must be converted to soluble sugars, using a combination of chemical and enzymatic processes (40, 42, 43). Chemical processes are accompanied by side reactions that produce a mixture of minor products such as alcohols, acids, and aldehydes that have a negative effect on the metabolism of microbial biocatalysts. Alcohols (catechol, syringol, etc.) have been shown to act by permeabilizing the cell membrane and toxicity correlated well with the hydrophobicity of the molecule (47). Organic acids (acetate, formate, etc.) are thought to cross the membrane in neutral form and ionize within the cytoplasm, inhibiting growth by acidifying the cytoplasm and collapsing the proton motive force (32, 48). The inhibitory mechanisms of aldehydes are more complex. Aldehydes can react to form products with many cellular constituents, in addition to direct physical and metabolic effects (27, 36). In aggregate, these side products from chemical pretreatments can retard cell growth and slow the fermentation of biomass-derived sugars (10, 31).
Furfural (a dehydration product of pentose sugars) is of particular importance. Furfural content in dilute acid hydrolysates of hemicellulose has been correlated with toxicity (23). Removal of furfural by lime addition (pH 10) rendered hydrolysates readily fermentable while readdition of furfural restored toxicity (22). Furfural has also been shown to potentiate the toxicity of other compounds known to be present in acid hydrolysates of hemicellulose (47-49). Furfural has been reported to alter DNA structure and sequence (3, 17), inhibit glycolytic enzymes (6), and slow sugar metabolism (11).
The ability of fermenting organisms to function in the presence of these inhibitors has been researched extensively. Encapsulation of Saccharomyces cerevisiae in alginate has been shown to be protective and improve fermentation in acid hydrolysates of hemicellulose (38). Strains of S. cerevisiae have been previously described with improved resistance to hydrolysate inhibitors (1, 20, 29). Escherichia coli (7), S. cerevisiae (2, 19), and other microorganisms (4) have been shown to contain enzymes that catalyze the reduction of furfural to the less toxic product, furfuryl alcohol (49). In E. coli, furfural reductase activity appears to be NADPH-dependent (7). An NADPH-dependent furfural reductase was purified from E. coli although others may also be present. Two NADPH-dependent enzymes capable of reducing 5-hydroxymethyl furfural (a dehydration product of hexose sugars) have been characterized in S. cerevisiae and identified as the ADH6 gene product (2, 34) and a mutant form of ADH1 (2).
In this paper, we describe the isolation of a furfural-resistant E. coli mutant (EMFR9) in which furfural reductase activity is lower than that of the parent (LY180) due to decreased expression of yqhD and dkgA. The reduction of furfural by these two NADPH-dependent oxidoreductases is proposed to inhibit growth by depleting the NADPH needed for biosynthesis.
MATERIALS AND METHODS
Strains, media, and growth conditions.
Strains and plasmids used in this study are listed in Table 1. Plasmid and strain constructions were made using Luria broth (26). Antibiotics were included as appropriate. Temperature-conditional plasmids were grown at 30°C; all others were grown at 37°C. Ethanologenic strains were maintained in AM1 mineral salts medium (24) supplemented with 20 g liter−1 xylose for solid medium and ≥50 g liter−1 xylose for liquid medium used in fermentation experiments. E. coli strain LY168 (13, 44, 45) is a derivative of KO11 and served as the starting point for this investigation. Note that E. coli W (ATCC 9637) is the parent for strain KO11, initially reported to be a derivative of E. coli B (30).
TABLE 1.
Bacterial strains, plasmids, and primers
| Strain, plasmid, or primer | Relevant characteristic(s)c | Reference or source |
|---|---|---|
| E. coli strains | ||
| LY168 | frdA::(Zm frg celYEc FRT) ΔldhA::FRT ΔadhE::(Zm frg estZPp FRT) ΔackA::FRT rrlE::(pdc adhA adhB FRT) lacY::FRT ΔmgsA::FRT | 13, 44, 45 |
| LY180 | ΔfrdBC::(Zm frg celYEc) ΔldhA::(Zm frg casABKo) adhE::(Zm frg estZPp FRT) ΔackA::FRT rrlE::(pdc adhA adhB FRT) ΔmgsA::FRT | This study |
| EMFR9 | LY180 improved for furfural tolerance | This study |
| EMFR9 ΔyqhD | EMFR9 ΔyqhD::kan | This study |
| EMFR9 ΔdkgA | EMFR9 ΔdkgA::cat sacB | This study |
| EMFR9 ΔyqhD ΔdkgA | EMFR9 ΔyqhD::kan, ΔdkgA::cat sacB | This study |
| BL21(λDE3) | F−ompT gal dcm lon hsdSB(rB− mB−) λ(DE3 [lacI lacUV5-T7 gene 1 ind1 sam7 nin5]) | Promega (Madison, WI) |
| TOP10F′ | F′{lacIq Tn10 (Tetr)} mcriA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL endA1 nupG | Invitrogen (Carlsbad, CA) |
| Plasmidsa | ||
| pCR 2.1 TOPO | bla kan lacZ Plac | Invitrogen (Carlsbad, CA) |
| pLOI4301 | yqhD gene in pCR 2.1 TOPO | This study |
| pLOI4302 | yjjN gene in pCR 2.1 TOPO | This study |
| pLOI4303 | dkgA gene in pCR 2.1 TOPO | This study |
| pLOI4304 | yqfA gene in pCR 2.1 TOPO | This study |
| pLOI4305 | yajO gene in pCR 2.1 TOPO | This study |
| pLOI4306 | ydhU gene in pCR 2.1 TOPO | This study |
| pLOI4307 | ydhV gene in pCR 2.1 TOPO | This study |
| pLOI4308 | ygcW gene in pCR 2.1 TOPO | This study |
| pLOI4309 | nemA gene in pCR 2.1 TOPO | This study |
| pLOI4310 | yjgB gene in pCR 2.1 TOPO | This study |
| pLOI4311 | ydhS gene in pCR 2.1 TOPO | This study |
| pLOI4312 | ydhY gene in pCR 2.1 TOPO | This study |
| pLOI4313 | His-tagged yqhD in pET15b | This study |
| PLOI4314 | His-tagged dkgA in pET15b | This study |
| pET15b | T7 promoter, bla, His-tagged vector | Novagen (Madison, WI) |
| pKD4 | FRT kan FRT | 5 |
| PKD46 | Para bla Red recombinase (γ,β,exo) | 5 |
| Primersb | ||
| yqhD cloning | For-ACATCAGGCAGATCGTTCTC | This study |
| Rev-CCACAGCTTAGTGGTGATGA | ||
| yjjN cloning | For-GGAGAGCCGAATCATGTCTA | This study |
| Rev-CCGGAACCTGTCTCAACCAA | ||
| dkgA cloning | For-GCCTGCTCCGGTGAGTTCAT | This study |
| Rev-CCGGCTCTGCATGATGATGT | ||
| yqfA cloning | For-GCTGGAGAGGTATACATGTG | This study |
| Rev-GCCGTATTCGCTCGAAGAGT | ||
| yajO cloning | For-CCGCAGCACATGCAACTTGA | This study |
| Rev-ATGGCGCTGCCGACCAATGA | ||
| ydhU cloning | For-CCGCATCTGTATCGCCGGTT | This study |
| Rev-GCCGATGCGAGCATGATTCGT | ||
| ydhV cloning | For-ATTATCGAGTGGAAAGATAT | This study |
| Rev-CGTAGTCTCCGTTCTGCTTA | ||
| ygcW cloning | For-ACCTTTCTTTTTTTTTGCCT | This study |
| Rev-TTACGACCGCTGCCGGAATC | ||
| nemA cloning | For-TTATTGCGACGCCTGCCGTT | This study |
| Rev-GTTCAATCACCGCTTCTTCG | ||
| yjgB cloning | For-CCTGCCATGCTCTACACTTC | This study |
| Rev-CTGGTTAGATGGCGACTATG | ||
| ydhS cloning | For-AACTTATCTGATAACACTAA | This study |
| Rev-CCAACAGCGGCGACAATGTA | ||
| ydhY cloning | For-TCAGGCTGCTGAATTGTCAG | This study |
| Rev-GGCACCAGATCCAGTTAATG | ||
| Deletion of yqhD | For-GTTCTCTGCCCTCATATTGGCCCAGCAAAGGGAGCAAGTAGTGTAGGCTGGAGCTGCTTC | This study |
| Rev-GACGAAATGCCCGAAAACGAA AGTTTGAGGCGTAAAAAGCCATATGAATATCCTCCTTA | ||
| Deletion of dkgA | Outward 1-ACGGTTGGATTAGCCATACG | This study |
| Outward 2-GACCAGTTCGGCGGCTAACA | ||
| For-GCCTGCTCCGGTGAGTTCAT | ||
| Rev-CCGGCTCTGCATGATGATGT | ||
| yqhD cloning into pET15b | For-TGACTCTCGAGATGAACAACTTTAATCTGCA | This study |
| Rev-AGTCAGGATCCTTAGCGGGCGGCTTCGTATA | ||
| dkgA cloning into pET15b | For-ATATGCCTCGAGATGGCTAATCCAACCGTTAT | This study |
| Rev- CCGATAGGATCCTTAGCCGCCGAACTGGTCAGG | ||
| Sequencing yqhD | yqhD_for1 CGGCGAGGTACTGGTGAC | This study |
| yqhD_rev1 CATGTTAGCCGCCGAACT | ||
| yqhD_seq1 TCATGTTGGCTTCTGCCG | ||
| yqhD_seq2 GCGCAATCGCTGGTTTAC | ||
| yqhD_seq3 GTTCCGATGATGAGCGTATTG | ||
| yqhD_seq4 AGGCGTTTTCGATCAGAAAG | ||
| Sequencing dkgA | dkgA_for1 CCAGCAACCGGTTCAGAAT | This study |
| dkgA_rev1 AACGCGTGAAAATAGCGACT | ||
| dkgA_seq1 GCGGTAAAGAGATTAAAAGCGC | ||
| dkgA_seq2 TATGGCTAATCCAACCGTTATTAAG | ||
| dkgA_seq3 CCCGCCCGTTGTTACTCT | ||
| Sequencing of yqfA | pcr_for CCATCCGCGACGAGTCTGAA | This study |
| pcr_rev GGTGAAGCGGAACTGAACAA | ||
| seq1 CCATCCGCGACGAGTCTGAA | ||
| seq2 CGACGCTCTATCACGCCATT | ||
| Sequencing of yjjN | pcr_for TGCGCTGTTTAAGATCGCT | This study |
| pcr_rev CATGATTGCCTTCTCGGG | ||
| seq1 ACTGAGATGATCTCAAGCGATTG | ||
| seq2 GGAAACAACGCGAGATACCT | ||
| seq3 CCACGCTGGCAGAAACCTA |
The genes inserted into pCR 2.1 TOPO include a native ribosomal binding site gene and a transcriptional terminator. Expression is from the plasmid promoter (Plac).
Orientation of genes cloned into pCR 2.1 TOPO was verified by PCR analysis.
Data for primers are 5′-to-3′ sequences.
Construction of strain LY180.
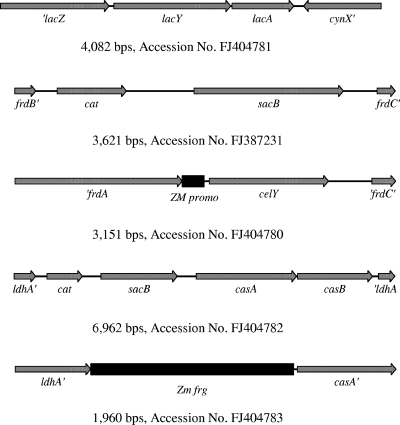
Strain LY168 has been previously described for the fermentation of sugars in hemicellulose hydrolysates (13). Several modifications were made to improve the substrate range (restoration of lactose utilization, integration of an endoglucanase, and integration of cellobiose utilization) resulting in LY180. Red recombinase technology (Gene Bridges GmbH, Dresden, Germany) was used to facilitate chromosomal integration. Linear DNA fragments used for integration are shown in Fig. 1 and have been deposited in GenBank.
FIG. 1.
Linear DNA fragments used for construction of LY180.
The FLP recombination target (FRT) region in lacY was replaced with the native E. coli ATCC 9637 sequence by double homologous recombination using fragment A, containing lacZ lacY lacA cynX′ (5, 12). Integrated strains were selected directly for lactose fermentation. The frdBC region downstream from frdA::Zm frg celYEc (Erwinia chrysanthemi) was deleted by double homologous recombination using a two-step process (12). Fragment B (frdB′, a cat sacB cassette, and frdC′) was integrated first with selection for chloramphenicol resistance. The cat sacB cassette was then replaced with fragment C, consisting of frdA′, a Zymomonas mobilis promoter fragment, E. chrysanthemi celY, and frdC′ by selecting for resistance to sucrose. This replacement also deleted an FRT site. The Klebsiella oxytoca genes encoding cellobiose utilization (casAB) were inserted into ldhA by double homologous recombination also using a two-step process (12). Fragment D (ldhA′, a cat sacB cassette, casAB, and ′ldhA) was used to replace the FRT site in ldhA with selection for resistance to chloramphenicol. The cat sacB cassette was then replaced with fragment E, consisting of ldhA′, a promoter fragment from Z. mobilis, and K. oxytoca casA′. Integrated strains were isolated by selecting directly for cellobiose fermentation. All constructs were verified by analyses of phenotypes and PCR products.
Growth-based selection for a furfural-resistant strain.
LY180 was inoculated into a 500-ml vessel (inoculum of 50 mg dry cell weight [dcw] liter−1) containing 350 ml of AM1 supplemented with 100 g liter−1 xylose and 0.5 g liter−1 furfural (37°C, 150 rpm, pH 6.5). Cultures were serially diluted into new fermenters at 24-h intervals, or when cell mass exceeded 330 mg dcw liter−1. The amount of furfural was gradually increased to 1.3 g liter−1 as growth permitted. After 54 serial transfers, a resistant strain was isolated and designated EMFR9.
Furfural resistance and metabolism during fermentation.
Furfural resistance was compared in small fermentors (37°C, 150 rpm, pH 6.5, 350-ml working volume) using AM1 medium (24) containing 100 g liter−1 xylose. Seed cultures were inoculated to approximately 33 mg dcw liter−1. Samples were removed periodically to measure cell mass, ethanol, and furfural.
Furfural toxicity and MIC were examined using tube cultures (13 by 100 mm) containing 4 ml of AM1 broth with 50 g liter−1 (wt/vol) filtered-sterilized sugar, furfural, and other supplements. Cultures were inoculated to an initial density of 17 mg dcw liter−1. Cell mass was measured after incubation at 37°C for 24 h and 48 h.
Comparison of hydrolysate toxicity.
A hemicellulose hydrolysate of sugar cane bagasse was produced using dilute sulfuric acid at elevated temperature and pressure and supplied by Verenium Corporation (Boston, MA). This hydrolysate contained 82 g liter−1 total sugar (primarily xylose), 1.4 g liter−1 furfural, and other constituents. Hydrolysate was supplemented with the mineral components of AM1 medium, adjusted to pH 6.5 using 45% KOH, and diluted with complete AM1 (80 g liter−1 xylose). Diluted samples of hydrolysate were distributed into 13-mm by 100-mm culture tubes (4 ml each), inoculated to an initial cell density of 17 g dcw liter−1, and incubated at 37°C. Cell mass (after centrifugation and resuspending in broth) and ethanol concentration were measured after 48 h.
Microarray analysis.
Cultures were grown in small fermentors to a density of 670 mg dcw liter−1. Furfural (0.5 g liter−1) was added and incubation continued for 15 min prior to harvesting. All samples were immediately cooled in an ethanol-dry-ice bath, harvested by centrifugation, resuspended in Qiagen RNALater reagent (Valencia, CA) and stored at −80°C until purification. RNA was purified using a Qiagen RNeasy mini kit and sent to NimbleGen (Madison, WI) for microarray comparisons. Data were analyzed with ArrayStar software (DNA Star, Madison, WI).
Cloning and deletion of oxidoreductases.
Oxidoreductase genes for expression studies (ribosomal-binding sites, coding regions, and 200-bp terminator regions) were amplified from strain LY180 genomic DNA by using a Bio-Rad iCycler (Hercules, CA), ligated into pCR2.1 TOPO vector, and cloned into E. coli TOP10F′ by using an Invitrogen TOPO TA cloning kit (Carlsbad, CA). Plasmids were purified using a QiaPrep Spin mini prep kit. Gene orientation was established by PCR.
A yqhD deletion was constructed in LY180 as described by Datsenko and Wanner (5), using the plasmids pKD4 and pKD46. A dkgA deletion in LY180 was constructed as described by Jantama et al. (12). A double mutant with deletions of both yqhD and dkgA was also constructed. Repeated attempts to delete the yqfA gene were not successful.
Purification and kinetic analysis of YqhD and DkgA.
Both the yqhD and dkgA genes were cloned into a Novagen pET-15b vector and expressed as a His-tagged protein in E. coli BL21(DE3). Cells were grown with IPTG (isopropyl-β-d-thiogalactopyranoside) to approximately 1.3 g dcw liter−1, washed with 100 mM phosphate buffer, and lysed using an MP FastPrep-24 cell disruptor (MP Biomedical, Solon, OH) and lysing matrix B tubes. Crude extracts were passed through a 0.22-μm polyvinylidene difluoride filter and further purified using a 1-ml HiTrap nickel column. Purified enzymes were dialyzed in 100 mM phosphate buffer using a Thermo Slide-A-Lyzer and quantified using a Thermo BCA protein assay kit. The purity of YqhD and DkgA was estimated to be greater than 90% by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. A single band was observed for each in a sodium dodecyl sulfate-polyacrylamide gel. Estimated sizes of the purified proteins were in agreement with the predicted values of 43 kDa and 31 kDa, respectively. Apparent Kcat and apparent Km values were determined for both purified enzymes using NADPH and furfural.
Whole-cell assays of furfural metabolism in vivo during fermentation.
Whole-cell furfural metabolism was measured using fermentors in which cultures were grown to a density of 670 mg dcw liter−1 (mid-log phase). Furfural was added to an initial concentration of 0.5 g liter−1. Samples were removed at zero time and after 15, 30, and 60 min of incubation for the measurement of furfural and cell mass. The specific rate of furfural metabolism was calculated using the average cell mass during each assay interval. Results are expressed as μmol min−1 mg dcw−1.
In vitro assay of furfural reduction.
Anaerobic tube cultures were grown in AM1 medium containing 50 g liter−1 xylose and harvested in mid-log phase (0.7 to 1.0 g dcw liter−1). Cells were washed once with 20 ml 100 mM potassium phosphate buffer (pH 7.0), resuspended in phosphate buffer to approximately 6.5 g dcw liter−1, chilled on ice, and lysed for 20 s using a FastPrep-24 cell disruptor and lysing matrix B tubes. Debris was removed by centrifugation (13,000 × g; 10 min) and the supernatant used to measure furfural-dependent oxidation of NADH and NADPH. Assays contained 100 mM phosphate buffer (pH 7.0), 20 mM furfural, and 0.2 mM reductant (NADPH or NADH). Furfural-dependent activity (μmol min−1 mg protein−1) was measured as the change in absorbance at 340 nm. Greater than 80% of activity was NADPH dependent.
Data and analyses.
With the exception of gene expression array measurements, all other experimental data are presented as an average of three or more measurements with standard deviations.
Ethanol was measured using an Agilent 6890N gas chromatograph (Palo Alto, CA) equipped with flame ionization detectors and a 15-meter HP-Plot Q Megabore column. Dry cell weight was estimated by measuring the optical density at 550 nm (OD550) using a Bausch & Lomb Spectronic 70 spectrophotometer. Each OD550 value is equivalent to approximately 333.3 mg dcw liter−1.
Furfural levels in AM1 medium were measured by absorbance at OD284 and OD320 (21). The accuracy of this method was confirmed by high-pressure liquid chromatography analysis. Furfural content of bagasse hemicellulose hydrolysate was measured using an Agilent LC1100 liquid chromatograph (refractive index monitor and UV detector) and an Aminex HPX-87P ion exclusion column (Bio-Rad, Hercules, CA) with water as the mobile phase.
RESULTS
Isolation and initial characterization of a furfural-resistant mutant.
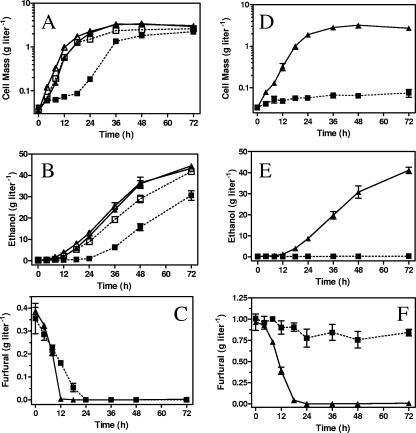
A furfural-resistant derivative of LY180 was isolated after 53 serial transfers in pH-controlled fermentors containing AM1 mineral salts medium with 100 g liter−1 xylose and increasing concentrations of furfural (0.5 g liter−1 initially to final concentration of 1.3 g liter−1). Attempts to directly isolate mutants resistant to 1.0 g liter−1 furfural in a single step (solid medium and broth) were not successful. Stepwise improvement in furfural tolerance was observed during serial transfers, consistent with multiple changes. The resulting strain, EMFR9, grew and fermented xylose in the presence of 1.0 g liter−1 furfural at a rate equivalent to that of the parent, LY180, in the absence of furfural (Fig. 2). Growth and ethanol production by EMFR9 also exceeded that of the parent, LY180, in the absence of furfural.
FIG. 2.
Effect of furfural on the pH-controlled fermentation of 100 g liter−1 xylose. Fermentation with 0.4 g liter−1 furfural (A, B, and C). Fermentation with 1.0 g liter−1 furfural (D, E, and F). For clarity, data for EMFR9 and LY180 are connected by solid and broken lines, respectively. ▪, LY180 with furfural; ▴, EMFR9 with furfural; □, LY180 without furfural; ▵, EMFR9 without furfural.
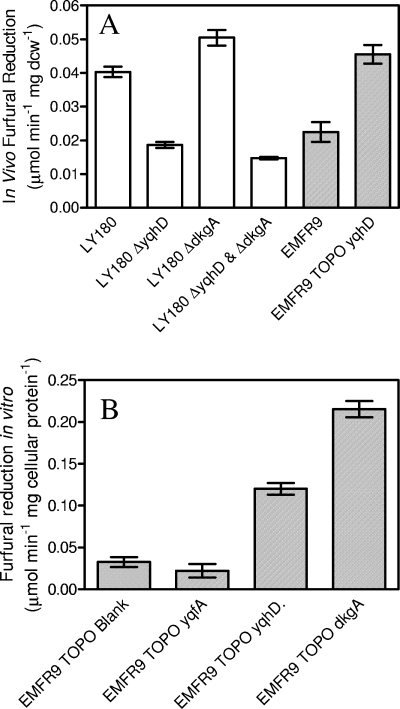
Addition of a low furfural concentration (0.4 g liter−1) to the parent, LY180, caused an initial lag in growth and ethanol production (Fig. 2A and B). During this lag, furfural was chemically reduced to the less toxic furfuryl alcohol (47, 49) (Fig. 2C). Growth and fermentation increased by more than threefold immediately following the complete removal of furfural. Growth and ethanol production by LY180 were strongly inhibited by 1.0 g liter−1 furfural throughout the 72-h incubation (Fig. 2D and E). During this time, approximately 20% of the furfural was reduced, indicating that LY180 remained metabolically active (Fig. 2F). In contrast to LY180, EMFR9 was virtually unaffected by the presence of furfural (0.4 g liter−1 or 1.0 g liter−1) (Fig. 2). The volumetric rate of furfural reduction was higher for EMFR9 than it was for LY180 at both furfural concentrations (Fig. 2C and F), primarily due to the larger amount of cell mass (Fig. 2A). This was confirmed by further experiments in which the in vivo rate of NADPH-dependent furfural reduction by EMFR9 (per mg dcw) was found to be about half that of the parent, LY180 (Fig. 3). In contrast to LY180, growth and fermentation of EMFR9 did not require prior reduction of furfural. With EMFR9, both 0.4 g liter−1 and 1.0 g liter−1 furfural were reduced to furfuryl alcohol concurrently with growth. Reduction by EMFR9 was complete after 12 h and 18 h, respectively (Fig. 2C and F).
FIG. 3.
Comparison of furfural-reducing activities. (A) In vivo activity of whole cells during fermentation. LY180 and deleted derivatives are shown as white bars. The furfural-resistant mutant EMFR9 and EMFR9(pLOI4301) expressing yqhD are shown as gray-shaded bars. (B) Comparison of in vitro furfural-reducing activities in cell extracts of EMFR9 harboring plasmids expressing cloned genes (forward direction, induced with 0.1 mM IPTG).
Together, these results suggest that the process of reducing furfural may be the basis for growth inhibition at low concentrations. The loss of function, i.e., a decrease in furfural-reducing activity, correlated with an increase in furfural tolerance in the mutant. Based on these results, we propose that the inhibition of growth by furfural results from competition between biosynthetic needs and furfural reduction for a limited pool of NADPH.
Effect of medium composition on furfural resistance (MIC).
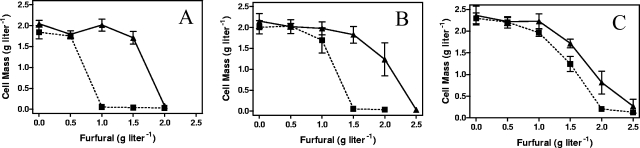
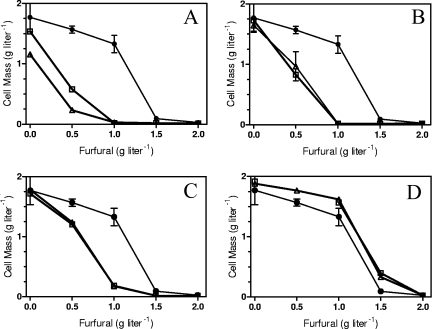
Unlike glucose, the production of NADPH is problematic during xylose fermentation (41) and offers an approach to test the NADPH competition hypothesis by measuring the MIC for furfural in different media. In mineral salts media with 50 g liter−1 xylose (Fig. 4A), the MIC of furfural was approximately 1.0 g liter−1 for LY180 (parent) and 2.0 g liter−1 for the mutant EMFR9. Replacement of xylose with glucose would be expected to increase the pool of NADPH. This change (Fig. 4B) increased the furfural MIC by 50% for LY180 (1.5 g liter−1) and by 25% for EMFR9 (2.5 g liter−1). Addition of a small amount of yeast extract (1.0 g liter−1) to xylose-mineral salts medium would be expected to decrease biosynthetic demands for NADPH. This supplement (Fig. 4C) doubled the furfural MIC for the parent, LY180 (2.0 g liter−1), and increased the MIC for EMFR9 (2.5 g liter−1) by 25%. With all media, EMFR9 was more resistant to furfural than was the parent, LY180. Both glucose (increased NADPH production) and yeast extract (decreased need for biosynthesis) increased furfural tolerance. However, this benefit was more pronounced for the parent, strain LY180, than it was for the mutant, EMFR9, consistent with the lower level of furfural reductase activity in EMFR9.
FIG. 4.
Effect of medium composition on furfural tolerance. (A) AM1 medium containing xylose (50 g liter−1). (B) AM1 medium containing glucose. (C) AM1 medium containing xylose and yeast extract. ▪, LY180 (dashed lines); ▴, EMFR9 (solid lines) after incubation for 48 h.
The MICs for three other compounds known to be present in hemicellulose hydrolysates were also examined: 2-hydroxymethyl furfural (analogue, dehydration product of hexose sugars), furfuryl alcohol (reduced product of furfural), and syringaldehyde (degradation product of lignin). EMFR9 was slightly more tolerant to 2-hydroxymethyl furfural (MIC of 3.0 g liter−1) than was LY180 (MIC of 2.5 g liter−1). Both strains were equally sensitive to syringaldehyde (MIC of 2.0 g liter−1) and furfuryl alcohol (15 g liter−1) (data not shown). The absence of an increase in tolerance to other compounds in EMFR9 is consistent with a specific site or target for furfural toxicity.
Comparison of oxidoreductase expression by mRNA microarray analysis.
Previous studies have demonstrated that E. coli contains an NADPH-dependent enzyme(s) capable of reducing furfural to a less toxic compound (furfuryl alcohol), but no gene was identified (7). The dependence of the parent, LY180, on the complete reduction of furfural prior to growth and the loss of this dependence by EMFR9 further implicate oxidoreductases as being of primary importance for furfural sensitivity.
Microarray analysis of mRNA was used to identify candidate oxidoreductase genes for furfural reduction. Cultures of LY180 and EMFR9 were grown to mid-log phase in pH-controlled fermentations with 100 g liter−1 (wt/vol) xylose. For this comparison, RNA was isolated 15 min after the addition of 0.5 g liter−1 furfural. A total of 12 known and putative oxidoreductases were found that differed by approximately twofold or higher (Table 2).
TABLE 2.
Oxidoreductase genes that were differentially expressed during growth in the presence of furfural (0.5 g liter−1)
| Gene | Accession no. | Fold increase or decrease | Effect of overexpression of cloned genes on MIC for furfural |
|---|---|---|---|
| ≥2-Fold greatera | |||
| yajO | b0419 | 1.9 | No increase |
| ydhU | b1670 | 1.8 | No increase |
| ydhV | b1673 | 2.0 | No increase |
| ygcW | b2774 | 2.1 | No increase |
| nemA | b1650 | 4.5 | No increase |
| yjgB | b4269 | 2.0 | No increase |
| ydhS | b1668 | 1.9 | No increase |
| ydhY | b1674 | 1.9 | No increase |
| ≥2-Fold lowerb | |||
| yqhD | b3011 | −48 | Reduced MIC |
| dkgA | b3012 | −12 | Reduced MIC |
| yjjN | b4358 | −4.4 | No effect on MIC |
| yqfA | b2899 | −2.5 | Reduced MIC |
Transcripts that were approximately ≥2-fold greater in EMFR9 relative to those in LY180. Effects on MIC shown are for expression in LY180.
Transcripts that were approximately ≥2-fold lower in EMFR9 relative to those in LY180. Effects on MIC shown are for expression in EMFR9.
Four oxidoreductases that were expressed at lower levels in EMFR9 were identified (Table 2). Each of these four genes was cloned into plasmids and transformed into EMFR9. When expressed from plasmids, three of these genes (dkgA, yqhD, and yqfA) were found to decrease furfural tolerance (Fig. 5). Expression of yqhD and dkgA were most detrimental and both were shown to increase furfural reductase activity in EMFR9 (Fig. 3B). Expression of yqfA did not restore the furfural reductase activity of EMFR9, and its effect on growth inhibition may be related to other functions. No detrimental effect on growth was observed for yjjN. Thus, the decrease in expression of yqhD, dkgA, and yqfA in EMFR9 can be inferred to be beneficial for furfural tolerance. Silencing of yqhD and dkgA in EMFR9 would decrease the competition with biosynthesis for NADPH during furfural reduction. Effects observed without induction can be attributed to leaky regulation with this high-copy vector (pCR 2.1 TOPO).
FIG. 5.
Effect of gene expression in EMFR9 on furfural tolerance. (A) Expression of dkgA. (B) Expression of yqhD. (C) Expression of yqfA. (D) Expression of yjjN. •, pCR 2.1 control without insert; □, uninduced expression; ▵, expression induced with 0.1 mM IPTG.
The other eight genes were cloned from LY180 into pCR 2.1 TOPO for expression. Eight of these oxidoreductases had increased expression in EMFR9 (1.8-fold to 4.5-fold) relative to that in the parent, LY180. Plasmids containing each of these genes were transformed into LY180. However, none of these eight caused an increase in furfural tolerance (data not shown).
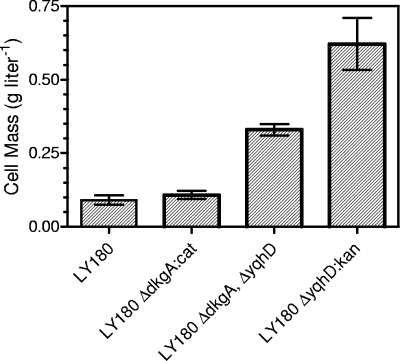
To further examine the potential importance of yqhD, dkgA, and yqfA silencing, attempts were made to delete each of these genes from LY180. Although deletions of both yqhD and dkgA were readily recovered, similar methods were not successful with yqfA. In LY180, deletion of yqhD alone or in combination with dkgA caused an increase in furfural tolerance (Fig. 6) and a decrease in furfural reductase activity in vivo, similar to that in EMFR9 (Fig. 3A). Since deletion of dkgA alone in LY180 did not lower the in vivo reductase activity or increase furfural tolerance, YqhD is presumed to be the more important activity for growth inhibition by low concentrations of furfural. The lowest furfural reductase activity was found after deletion of both genes (Fig. 3).
FIG. 6.
Effect of gene deletions in LY180 on growth in the presence of 1.0 g liter−1 furfural (48 h incubation).
Characterization of YqhD and DkgA.
The largest changes in gene expression among oxidoreductases were the silencing of yqhD and dkgA. Both YqhD and DkgA were expressed as His-tagged proteins in BL21(λDE3) and purified to discernible homogeneity. Both enzymes catalyzed the NADPH-dependent reduction of furfural to furfuryl alcohol. The apparent Km values for furfural were relatively high for YqhD (9.0 mM) and DkgA (>130 mM). With such values, it is unlikely that furfural is the native substrate for either enzyme. Assuming that cells are permeable to furfural, the intracellular activities of YqhD and DkgA would be expected to vary over the range of furfural concentrations used for selection (5 to 14 mM; 0.5 to 1.3 g liter−1). The apparent Km values for NADPH were quite low for both YqhD (8 μM) and DkgA (23 μM). In the presence of furfural, the high affinity of both enzymes for NADPH would compete effectively with biosynthetic reactions for NADPH. Partitioning of NADPH among pathways would be determined by the Km value for NADPH, the steady-state pool size of NADPH, and the relative abundances of competing oxidoreductase activities.
Tolerance to acid hydrolysate of hemicellulose.
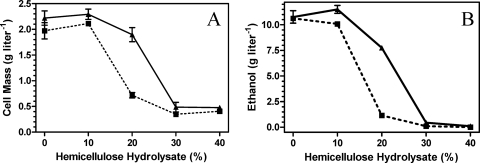
Hemicellulose hydrolysates contain a mixture of compounds that act in combination to inhibit microbial growth and fermentation (22, 23, 47-49). Growth and fermentation were examined in dilutions of a neutralized hydrolysate that contained 1.4 g liter−1 furfural (Fig. 7). Although the MICs for growth and ethanol production were similar (30% hydrolysate), EMFR9 grew to a threefold higher density and produced over 10-fold more ethanol in 20% hydrolysate than did the parent, LY180. Selection of EMFR9 for increased resistance to furfural was accompanied by an increase in resistance to hemicellulose hydrolysate, confirming the importance of furfural as a component of hydrolysate toxicity.
FIG. 7.
Effect of hemicellulose hydrolysate on growth (A) and ethanol production (B). ▪, LY180 (dashed line); ▴, EMFR9 (solid line) after incubation for 48 h.
DISCUSSION
Furfural is a natural product of lignocellulosic decomposition. Furfural is also formed by the dehydration of pentose sugars during the depolymerization of cellulosic biomass under acidic conditions (22). This compound is an important contributor to the toxicity of hemicellulose syrups and increases the toxicity of other compounds (47). Selection for a furfural-resistant mutant of E. coli during growth in xylose-mineral salts medium resulted in a strain (EMFR9) with improved resistance to hemicellulose hydrolysate, confirming the practical importance of this compound as the dominant inhibitor (21, 22).
The ability to reduce furfural into the less toxic furfuryl alcohol is widely distributed in nature (4). An enzyme has been purified from E. coli that catalyzes this reaction (7), and a gene that reduces the analogue 5-hydroxymethyl furfural has been identified in S. cerevisiae (2). The mRNA levels of oxidoreductases in the furfural-resistant mutant EMFR9 and those in the parent, LY180, were compared. Twelve differed by twofold or more. Of these, eight were higher in EMFR9, and four were lower in EMFR9. All were cloned and tested for their ability to either confer furfural tolerance in LY180 or decrease furfural tolerance in EMFR9. None of these gene products increased furfural tolerance when overexpressed from plasmids. Expression of three genes (yqhD, dkgA, and yqfA) decreased the furfural tolerance of EMFR9. Contrary to initial expectations that furfural tolerance would be improved by increased expression of reductase activity, these results demonstrated that the increased tolerance in EMFR9 results in large part from gene silencing (yqhD, dkgA) that decreased the level of NADPH-dependent furfural reductase activities. The deletion of yqhD encoding the lower Km oxidoreductase (NADPH) increased furfural tolerance in LY180, while the deletion of dkgA had no effect. No change in furfural reductase activity was detected from the overexpression of yqfA, and the role of this gene in furfural tolerance remains unknown. No mutation was found in these genes or the surrounding regions in EMFR9, and the mechanism of this gene silencing is unknown (under investigation).
The yqhD gene has been previously shown to encode an NADPH-dependent aldehyde oxidoreductase (37) that can be used for the production of propanediol (28, 50). This gene has also been shown to confer resistance to damage by reactive species of oxygen (33). The dkgA gene has been shown to catalyze the reduction of 2,5-diketo-d-gluconic acid, a key step in the production of ascorbic acid (8, 46). This enzyme is also thought to function in the reduction of methylglyoxal (14, 18). The function of the yqfA gene is unknown but is proposed to be a membrane subunit of an oxidoreductase (15), which may be involved in fatty acid metabolism (25).
Enzymes encoded by yqhD and dkgA were purified and demonstrated to have NADPH-dependent furfural reductase activities. Both YqhD and DkgA have low Km values for NADPH that would allow competition with biosynthetic reactions. Many key metabolic enzymes have a higher Km for NADPH than does YqhD (8 μM), including the following: CysJ (80 μM), which is required for sulfate assimilation to form cysteine and methionine; ThrA (90 μM), required for the formation of threonine; and DapB (17 μM), required for lysine formation (35). Competition between YqhD and biosynthetic enzymes for NADPH appears to be the primary basis for growth inhibition by furfural. Growth of the parent resumed upon complete reduction of added furfural. Replacing xylose with glucose and adding yeast extract to xylose medium would be expected to increase the availability of NADPH, and both changes increased furfural tolerance. Deleting yqhD and dkgA in the parent, LY180, increased furfural tolerance, but not to the full extent present in the mutant EMFR9. Additional mutations may also contribute to increased furfural tolerance in EMFR9.
In summary, these results show that the low concentration of furfural (up to about 1.5 g liter−1) found in hemicellulose hydrolysates of sugar cane bagasse is not inhibitory to the growth or fermentation of ethanologenic strain EMFR9. The observed growth inhibition of the parent, LY180, appears to be due to the diversion of NADPH away from biosynthesis by enzymes such as YqhD and DkgA.
Acknowledgments
We gratefully acknowledge research support by grants from the U.S. Department of Energy (DE-FG02-96ER20222, DE-FG36-08GO88142, and DE-FC36-GO17058) and by the Verenium Corporation. This work was facilitated by the BioCyc databases (15).
Footnotes
Published ahead of print on 8 May 2009.
REFERENCES
- 1.Almeida, J. R. M., T. Modig, A. Petersson, B. Hahn-Hagerdal, G. Liden, and M. F. Gorwa-Grauslund. 2007. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 82:340-349. [Google Scholar]
- 2.Almeida, J. R. M., A. Roder, T. Modig, B. Laddan, G. Lidén, and M. F. Gorwa-Grauslund. 2008. NADH- vs. NADPH-coupled reduction of 5-hydroxymethyl furfural (HMF) and its implications on product distribution in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 78:939-945. [DOI] [PubMed] [Google Scholar]
- 3.Barciszewski, J., G. E. Siboska, B. O. Pedersen, B. F. C. Clark, and S. I. S. Ratten. 1997. A mechanism for the in vivo formation of N-6-furfuryladenine, kinetin, as a secondary oxidative damage product of DNA. FEBS Lett. 414:457-460. [DOI] [PubMed] [Google Scholar]
- 4.Boopathy, R., H. Bokang, and L. Daniels. 1993. Biotransformation of furfural and 5-hydroxymethyl furfural by enteric bacteria. J. Ind. Microbiol. 11:147-150. [Google Scholar]
- 5.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorsich, S. W., B. S. Dien, N. N. Nichols, P. J. Slinger, Z. L. Liu, and C. D. Skory. 2006. Tolerance to furfural-induced stress is associated with pentose phosphate pathway genes ZWF1, GND1, RPE1, and TKL1, in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 71:339-349. [DOI] [PubMed] [Google Scholar]
- 7.Gutiérrez, T., L. O. Ingram, and J. F. Preston. 2006. Purification and characterization of a furfural reductase (FFR) from Escherichia coli strain LY01—an enzyme important in the detoxification of furfural during ethanol production. J. Biotechnol. 121:154-164. [DOI] [PubMed] [Google Scholar]
- 8.Habrych, M., S. Rodriguez, and J. Stewart. 2002. Purification and identification of an Escherichia coli beta-keto ester reductase as 2,5-diketo-d-gluconate reductase YqhE. Biotechnol. Prog. 18:257-261. [DOI] [PubMed] [Google Scholar]
- 9.Hahn-Hägerdal, B., M. Galbe, M. F. Gorwa-Grauslund, G. Liden, and G. Zacchi. 2006. Bio-ethanol—the fuel of tomorrow from the residues of today. Trends Biotechnol. 24:549-556. [DOI] [PubMed] [Google Scholar]
- 10.Horváth, I. S., M. J. Taherzadeh, C. Niklasson, and G. Liden. 2001. Effects of furfural on anaerobic continuous cultivation of Saccharomyces cerevisiae. Biotechnol. Bioeng. 75:540-549. [DOI] [PubMed] [Google Scholar]
- 11.Hristozova, T. S., A. Angelov, B. Tzvetkova, D. Paskaleva, V. Gotcheva, S. Gargova, and K. Pavlova. 2006. Effect of furfural on carbon metabolism key enzymes of lactose-assimilating yeasts. Enzyme Microbiol. Technol. 39:1108-1112. [Google Scholar]
- 12.Jantama, K., X. Zhang, J. C. Moore, K. T. Shanmugam, S. A. Svoronos, and L. O. Ingram. 2008. Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C. Biotechnol. Bioeng. 30:881-893. [DOI] [PubMed] [Google Scholar]
- 13.Jarboe, L. R., T. B. Grabar, L. P. Yomano, K. T. Shanmugan, and L. O. Ingram. 2007. Development of ethanologenic bacteria. Adv. Biochem. Eng. Biotechnol. 108:237-261. [DOI] [PubMed] [Google Scholar]
- 14.Jeudy, S., V. Monchois, C. Maza, J. M. Claverie, and C. Abergel. 2006. Crystal structure of Escherichia coli DkgA, a broad-specificity aldo-keto reductase. Proteins 62:302-307. [DOI] [PubMed] [Google Scholar]
- 15.Karp, P. D., I. M. Keseler, A. Shearer, M. Latendresse, M. Krummenacker, S. M. Paley, I. Paulsen, J. Collado-Vides, S. Gama-Castro, M. Peralta-Gil, A. Santos-Zavaleta, M. I. Penaloza-Spinola, C. Bonavides-Martinez, and J. Ingraham. 2007. Multidimensional annotation of the Escherichia coli K12 genome. Nucleic Acids Res. 35:7577-7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katahira, S., A. Mizuike, H. Fukuda, and A. Kondo. 2006. Ethanol fermentation from lignocellulosic hydrolysate by a recombinant xylose and cellooligosaccharide-assimilating yeast strain. Appl. Microbiol. Biotechnol. 72:1136-1143. [DOI] [PubMed] [Google Scholar]
- 17.Khan, Q. A., F. A. Shamsi, and S. M. Hadi. 1995. Mutagenicity of furfural in plasmid DNA. Cancer Lett. 89:95-99. [DOI] [PubMed] [Google Scholar]
- 18.Ko, J., I. Kim, S. Yoo, B. Min, K. Kim, and C. Park. 2005. Conversion of methylglyoxal to acetol by Escherichia coli aldo-keto reductases. J. Bacteriol. 187:5782-5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, Z. L., J. Moon, B. J. Andersh, P. J. Slininger, and S. Weber. 2008. Multiple gene-mediated NAD(P)H-dependent aldehyde reduction is a mechanism of in situ detoxification of furfural and 5-hydroxyfurfural by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 81:743-753. [DOI] [PubMed] [Google Scholar]
- 20.Martín, C., M. Marcet, O. Almazan, and L. J. Jonsson. 2007. Adaptation of a recombinant xylose-utilizing Saccharomyces cerevisiae strain to a sugarcane bagasse hydrolysate with high content of fermentation inhibitors. Bioresour. Technol. 98:1767-1773. [DOI] [PubMed] [Google Scholar]
- 21.Martinez, A., M. E. Rodriguez, S. W. York, J. F. Preston, and L. O. Ingram. 2000. Use of UV absorbance to monitor furans in dilute acid hydrolysates of biomass. Biotechnol. Prog. 16:637-641. [DOI] [PubMed] [Google Scholar]
- 22.Martinez, A., M. E. Rodriguez, M. L. Wells, S. W. York, J. F. Preston, and L. O. Ingram. 2001. Detoxification of dilute acid hydrolysates of lignocellulose with lime. Biotechnol. Prog. 17:287-293. [DOI] [PubMed] [Google Scholar]
- 23.Martinez, A., M. E. Rodriguez, S. W. York, J. F. Preston, and L. O. Ingram. 2000. Effects of Ca(OH)2 treatments (“overliming”) on the composition and toxicity of bagasse hemicellulose hydrolysates. Biotechnol. Bioeng. 69(5):526-536. [DOI] [PubMed] [Google Scholar]
- 24.Martinez, A., T. B. Grabar, K. T. Shanmugam, L. P. Yomano, S. W. York, and L. O. Ingram. 2007. Low salt medium for lactate and ethanol production by recombinant Escherichia coli B. Biotechnol. Lett. 29:397-404. [DOI] [PubMed] [Google Scholar]
- 25.McCue, L. A., W. Thompson, C. S. Carmack, M. P. Ryan, J. S. Liu, V. Derbyshire, and C. E. Lawrence. 2001. Phylogenetic footprinting of transcription factor binding sites in proteobacterial genomes. Nucleic Acids Res. 29:774-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Plainview, NY.
- 27.Modig, T., G. Liden, and M. J. Taherzadeh. 2002. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase, and pyruvate dehydrogenase. Biochem. J. 363:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura, C. E., and G. M. Whited. 2003. Metabolic engineering for the microbial production of 1,3-propandiol. Curr. Opin. Biotechnol. 14:454-459. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson, A., M. F. Gorwa-Grauslund, B. Hahn-Hagerdal, and G. Liden. 2005. Cofactor dependence in furan reduction by Saccharomyces cerevisiae in fermentation of acid-hydrolyzed lignocellulose. Appl. Environ. Microbiol. 71:7866-7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohta, K., D. S. Beall, K. T. Shanmugam, and L. O. Ingram. 1991. Genetic improvement of Escherichia coli for ethanol production: chromosomal integration of Zymomonas mobilis genes encoding pyruvate decarboxylase and alcohol dehydrogenase II. Appl. Environ. Microbiol. 57:893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmqvist, E., and B. Hahn-Hagerdal. 2000. Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour. Technol. 74:17-24. [Google Scholar]
- 32.Palmqvist, E., and B. Hahn-Hagerdal. 2000. Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour. Technol. 74:25-33. [Google Scholar]
- 33.Pérez, J. M., F. A. Arenas, G. A. Pradenas, J. M. Sandoval, and C. C. Vásquez. 2008. Escherichia coli YqhD exhibits aldehyde reductase activity and protects from the harmful effect of lipid peroxidation-derived aldehydes. J. Biol. Chem. 283:7346-7353. [DOI] [PubMed] [Google Scholar]
- 34.Petersson, A., J. R. M. Almeida, T. Modig, K. Karhumaa, B. Hahn-Hagerdal, M. F. Gorwa-Grauslund, and G. Liden. 2006. A 5-hydroxymethyl furfural reducing enzyme encoded by Saccharomyces cerevisiae ADH6 gene conveys HMF tolerance. Yeast 23:455-464. [DOI] [PubMed] [Google Scholar]
- 35.Schomburg, I., A. Chang, O. Hofmann, C. Ebeling, F. Ehrentreich, and D. Schomburg. 2002. BRENDA: a resource for enzyme data and metabolic information. Trends Biochem. Sci. 27:54-56. [DOI] [PubMed] [Google Scholar]
- 36.Singh, N. P., and A. Khan. 1995. Acetaldehyde: genotoxicity and cytotoxicity in human lymphocytes. Mutat. Res. 337:9-17. [DOI] [PubMed] [Google Scholar]
- 37.Sulzenbacher, G., K. Alvarez, R. Heuvel, C. Versluis, S. Spinelli, V. Campanacci, C. Valencia, C. Cambillau, H. Eklund, and M. Tegoni. 2004. Crystal structure of E. coli alcohol dehydrogenase YqhD: evidence of a covalently modified NADP coenzyme. J. Mol. Biol. 342:489-502. [DOI] [PubMed] [Google Scholar]
- 38.Talebnia, F., and M. J. Taherzadeh. 2006. In situ detoxification and continuous cultivation of dilute-acid hydrolysate to ethanol by encapsulated S. cerevisiae. J. Biotechnol. 125:377-384. [DOI] [PubMed] [Google Scholar]
- 39.Tokiwa, Y., and B. P. Calabia. 2008. Biological production of functional chemicals from renewable resources. Can. J. Chem. 86:548-555. [Google Scholar]
- 40.Um, B. H., M. N. Karim, and L. L. Henk. 2003. Effect of sulfuric and phosphoric acid pretreatments on enzymatic hydrolysis of corn stover. Appl. Biochem. Biotechnol. 105-108:115-125. [DOI] [PubMed] [Google Scholar]
- 41.White, D. 2000. The physiology and biochemistry of prokaryotes, 2nd edition. Oxford University Press, New York, NY.
- 42.Wyman, C. E., B. E. Dale, R. T. Elander, M. Holtzapple, M. R. Ladisch, and Y. Y. Lee. 2005. Coordinated development of leading biomass pretreatment technologies. Bioresour. Technol. 96:1959-1966. [DOI] [PubMed] [Google Scholar]
- 43.Wyman, C. E., B. E. Dale, R. T. Elander, M. Holtzapple, M. R. Ladisch, and Y. Y. Lee. 2005. Comparative sugar recovery data from laboratory scale application of leading pretreatment technologies to corn stover. Bioresour. Technol. 96:2026-2032. [DOI] [PubMed] [Google Scholar]
- 44.Yomano, L. P., S. W. York, K. T. Shanmugam, and L. O. Ingram. Deletion of methylglyoxal synthase gene (mgsA) increased sugar co-metabolism in ethanol-producing Escherichia coli. Biotechnol. Lett., in press. [DOI] [PMC free article] [PubMed]
- 45.Yomano, L. P., S. W. York, S. Zhou, K. T. Shanmugam, and L. O. Ingram. 2008. Re-engineering Escherichia coli for ethanol production. Biotechnol. Lett. 30:2097-2103. [DOI] [PubMed] [Google Scholar]
- 46.Yum, D., B. Lee, and J. Pan. 1999. Identification of the yqhE and yafB genes encoding two 2,5-diketo-d-gluconate reductases in Escherichia coli. Appl. Environ. Microbiol. 65:3341-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaldivar, J., A. Martinez, and L. O. Ingram. 1999. Effect of selected aldehydes on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol. Bioeng. 65:24-33. [DOI] [PubMed] [Google Scholar]
- 48.Zaldivar, J., and L. O. Ingram. 1999. Effect of organic acids on the growth and fermentation of ethanologenic Escherichia coli LY01. Biotechnol. Bioeng. 66:203-210. [DOI] [PubMed] [Google Scholar]
- 49.Zaldivar, J., A. Martinez, and L. O. Ingram. 2000. Effect of alcohol compounds found in hemicellulose hydrolysate on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol. Bioeng. 68:524-530. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, X. M., Y. Li, B. Zhuge, X. M. Tang, W. Shen, Z. M. Rao, H. Y. Fang, and J. Zhuge. 2006. Construction of a novel Escherichia coli strain capable of producing 1,3-propanediol and optimization of fermentation parameters by statistical design. World J. Microbiol. Biotechnol. 22:945-952. [Google Scholar]