Abstract
Listeria monocytogenes has a remarkable ability to survive and persist in food production environments. The purpose of the present study was to determine if cells in a population of L. monocytogenes differ in sensitivity to disinfection agents as this could be a factor explaining persistence of the bacterium. In situ analyses of Listeria monocytogenes single cells were performed during exposure to different concentrations of the disinfectant Incimaxx DES to study a possible population subdivision. Bacterial survival was quantified with plate counting and disinfection stress at the single-cell level by measuring intracellular pH (pHi) over time by fluorescence ratio imaging microscopy. pHi values were initially 7 to 7.5 and decreased in both attached and planktonic L. monocytogenes cells during exposure to sublethal and lethal concentrations of Incimaxx DES. The response of the bacterial population was homogenous; hence, subpopulations were not detected. However, pregrowth with NaCl protected the planktonic bacterial cells during disinfection with Incimaxx (0.0015%) since pHi was higher (6 to 6.5) for the bacterial population pregrown with NaCl than for cells grown without NaCl (pHi 5 to 5.5) (P < 0.05). The protective effect of NaCl was reflected by viable-cell counts at a higher concentration of Incimaxx (0.0031%), where the salt-grown population survived better than the population grown without NaCl (P < 0.05). NaCl protected attached cells through drying but not during disinfection. This study indicates that a population of L. monocytogenes cells, whether planktonic or attached, is homogenous with respect to sensitivity to an acidic disinfectant studied on the single-cell level. Hence a major subpopulation more tolerant to disinfectants, and hence more persistent, does not appear to be present.
Listeria monocytogenes is a food-borne, human pathogen that has a remarkable ability to colonize food-processing environments (5, 16, 20, 21, 26, 29). Some L. monocytogenes strains can persist for years in food-processing plants (11, 14, 20, 27), and specific molecular subtypes can repeatedly be isolated from the processing environment (29) despite being very infrequent in the outdoor environment (9). This ability to persist has, hitherto, not been linked to any specific genetic or phenotypic trait.
It has been suggested that persistent L. monocytogenes strains may be more tolerant or resistant to cleaning and especially disinfectants used in the food industry. Aase et al. (1) found increased tolerance to both benzalkonium chloride and ethidium bromide in L. monocytogenes isolates that had persisted for more than 4 years; however, other studies have not been able to link persistence and tolerance to disinfectants (6, 10, 11, 13). We recently compared disinfection sensitivities of persistent and presumed nonpersistent L. monocytogenes strains using viable-cell counts and did not find the latter group more sensitive to the two disinfectants Triquart SUPER and Incimaxx DES than persistent strains (13). However, we found that for all subtypes of L. monocytogenes, growth with NaCl increased the tolerance of planktonic L. monocytogenes cells to Incimaxx DES, whereas spot-inoculated, dried L. monocytogenes cells were not protected by NaCl against disinfection.
There is no doubt that L. monocytogenes will be completely inactivated at the disinfectant concentrations recommended for use in the food industry; however, the efficiency of the disinfectant is very much influenced by the presence of organic material being inactivated by the presence of food debris. Hence, it is likely that the bacterial cell in a food production environment may be exposed to concentrations at a sublethal level. It is currently not known if treatment with a sublethal concentration of disinfectant affects the entire bacterial population or only attacks a fraction of the cell population, leaving another fraction of cells unaffected. In case of the latter, some bacterial cells may be able to survive the disinfection treatment. The potential presence of such tolerant subpopulations could, ultimately, ensure that the genome is propagated, leading to persistence.
The presence of a more tolerant subpopulation can be determined on the single-cell level. Flow cytometry is a rapid method useable for measurements at the single-cell resolution (22); however, it cannot monitor the same single cells over time. Optical microscopy combined with microfluidic devices that allow measurement of growth of single cells is a useful technique (2), and in situ analyses of the physiological condition of single cells by the fluorescence ratio imaging microscopy (FRIM) technique represents another elegant approach (25). FRIM enables studies of dynamic changes with high sensitivity and on the single-cell level in important physiological parameters: e.g., intracellular pH (pHi). Listeria maintains its pHi within a narrow range of 7.6 to 8 at extracellular pH (pHex) values of 5.0 to 8.0 (4, 25) and at pHex 4.0 with the presence of glucose (23). It is believed that viable cells need to maintain a transmembrane pH gradient with their pHi above the pHex, and failure to maintain pHi homeostasis indicates that the bacterial cell is severely stressed and ultimately leads to loss of cell viability. FRIM has been used to determine the pHi of L. monocytogenes after exposure to osmotic and acid stress (7, 23). Also, the dissipation of the pH gradient in L. monocytogenes after exposure to different bacteriocins has been determined with FRIM (4, 12). Hornbæk et al. (12) found that treatment with subinhibitory concentrations of leucocin and nisin gave rise to two subpopulations: one consisting of cells with a dissipated pH gradient (ΔpH) and the other consisting of cells that maintained ΔpH, which could indicate phenotypic heterogeneity.
The aim of the present study was to investigate the physiological effects of the disinfectant Incimaxx DES at sublethal and lethal concentrations on single cells and the population level of a persistent L. monocytogenes strain to study a possible subdivision of sensitivity in the population. We also addressed the potential protective effect of NaCl against disinfection and compared sensitivities in a population of planktonic and attached bacteria. We applied the in situ technique FRIM and compared the pHi measurements with the traditional viable-cell-count method.
(Part of the results have been presented at a poster session at the 95th International Association for Food Protection annual meeting in Columbus, OH, 3 to 6 August 2008.)
MATERIALS AND METHODS
Bacterial strains and media.
Listeria monocytogenes strain N53-1 was isolated from equipment in a fish smokehouse (29). It belongs to the randomly amplified polymorphic DNA type 9 cluster, a molecular subtype that has been persistent for many years in several fish-processing industries (28). Stock cultures were stored at −80°C in 4% (wt/vol) glycerol, 3% (wt/vol) tryptone soya broth (TSB) (CM129; Oxoid, Basingstoke, United Kingdom), 2% (wt/vol) skim milk powder, and 0.5% (wt/vol) glucose. The bacteria were cultivated on brain heart infusion (BHI) agar (CM0225 supplemented with 1.5% agar; Oxoid, Basingstoke, United Kingdom) at 30°C and kept at 4°C for maximum of a month. Subsequent cultures were prepared in TSB supplemented with glucose to a final concentration of 1% (wt/vol), plus in some trials, NaCl was added to a final concentration of 5% (wt/wt). The bacteria were grown overnight at 30°C, diluted 1,000 times, and grown at 30°C for 22 h.
Fluorescence labeling of bacterial cells.
Fluorescent labeling of L. monocytogenes N53-1 with 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFDA-SE; Molecular Probes, Inc., Eugene, OR) was carried out as follows. Bacterial cells were harvested by centrifugation at 14,000 × g for 2 min and resuspended in sterile-filtered (pore size, 0.22 μm; GP Express Membrane Filter; Millipore, Bedford, Mass.) citric acid-phosphate buffer solution (pH 7.0) containing 0.37% (wt/vol) citric acid monohydrate and 2.93% (wt/vol) Na2HPO4 (19). Glucose and the fluorophor CFDA-SE were added to obtain final concentrations of 10 mM and 10 μM, respectively, and the cells were incubated at 30°C for 30 min. Cells were harvested by centrifugation at 14,000 × g for 2 min. For studies of planktonic bacteria, the pellet was resuspended and adjusted in 0.9% NaCl (wt/vol) with 10 mM glucose to an optical density at 600 nm of 0.4 to standardize the biomass. For studies of spot-inoculated and dried L. monocytogenes cells, the cells were resuspended and adjusted in sterile growth media to an optical density at 600 nm of 1.0. Bacteria were kept under these conditions for no longer than 2 h.
Immobilization chambers for planktonic bacteria.
Planktonic L. monocytogenes cells were immobilized on a glass surface essentially as described by Shabala et al. (24). Briefly, a glass coverslip was cleaned with a 70% ethanol-1% HCl mixture, rinsed with sterile Milli-Q water, and dried. Poly-l-lysine solution (0.1% [wt/vol] aqueous solution, P 8920; Sigma Diagnostics, St. Louis, MO) was used to attach cells to the glass surface. One drop (∼30 μl) of the poly-l-lysine solution was applied to the coverslip and left for about 5 min to dry. Subsequently, a CoverWell perfusion chamber gasket (C18128; Molecular Probes, Inc., Eugene, OR) was attached and the coverslip and chamber were mounted on a platform designed for the purpose.
Measurement of pHi of individual bacterial cells.
The pHi of individual L. monocytogenes N53-1 bacterial cells was measured by FRIM as described by Guldfeldt and Arneborg (8). This setup consisted of an inverted epifluorescence microscope (Axiovert 135 TV; Zeiss, Birkerød, Denmark) equipped with a Zeiss Fluar ×100 objective (numerical aperture, 1.3), a dichroic mirror (510 nm), and an emission band-pass filter (515 to 565 nm). Bacterial cells were excited at 488 and 435 nm with an exposure time of 1,000 ms by a Monochromator equipped with a 75-W xenon lamp (Monochromator B; TILL Photonics GmbH, Planegg, Germany). To minimize photobleaching of the stained cells, a 10% neutral-density filter was inserted between the optical fiber and the microscope for the experiments done with the planktonic bacteria, whereas a 2.5% neutral-density filter was used for the experiments with spot-inoculated and dried bacteria. Fluorescence emission was collected with a cool charge-coupled device camera (Coolsnap FX; Photometrics, Roper Scientific), and images were analyzed by using Metavue 6.1 software (Molecular Devices, Dowington, PA). Regions were defined around approximately 40 to 50 individual cells for each experiment by using Metavue. A region near but without the cell was subtracted for each cell as background. The ratio value (R488/435) for each cell examined was obtained by dividing the fluorescence intensity at 488 nm (pH-sensitive wavelength) by the fluorescence intensity at 435 nm (pH-insensitive wavelength). The ratio values were transformed to pHi values by using a calibration curve. To construct this, ethanol (70% [vol/vol]) was added to CFDA-SE-stained L. monocytogenes N53-1 cells for 5 min to permeabilize the membrane irreversibly. Subsequently, the bacterial cells were harvested by centrifugation at 14,000 × g for 2 min and resuspended in citric acid-phosphate buffers having pH values ranking from 5.0 to 7.7 (19). The calibration curve used for spot-inoculated, dried L. monocytogenes N53-1 cells was constructed for bacterium permeabilized and resuspended in TSB with pH values ranging from 5.0 to 9.5.
Preparation of disinfectant solutions.
The disinfectant Incimaxx DES (Ecolab Denmark ApS, Valby, Denmark), commonly used in the food industry as a disinfectant and for decalcification, contains a mixture of peroxy acids and hydrogen peroxide as active ingredients. Disinfectant solutions were prepared by dilution in sterilized, demineralized water to obtain concentrations where differences in pHi were seen. Disinfectant solutions were prepared at 10 times the strength of desired treatment right before use for testing planktonic bacteria and at the desired concentrations for disinfection of spot-inoculated bacteria.
Exposure of planktonic L. monocytogenes N53-1 cells to Incimaxx DES.
At time zero, 60 μl of a disinfection solution was mixed thoroughly with 540 μl of bacterial cell suspension. Seventy microliters was transferred to the CoverWell perfusion chamber gasket, and microscopic images were acquired every 2.5 min for 20 min in at least three defined positions. Sterile demineralized water was used as the control. Also, to determine the number of viable L. monocytogenes cells during disinfection, 40 μl of cell suspension was transferred to 160 μl Dey-Engly (DE) neutralizing broth (281910; Difco, BD, Sparks, France) every 5 min for 20 min. This was done in duplicate. Preliminary experiments showed that DE broth neutralized disinfectants also in higher concentrations of disinfectants than used and that viability of L. monocytogenes was unaffected when cells were suspended in DE broth (13). Bacterial numbers were determined by surface plating on BHI agar plates that were incubated at 37°C for 2 days. The complete experiment was repeated twice. Based on a set of preliminary experiments of disinfection sensitivity, L. monocytogenes cells were exposed to Incimaxx DES concentrations of 0.0062%, 0.0031%, and 0.0015% (vol/vol). To monitor the pHex in the bacterial disinfection mixture, pHs were determined every fifth minute for 20 min. Before each experiment, microscopic images of the bacterial cell suspension of L. monocytogenes N53-1 were acquired to obtain an initial (t = 0) pHi value.
Exposure of spot-inoculated and dried L. monocytogenes N53-1 cells to Incimaxx DES.
Sterile coverslips were placed on a wire screen in a laminar-flow biosafety cabinet. Twenty microliters of CFDA-stained L. monocytogenes N53-1 cells suspended in sterile growth media was deposited on each coupon and dried for 20 h in the laminar-flow biosafety cabinet. Spot-inoculated slips were covered with a CoverWell perfusion chamber gasket (C18120; Molecular Probes, Inc., Eugene, OR), and 320 μl Incimaxx DES was added. Sterilized demineralized water was used as the control. To monitor pHi during disinfection, microscopic images were acquired every 2.5 min for 20 min in three defined positions. To monitor the viability of spot-inoculated, dried bacteria, coverslips were disinfected in parallel in triple determinations for 20 min. After 20 min of incubation, the coupons were transferred with the disinfectant to 10 ml DE neutralizing broth, and bacteria were detached by sonication for 4 min (28-kHz, 2× 150-W sonication bath in a Delta 220; Deltasonic, Meaux, France) (15), vortexed at maximum speed for 15 s and diluted 10-fold serially. Also, three untreated coupons were transferred directly to DE neutralizer broth and sonicated. Cell numbers were determined by surface plating on BHI agar plates that were incubated at 37°C for 2 days. The experiment was repeated at least twice. L. monocytogenes was exposed to Incimaxx DES concentrations of 0.062%, 0.031%, and 0.015% (vol/vol). Before each experiment, microscopic images were acquired of a chamber with spot-inoculated and dried L. monocytogenes N53-1 cells with 0.9% NaCl added to obtain an initial pHi value. The pH values of the disinfectants were determined. This equals the pHex in the bacterial disinfection mixture, as the disinfectant volume used is 16 times the bacterial volume.
Statistical analysis.
Statistical comparisons were made between means of pHi or log10-transformed bacterial counts using Student's t test with a significance level of P < 0.05.
RESULTS
Construction of calibration curves.
Calibration curves (R488/435 versus pHi) were prepared using ethanol-treated cells of L. monocytogenes N53-1 suspended at different pH values in citric acid-phosphate buffer (planktonic bacteria) or TSB medium (attached bacteria) (Fig. 1). The two calibration curves differed at the low-ratio values and were more uniform at the high-ratio values. The probe is very pH sensitive at pH 6.0 to 9.0, while values below pH 6.0 can be difficult to distinguish (Fig. 1). Hence, ratio values less than 1.78 or 1.88 were recorded as pH 5.0 for planktonic and attached bacteria, respectively.
FIG. 1.
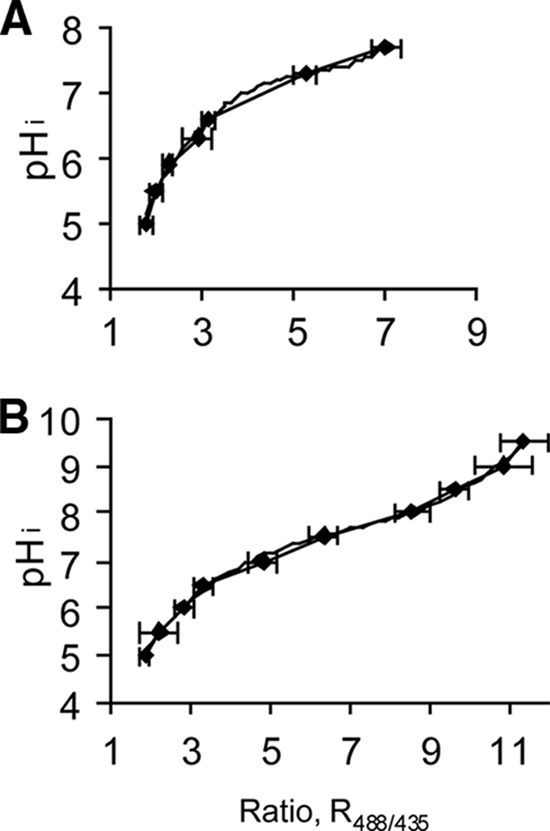
Relationship between R488/435 of the individual cells of Listeria monocytogenes N53-1 and pHi. The pHi was equilibrated to pHex by incubating preparations with 70% ethanol and dissolving them at different pH values in buffer (A) or TSB medium (B). The ratio values are averages based on at least 40 single cells. The error bars indicate standard deviations.
pHi of planktonic L. monocytogenes N53-1 exposed to Incimaxx DES.
The viable-cell count and pHi of planktonic L. monocytogenes N53-1 cells grown in TSB with 1% glucose were determined following 20 min of exposure to three different concentrations of Incimaxx DES or demineralized water as control (Fig. 2 and 3). pHi decreased during the 20-min treatment to pH 5 for all three concentrations of Incimaxx DES, indicating that the bacterial cells were stressed (Fig. 2). The rapidity in change in pHi differed between the three Incimaxx DES concentrations. pHi decreased to less than 5.5 in 5 min of treatment with a 0.0062% concentration. The change was slower but similar for concentrations of 0.0031% and 0.0015%, where pHi decreased to less than 5.5 in 10 min. Demineralized water caused only a minor decrease from pHi 7.33 ± 0.06 to pHi 6.75 ± 0.28 during the 20-min treatment. The extracellular pH in the bacterial disinfection solutions was 4.0 to 4.1 during 20 min with all three concentrations of Incimaxx DES, including water (data not shown). This indicates that the decrease in pHi during disinfection is due to Incimaxx DES and not just an effect of low external pH. The changes in pHi were similar in all cells measured, indicating that the response of the bacterial population was homogenous (Fig. 2). During the early treatment with Incimaxx DES, the pHi of the bacterial cells was distributed over 1 to 1.5 pH units; however, the pHi of the bacterial cells decreased to the same pHi ± 0.5 and did not split into subpopulations.
FIG. 2.
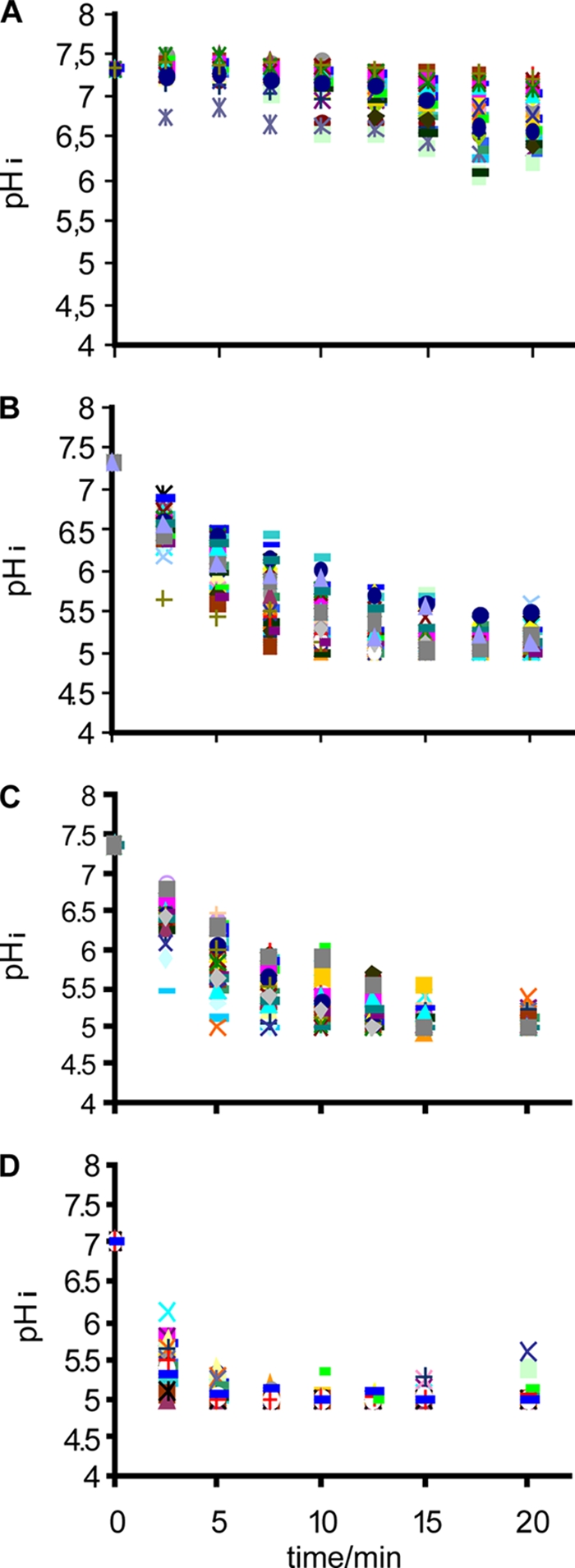
Change in the pHi of Listeria monocytogenes N53-1 grown in TSB with 1% glucose during disinfection with (A) water (control) or (B) 0.0015%, (C) 0.0031%, or (D) 0.0062% Incimaxx DES. Each point shows the pHi of a single cell.
FIG. 3.
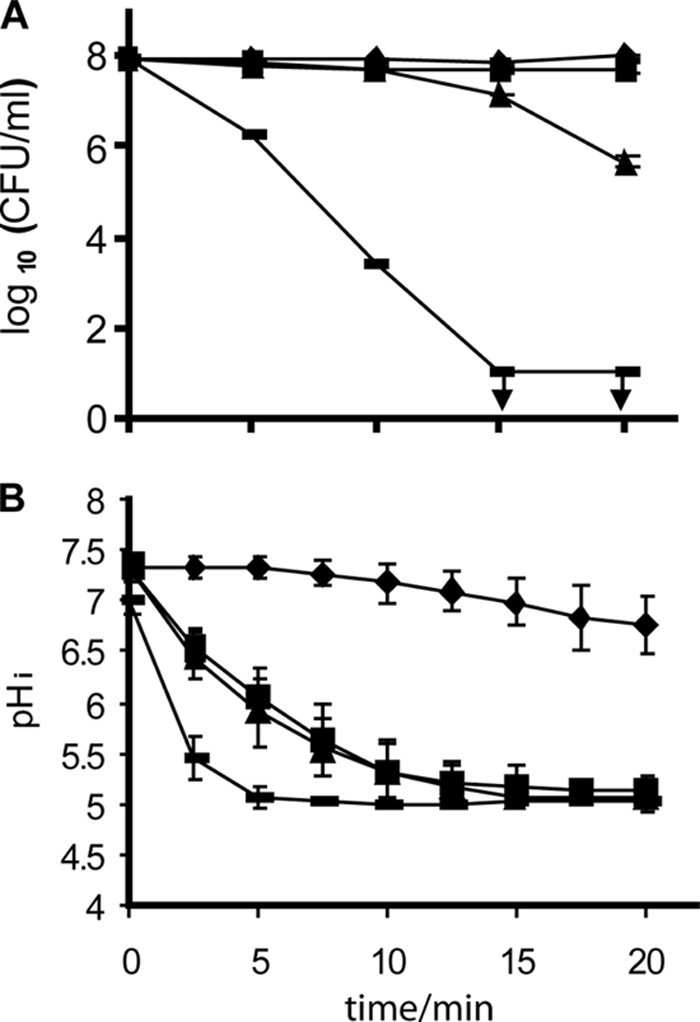
Viable-cell counts (A) and pHi (B) of Listeria monocytogenes N53-1 grown in TSB with 1% glucose and resuspended in 0.9% NaCl with 10 mM glucose during treatment with water (⧫) or 0.0015% (▪), 0.0031% (▴), or 0.0062% ( ) Incimaxx DES. The viable-cell counts are averages of duplicate determinations. Error bars are based on standard deviations from the duplicate determinations. pHis are averages based on at least 40 single cells from Fig. 2, and error bars indicate population variation. Arrows indicate that CFU were under the detection limit in the plating assay (10 CFU/ml). The results are representative of two independent experiments.
) Incimaxx DES. The viable-cell counts are averages of duplicate determinations. Error bars are based on standard deviations from the duplicate determinations. pHis are averages based on at least 40 single cells from Fig. 2, and error bars indicate population variation. Arrows indicate that CFU were under the detection limit in the plating assay (10 CFU/ml). The results are representative of two independent experiments.
The viable-cell counts remained constant over time when L. monocytogenes N53-1 was treated with water, indicating that the minor decrease from pHi 7.33 ± 0.06 to pHi 6.75 ± 0.28 did not affect viability (Fig. 3). Disinfection with 0.0015% Incimaxx DES caused only a minor decrease in CFU/ml after 20 min, and this decrease was not significantly different (P > 0.05) from the counts of bacteria treated with water. Hence, despite the low pHi of 5 to 5.5 caused by 0.0015% Incimaxx DES, the stressed bacterial cells were able to recover and grow on agar. A more pronounced reduction in viable cells of L. monocytogenes N53-1 was seen during treatment with 0.0031% and especially 0.0062% Incimaxx DES. The counts of bacteria treated with 0.0062% were already after 5 min significantly different (P < 0.05) from the counts of bacteria treated with water or 0.0015% Incimaxx DES.
The counts of L. monocytogenes N53-1 cells pregrown with 5% NaCl did not decrease (CFU/ml) when the bacteria were exposed to 0.0015% Incimaxx DES as compared to water. A slight reduction in viable-cell counts was seen for bacteria exposed for 20 min to 0.0031% Incimaxx (Fig. 3A and Fig. 4A). This protective effect by NaCl was reflected in the pHi of the bacterial cells, and the pHi of L. monocytogenes N53-1 remained at 6 to 6.5 when treated with Incimaxx DES at 0.0015%. In comparison, the pHi of 5.5 for bacteria grown without NaCl was significantly lower (P < 0.05). The responses of the bacterial population when grown with 5% NaCl were homogenous, and no subpopulations were detected (data not shown).
FIG. 4.
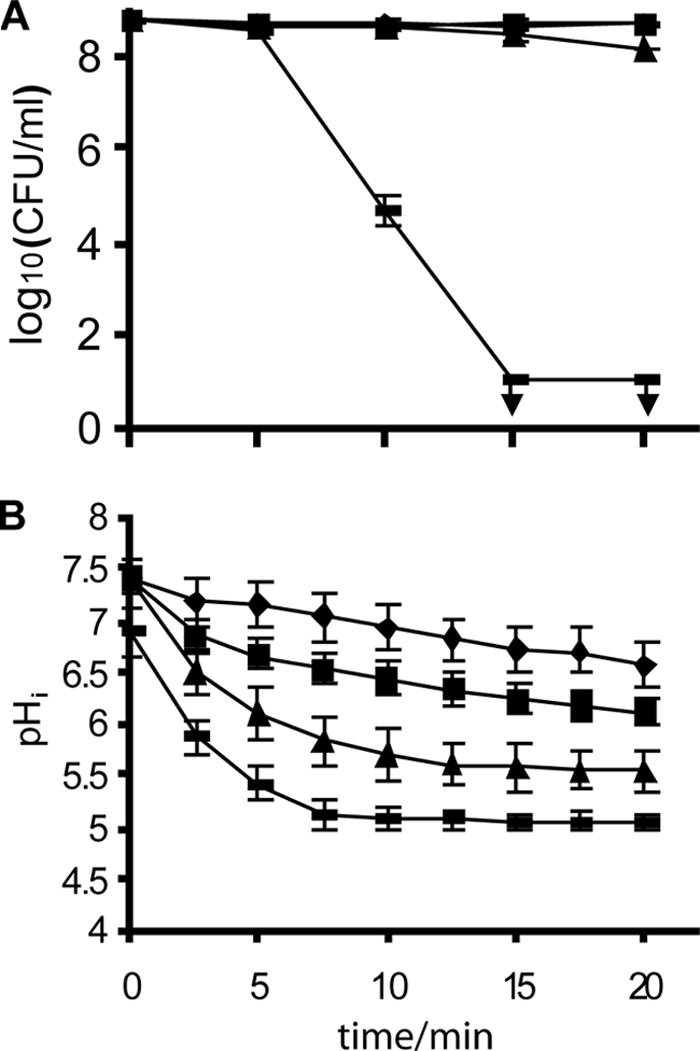
Viable-cell counts (A) and pHi (B) of Listeria monocytogenes N53-1 grown in TSB with 1% glucose and 5% NaCl and resuspended in 0.9% NaCl with 10 mM glucose during treatment with water (⧫) or 0.0015% (▪), 0.0031% (▴), or 0.0062% ( ) Incimaxx DES. The viable-cell counts are averages of duplicate determinations. Error bars are based on standard deviations from the duplicate determinations. pHis are averages based on at least 40 single cells, and error bars indicate population variation. Arrows indicate that CFU were under the detection limit in the plating assay (10 CFU/ml). The results are representative of two independent experiments.
) Incimaxx DES. The viable-cell counts are averages of duplicate determinations. Error bars are based on standard deviations from the duplicate determinations. pHis are averages based on at least 40 single cells, and error bars indicate population variation. Arrows indicate that CFU were under the detection limit in the plating assay (10 CFU/ml). The results are representative of two independent experiments.
pHi of spot-inoculated and dried L. monocytogenes N53-1 cells exposed to Incimaxx DES.
The response of a bacterial population of L. monocytogenes N53-1 cells grown in TSB with 1% glucose and spot inoculated was homogenous during treatment with Incimaxx DES (Fig. 5). However, the pHi of the population was distributed over a broader range of pH values over time compared to the planktonic population (Fig. 2). There was no indication of formation of subpopulations of cells with increased tolerance. pHi decreased rapidly to less than 5.5 after 5 min of treatment with 0.062% Incimaxx DES (Fig. 6B). Exposure to 0.031% and 0.015% Incimaxx DES caused the same decline in pHi from 7.0 to 5.7 during 20 min of treatment. Only a minor decrease in pHi from 7.0 to 6.5 was seen over time when cells were treated with demineralized water.
FIG. 5.
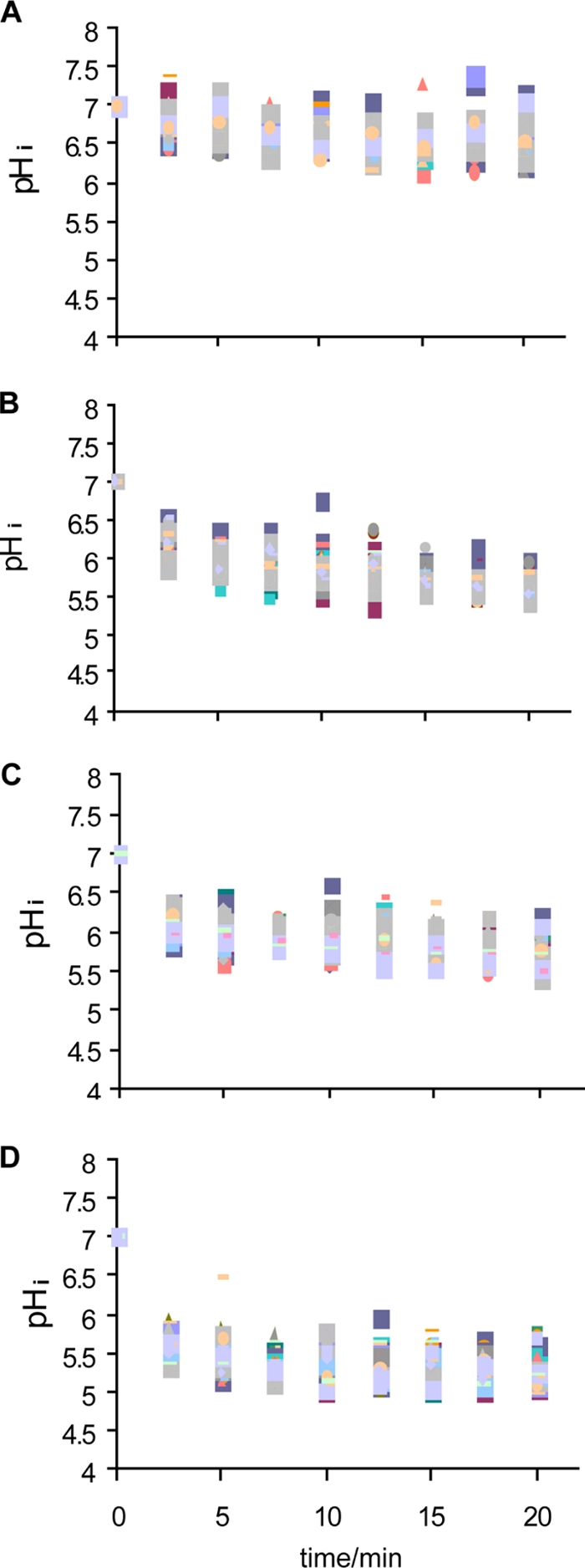
Change in the pHi of Listeria monocytogenes N53-1 grown, spot inoculated, and dried in TSB with 1% glucose during disinfection with water (control) (A) or 0.015% (B), 0.031% (C), or 0.062% (D) Incimaxx DES. Each point shows the pHi of a single cell.
FIG. 6.
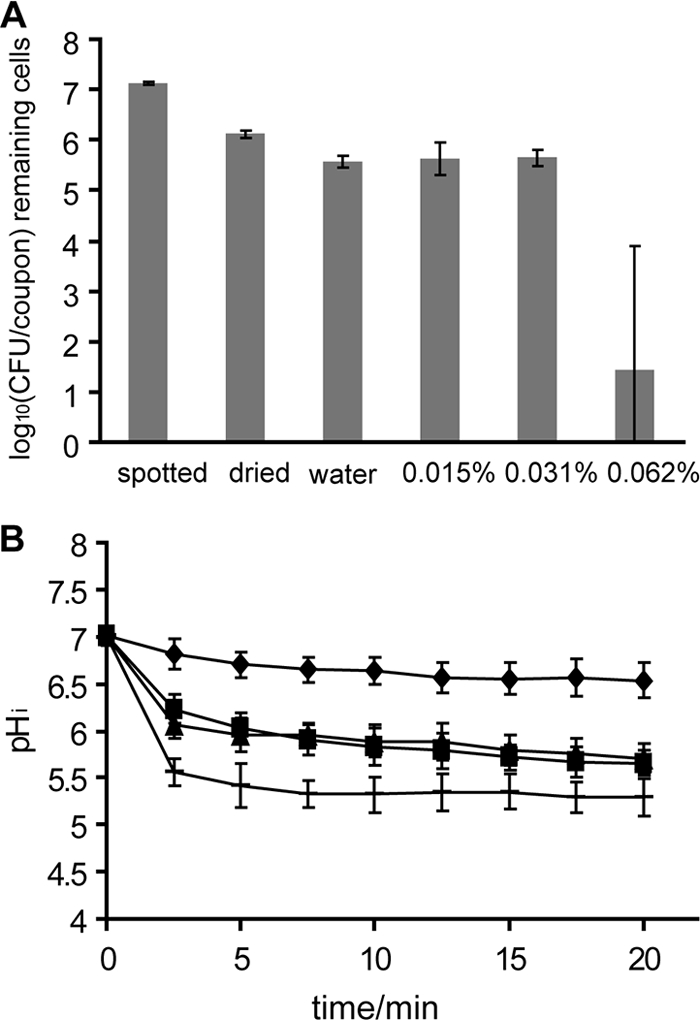
Viable-cell counts (A) and pHi (B) of Listeria monocytogenes N53-1 grown, spot inoculated, and dried in TSB with 1% glucose during treatment with water (⧫) or 0.015% (▪), 0.031% (▴), or 0.062% ( ) Incimaxx DES. The viable-cell counts are averages of triplicate determinations. Error bars are based on standard deviations from the triplicate determinations. pHis are averages based on at least 40 single cells, and error bars indicate population variation. The results are representative of two independent experiments.
) Incimaxx DES. The viable-cell counts are averages of triplicate determinations. Error bars are based on standard deviations from the triplicate determinations. pHis are averages based on at least 40 single cells, and error bars indicate population variation. The results are representative of two independent experiments.
The cell numbers of L. monocytogenes N53-1 on glass surfaces were equal after treatment with water or 0.015% or 0.031% Incimaxx DES. However, 0.062% Incimaxx DES caused a marked decrease in bacterial numbers (Fig. 6A).
Addition of NaCl to L. monocytogenes N53-1 caused more cells to survive the drying process, since the numbers of CFU/coupon were significantly higher (P < 0.05) than those of L. monocytogenes N53-1 grown, spot-inoculated, and dried in TSB with 1% glucose (Fig. 6A and Fig. 7A). This indicates that NaCl protects the bacterial cells during drying. Also, this protective effect could be seen from the pHi measurement during treatment with water, since the pHi of L. monocytogenes N53-1 grown and spotted with 5% NaCl was 7.1 after 20 min, while it was 6.5 for L. monocytogenes N53-1 grown and spotted without NaCl. This indicates that preculture with NaCl enable the bacterial cells to keep the pHi more constant in water. However, when disinfecting L. monocytogenes N53-1 cells dried with 5% NaCl, the number of remaining bacterial cells decreased as rapidly as the non-NaCl-grown cells with increasing concentrations of Incimaxx DES, indicating that preculture and spotting with NaCl render the attached, dried bacterial cells more sensitive than or as sensitive to Incimaxx DES as N53-1 cells grown and spotted without NaCl.
FIG. 7.
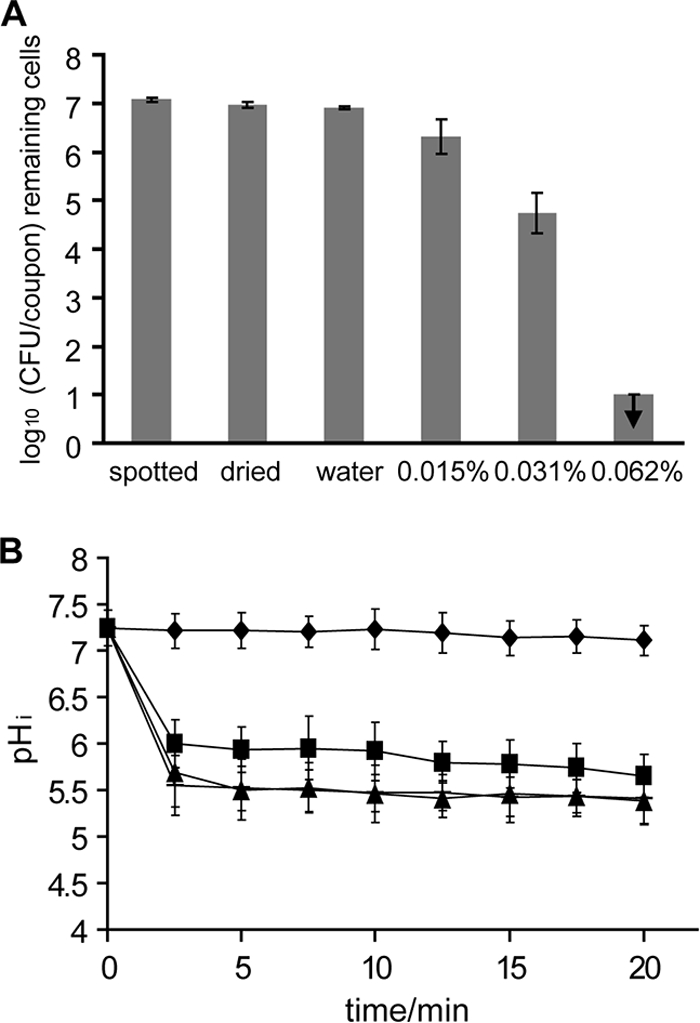
Viable-cell counts (A) and pHi (B) of Listeria monocytogenes N53-1 grown, spot inoculated, and dried in TSB with 1% glucose and 5% NaCl during treatment with water (⧫) or 0.015% (▪), 0.031% (▴), or 0.062% ( ) Incimaxx DES. The viable-cell counts are averages of triplicate determinations. Error bars are based on standard deviations from the triplicate determinations. pHis are averages based on at least 40 single cells, and error bars indicate population variation. Arrows indicate that CFU were under the detection limit in the plating assay (100 CFU/coupon). The results are representative of two independent experiments.
) Incimaxx DES. The viable-cell counts are averages of triplicate determinations. Error bars are based on standard deviations from the triplicate determinations. pHis are averages based on at least 40 single cells, and error bars indicate population variation. Arrows indicate that CFU were under the detection limit in the plating assay (100 CFU/coupon). The results are representative of two independent experiments.
DISCUSSION
Some subtypes of L. monocytogenes are able to persist in food-processing plants for many years (11, 14, 20, 27, 29), and this is a major food safety issue as they constitute a constant reservoir of food product contamination. The seriousness of this is highlighted by a recent (summer 2008) outbreak of listeriosis in Canada that has been caused by meat products contaminated with L. monocytogenes on two processing lines in a meat factory. The ability to persist has, hitherto, not been linked to any specific genetic or phenotypic trait. It has been hypothesized that persistent L. monocytogenes strains were less susceptible to disinfection than nonpersistent strains; however, most studies have not been able to confirm this hypothesis (6, 10, 11, 13). However, the persistent strains may reside in the processing environment due to the constant maintained tolerant subpopulation, and such heterogeneity at the single-cell level would be masked in a study of a sensitivity and killing kinetics in the complete bacterial population. In the present study, we have for the first time used FRIM to study the physiological effect of disinfectants at the single-cell level by measuring pHi. We did not, however, find an indication that sublethal concentrations of the disinfectant studied caused division of the population in subpopulations.
As mentioned, the present study is to our knowledge the first study using measurement of pHi to detect the appearance of subpopulations during disinfection. The technique detected the single-cell physiological response to the stress factor at concentrations where the determination of bacterial density (CFU/ml or CFU/coupon) did not reveal any effects. Other studies have monitored pHi over time for exponential- and stationary-phase cells of L. monocytogenes during exposure to benzalkonium chloride (17). However, in these studies, the pHi was measured on a population basis using spectrophotometry and single cells could not be monitored. In the present study, we did not detect any more tolerant subpopulation, neither in the planktonic nor in the attached population, since the decrease in pHi was very homogenous for all cells over time. Earlier, the FRIM method had successfully been used to detect subpopulations in L. monocytogenes cultures exposed to bacteriocins (12). Similarly, L. monocytogenes cells previously grown on agar plates were heterogeneous with respect to sensitivity to nisin when measuring pHi, and some of the cells appeared to be more tolerant than others (4).
A pHi value of 5.0 to 5.5 is the lower limit of the sensitivity for CFDA (3), which was also seen from the calibration curves. As the external pH is around 4, the pHi measurement can be used for information on stress conditions of the cells but not on viability. Another dye with higher sensitivity at lower pH could have been used instead. However, the low-pH-sensitive dye would not cover the neutral-pH range, leading to loss of information. Instead we combined the pHi measurement with viability measured as CFU on agar plates.
We found that growth with a typical food component, NaCl, protected the bacteria against drying and disinfection. When using pHi as a measure of bacterial response, the protective effect was seen at treatments with 0.0015% Incimaxx, whereas it was reflected by viable-cell counts at a higher concentration of Incimaxx (0.0031%), in accordance with earlier results (13). As pHi decreased very rapidly to below 5.5 during disinfection with 0.0031% Incimaxx DES, one may speculate if using a probe more sensitive in the lower-pH area could have indicated a protective effect of NaCl. Bacteria that were spot inoculated and dried on surfaces were only protected by NaCl during the drying stage but not when exposed to disinfectants. This is in agreement with our previous data using colony counts to measure the effect of disinfectants (13). A higher concentration of Incimaxx DES was needed to eliminate attached L. monocytogenes cells compared to planktonic bacteria cells. However, this is more likely due to the higher biological load introduced by the setup than to increased tolerance of the attached bacteria per se.
In the present study, L. monocytogenes survived a pHi of <5.5 for more than 10 min as planktonic cells during disinfection with 0.0015% Incimaxx DES, and cell viability was not significantly different from the viability in water. Shabala et al. (23) measured a pHi of ≤5 after 2 h for L. monocytogenes maintained at pHex 3.0, and the cells remained viable as the organism recovered immediately and remained constant at pHi 7.3, when returning to pHex 6.0. Hence, the ability of this organism to sustain a low pHi, even though it is critical for many cellular processes, such as DNA transcription, protein synthesis, and enzyme activities, may contribute to the survival of L. monocytogenes in acidified environments.
pHi was a more sensitive measure of adverse effects on L. monocytogenes than viability (CFU). Thus, a concentration of Incimaxx DES (0.0015%) that did not significantly affect cell counts had a marked effect on pHi (Fig. 3). Similarly, Luppens et al. (18) found that the ability to maintain a pH gradient was largely affected by benzalkonium chloride and hydrogen peroxide before a major loss in viability (according to plate counts) was detected. This indicates that antibacterial components may clearly stress bacterial cells even at levels where no effect is seen on viable-cell counts. Measurements of pHi allow an online indication of the physiological status of bacterial cells and can be used to monitor both individual bacterial cells and a population of bacteria. Due to the high sensitivity of the pHi measurement, it is useful for determination of subinhibitory concentrations of disinfectants: i.e., concentrations that do stress the bacteria by decreasing pHi but do not affect viability. This is highly useful for further studies of the impact of subinhibitory stress on L. monocytogenes in relation to, for example, gene expression and virulence. Furthermore, the method may be useful for studying subinhibitory concentrations of antibiotics.
In conclusion, we have shown that the response of a persistent strain of L. monocytogenes, whether planktonic or attached, is homogenous on the single-cell level to an acidic disinfectant; hence, subpopulations do not appear. It is not likely that the persistent strain of L. monocytogenes survives in the production environment due to presence of a more tolerant subpopulation. The pHi measurement is useful for determination of subinhibitory concentrations of disinfectants and is relevant for further studies of the impact of subinhibitory stress on L. monocytogenes.
Acknowledgments
The work was financed by the European Commission within the VI Framework Program, contract no. 007081, “Pathogen combat: control and prevention of emerging and future pathogens at cellular and molecular level throughout the food chain.”
We thank Søs Inger Nielsen for excellent technical assistance.
Footnotes
Published ahead of print on 1 May 2009.
REFERENCES
- 1.Aase, B., G. Sundheim, S. Langsrud, and L. M. Rørvik. 2000. Occurrence of and a possible mechanism for resistance to a quaternary ammonium compound in Listeria monocytogenes. Int. J. Food Microbiol. 62:57-63. [DOI] [PubMed] [Google Scholar]
- 2.Balaban, N. Q., J. Merrin, R. Chait, L. Kowalik, and S. Leibler. 2004. Bacterial persistence as a phenotypic switch. Science 305:1622-1625. [DOI] [PubMed] [Google Scholar]
- 3.Breeuwer, P., J.-L. Drocourt, F. M. Rombouts, and T. Abee. 1996. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-)-carboxyfluorescein succinimidyl ester. Appl. Environ. Microbiol. 62:178-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budde, B. B., and M. Jakobsen. 2000. Real-time measurements of the interaction between single cells of Listeria monocytogenes and nisin on a solid surface. Appl. Environ. Microbiol. 66:3586-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chasseignaux, E., M.-T. Toquin, C. Ragimbeau, G. Salvat, P. Colin, and G. Ermel. 2001. Molecular epidemiology of Listeria monocytogenes isolates collected from the environment, raw meat and raw products in two poultry- and pork-processing plants. J. Appl. Microbiol. 91:888-899. [DOI] [PubMed] [Google Scholar]
- 6.Earnshaw, A. M., and L. M. Lawrence. 1998. Sensitivity to commercial disinfectants, and the occurrence of plasmids within various Listeria monocytogenes genotypes isolated from poultry products and the poultry processing environment. J. Appl. Microbiol. 84:642-648. [DOI] [PubMed] [Google Scholar]
- 7.Fang, W., H. Siegumfeldt, B. B. Budde, and M. Jakobsen. 2004. Osmotic stress leads to decreased intracellular pH of Listeria monocytogenes as determined by fluorescence ratio-imaging microscopy. Appl. Environ. Microbiol. 70:3176-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guldfeldt, L. U., and N. Arneborg. 1998. Measurement of the effects of acetic acid and extracellular pH on intracellular pH of nonfermenting, individual Saccharomyces cerevisiae cells by fluorescence microscopy. Appl. Environ. Microbiol. 64:530-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen, C. H., B. F. Vogel, and L. Gram. 2006. Prevalence and survival of Listeria monocytogenes in Danish aquatic and fish-processing environments. J. Food Prot. 69:2113-2122. [DOI] [PubMed] [Google Scholar]
- 10.Heir, E., B. A. Lindstedt, O. J. Rotterud, T. Vardund, G. Kapperud, and T. Nesbakken. 2004. Molecular epidemiology and disinfectant susceptibility of Listeria monocytogenes from meat processing plants and human infections. Int. J. Food Microbiol. 96:85-96. [DOI] [PubMed] [Google Scholar]
- 11.Holah, J. T., J. H. Taylor, D. J. Dawson, and K. E. Hall. 2002. Biocide use in the food industry and the disinfectant resistance of persistent strains of Listeria monocytogenes and Escherichia coli. J. Appl. Microbiol. 92:111S-120S. [PubMed] [Google Scholar]
- 12.Hornbæk, T., P. B. Brockhoff, H. Siegumfeldt, and B. B. Budde. 2006. Two subpopulations of Listeria monocytogenes occur at subinhibitory concentrations of leucocin 4010 and nisin. Appl. Environ. Microbiol. 72:1631-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kastbjerg, V., and L. Gram. 2009. Model systems allowing quantification of sensitivity to disinfectants and comparison of disinfection susceptibility of persistent and presumed non-persistent strains of Listeria monocytogenes. J. Appl. Microbiol. 106:1667-1681. [DOI] [PubMed] [Google Scholar]
- 14.Keto-Timonen, R., R. Tolvanen, J. Lundén, and H. Korkeala. 2007. An 8-year surveillance of the diversity and persistence of Listeria monocytogenes in a chilled food processing plant analyzed by amplified fragment length polymorphism. J. Food Prot. 70:1866-1873. [DOI] [PubMed] [Google Scholar]
- 15.Leriche, V., and B. Carpentier. 1995. Viable but nonculturable Salmonella Typhimurium in single-species and binary-species biofilms in response to chlorine treatment. J. Food Prot. 58:1186-1191. [DOI] [PubMed] [Google Scholar]
- 16.Lundén, J. M., T. J. Autio, A.-M. Sjöberg, and H. J. Korkeala. 2003. Persistent and nonpersistent Listeria monocytogenes contamination in meat and poultry processing plants. J. Food Prot. 66:2062-2069. [DOI] [PubMed] [Google Scholar]
- 17.Luppens, S. B. I., T. Abee, and J. Oosterom. 2001. Effect of benzalkonium chloride on viability and energy metabolism in exponential- and stationary-growth-phase cells of Listeria monocytogenes. J. Food Prot. 64:476-482. [DOI] [PubMed] [Google Scholar]
- 18.Luppens, S. B. I., B. Barbaras, P. Breeuwer, F. M. Rombouts, and T. Abee. 2003. Selction of fluorescent probes for flow cytometric viability assessment of Listeria monocytogens exposed to membrane-active and oxidizing disinfectants. J. Food Prot. 66:1393-1401. [DOI] [PubMed] [Google Scholar]
- 19.McIlvaine, T. C. 1921. A buffer solution for colorimetric comparison. J. Biol. Chem. 49:183-186. [Google Scholar]
- 20.Miettinen, M. K., K. J. Björkroth, and H. J. Korkeala. 1999. Characterization of Listeria monocytogenes from an ice cream plant by serotyping and pulsed-field gel electrophoresis. Int. J. Food Microbiol. 46:187-192. [DOI] [PubMed] [Google Scholar]
- 21.Norton, D. M., M. A. McCamey, K. L. Gall, J. M. Scarlett, K. J. Boor, and M. Wiedmann. 2001. Molecular studies on the ecology of Listeria monocytogenes in the smoked fish processing industry. Appl. Environ. Microbiol. 67:198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roostalu, J., A. Joers, H. Luidalepp, N. Kaldalu, and T. Tenson. 2008. Cell division in Escherichia coli cultures monitored at single cell resolution. BMC Microbiol. 8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shabala, L., B. B. Budde, T. Ross, H. Siegumfeldt, and T. McMeekin. 2002. Responses of Listeria monocytogenes to acid stress and glucose availability monitored by measurements of intracellular pH and viable counts. Int. J. Food Microbiol. 75:89-97. [DOI] [PubMed] [Google Scholar]
- 24.Shabala, L., T. Ross, I. Newmann, T. McMeekin, and S. Shabala. 2001. Measurements of net fluxes and extracellular changes of H+, Ca2+, K+, and NH4+ in Escherichia coli using ion-selective microelectrodes. J. Microbiol. Methods 46:119-129. [DOI] [PubMed] [Google Scholar]
- 25.Siegumfeldt, H., K. B. Rechinger, and M. Jakobsen. 1999. Use of fluorescence ratio imaging for intracellular pH determination of individual cells in mixed cultures. Microbiology 145:1703-1709. [DOI] [PubMed] [Google Scholar]
- 26.Unnerstad, H., E. Bannerman, J. Bille, M.-L. Danielsson-Tham, E. Waak, and W. Tham. 1996. Prolonged contamination of a dairy with Listeria monocytogenes. Neth. Milk Dairy J. 50:493-499. [Google Scholar]
- 27.Vogel, B. F., H. H. Huss, B. Ojeniyi, P. Ahrens, and L. Gram. 2001. Elucidation of Listeria monocytogenes contamination routes in cold-smoked salmon processing plants detected by DNA-based typing methods. Appl. Environ. Microbiol. 67:2586-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogel, B. F., L. V. Jørgensen, B. Ojeniyi, H. H. Huss, and L. Gram. 2001. Diversity of Listeria monocytogenes isolates from cold-smoked salmon produced in different smokehouses as assessed by random amplified polymorphic DNA analyses. Int. J. Food Microbiol. 65:83-92. [DOI] [PubMed] [Google Scholar]
- 29.Wulff, G., L. Gram, P. Ahrens, and B. F. Vogel. 2006. One group of genetically similar Listeria monocytogenes strains frequently dominates and persists in several fish slaughter- and smokehouses. Appl. Environ. Microbiol. 72:4313-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]


