Abstract
Transposon mutagenesis of Pseudomonas syringae Lz4W, a psychrophilic bacterium capable of growing at temperatures between 2 and 30°C, yielded 30 cold-sensitive mutants, and CSM1, one of these cold-sensitive mutants, was characterized. Growth of CSM1 was retarded when it was cultured at 4°C but not when it was cultured at 22°C and 28°C compared to the growth of wild-type cells, indicating that CSM1 is a cold-sensitive mutant of P. syringae Lz4W. The mutated gene in CSM1 was identified as trmE (coding for tRNA modification GTPase), and evidence is provided that this gene is induced at low temperatures. Further, the cold-inducible nature of the trmE promoter was demonstrated. In addition, the transcription start site and the various regulatory elements of the trmE promoter, such as the −10 region, −35 region, UP element, cold box, and DEAD box, were identified, and the importance of these regulatory elements in promoter activity were confirmed. The importance of trmE in rapid adaptation to growth at low temperatures was further highlighted by plasmid-mediated complementation that alleviated the cold-sensitive phenotype of CSM1.
Psychrophilic bacteria (57) constitute a sizeable proportion of bacterial diversity because a large proportion of Earth's biosphere (75%) is either transiently or permanently cold (temperature, <5°C) (2). Studies have indicated that psychrophiles adapt to low temperatures by being able to sense changes in temperature (41, 48, 59), by modulating membrane fluidity (11-13, 28, 29), and because they possess enzymes and genes which are active at low temperatures (8, 10, 19, 35, 50, 60, 64). In psychrophilic bacteria pnp (encoding polynucleotide phosphorylase) (23), oppA (mediation of the transport of oligonucleotides) (5), and recD (51) have been identified as genes required for low-temperature growth. In contrast, in mesophilic bacteria many genes are induced following a downshift in temperature; these genes include genes for fatty acid desaturases and other enzymes (26, 32, 62), cold shock genes (33, 47), and genes involved in replication transcription and translation (3, 9, 26, 33, 69). The question is whether such genes are induced in psychrophiles, which, unlike mesophiles, are not cold stressed but are cold adapted. The present study investigated the role of trmE in low-temperature growth.
MATERIALS AND METHODS
Generation of cold-sensitive mutants.
Psychrophilic Pseudomonas syringae Lz4W (referred to as P.syringae below) and Escherichia coli strains DH-5α and S-17-1 (Table 1) were grown in Antarctic bacterial medium (58) or Luria-Bertani medium (66). P. syringae was mutagenized with a Tn5 transposon-based suicide plasmid vector (pOT182) (35, 42), and cold-sensitive mutants were identified based on their inability to grow or their delayed growth on plates incubated at 4°C for 1 week. Growth characteristics were also analyzed using a UV-visible spectrophotometer (Shimadzu, Kyoto, Japan) (36, 37).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli DH5α | supE44 ΔlacU169 (φ80lacZM15) hsdR17 recA endA1 gyrA96 thi-1 relA1 | Laboratory stock |
| Escherichia coli S-17-1 | RP4-2Tc::Mu-Kn::Tn7 pro hsdR recA | 61 |
| Pseudomonas syringae Lz4W | Wild type, Antarctic isolate | 58 |
| Plasmids | ||
| pMOSBlue | Cloning vector, Ampr | Amersham Life Sciences (Buckinghamshire, United Kingdom) |
| pGL10 | Broad-host-range cloning vector, IncP replicon, mob+ Kanr | 3 |
| pKZ27 | lacZ transcription fusion vector, KanrincQ oriT | 68 |
Identification of the disrupted gene.
Southern analysis using genomic DNA of CSM1 (one of the cold-sensitive mutants) and α-32P-labeled pOT182 (35, 36) showed that CSM1 produced two bands, confirming that a single copy of the transposon was integrated (data not shown). The disrupted gene was identified by inverse PCR (46) using 35 cycles and transposon-specific outward primers (primers Ash 1 and Ash 3 for upstream regions and primers Ash 1 and Ash 2 for downstream regions) (Table 2). PCR-amplified products were purified and sequenced with a ABI Prism 3700 DNA sequencer (Applied Biosystems, California) (12, 13, 36, 37) using Ash 1 and Ash 3.
TABLE 2.
Primers used in this studya
| Primer | Oligonucleotide sequence | Gene |
|---|---|---|
| Ash 1 | 5′CTGCAAGGCGATTAAGTTGGG3′ | Transposon |
| Ash 2 | 5′CCATGTTAGGAGGTCACATGG3′ | Transposon |
| Ash 3 | 5′CCTGATGCAGTAATCCTACGG3′ | Transposon |
| Ash 5 | 5′CAATCGCAGCTTCGACGTAG3′ | trmE |
| Ash 44 | 5′CCGGAATTCATGAGTGTTGCTGCTGAA3′ | trmE |
| Ash 47 | 5′CCGGAATTCCTATTTACCGATACAGA3′ | trmE promoter |
| Ash 57 | 5′GCTGCAGCAGCTACAAGTCGATGGCCC3′ | trmE promoter |
| Ash 59 | 5′GGGGTACCCCACACCACCTCGGCCTTG3′ | trmE |
| Ash 60 | 5′ATCAAGGACCCGTTCTTGCTTTCCATCACT3′ | trmE promoter |
| Ash 61 | 5′AGTGATGGAAAGCAAGAACGGGTCCTTGAT3′ | trmE promoter |
| Ash 62 | 5′ACTTCTGGAAAGTGTGCGCCAGGCGCCGTT3′ | trmE promoter |
| Ash 63 | 5′AACGGCGCCTGGCGCACACTTTCCAGAAGT3′ | trmE promoter |
| Ash 64 | 5′GCGCCGTTCATGCTCACCGACCTGTCGATC3′ | trmE promoter |
| Ash 65 | 5′GATCGACAGGTCGGTGAGCATGAACGGCGC3′ | trmE promoter |
| Ash 66 | 5′GCCTGTCCATCGCCCGGTCGAAGCTGC TAC3′ | trmE promoter |
| Ash 67 | 5′GTAGCAGCTTCGACCGGGCGATGGACAGGC3′ | trmE promoter |
| Ash 71 | 5′ACCGACTACACAAATCAGCGA T3′ | β-gal |
| Ash 72 | 5′TTCATTCCCCAGCGACCAGAT3′ | β-gal |
| Ash 77 | 5′GGTCGAAGCTGCTACGCAGCCGAGTAACTT3′ | trmE promoter |
| Ash 78 | 5′AAGTTACTCGGCTGCGTAGCAGCTTCGACC3′ | trmE promoter |
| PextF | 5′ATGCCGGTCTTCCTGTCCTTGTAC3′ | trmE promoter |
| PextR2 | 5′TGCATCGGATCCGGAGGAGTCGG3′ | trmE promoter |
PCR primers were designed using SeWeR (http://iubio.bio.indiana.edu/webapps/SeWeR.xx).
Molecular biology techniques.
Genomic DNA of P. syringae was isolated (36), fragmented (35 to 40 kb), and cloned in pCC1FOS (Epicentre Technologies, Madison, WI). All other DNA manipulation techniques, including colony hybridization, were performed as described by Sambrook et al. (54).
For reverse transcription PCR RNA was isolated from P. syringae (1, 13, 36), and first-strand cDNA was synthesized by RT. The cDNA was then amplified using Ash 71 and Ash 72 for the β-galactosidase gene and Ash 5 and Ash 44 for trmE for 21 cycles (Table 2).
Primer extension analysis and cloning of trmE promoter.
Transcription start site mapping of trmE was carried out by performing primer extension analysis (37) using [γ-32P]ATP-end-labeled PextR2 (Table 2) and total RNA of P. syringae grown at 4°C and 22°C. The putative trmE promoter of P. syringae was amplified by PCR using Ash57 and Ash59 (Table 2) and then cloned in pKZ27 with the β-galactosidase gene as the reporter gene (Promega Corporation, Madison, WI). The resulting pKZ27::promoter construct was then electroporated into P syringae (37).
Deletions of a specific promoter element were obtained by performing overlap extension PCR (65) (see Fig. 4A) using primers listed in Table 2. PCR products were cloned in pKZ27, and deletions were confirmed by DNA sequencing. The promoter constructs were electroporated into P. syringae, and transformants positive for β-galactosidase activity (43) were selected.
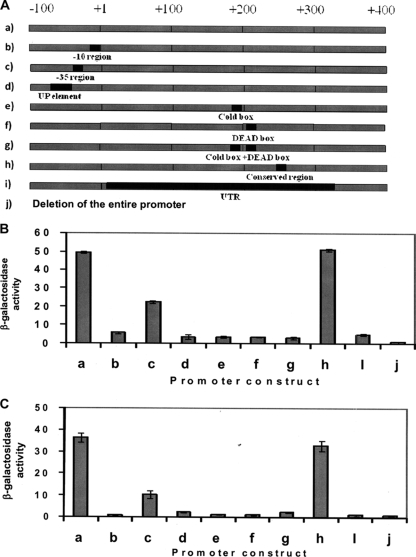
FIG. 4.
(A) Schematic diagrams of trmE promoter fragment deletion constructs of P. syringae. The deleted regions are indicated by dark gray boxes. The intact trmE promoter is shown on line a, whereas lines b to j show the regions that were deleted, as follows: line b, −10 region (deletion from position −5 to position −10); line c, −35 region (deletion from position −33 to position −38); line d, UP element region (deletion from position −41 to position −54); line e, cold box region (deletion from position 184 to position 194); line f, DEAD box (deletion from position 208 to position 217); line g, cold box and DEAD box regions (deletion from position 184 to position 194 and from position 208 to position 217); line h, conserved region (deletion from position 246 to position 252); line i, long 5′-UTR (deletion from position 9 to position 322); and line j, the entire promoter. (B and C) trmE promoter activities in P. syringae harboring the promoter deletions indicated above, assayed using cells grown at 22°C (B) and at 4°C (C), based on the activity of the β-galactosidase gene, the reporter gene.
Complementation of CSM1 with trmE.
The open reading frame (ORF) of trmE, along with its promoter, was amplified from P. syringae using primers Ash 57 and Ash 47 and cloned in pGL10 (3). The resulting pGL10::promoter-trmE construct was mobilized into CSM1 (52), and the complemented strain was checked for recovery of the phenotype.
RESULTS AND DISCUSSION
Cold-sensitive mutants of P. syringae.
A total of 3,500 mutants of P. syringae were generated using a Tn5 transposon-based suicide plasmid vector (pOT182) (47). Thirty of these mutants were cold sensitive and exhibited delayed growth at 4°C but not at 22°C or 28°C (Fig. 1). Growth analysis of CSM1, one of the cold-sensitive mutants, indicated that its growth is not altered at 22°C and 28°C compared to the growth of the P. syringae wild-type strain (Fig. 1). But at 4°C, CSM1 exhibited a prolonged 60-h lag period, compared to the 20-h lag period for the wild type. Thus, CSM1, like the pnp mutants of the psychrophile Yersinia enterocolitica (24), is a “psychrotrophy-defective” mutant since it exhibits delayed growth at 4°C but not at 22°C and 28°C compared to the growth of the parental strain.
FIG. 1.
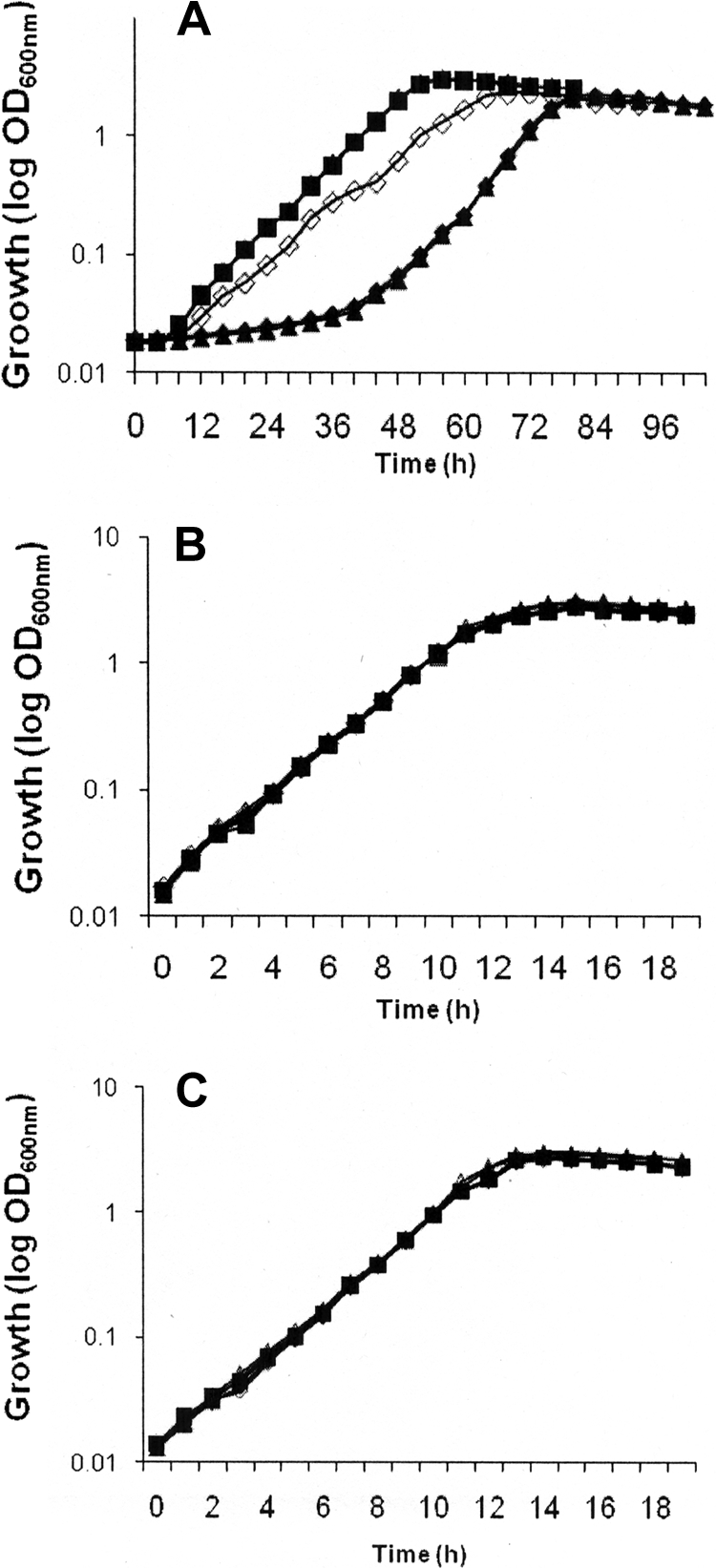
Growth of the psychrophile P. syringae (▪), P. syringae harboring pGL10 (▵), a cold-sensitive mutant of P. syringae (CSM1) (▴), complemented P. syringae cold-sensitive mutant CSM1 harboring the pGL10::trmE promoter plus trmE (□), and P. syringae cold-sensitive mutant CSM1 harboring only pGL10 (⧫) cultured at 4°C (A), 22°C (B), and 28°C (C). OD600nm, optical density at 600 nm.
The gene that was disrupted in CSM1 following amplification yielded a 700-bp fragment which, as determined by sequencing, exhibited high levels of similarity (98 to 99%) with trmE (encoding tRNA modification GTPase) of Halomonas cupida, Brevibacterium linens, and Bacillus megaterium.
Characteristics of trmE of P. syringae.
Colony hybridization screening of a genomic library of P. syringae using the 32P-labeled 700-bp PCR product of trmE identified two positive clones (5pCC1FOS and 11pCC1FOS). Sequencing of the insert of 5pCC1FOS (35 to 40 kb) using Ash 5, Ash 44, and Ash 59 yielded a 2,371-bp sequence corresponding to the complete ORF of trmE (1,377 bp) (accession number AM944531), an upstream incomplete ORF of the putative inner membrane protein translocase component yidC gene subunit (587 bp), and a downstream incomplete ORF of the abc transporter ATP-binding protein gene (407 bp). abc was in the same orientation as trmE, and transcriptional terminators were not detected downstream of trmE. The transposon insertion site was identified between bp 829 and 830 in trmE.
trmE codes for a 458-amino-acid protein (accession number CAQ16331). BLAST analysis (www.ncbi.nlm.nih.gov) showed that the amino acid and nucleotide sequences of trmE of P. syringae were similar to the trmE sequences of various bacterial species, and the highest level of similarity was 99% similarity with H. cupida (nucleotide sequence accession number AM944535 and amino acid sequence accession number CAQ16333).
CLUSTAL W analysis (www.chembnet.org/software/clustals.html) indicated that in TrmE of P. syringae the GTP binding (G-1, G-3, and G-4) and effector molecule binding (G-2) motifs involved in GTPase activity are conserved (8). In addition, four other regions (regions I, II, III, and IV) are conserved (see Fig. S1 in the supplemental material).
Complementation of trmE in CSM1.
The growth of complemented strain CSM1 (harboring pGL10::promoter-trmE) matched the growth of the wild type and the mutant at 22°C and 28°C. But at 4°C the complemented strain grew faster than the mutant, and the phenotype was rescued significantly (Fig. 1). The lag period for the complemented strain was 32 h, compared to 60 h for the mutant. Thus, it appears that the cold-sensitive phenotype was due to inactivation of trmE. However, the mutant did not recover totally compared to the wild type (Fig. 1). The slower growth of the complemented mutant was not due to the vector since analysis of wild-type cells transformed either with the vector (Fig. 1) or with the vector with the insert did not show any effect on the growth kinetics (data not shown). The inability of the complemented mutant to recover totally may have been due to the polar effect of another gene that is downstream of trmE (22, 53) or due to a gene dosage effect, or trmE may not have been expressed properly.
TrmE helps modify the uridine (U34) at the wobble position to 5-methylaminomethyl-uridine (72). This modification influences frameshift during the translation process (4, 6), thus causing a transient block in initiation of translation, as a consequence of which growth at low temperatures is affected, as observed for E. coli. Further, many genes involved in translation, such as fus and genes for RNA helicases (63) and ribosomal proteins (14, 26, 33, 69), have been implicated as genes that may be required for growth of mesophilic bacteria at low temperatures. trmE should be added to this list of genes.
Studies of E. coli have led to identification of a number of cold shock proteins and cold-inducible genes (20, 24, 25). CspA, the major cold shock protein in E. coli, belongs to a family of nine homologous proteins (27), but not all nine proteins are cold inducible (38, 45, 56). cspA has been identified in various psychrophilic bacteria, and this gene is constitutively expressed at 4°C and 22°C (49). Interestingly, none of the cold-shock-inducible genes are singularly responsible for cold adaptation (70).
Expression of trmE and mapping of the transcription start site.
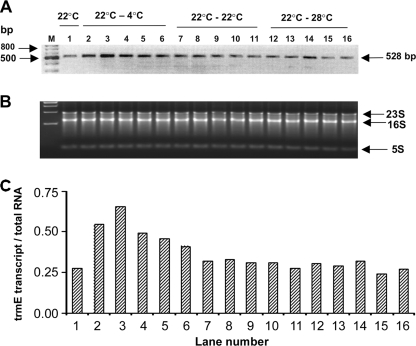
In vivo expression of trmE in P. syringae that was shifted from 22°C to 4°C increased transiently more than twofold up to 30 min after the shift (Fig. 2A to C), and the maximum increase, about 2.5-fold, was observed at 20 min after the shift. This temporal increase was consistently observed in three independent experiments using RNA isolated specifically in each experiment. In contrast, cells which were grown only at 22°C (control) or cells which were shifted from 22°C to 28°C did not show any change in the transcript levels for up to 60 min after the shift. These results imply that trmE expression is under the control of a cold-inducible promoter. Expression of pnp and oppA in the psychrophiles Y. enterocolitica and Listeria monocytogenes, respectively, was also reported to increase in cells growing at 5°C compared to cells growing at 30°C (5, 23).
FIG. 2.
RT-PCR analysis of trmE expression in P. syringae. RT-PCR was carried out using total RNA isolated from 3 ml of P. syringae grown to an optical density at 600 nm of ∼1.1 at 22°C and shifted to 4°C, 22°C, and 28°C for 0, 10, 20, 30, 45, and 60 min. Lane 1 contained the culture grown at 22°C (before the shift); lanes 2 to 6 contained the culture grown at 22°C and shifted to 4°C for 10, 20, 30, 45, and 60 min, respectively; lanes 7 to 11 contained the culture grown at 22°C and shifted to 22°C for 10, 20, 30, 45, and 60 min, respectively; and lanes 12 to 16 contained the culture grown at 22°C and shifted to 28°C for 10, 20, 30, 45, and 60 min, respectively. Lane M contained markers. (B) Amount of total RNA used in each of the RT-PCRs. (C) Ratios of the trmE transcript to total RNA (as determined by densitometry) for lanes 1 to 16 in panels A and B. The experiment was done three times, and the data are the results of a representative experiment.
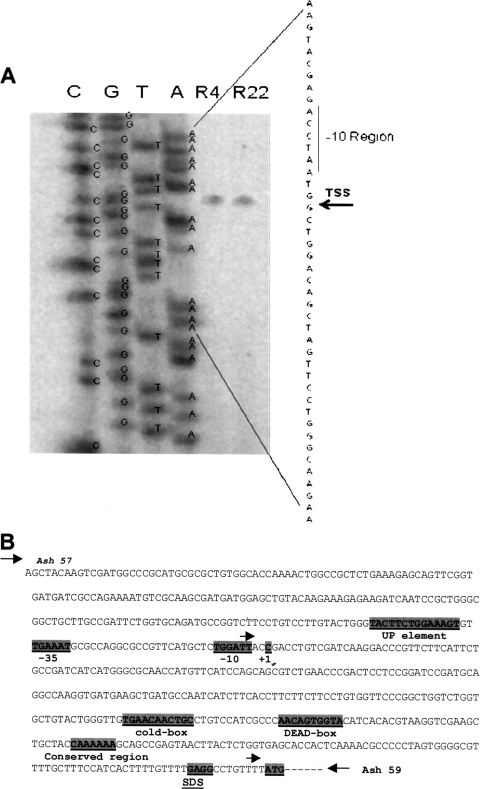
Primer extension analysis revealed a primer extension product of an expected size (100 bp) in P. syringae grown either at 4°C or at 22°C (Fig. 3A). The transcription start site was identified as a C (Fig. 3B).
FIG. 3.
(A) Primer extension analysis of the trmE transcript of P. syringae cells grown to mid-log phase (optical density at 600 nm, ∼1.1) at 4°C and 22°C. The primer extension reactions were carried out using 5′-end-labeled primer PextR2 and reverse transcriptase. Primer extension products R4 and R22 are from cultures grown at 4°C and 22°C, respectively, and represent the transcription start site (TSS) (arrow). (B) Nucleotide sequence of the trmE promoter of P. syringae and its regulatory elements. The transcription start site at position +1 (C), −10 region (TGGATT), −35 region (TGAAAT), UP element (TACTTCTGGAAAGT), cold box (TGAACAACTGC), DEAD box (AACAGTGGTA), conserved region (CAAAAA), Shine-Dalgarno sequence (SDS) (GAGG), and translation start site (ATG) are highlighted. The arrows indicate the direction of the primers, the transcription start site, and the translation start site.
Activity and expression of the trmE promoter.
Primers Ash 57 and Ash 59 amplified a 655-bp product, which corresponded to 591 bp upstream and 64 bp downstream of the translation start site (ATG). Between the transcription and translation start sites, a long 345-bp 5′ untranslated region (5′-UTR) was identified (Fig. 3B), which is one of the longest 5′-UTRs in cold-inducible genes (Table 3). Further, the −10 region, the −35 region, the UP element, the cold box, the DEAD box, and a conserved region (CAAAAAA) were identified (Fig. 3B; see Fig. S2 in the supplemental material). These promoter elements are highly conserved in the trmE promoters of other bacteria (see Fig. S2 in the supplemental material). When the putative promoter (655-bp fragment) upstream of the trmE gene was ligated upstream of the β-galactosidase gene and cloned in P. syringae, it exhibited β-galactosidase activity (Fig. 4A to C). But when the promoter elements were deleted (except for deletion of the −35 region, which resulted in 50 and 70% reductions in activity at 22 and 4°C, and deletion of CAAAAA, which had no effect), a drastic reduction in β-galactosidase activity was observed irrespective of whether the transformants were cultured at 4°C or 22°C (Fig. 4A to C). The CAAAAAA sequence was reported previously for the hut (30) and cti (37) promoters of P. syringae (see Fig. S2 in the supplemental material), but it does not appear to influence the promoter activity of trmE. The present study demonstrates that the 5′-UTR, −10 region, −35 region, UP element, cold box, and DEAD box (Fig. 3B) are essential for the activity of the trmE promoter, as they are for other genes (18, 31, 39, 44, 47, 66, 67) (Fig. 4A to C). Further, the trmE promoter elements of P. syringae had sequences identical to those in B. linens and Marinomonas primoryensis, which are psychrophiles (see Fig. S2 in the supplemental material).
TABLE 3.
Length of 5′-UTRs in some cold-inducible genes of bacteria
| Microorganism | Gene | 5′-UTR length (bp) | Reference |
|---|---|---|---|
| Pseudomonas syringae Lz4W | trmE | 345 | This study |
| Pseudomonas syringae Lz4W | hutU | 170 | 30 |
| Pseudomonas putida | rpoS | 368 | 34 |
| Escherichia coli | cspB | 161 | 38 |
| Escherichia coli | cspA | 159 | 20 |
| Escherichia coli | csdA | 226 | 63 |
| Escherichia coli | cspG | 161 | 45 |
| Escherichia coli | cspI | 145 | 6 |
| Anabaena sp. | chrC | 116 | 9 |
| Methanococcoides burtonii | deaD | 113 | 39 |
| Sinorhizobium meliloti | cspA | 119 | 47 |
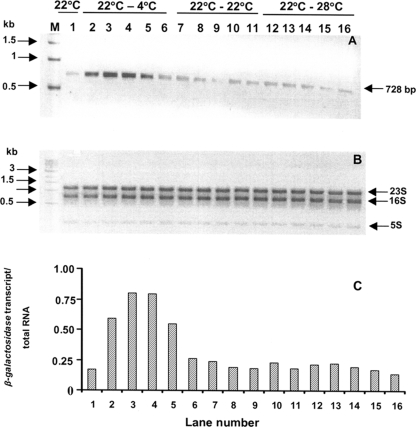
The sequences of the −10 and −35 regions were similar to the sequences of the −10 (TATA/GAT/C) and −35 (TA/GGA/GTA/T) regions of 11 promoters of the psychrophile Pseudoalteromonas haloplanktis but differed from the consensus sequences of the E. coli −10 and −35 regions (TATAAT and TTGACA, respectively) based on 300 promoters (40). Whether the observed similarity is a reflection of the cold-inducible nature of the promoter is difficult to predict. The UP element (17) in P. syringae is an AT-rich region (64.3%) and shows limited similarity with E. coli (21) and P. haloplanktis sequences (15) (see Fig. S2 in the supplemental material). The activity of the trmE promoter was also analyzed by monitoring the levels of β-galactosidase (reporter) transcripts in P. syringae harboring pKZ27::promoter. The β-galactosidase gene transcript level was unaltered in control cultures, which were cultured continuously at 22°C, and in cultures shifted from 22°C to 28°C (Fig. 5A to C). But when cultures were shifted from 22°C to 4°C, a transient increase in the β-galactosidase gene transcript level was observed for up to 30 min (Fig. 5A to C), confirming that the trmE promoter is under transcriptional regulation at low temperatures.
FIG. 5.
RT-PCR analysis of the expression of the trmE promoter based on expression of the β-galactosidase gene, the reporter gene in P. syringae (harboring pKZ27::promoter). (A) Lane 1, β-galactosidase gene transcript level in a culture grown at 22°C (before a shift); lanes 2 to 6, transcript levels in cultures grown at 22°C and shifted to 4°C for 10, 20, 30, 45, and 60 min, respectively; lanes 7 to 11, transcript levels in cultures grown at 22°C and shifted to 22°C for 10, 20, 30, 45, and 60 min; lanes 12 to 16, cultures grown at 22°C and shifted to 28°C for 10, 20, 30, 45, and 60 min, respectively; lane M, markers. (B) Amount of total RNA used in each of the RT-PCRs in panel A. (C) Ratio of the β-galactosidase gene transcript to the total RNA (as determined by densitometry) for lanes 1 to 16 in panels A and B. The experiment was done three times, and the data are the results of a representative experiment.
This temperature-dependent regulation is consistent with the conclusion that trmE plays a significant role in the adaptation to cold temperatures. Whereas previous studies have shown that TrmE modifies tRNA and has GTPase activity, this report is the first report to identify a low-temperature function (7, 55, 71). When TrmE activity is inhibited in E. coli, the loss of U34 modification causes a transient block in the initiation of translation (16). Additional studies are needed to determine the exact relationship between protein translation and the TrmE-mediated mechanism of cold resistance. This report is the first report demonstrating the importance of trmE for growth at low temperatures.
Supplementary Material
Acknowledgments
A.K.S. thanks the Council of Scientific and Industrial Research (Government of India, New Delhi, India) for a Junior and Senior Research Fellowship. S.S. thanks CSIR for funding under the Network Project “Exploitation of India's rich microbial diversity.”
Footnotes
Published ahead of print on 8 May 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aiba, H., S. Adhya, and B. de Crombrugghe. 1981. Evidence for the functional gal promoters in intact Escherichia coli cells. J. Biol. Chem. 256:11905-11910. [PubMed] [Google Scholar]
- 2.Baross, J. A., and R. Y. Morita. 1978. Microbial life at low temperatures: ecological aspects, p. 9-71. In D. J. Kushner (ed.), Microbial life in extreme environments. Academic Press, New York, NY.
- 3.Bidle, K. A., and D. H. Bartlett. 1999. RecD function is required for high-pressure growth of a deep-sea bacterium. J. Bacteriol. 181:2330-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjork, G. R., J. M. Durand, T. G. Hagervall, R. Leipuviene, H. K. Lundgren, K. Nilsson, P. Chen, Q. Qian, and J. Urbonavicius. 1999. Transfer RNA modification: influence on translational frameshifting and metabolism. FEBS Lett. 452:47-51. [DOI] [PubMed] [Google Scholar]
- 5.Borezee, E., E. Pellegrini, and P. Berche. 2000. OppA of Listeria monocytogenes, an oligopeptide-binding protein required for bacterial growth at low temperature and involved in intracellular survival. Infect. Immun. 68:7069-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brierley, I., M. R. Meredith, A. J. Bloys, and T. G. Haggervall. 1997. Expression of a coronavirus ribosomal frameshift signal in Escherichia coli: influence of tRNA anticodon modification on frameshifting, J. Mol. Biol. 270:360-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabedo, H., F. Macian, M. Villarroya, J. C. Escudero, M. Martinez-Vicente, E. Knecht, and M. E. Armengod. 1999. The Escherichia coli trmE (mnmE) gene, involved in tRNA modification, codes for an evolutionarily conserved GTPase with unusual biochemical properties. EMBO J. 18:7063-7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavicchioli, R., K. S. Siddiqui, D. Andrews, and K. R. Sowers. 2002. Low-temperature extremophiles and their applications. Curr. Opin. Biotechnol. 13:253-261. [DOI] [PubMed] [Google Scholar]
- 9.Chamot, D., W. C. Magee, E. Yu, and G. W. Owttrim. 1999. A cold shock-induced cyanobacterial RNA helicase. J. Bacteriol. 181:1728-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chattopadhyay, M. K., K. Uma Devi, Y. Gopisankar, and S. Shivaji. 1995. Thermolabile alkaline phosphatase from Sphingobacterium antarcticus, a psychrotrophic bacterium from Antarctica. Polar Biol. 15:215-219. [Google Scholar]
- 11.Chattopadhyay, M. K., M. V. Jagannadham, M. Vairamani, and S. Shivaji. 1997. Carotenoid pigments of an antarctic psychrotrophic bacterium, Micrococcus roseus: temperature dependent biosynthesis, structure and interaction with synthetic membranes. Biochem. Biophys. Res. Commun. 239:85-90. [DOI] [PubMed] [Google Scholar]
- 12.Chintalapati, S., J. S. S. Prakash, P. Gupta, S. Ohtani, I. Suzuki, T. Sakamoto, N. Murata, and S. Shivaji. 2006. A novel Δ9 acyl-lipid desaturase, DesC2 from cyanobacteria acts on fatty acids esterified to the sn-2 position of glycerolipids. Biochem. J. 398:207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chintalapati, S., J. S. S. Prakash, A. K. Singh, S. Ohtani, I. Suzuki, N. Murata, and S. Shivaji. 2007. Desaturase genes in a psychrotolerant Nostoc sp. are constitutively expressed at low temperature. Biochem. Biophys. Res. Commun. 362:81-87. [DOI] [PubMed] [Google Scholar]
- 14.Dammel, C. S., and H. F. Noller. 1995. Suppression of a cold sensitive mutation in 16S rRNA by overexpression of a novel ribosome-binding factor, RbfA. Genes Dev. 9:626-637. [DOI] [PubMed] [Google Scholar]
- 15.Duilio, A., S. Madonna, M. L. Tutino, M. Pirozzi, G. Sannia, and G. Marino. 2004. Promoters from a cold adapted bacterium: definition of a consensus motif and molecular characterization of UP regulative elements. Extremophiles 8:125-132. [DOI] [PubMed] [Google Scholar]
- 16.Elseviers, D., L. A. Petrullo, and P. Gallagher. 1984. Novel E. coli mutants deficient in biosynthesis of 5-methylaminomethyl-2-thiouridine. Nucleic Acids Res. 12:3521-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estrem, S. T., T. Gaal, W. Ross, and R. L. Gourse. 1998. Identification of an UP element consensus sequence for bacterial promoters. Proc. Natl. Acad. Sci. USA 95:9761-9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang, L., Y. Hou, and M. Inouye. 1998. Role of cold-box region in the 5′ untranslated region of the cspA mRNA in its transient expression at low temperature in Escherichia coli. J. Bacteriol. 180:90-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feller, G., and C. Gerday. 2003. Psychrophilic enzymes: hot topics in cold adaptation. Nat. Rev. Microbiol. 1:200-208. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein, J., N. S. Pollitt, and M. Inouye. 1990. Major cold shock proteins of Escherichia coli. Proc. Natl. Acad. Sci. USA 87:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gourse, R. L., W. Ross, and T. Gaal. 2000. Ups and downs in bacterial transcription initiation: the role of the alpha subunit of RNA polymerase in promoter recognition. Mol. Microbiol. 37:687-695. [DOI] [PubMed] [Google Scholar]
- 22.Goverde, R. L., J. G. Kusters, and V. J. H. Huis. 1994. Growth rate and physiology of Yersinia enterocolitica: influence of temperature and presence of the virulence plasmid. J. Appl. Bacteriol. 77:96-104. [DOI] [PubMed] [Google Scholar]
- 23.Goverde, R. L., J. H. J. Veld, J. G. Kusters, and F. R. Mool. 1998. The psychrotrophic bacterium Yersinia enterocolitica requires expression of pnp, the gene for polynucleotide phosphorylase, for growth at low temperature (5°C). Mol. Microbiol. 28:555-569. [DOI] [PubMed] [Google Scholar]
- 24.Graumann, P., T. M. Wendrich, M. H. Weber, K. Schroder, and M. A. Marahiel. 1997. A family of cold shock proteins in Bacillus subtilis is essential for cellular growth and for efficient protein synthesis at optimal and low temperature. Mol. Microbiol. 25:741-756. [DOI] [PubMed] [Google Scholar]
- 25.Graumann, P. L., and M. A. Marahiel. 1998. A superfamily of proteins that contain the cold shock domain. Trends Biochem. Sci. 23:286-290. [DOI] [PubMed] [Google Scholar]
- 26.Graumann, P. L., and M. A. Marahiel. 1999. Cold shock response in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 1:203-209. [PubMed] [Google Scholar]
- 27.Inouye, M., and S. Phadtare. 2007. The cold shock response, p. 182-193. In C. Gerday and N. Glandsdorff (ed.), Physiology and biochemistry of extremophiles. ASM Press, Washington, DC.
- 28.Jagannadham, M. V., V. Jayathirtha Rao, and S. Shivaji. 1991. The major carotenoid pigment of a psychrotrophic Micrococcus roseus: purification, structure, and interaction of the pigment with synthetic membranes. J. Bacteriol. 173:7911-7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jagannadham, M. V., M. K. Chattopadhyay, and S. Shivaji. 1996. The major carotenoid pigment of a psychrotrophic Micrococcus roseus strain: fluorescence properties of the pigment and its binding to membranes. Biochem. Biophys. Res. Commun. 220:724-728. [DOI] [PubMed] [Google Scholar]
- 30.Janiyani, K. L., and M. K. Ray. 2002. Cloning, sequencing, and expression of the cold-inducible hutU gene from the Antarctic psychrotrophic bacterium Pseudomonas syringae. Appl. Environ. Microbiol. 68:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang, W., L. Fang, and M. Inouye. 1996. The role of the 5′-end untranslated region of the mRNA for CspA, the major cold-shock protein of Escherichia coli, in cold-shock adaptation. J. Bacteriol. 178:4919-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones, P. G., and M. Inouye. 1994. The cold shock response: a hot topic. Mol. Microbiol. 11:811-818. [DOI] [PubMed] [Google Scholar]
- 33.Jones, P. G., R. A. VanBogelan, and F. C. Neidhardt. 1987. Induction of proteins in response to low temperature in Escherichia coli. J. Bacteriol. 169:2092-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jovcic, B., I. Bertani, V. Venturi, L. Topoisirovic, and M. Kojic. 2008. 5′ Untranslated region of Pseudomonas putida WS358 stationary phase sigma factor rpoS mRNA is involved in RpoS translation regulation. J. Microbiol. 46:56-61. [DOI] [PubMed] [Google Scholar]
- 35.Kannan, K., K. Janiyani, S. Shivaji, and M. K. Ray. 1998. Histidine utilization operon (hut) is upregulated at low temperature in the Antarctic psychrotrophic bacterium Pseudomonas syringae. FEMS Microbiol. Lett. 161:7-14. [DOI] [PubMed] [Google Scholar]
- 36.Kiran, M. D., J. S. S. Prakash, S. Annapoorni, S. Dube, T. Kusano, H. Okuyama, N. Murata, and S. Shivaji. 2004. Psychrophilic Pseudomonas syringae required trans monounsaturated fatty acid for growth at higher temperature. Extremophiles 8:401-410. [DOI] [PubMed] [Google Scholar]
- 37.Kiran, M. D., S. Annapoorni, I. Suzuki, N. Murata, and S. Shivaji. 2005. cis-trans isomerase gene in psychrophilic Pseudomonas syringae is constitutively expressed during growth and under conditions of temperature and solvent stress. Extremophiles 9:117-125. [DOI] [PubMed] [Google Scholar]
- 38.Lee, S. J., A. Xie, W. Jiang, J. P. Etchegary, P. G. Jones, and M. Inouye. 1994. Family of the major cold shock protein, CspA (CS7.4) of E. coli, whose members show a high sequence similarity with eukaryotic Y-box binding protein. Mol. Microbiol. 11:833-839. [DOI] [PubMed] [Google Scholar]
- 39.Lim, J., T. Thomas, and R. Cavicchioli. 2000. Low temperature regulated DEAD-box RNA helicase from the Antarctic archaeon, Methanococcoides burtoni. J. Mol. Biol. 297:553-567. [DOI] [PubMed] [Google Scholar]
- 40.Lisser, S., and H. Maragalit. 1993. Compilation of E. coli mRNA promoter sequences. Nucleic Acids Res. 21:1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Margesin, R. 2007. Alpine microorganisms: useful tools for low temperature bioremediation. J. Microbiol. 45:281-285. [PubMed] [Google Scholar]
- 42.Merriman, T. R., and I. L. Lamont. 1993. Construction and use of a self-cloning promoter probe vector for Gram-negative bacteria. Gene 126:17-23. [DOI] [PubMed] [Google Scholar]
- 43.Miller, J. H. 1972. Experiments in molecular genetics, p. 443. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 44.Mitta, M., L. Fang, and M. Inouye. 1997. Deletion analysis of cspA of Escherichia coli: requirement of the AT-rich UP element for cspA transcription and the downstream box in the coding region for its cold shock induction. Mol. Microbiol. 26:321-335. [DOI] [PubMed] [Google Scholar]
- 45.Nakashima, K., K. Kanamaru, T. Mizuno, and K. Horikoshi. 1996. A novel member of CspA family genes that is induced by cold shock in Escherichia coli. J. Bacteriol. 178:2994-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ochman, H., A. S. Gerber, and D. L. Hartl. 1988. Genetic application of an inverse polymerase chain reaction. Genetics 120:621-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Connell, K. P., and F. Thomashow. 2000. Transcriptional organization and regulation of a polycistronic cold shock operon in Sinorhizobium meliloti RM1021 encoding homologs of the Escherichia coli major cold shock gene cspA and ribosomal protein gene rpsU. Appl. Environ. Microbiol. 66:392-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray, M. K., G. Seshu Kumar, and S. Shivaji. 1994. Phosphorylation of membrane proteins in response to temperature in an Antarctic Pseudomonas syringae. Microbiology 140:3217-3223. [DOI] [PubMed] [Google Scholar]
- 49.Ray, M. K., T. Sitaramamma, S. Ghandhi, and S. Shivaji. 1994. Occurrence and expression of cspA, a cold shock gene, in Antarctic psychrotrophic bacteria. FEMS Microbiol. Letts. 116:55-60. [DOI] [PubMed] [Google Scholar]
- 50.Reddy, G. S. N., G. Rajagopalan, and S. Shivaji. 1994. Thermolabile ribonucleases from antarctic psychrotrophic bacteria: detection of the enzyme in various bacteria and purification from Pseudomonas fluorescens. FEMS Microbiol. Lett. 122:122-126. [Google Scholar]
- 51.Regha, K., A. K. Satapathy, and M. K. Ray. 2005. RecD plays an essential function during growth at low temperature in the Antarctic bacterium Pseudomonas syringae (Lz4W). Genetics 170:1473-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roine, E., D. N. Nunn, I. Paulin, and M. Romantschuk. 1996. Characterization of genes required for pilus expression in Pseudomonas syringae pathovar phaseolicola. J. Bacteriol. 178:410-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ross, W., S. E. Aiyar, and J. Salomon. 1998. Escherichia coli promoters with UP elements of different strengths: modular structure of bacterial promoters. J. Bacteriol. 180:5375-5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 55.Scrima, A., R. I. Vetter, M. E. Armengod, and A. Wittinghofer. 2005. The structure of the TrmE GTP-binding protein and its implications for tRNA modification. EMBO J. 24:23-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serror, P., R. Dervyn, S. D. Ehrlich, and E. Maguin. 2003. Csp-like genes of Lactobacillus delbrueckii ssp. bulgaricus and their response to cold shock. FEMS Microbiol. Lett. 226:323-330. [DOI] [PubMed] [Google Scholar]
- 57.Shivaji, S. 2005. Microbial diversity and molecular basis of cold adaptation in Antarctic bacteria, p. 3-24. In T. Satyanarayana and B. N. Johri (ed.), Microbial diversity: current perspectives and potential applications. I.K. International Pvt. Ltd., New Delhi, India.
- 58.Shivaji, S., N. S. Rao, L. Saisree, V. Sheth, G. S. N. Reddy, and P. M. Bhargava. 1989. Isolation and identification of Pseudomonas spp. from Schirmacher Oasis, Antarctica. Appl. Environ. Microbiol. 55:767-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shivaji, S., M. D. Kiran, and S. Chintalapati. 2007. Perception and transduction of low temperature in bacteria, p. 194-207. In C. Gerday and N. Glansdorff (ed.), Physiology and biochemistry of extremophiles. ASM Press, Washington, DC.
- 60.Siddiqui, K. S., and R. Cavicchioli. 2006. Cold adapted enzymes. Annu. Rev. Biochem. 75:403-433. [DOI] [PubMed] [Google Scholar]
- 61.Simon, R., U. B. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vitro genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 62.Suzuki, I., Y. Kanasaki, K. Mikami, M. Kanehisa, and N. Murata. 2001. Cold regulated genes under the control of cold sensor hik33 in Synechocystis. Mol. Microbiol. 40:235-245. [DOI] [PubMed] [Google Scholar]
- 63.Toone, W. M., K. E. Rudd, and J. D. Friesen. 1991. deaD, a new Escherichia coli gene encoding a presumed ATP-dependent RNA helicase, can suppress a mutation in rpsB, the gene encoding ribosomal protein S2. J. Bacteriol. 173:3291-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uma, S., R. S. Jadhav, G. Seshukumar, S. Shivaji, and M. K. Ray. 1999. A RNA polymerase with transcriptional activity at 0°C from the Antarctic bacterium Pseudomonas syringae. FEBS Lett. 453:313-317. [DOI] [PubMed] [Google Scholar]
- 65.Urban, A., S. Neukirchen, and K. E. Jaeger. 1997. A rapid and efficient method for site directed mutagenesis using one step overlap extension PCR. Nucleic Acids Res. 25:2227-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang, N., K. Yamanaka, and M. Inouye. 1999. CspI, the ninth member of the CspA family of Escherichia coli, is induced upon cold shock. J. Bacteriol. 181:1603-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong, K. K., H. G. Bouwer, N. E. Freitag. 2004. Evidence implicating the 5′ untranslated region of Listeria monocytogenes actA in the regulation of bacterial actin-based motility. Cell. Microbiol. 6:155-166. [DOI] [PubMed] [Google Scholar]
- 68.Wu, H., J. Kato, A. Kuroda, T. Ikeda, N. Takiguchi, and H. Ohtake. 2000. Identification and characterization of two chemotactic transducers for inorganic phosphate in Pseudomonas aeruginosa. J. Bacteriol. 182:3400-3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xia, B., H. Ke, U. Shinde, and M. Inouye. 2003. The role of RbfA in 16S rRNA processing and cell growth at low temperature in Escherichia coli. J. Mol. Biol. 332:575-584. [DOI] [PubMed] [Google Scholar]
- 70.Xia, B., H. Ke, and M. Innouye. 2001. Acquirement of cold sensitivity by quadruple deletion of the cspA family and its suppression by PNPase S1 domain in Escherichia coli. Mol. Microbiol. 40:179-188. [DOI] [PubMed] [Google Scholar]
- 71.Yim, L., I. Moukadiri, G. R. Bjork, and M. E. Armengod. 2006. Further insights into the tRNA modification process controlled by proteins MnmE and GidA of Escherichia coli. Nucleic Acids Res. 34:5892-5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yokoyama, S., T. Watanabe, K. Murao, H. Ishikura, Z. Yamaizumi, S. Nishimura, and T. Miyazawa. 1985. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl. Acad. Sci. USA 82:4905-4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.