Abstract
Cryptosporidium parvum oocysts accumulate on biofilm surfaces. The percentage of oocysts attached to biofilms remained nearly constant while oocysts were supplied to the system but decreased to a new steady-state level once oocysts were removed from the feed. More oocysts attached to summer biofilm cultures than winter biofilm cultures.
Cryptosporidium causes a potentially life-threatening gastrointestinal disease. Because conventional water treatment may not effectively target Cryptosporidium, source water monitoring and protection are important to avoid infection outbreaks.
Biofilms can accumulate pathogens at densities that are much higher than water column densities, with the potential for pathogen release long after entrapment (5, 13, 15, 19). Biofilms have been identified as a drinking water contamination source (7), causing infections for which the source cannot be identified (4, 6).
Several previous studies examined pathogen transport in biofilms using Cryptosporidium parvum oocysts (2, 6, 15, 16) or beads as pathogen surrogates (3, 5, 11, 12). The former studies did not use natural microbial assemblages (2, 16) or quantify oocyst attachment or sloughing (6, 15). The current study provides novel information about C. parvum oocyst attachment to environmental biofilms, including a mass balance analysis to identify the daily number of oocysts that (i) remained in the flowing water or were sloughed from the biofilm and (ii) were attached to the biofilm. We imaged biofilms using scanning confocal laser microscopy, as used in other studies (9, 17, 20, 21), to identify spatial patterns of oocyst attachment.
Biofilms were scraped from rocks found in Monocacy Creek (Bethlehem, PA) into 1 liter of creek water in January 2007 (winter biofilm culture) and July 2008 (summer biofilm culture). The biofilm suspension was vacuum filtered through a 6-μm cellulose filter. The filtrate was centrifuged (1,754 × g for 15 min), and the resulting biofilm pellet was resuspended in 1 ml of raw creek water. The cell concentration was quantified by DAPI (4′,6-diamidino-2-phenylindole) staining (14). Cells were split into aliquots (5 × 106 cells each) and stored at −80°C in cryovials containing 30% glycerol.
Single-channel flow chambers (length by width by height, 24 mm by 8 mm by 4 mm) with glass coverslips (Stovall Life Science, Inc., Greensboro, NC) were inoculated with 5 × 106 biofilm cells for 24 h before the flow was started. Filter-sterilized creek water was used as the flow medium. A 12-channel peristaltic pump (Ismatec, Glattbrugg, Switzerland) maintained a constant flow of 0.2 mm/s (1).
For biofilm imaging, the following two setups were used: (i) 1 × 104 C. parvum oocysts (Iowa isolate; Waterborne, Inc., New Orleans, LA) (all oocysts were used within 3 weeks of shedding) in the influent each day for 3 days and (ii) 3 × 104 C. parvum oocysts added to the influent for the last 24 h of a 3-day flow experiment. Biofilms were imaged with a Zeiss LSM 510 META laser scanning microscope, using an argon laser (458-nm, 477-nm, 488-nm, and 514-nm excitation wavelengths) and a HeNe1 laser (543-nm excitation wavelength). Biofilms were fixed with methanol, blocked using a 1:10 dilution of fetal bovine serum, and stained with 20 μM SYTO 9 (Invitrogen, Molecular Probes, Eugene, OR) (16). C. parvum oocysts in the biofilm were stained with a Cy3-conjugated monoclonal antibody solution specific for Cryptosporidium (Waterborne, Inc.) (16).
For the mass balance analysis, C. parvum oocysts (1 × 104 per day for 3 days) were added to 500 ml constantly stirred influent water to keep oocysts in suspension. Influent water was replaced each day. Experiments to quantify sloughing included 2 or 5 additional days with oocyst-free feed water, for a total of 5 or 8 days. Biofilms used for the 3- and 5-day experiments were grown with the winter biofilm culture; biofilms used for the 8-day experiments were grown with the summer biofilm culture.
After each 24-hour period, the remaining influent and effluent waters were processed by membrane filtration (MF) and immunomagnetic separation (IMS) to recover the oocysts. On the last day of each experiment, biofilms were scraped from the flow chambers, resuspended in sterile creek water, and also processed by MF and IMS. MF was performed according to the method of Oda et al. (10), using the 3-μm filter only. IMS was performed on the filtrate using the Aureon IMS kit (ImmTech, Inc., New Windsor, MD), and oocysts were dissociated from the magnetic beads with 0.05 M HCl. IMS products were counted by hemocytometry and corrected for MF and IMS processing losses. An average IMS recovery of 65% ± 4.2% standard error (SE) (determined by four trials using 1 × 104 oocysts in deionized water) was used. MF recoveries were consistent within each day but varied between days. Therefore, an MF recovery control was performed each day using 1 × 104 oocysts in 1 liter deionized water to obtain a daily MF correction factor.
The mass balance analysis demonstrated that these methods were effective for tracking oocysts throughout the flow system for the experiment's duration, accounting for all the oocysts within 8% (Table 1). In a control flow chamber with no biofilm growth (i.e., a clean glass surface), oocyst loss within the system was 1% or less, indicating that very few oocysts attached to any abiotic surface within the flow system. Laboratory biofilms composed of natural microbial assemblages were successfully created, although grazing impacts that would affect biofilm dynamics in the environment were eliminated. The thicknesses of laboratory biofilms (average thickness, 39.6 μm; SD, 4.7 μm; n = 16) were not statistically different (P of 0.17 by independent t test) than those of natural biofilms in Monocacy Creek (average thickness, 35.8 μm; SD, 10.2 μm; n = 36).
TABLE 1.
Mass balance analysis of biofilms grown for 3, 5, and 8 days, with 3-day oocyst dosinga
| Biofilm growth | No. of oocysts ± % SE
|
% of oocysts ± % SE
|
Avg biofilm thickness ± SE (μm) | ||||
|---|---|---|---|---|---|---|---|
| Influent | Effluent | Biofilmb | In biofilm at end of oocyst dosing (day 3) | In biofilm at end of experimentc | Accounted for in system | ||
| Day 3 (n = 3) | 1.5 × 104 ± 1.8 | 8.6 × 103 ± 9.3 | 6.4 × 103 ± 7.1 | 43 ± 5.6 | 43 ± 5.6 | 100 ± 1.9 | 31 ± 6.1 |
| Day 5 (n = 2) | 1.9 × 104 ± 2.4 | 1.8 × 104 ± 3.5 | 3.2 × 103 ± 5.3 | 40 ± 4.5 | 4.8 ± 2.1 | 108 ± 1.0 | 37 ± 3.8 |
| Day 8 (n = 2) | 2.0 × 104 ± 3.1 | 1.5 × 104 ± 2.9 | 7.6 × 103 ± 1.8 | 64 ± 5.0 | 28 ± 0.2 | 107 ± 0.9 | 42 ± 3.6 |
Data from two or three replicate experiments are presented.
Data determined from direct hemacytometer counts of scraped biofilm at the end of the experiment.
Calculated from influent and effluent data [(influent − effluent)/influent].
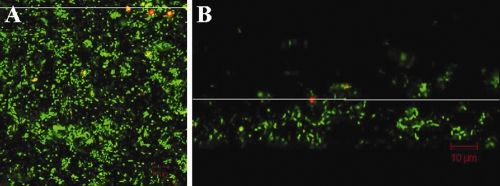
Oocyst attachment location within the biofilm is important for transport dynamics. Oocyst attachment at the biofilm surface may be followed by (i) no transport into the biofilm depth, (ii) burial by biofilm overgrowth, or (iii) transport into the biofilm depth through water channels. In these experiments, oocysts attached to the biofilm surface and were not observed to move to depths or be buried by biofilm overgrowth (Fig. 1). In the 28 biofilms examined, no difference in oocyst attachment location was seen whether oocysts were present in the flow for the entire study duration (n = 14) or whether oocysts were added to the flow on the last study day (n = 14).
FIG. 1.
Top-down projection (A) and cross-sectional view (B) of a summer biofilm culture, with C. parvum oocysts attached at the biofilm surface. Biofilm cells are stained green with SYTO 9; oocysts are stained red with Cy3. The white line in panel A indicates the location of the cross section shown in panel B. Direction of water flow is from right to left. The biofilm is approximately 24-μm thick; oocysts are located 16 μm above the biofilm base.
Previous studies (11, 12) also reported that particle attachment and detachment occurred at the biofilm surface. The inner biofilm was denser, with less pore space, while the biofilm surface had more water channels, providing more surface area for particle attachment. The mean pore size in a variety of biofilms was reported as 1.7 to 2.7 μm at the water surface and 0.3 to 0.4 μm at the substrate surface (11), which would restrict larger particle movement, including oocysts (4 to 7 μm).
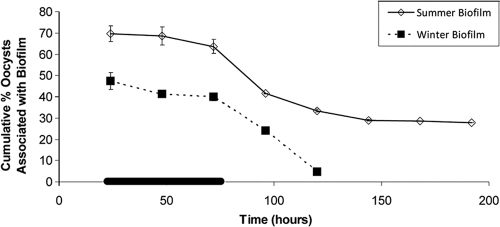
Oocysts became attached to biofilms and rapidly reached a steady state (Fig. 2), as seen in other studies (5, 6). The percentage of oocysts attached to the biofilm remained nearly constant while oocysts were supplied to the system. Once the oocyst supply was removed, the percentage of oocysts in the biofilm decreased to a new steady state. For winter biofilm cultures, the cumulative percentage of oocysts attached to the biofilm at day 3 (i.e., the end of the dosing period; average, 40.0%; SD, 25%; n = 2) was statistically higher (P of 0.003 by independent t test) than the cumulative percentage of oocysts attached to the biofilm at day 5 (average, 4.8%; SD, 1.4%; n = 2). For the summer biofilm cultures, the cumulative percentage of oocysts attached to the biofilm at day 3 (average, 63.7%; SD, 4.5%; n = 2) was also statistically higher (P of 0.01) than the cumulative percentage of oocysts attached to the biofilm at day 5 (average, 33.5%; SD, 1.1%; n = 2). The oocysts that remained in the biofilm at day 5 likely attached to more-stable or sheltered portions of the biofilm that did not slough.
FIG. 2.
Cumulative percentage of oocysts (±SE; n = 2) associated with the biofilm. The cumulative number of oocysts in the biofilm each day was calculated by adding the daily differences between the number of oocysts in the effluent and influent. This number was converted to a percentage by dividing by the cumulative number of influent oocysts. The biofilm accumulation on the last day was determined from the oocysts collected and counted directly from the biofilm, which agreed with the number calculated using the above-described method. Time zero indicates when the flow began; biofilm growth began 24 h earlier by seeding with microbial concentrate at zero flow. The solid black line on the x axis indicates the period of oocyst addition to the inflow. Error bars are smaller than symbols where not visible.
The cumulative percentage of oocysts attached to summer biofilm cultures was statistically higher (P of 0.02 and 0.002 at days 3 and 5, respectively, by independent t test) than the cumulative percentage of oocysts attached to the winter biofilm cultures (Fig. 2). In addition, the thickness of summer biofilm cultures (average thickness, 42.1 μm; SD, 4.2 μm; n = 8) was statistically higher (P of 0.03 by independent t test) than that of winter biofilm cultures (average, 37.0 μm; SD, 3.9 μm; n = 8). However, it is unlikely that biofilm thickness explains the increased oocyst attachment to summer biofilm cultures, because all oocysts were observed to attach at the biofilm surface and no oocysts were ever observed within biofilm depths. These observations are in agreement with those of other studies (3, 8, 16) and suggest that other biofilm characteristics (e.g., surface roughness or pore size) may (i) be more important than biofilm thickness for oocyst attachment and (ii) vary with seasonal differences in water chemistry or microbial community caused by water quality differences, such as temperature, pH, or dissolved organic carbon (16).
Biofilms are significant reservoirs for oocysts compared to abiotic surfaces (5, 12, 15, 16). Oocysts that remain in the biofilm have important public health implications because they may persist in the biofilm and eventually be released, resulting in potential human exposure.
These results confirm that C. parvum oocysts quickly attach to natural microbial biofilms and can be released into the flowing water over time. Oocyst attachment and release dynamics are important for assessing and potentially reducing the risk of human exposure and infection. Although this study used natural stream biofilms, these transport dynamics have important implications for the drinking water industry. Biofilms in the raw water source, represented here by stream biofilms, are linked to drinking water intakes, where any disturbance event can affect water quality. For this reason, a better understanding of the environmental transport of oocysts is important for tracking oocyst contamination, which ultimately affects the drinking water industry. Further investigation is necessary to understand the differences between the summer and winter biofilm cultures as well as the pathogen reservoir that forms in the biofilm.
Acknowledgments
This research was funded by a National Science Foundation CAREER award (0545687) to K. L. Jellison.
Footnotes
Published ahead of print on 15 May 2009.
REFERENCES
- 1.Christensen, B. B., C. Sternberg, J. B. Anderson, R. J. Palmer, A. T. Nielsen, M. Givskov, and S. Molin. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20-42. [DOI] [PubMed] [Google Scholar]
- 2.Dai, X., and R. M. Hozalski. 2002. Effect of NOM and biofilm on the removal of Cryptosporidium parvum oocysts in rapid filters. Water Res. 36:3523-3532. [DOI] [PubMed] [Google Scholar]
- 3.Drury, W. J., W. G. Characklis, and P. S. Stewart. 1993. Interactions of 1μm latex particles with Pseudomonas aeruginosa biofilms. Water Res. 27:1119-1126. [DOI] [PubMed] [Google Scholar]
- 4.Flemming, H. C., S. L. Percival, and J. T. Walker. 2002. Contamination potential of biofilms in water distribution systems. Water Sci. Technol. 2:271-280. [Google Scholar]
- 5.Flood, J. A., and N. J. Ashbolt. 2000. Virus-sized particles can be entrapped and concentrated one hundred fold within wetland biofilms. Adv. Environ. Res. 3:403-411. [Google Scholar]
- 6.Helmi, K., S. Skraber, C. Gantzer, R. Willame, L. Hoffman, and H. Cauchie. 2008. Interactions of Cryptosporidium parvum, Giardia lamblia, vaccinal poliovirus type I, and bacteriophages ϕX174 and MS2 with a drinking water biofilm and a wastewater biofilm. Appl. Environ. Microbiol. 74:2079-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howe, A. D., S. Forster, S. Morton, R. Marshall, K. S. Osborn, P. Wright, and P. R. Hunter. 2002. Cryptosporidium oocysts in a water supply associated with a cryptosporidiosis outbreak. Emerg. Infect. Dis. 8:619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Långmark, J., M. V. Storey, N. J. Ashbolt, and T. A. Stenström. 2005. Accumulation and fate of microorganisms and microshperes in biofilms formed in a pilot-scale water distribution system. Appl. Environ. Microbiol. 71:706-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence, J. R., D. R. Korber, B. D. Hoyle, J. W. Costerton, and D. E. Caldwell. 1991. Optical sectioning of microbial biofilms. J. Bacteriol. 173:6558-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oda, T., M. Sakagame, H. Ito, H. Yano, S. K. Rai, M. Kawabata, and S. Uga. 2000. Size selective continuous flow filtration method for detection of Cryptosporidium and Giardia. Water Res. 34:4477-4481. [Google Scholar]
- 11.Okabe, S., T. Yasuda, and Y. Watanabe. 1997. Uptake and release of inert fluorescence particles by mixed population biofilms. Biotechnol. Bioeng. 53:459-469. [DOI] [PubMed] [Google Scholar]
- 12.Okabe, S., H. Kuroda, and Y. Watanabe. 1998. Significance of biofilm structure on transport of inert particulates into biofilms. Water Sci. Technol. 38:163-170. [Google Scholar]
- 13.Percival, S. L., J. T. Walker, and P. R. Hunter. 2000. Microbiological aspects of biofilms and drinking water. CRC Press, New York, NY.
- 14.Porter, K. G., and Y. S. Keig. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25:943-948. [Google Scholar]
- 15.Rogers, J., and C. W. Keevil. 1995. Survival of Cryptosporidium parvum oocysts in biofilm and planktonic samples in a model system, p. 209-213. In W. B. Betts, D. Casemore, C. Fricker, H. Smith, and J. Watkins (ed.), Protozoan parasites and water. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 16.Searcy, K. E., A. I. Packman, E. R. Atwill, and T. Harter. 2006. Capture and retention of Cryptosporidium parvum oocysts by Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 72:6242-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sternberg, C., B. B. Christensen, T. Johansen, A. T. Nielsen, J. B. Andersen, M. Givskov, and S. Molin. 1999. Distribution of bacterial growth activity in flow-chamber biofilms. Appl. Environ. Microbiol. 65:4108-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reference deleted.
- 19.Szewzyk, U., R. Szewzyk, W. Manz, and K. H. Schleifer. 2000. Microbiological safety of drinking water. Annu. Rev. Microbiol. 54:81-127. [DOI] [PubMed] [Google Scholar]
- 20.Tolker-Nielsen, T., and S. Molin. 2000. Spatial organization of microbial biofilm communities. Microb. Ecol. 40:75-84. [DOI] [PubMed] [Google Scholar]
- 21.Wolfaardt, G. M., J. R. Lawrence, R. D. Roberts, S. J. Caldwell, and D. E. Caldwell. 1994. Multicellular organization in a degradative biofilm community. Appl. Environ. Microbiol. 60:434-446. [DOI] [PMC free article] [PubMed] [Google Scholar]