Abstract
Background
Glucagon like peptide-1 (GLP-1) and its analogue exendin-4 (Ex-4) enhance glucose stimulated insulin secretion (GSIS) and activate various signaling pathways in pancreatic β-cells, in particular cAMP, Ca2+ and protein kinase-B (PKB/Akt). In many cells these signals activate intermediary metabolism. However, it is not clear whether the acute amplification of GSIS by GLP-1 involves in part metabolic alterations and the production of metabolic coupling factors.
Methodology/Prinicipal Findings
GLP-1 or Ex-4 at high glucose caused release (∼20%) of the total rat islet insulin content over 1 h. While both GLP-1 and Ex-4 markedly potentiated GSIS in isolated rat and mouse islets, neither had an effect on β-cell fuel and energy metabolism over a 5 min to 3 h time period. GLP-1 activated PKB without changing glucose usage and oxidation, fatty acid oxidation, lipolysis or esterification into various lipids in rat islets. Ex-4 caused a rise in [Ca2+]i and cAMP but did not enhance energy utilization, as neither oxygen consumption nor mitochondrial ATP levels were altered.
Conclusions/Significance
The results indicate that GLP-1 barely affects β-cell intermediary metabolism and that metabolic signaling does not significantly contribute to GLP-1 potentiation of GSIS. The data also indicate that insulin secretion is a minor energy consuming process in the β-cell, and that the β-cell is different from most cell types in that its metabolic activation appears to be primarily governed by a “push” (fuel substrate driven) process, rather than a “pull” mechanism secondary to enhanced insulin release as well as to Ca2+, cAMP and PKB signaling.
Introduction
The mechanisms of glucose-stimulated insulin secretion (GSIS) in the β-cell remain to be defined. In addition to the triggering pathway involving a rise in ATP production, KATP channel closure and a Ca2+ rise [1], [2], [3], fuel signaling is thought to involve additional pathways, in particular anaplerosis/cataplerosis, pyruvate cycling processes, endogenous lipolysis and enhanced glycerolipid/fatty acid (GL/FFA) cycling [4], [5], [6], [7], [8], [9]. Besides the signals induced by calorigenic nutrients and their associated production of metabolic coupling factors [4], β-cell function is modulated by a variety of neurohormonal agents and glucoincretins [10], including glucagon like peptide-1 (GLP-1), an incretin hormone secreted by the L-cells of the distal intestine [10], [11]. GLP-1 levels in the plasma increase rapidly following a meal [12], and this hormone has a profound glucose-lowering effect through both central and peripheral actions [13], the latter effect being particularly at the level of the β-cell [10]. GLP-1 stimulates insulin gene expression [14], proinsulin biosynthesis [10], and it also potentiates GSIS [10], [14]. GLP-1 also has proliferative [15] and antiapoptotic actions on the β-cell [10]. The biologically active form of GLP-1 is derived from proglucagon via the action of prohormone convertase enzymes [10], [11], and circulating GLP-1 is rapidly removed from the circulation following its degradation by dipeptidyl peptidase-4 (DPP-4) [16].
GLP-1 exerts its cellular action by binding to its receptor, a G-protein coupled receptor (GLP-1R), expressed in β-cells, nervous system, heart and kidney [10], [11]. The activation of the GLP-1R leads to the induction of many signal transduction systems, including cAMP, Ca2+, PI3-Kinase and EGF receptor signaling [10], [11], [17], [18]. These multiple actions of GLP-1 are also observed upon exposure of β cells to Exendin-4 (Ex-4), a peptide that is an incretin mimetic and which lowers levels of blood glucose as a consequence of its ability to activate the GLP-1R [10]. GLP-1 induces insulin secretion during short-term exposure to the hormone, or after chronic exposure to the hormone [10], [19]. Even though the precise mechanisms of GLP-1 action are not fully understood, it is established that the stimulation of GSIS by GLP-1 involves activation of membrane-bound adenylyl cyclase and cAMP production, leading to protein kinase-A (PKA) and Epac [20] activation, and an increase in intracellular Ca2+ [10], [11], [21]. A rise in cytoplasmic and mitochondrial Ca2+ has been linked to the activation of mitochondrial dehydrogenases, in particular pyruvate dehydrogenase [22], α-ketoglutarate dehydrogenase and isocitrate dehydrogenase [3], [22], [23]. Additionally, islet tissue and the β-cell contain some glycogen [24], [25] that might be mobilized following a rise in cellular Ca2+ or cAMP [26], thus releasing glucose-1P that may enter the glycolytic pathway following its conversion to glucose-6-P. It is therefore attractive to hypothesize that GLP-1 may indirectly activate β-cell energy metabolism, thereby raising levels of cellular ATP, and possibly influencing other metabolic coupling factors, via its effect on cellular levels of Ca2+ and cAMP. Thus, it is generally believed that the cAMP and Ca2+ pathways cannot fully account for the complete magnitude of GLP-1 mediated GSIS enhancement [10], [11], [19].
Besides Ca2+ signaling, the binding of GLP-1 to its receptor also results in the activation of protein kinase-B (PKB/Akt) [27], [28], [29] and PKB activation in other cell types has been linked to various metabolic effects, including glucose transport in muscle [30] glycogen synthesis [31] and lipolysis [32]. It can also be hypothesized that an increase in cellular ATP content might inactivate AMP-activated protein kinase (AMPK) [33], as elevated glucose does [34], which may lead to reduced phosphorylation of hormone sensitive lipase and adipose triglyceride lipase [35], with subsequent enhanced lipolysis [36] and activation of the lipid amplification arm of glucose signaling for insulin secretion [36]. Thus, our previous work has established a role for lipolysis in GSIS [37], [38]. In addition, it was previously shown that orlistat, a pan-inhibitor of lipases, suppresses the incretin action of GLP-1 [39], and that GLP-1 enhances lipolysis in HIT (β) cells [40].
In the present study we examined whether the acute stimulation of GSIS by GLP-1 or Ex-4 involves the modulation of glucose, fatty acid and energy metabolism in the β-cells, as studied using isolated islets of both rats and mice. The study was designed to respond to four questions of general interest for β-cell neuropeptide and fuel signaling. 1) Does GLP-1 amplify GSIS in part by metabolic signaling? 2) Does the activation of major cellular signaling processes (Ca2+, cAMP, PKB etc) in response to a physiological peptide agonist changes β-cell metabolism? 3) Does enhanced insulin secretion contribute significantly to total energy consuming processes in the β-cell? 4) Is the β-cell similar or different from most tissues in term of metabolic activation, specifically whether it is primarily governed by a “push” (fuel substrate driven) process, rather than a “pull” mechanism secondary to enhanced activation of its major cellular function.
Materials and Methods
Animals and diets
Ethics Statement
All procedures were performed in accordance with the Institutional Committee for the Protection of Animals at the Centre Hospitalier de l'Université de Montréal or Institutional Guidelines for Animal Care (IACUC) at the Marine Biological Laboratory, in compliance with United States Public Health Service regulations.
Wistar rats (200–250 g; Charles River) and male Swiss-Webster mice or male CD-1 rats were housed under controlled temperature (21°C) and light conditions (12-h light/dark cycle) with free access to water and standard chow diet.
Isolation and culture of islets and islet cells
Pancreatic islets were isolated by collagenase digestion of the pancreas according to Gotoh et al [41]. After digestion and washing and separation by histopaque gradient centrifugation, islets were hand-picked and cultured overnight in a humidified incubator with 5% CO2. For measurements of insulin secretion, the cell culture medium was RPMI-1640 containing 11 mM glucose and supplemented with 10% foetal calf serum, 10 mM HEPES (pH 7.4), 1 mM sodium pyruvate, 100 U/ml penicillin and 100 µg/ml streptomycin (RPMI complete medium). For measurements of oxygen consumption, isolated rat or mouse islets were cultured overnight in RPMI-1640 complete medium containing 5 mM glucose. For single cell studies of mitochondrial ATP levels, the islets were dispersed by incubation in Ca2+/Mg2+ free phosphate buffered saline, 3 mM EGTA and 0.002% trypsin as previously described [42]. Islet cells obtained by dispersion of islets were plated on poly-D-lysine coated coverslips (MatTek, Ashland, MA) in 35 mm Petri dishes. After 24 h, single islet cells were transduced with Ad-MitoLuc-RFP at 50 MOI (multiplicity of infection) for 12 h, after which viral media were replaced with appropriate growth media. Transduction efficiency in single islet cells, determined from RFP fluorescence, reached more then 90% under these conditions.
Measurement of insulin secretion and insulin content
After overnight culture, rat islets were distributed in 12-well plates (10 islets/well) and incubated for 2 h in 1 ml RPMI complete medium containing 2.8 mM glucose. The islets were then washed and pre-incubated for 45 min at 37°C in KRBH/0.07% defatted BSA and 2.8 mM glucose, followed by incubation for 1 h in 1 ml KRBH/0.5% defatted BSA and 2.8, 8.3 or 16.7 mM glucose plus or minus 20 nM GLP-1-(7–36)-amide (Bachem Americas, Torrance, CA, USA) or 20 nM Ex-4 (Bachem Americas, Torrance, CA, USA), in the presence or absence of 0.3 mM palmitate. For whole mouse islets, or populations of single mouse islet cells (plated on the wells of 48-well plates), insulin secretion was measured at 4, 7.5 and 16.7 mM glucose in the presence or absence of 10 nM Ex-4. At the end of a 30 min static incubation, media were kept for insulin measurement by radioimmunoassay (Linco research, St. Charles, MO, USA). Islet total insulin content was measured following acid-ethanol (0.2 mM HCl in 75% ethanol) extraction.
Islet fatty acid oxidation and esterification
Fatty acid (FA) oxidation and esterification were determined in batches of 50 islets cultured as described above. After 2 h incubation in 2.8 mM glucose-RPMI complete medium, islets were washed in KRBH/0.25% BSA and pre-incubated for 45 min at 37°C in KRBH/0.25% defatted BSA and 2.8 mM glucose after which they were incubated for 2 h (FA oxidation) or 4 h (FA esterification) in 1 ml KRBH/0.25% defatted BSA containing 2.8, 8.3 or 16.7 mM glucose in the presence or absence of 20 nM GLP-1, 0.1 mM (oxidation) or 0.2 mM (esterification) palmitate, 1 µCi/ml [9,10(n)-3H] palmitate (51 Ci/mmol, GE Healthcare, Baie d'Urfé, QC, Canada), and 1 mM carnitine. At the end of the incubation, the media were collected for the determination of islet FA oxidation and total lipids were extracted from islets for the measurement of islet FA esterification [38].
Glucose oxidation and utilization
Groups of 20 islets cultured and pre-incubated as described above for the insulin secretion assay, were incubated in a 0.6 ml Eppendorf tube without capping, in a final volume of 70 µl KRBH/0.25% defatted BSA containing 2.8 to 16.7 mM glucose with D-[U-14C]-glucose for oxidation measurements (250 mCi/mmol, PerkinElmer, Canada) and D-[5-3H]-glucose for utilization measurements (16 Ci/mmol, GE Healthcare, Canada) with or without 20 nM GLP-1 [43]. For oxidation incubations, this incubation tube was placed upright in an airtight-sealed 20 ml scintillation vial, which also contained an empty 1.5 ml Eppendorf tube without capping. The reaction was stopped after 90 min incubation at 37°C with constant agitation by the addition of 50 µl of a mix consisting of metabolic poisons (400 mM citric acid, 10 µM rotenone, 10 µM antimycin and 3.5 mg KCN, pH 4.9). To the empty 1.5 ml tube in the scintillation vial, 250 µl of 5% (w/v) KOH was added to trap released 14CO2. Incubations were continued for 60 min at room temperature and glucose oxidation was determined by measuring the KOH-trapped 14CO2. For utilization measurements, the scintillation vial also contained 500 µl of 1 mM HCl at the bottom. After stopping the incubations as above, the tightly sealed vials were left at room temperature for 40 h and glucose utilization was determined by measuring the amount of 3H2O equilibrated into the 0.5 ml HCl in the vial.
Lipolysis
Batches of 60 islets, cultured as described above, were washed in KRBH/0.07% BSA and 2.8 mM glucose and were transferred into 0.2 mL of KRBH/0.07% BSA medium in a 48 well plate with 2.8, 8.3 or 16.7 mM glucose and 20 nM GLP-1 or 20 nM Ex-4. The plate was incubated for 3 h at 37°C in a humidified atmosphere containing 5% CO2, after which the media were collected for glycerol determination by an enzymatic assay [37]. Islet protein content was measured, as previously described [37].
Measurement of [Ca2+]i and cAMP content
Mouse islets were dispersed by mild digestion with trypsin-EDTA and the single islet cells were plated on glass cover-slips for [Ca2+]i measurement, or in 96-well cell culture plates for cAMP determination. After overnight culture, the islet cells were infected with Ad-MtLuc-RFP (m.o.i. equal to 50). Measurements of [Ca2+]i were performed after 48 h, using fura-2 loaded β-cells, imaged at 100X magnification using a dual excitation light source and a ratiometric imaging system (IonOptix Corp.) equipped with filter sets that minimize crossover between fura-2 and RFP [44]. The cAMP content was determined by immunoassay using a Direct Biotrak EIA kit (Amersham) as described earlier [45].
Oxygen Consumption
Oxygen consumption in single rat or mouse islets was measured at 37°C in the presence or absence of 10 nM Ex-4 or 10 µM forskolin by the self-referencing method based on an electrochemical oxygen sensor (BioCurrents Center, MBL, Woods Hole, MA) moving between a “near” and “far” position at the islet. The magnitude of the amperometric current used for the reduction of oxygen is proportional to the oxygen concentration at that particular point [46]. When islet respires, oxygen concentration is lower at near position. Thus, the current used for reduction of oxygen on the sensor will be greater at the far position, and the measured difference in the electric current between far and near position (Difference Current, DC) is greater than zero. When an islet further increases oxygen consumption (in response to a rise in glucose concentration), oxygen concentration at near position decreases even more and causes further increase in the DC. Oxygen consumption was measured in islets incubated in KRBH containing 4, 7.5, and 16.6 mM glucose.
Mitochondrial ATP
Changes of mitochondrial ATP levels (ATPm) were measured in a population of approximately 250,000 single rat or mouse islet cells infected with Ad-MitoLuc-RFP. This virus was generated using the mt-Luc coding sequence in plasmid VR102. mt-Luc is a fusion protein in which the 26 amino acid N-terminal signal peptide of cytochrome C oxidase subunit VIII (COX8) is fused to codon-optimized firefly luciferase [47]. Ad-MitoLuc-RFP infected islet cells were incubated with KRBH buffer (for 72 h) containing, either 4, 7.5 or 16.6 mM glucose without or with 10 nM Ex-4. Single islet cells, grown and infected on the PDL-coated glass coverslips inside a 35 mm dish (MatTek, Ashland, MA) were placed directly onto the surface of the photocathode optical window of a Hamamatsu R464 photomultiplier tube housed in a 37°C heated box. Luciferin was then added to the KRBH at a final concentration of 100 µM in order to allow the measurement of photoemissions resulting from luciferase-catalyzed oxidation of luciferin [48].
PKB/Akt phosphorylation
A group of 200 islets cultured and pre-incubated as described for insulin secretion experiments, was incubated for 30 min in 1 ml KRBH/0.5% defatted BSA containing 2.8 or 8.3 mM glucose in the presence or absence of 20 nM GLP-1. After 30 min, islets were lysed in 0.1 ml of 50 mM HEPES (pH 7.5), 2 mM sodium orthovanadate, 4 mM EDTA, 100 mM sodium fluoride, 10 mM sodium pyrophosphate, 1 mM PMSF, 1% (v/v) NP40 and protease inhibitors. Total cellular proteins were obtained after sonicating the islets for 10 s and centrifugation at 15,000×g at 4°C for 12 min. The supernatant was collected and the protein content assayed (Pierce). Proteins were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to nitrocellulose membranes (BioRad, Hercules, CA, USA). After overnight blocking with 5% non-fat milk, the membranes were probed with antibodies for pSer473-PKB and total PKB (Cell Signaling Technology, MA, USA) and the proteins were visualized by enhanced chemiluminescence (Pierce).
Statistical analysis
Data are expressed as means±SE. Significance was calculated for multiple comparisons by using one-way analysis of variance (ANOVA) with Bonferroni post-hoc testing. A P-value of<0.05 was considered significant.
Results
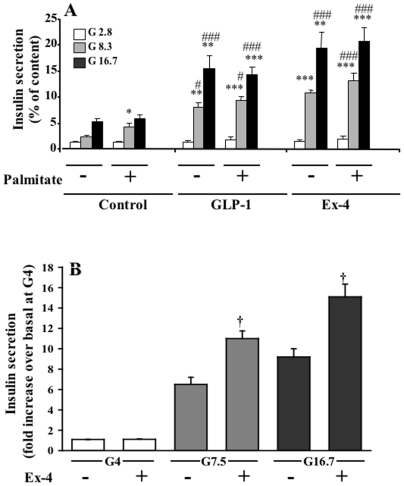
Glucose stimulated insulin secretion from isolated rat islets in a dose-dependent manner, and this effect was enhanced by 0.3 mM palmitate at an intermediate concentration (8.3 mM) of glucose (Fig. 1A). Both GLP-1 and Ex-4 markedly potentiated GSIS at 8.3 and 16.7 mM glucose, and palmitate did not further elevate insulin secretion. At 16.7 mM glucose, GLP-1 and Ex-4 stimulated insulin secretion 3–4 fold more than glucose alone. In the presence of GLP-1 or Ex-4 the amount of insulin released during the 45 min time period corresponded to approximately 20% of the total islet content of insulin. In mouse islets, Ex-4 enhanced GSIS at 7.5 and 16.7 mM glucose, but not at 4 mM glucose (Fig. 1B). Overall, Fig. 1 shows that the islets that were used in the current study were highly responsive to glucose, GLP-1 and Ex-4 and therefore suitable to be used to respond to the addressed questions. Inasmuch as both GLP-1 and Ex-4 are agonists of GLP-1 receptor on the β-cells, with near equal potency, most studies to date have not found any significant differences between these two agonists in in vitro experiments. In the present study, although most experiments were performed with both GLP-1 and Ex-4, because of the similar nature of the data, only either of these is illustrated.
Figure 1. Acute effects of GLP-1 and Ex-4 on GSIS in rat (A) and mouse (B) islets.
Pancreatic islets were isolated and cultured overnight prior to use as described in Methods. Islets were incubated for 1 h (A) or 30 min (B) as described in Methods for examining insulin secretion at indicated concentrations of glucose and GLP-1 (20 nM) or Ex-4 (20 nM) in A, or 10 nM Ex-4 in B, in the absence or presence of 0.3 mM palmitate. Insulin released into the media and the total islet insulin content were measured. Results shown are mean±SE from 3 independent experiments with quadruplicates (n = 12). For A, *p<0.05, **p<0.01, ***p<0.001 when compared with corresponding 2.8 mM glucose group. #p<0.05, ##p<0.01, ###p<0.001 when compared with corresponding groups without GLP-1 or Ex-4 treatment. For B, †p<0.01 when compared to corresponding minus Ex-4 group. Insulin secretion in mouse islets at basal glucose levels (4 mM) was 1.56±0.3 ng insulin/10 islets/30 min.
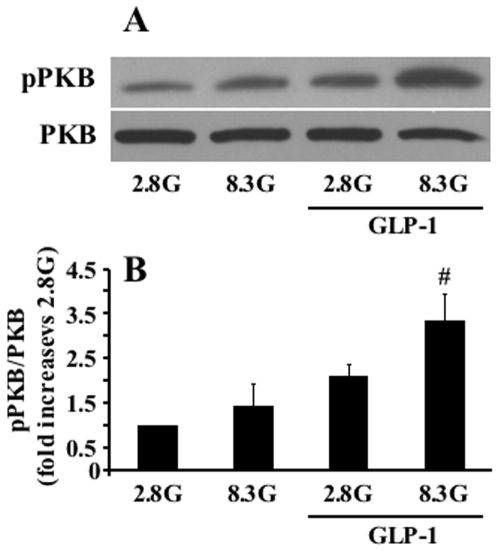
PKB has multiple metabolic effects in various cell types [49] and GLP-1 acutely activates PKB in INS cells [27] and human islets [29]. So far it has not been shown that GLP-1 activates PKB in normal rodent β-cells. We examined the effect of GLP-1 (20 nM) on PKB phosphorylation in rat islets after a 30 min exposure to the hormone, and observed that GLP-1 significantly increased Ser-473 phosphorylation of PKB in normal islet cells (Fig. 2).
Figure 2. PKB phosphorylation in response to GLP-1 in rat islets.
Islets were incubated for 30 min at 2.8 or 8.3 mM glucose in the presence or absence of 20 nM GLP-1. Activation of PKB by GLP-1 was assessed with antibodies specific for Ser473phospho-PKB and PKB, respectively. (A) Representative immunoblot of phospho- and total PKB in rat islets. (B) Quantitative measurement of PKB phosphorylation after 30 min treatment with GLP-1. Results are means±SE of 4 separate experiments. #p<0.05, when compared to the corresponding ‘minus GLP-1’ group.
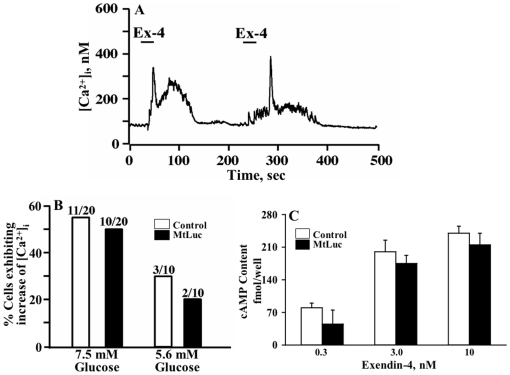
It was therefore of interest to determine whether changes in islet intermediate metabolism might be explained by influences of GLP-1 on PKB phosphorylation/activation [50], [51], in addition to its previously demonstrated stimulatory effects on islet cAMP and Ca2+ signaling shown in many studies employing both normal (human, rat, mouse) and tumoral β-cell (for reviews see [3], [10], [19], [20], [21]). Tsuboi and co-workers [52] reported that GLP-1 receptor activation increased [Ca2+]i, which caused an elevation of mitochondrial ATP in MIN6 insulin-secreting cells. In order to ascertain that Ex-4 increases [Ca2+]i and cAMP in normal mouse β-cells under our experimental conditions where mitochondrial ATP and O2 consumption were measured, single cell measurements of [Ca2+]i were performed using β-cells loaded with fura-2, infected with Ad-MtLuc-RFP, and equilibrated in KRB containing 5.6 mM glucose. Under these conditions, Ex-4 (10 nM) stimulated an increase of [Ca2+]i in these cells (Fig. 3A). Importantly, MtLuc expression had no effect on the percentage of cells exhibiting a >100 nM increase of [Ca2+]i (Fig. 3B). Thus, viral infection did not disrupt the stimulatory action of Ex-4 on intracellular Ca2+ signaling. Since Ex-4 is known to stimulate insulin secretion in a glucose-dependent manner, we examined whether glucose concentration influences Ex-4 stimulated intracellular Ca2+ signaling. This was in fact the case since the action of Ex-4 to increase [Ca2+]i was more prominent under conditions in which mouse β-cells were equilibrated in KRB containing 7.5 mM glucose as compared to 5.6 mM glucose (Fig. 3B).
Figure 3. Exendin-4 increases [Ca2+]i and cAMP content in mouse β-cells.
A, A single fura-2 loaded and Ad-MtLuc-RFP-infected mouse β-cell was imaged to determine the [Ca2+]I, at 5.6 mM glucose in KRBH. After establishment of a stable baseline [Ca2+]i, 10 nM Ex-4 was applied for 25 sec (indicated by horizontal bars). Note that a repeatable increase of [Ca2+]i was measured. B, Population study conducted at the single cell level in which the action of Ex-4 to increase [Ca2+]i was evaluated in β-cells not infected (open bars) or infected with Ad-MtLuc-RFP (filled bars). For these experiments, the KRB contained 5.6 or 7.5 mM glucose, as indicated. A response to Ex-4 was defined as a >100 nM increase of [Ca2+]i occurring in a single β-cell. C, Ex-4 caused a dose-dependent increase in cAMP content in mouse islet cells in KRB containing 7.5 mM glucose without or with Ad-MtLuc-RFP infection.
Since it is known that the increase of [Ca2+]i in response to Ex-4 is secondary to β-cell cAMP production [44], it was of interest to determine if viral infection altered the ability of Ex-4 to increase levels of cAMP in primary mouse islet cells. We found that Ex-4 stimulated cAMP production in a dose-dependent manner, in islet cells infected with Ad-MtLuc-RFP (Fig. 3C), without any significant difference from cells not infected with Ad-MtLuc-RFP (Fig. 3C). Thus, viral infection as described here for Ad-MtLuc-RFP, did not disrupt intracellular Ca2+ signaling and cAMP production in mouse β-cells.
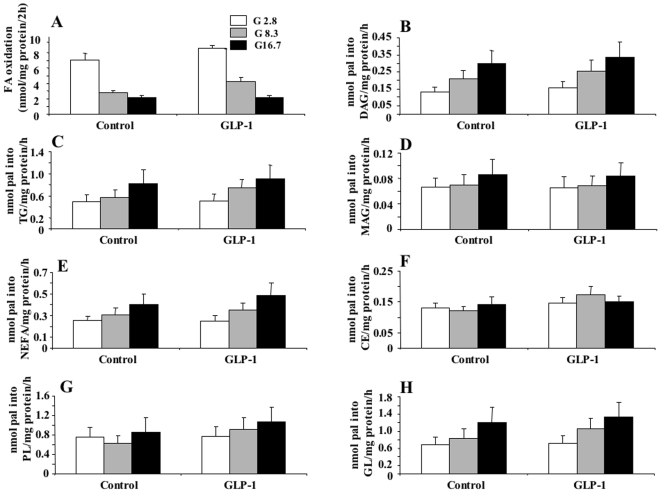
We have previously shown [53], [54] that GSIS in rat islets and INS cells is accompanied by reduced β-oxidation and increased partitioning of fatty acids into glycerolipids, an event that is thought to be coupled to β-cell activation of insulin release [5]. Therefore, we examined whether the acute stimulatory effect of GLP-1 on GSIS in islets might result from altered lipid metabolism. There was no significant effect of GLP-1 on palmitate oxidation or its incorporation into different classes of glycerolipids or cholesterol esters or phospholipids (Fig. 4) at the various tested glucose concentrations.
Figure 4. Palmitate β-oxidation and esterification into different lipids in rat islets in the absence or presence of GLP-1.
Islets were processed as described for insulin secretion (see [Fig. 1]) and after the pre-incubation step they were incubated for 2 h (FA oxidation) or 4 h (FA esterification) in 1 ml KRBH/0.25% defatted BSA containing medium with 1 mM carnitine and 1 µCi/ml [9,10(n)-3H] palmitate (51 Ci/mmol), at 2.8, 8.3 or 16.7 mM glucose in the presence or absence of 20 nM GLP-1. Cold palmitate (pal) was present at 0.1 mM for oxidation and 0.2 mM for esterification experiments. A, Palmitate oxidation; B—H, palmitate incorporation into diacylglycerol, DAG (B), triacylglycerol, TG (C), monoacylglycerol, MAG (D), non-esterified fatty acids, NEFA (E), cholesterol esters, CE (F), phospholipids, PL (G) and total glycerolipids, GL (H). Means±SE of 6–8 separate incubations in 3 independent experiments.
We next assessed whether the acute stimulatory effect of GLP-1 on GSIS is associated with changes in islet glucose metabolism. However, rat islet glucose utilization (Fig. 5A) or oxidation (Fig. 5B) was not significantly affected by GLP-1 at all tested glucose concentrations, except for a small increase in utilization at 16.7 mM glucose (Fig. 5A).
Figure 5. Glucose metabolism and lipolysis in rat islets in the absence or presence of GLP-1.
Islets were processed as described for insulin secretion (see [Fig. 1]) and after the pre-incubation step they were incubated in 70 µl KRBH/0.25% defatted BSA medium containing 2.8, 8.3 or 16.7 mM glucose plus or minus 20 nM GLP-1 in presence of D-[U-14C]-glucose (for oxidation) (A) and D-[5-3H]-glucose (for utilization) (B). Incubations were stopped after 90 min as described in Methods. Glucose oxidation was measured as 14CO2 released, and glucose utilization was determined by measuring the amount of released 3H2O. Results are means±SE of 15 determinations in 3 separate experiments. *p<0.05, **p<0.01, ***p<0.001 when compared to the corresponding 2.8 mM glucose group; #p<0.05 when compared to the corresponding ‘minus GLP-1’ group. For lipolysis determinations (C) overnight-cultured rat islets were washed in KRBH/0.07% BSA medium with 2.8 mM glucose and were transferred into 0.2 mL KRBH/0.07% BSA medium with 2.8, 8.3 or 16.7 mM glucose with or without 20 nM GLP-1. After incubation for 3 h at 37°C, glycerol released into the media and the islet protein content were determined. Means±SE from 4 independent experiments with pentaplicates.
Enhanced lipolysis and GL/FFA cycling are thought to play a role in the amplification (KATP-independent) arm of fuel induced insulin secretion [5], [35], and previous work in the HIT cell line showed that GLP-1 enhances glycerol release in this tumoral (β) cell [40]. However, GLP-1 did not enhance lipolysis in rat islets at low, intermediate and high glucose (Fig. 5C).
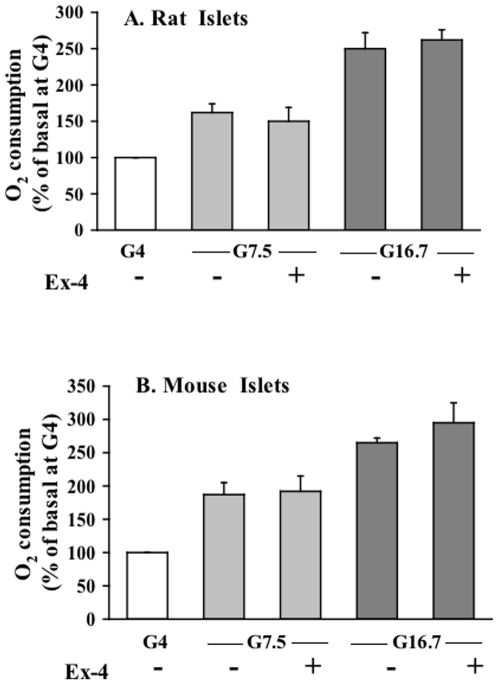
We also examined the effect of Ex-4 and GLP-1 on oxygen consumption at different glucose concentrations in rat and mouse islets since this parameter reflects overall fuel utilization and metabolic activation of a given tissue. Even though oxygen consumption increased with glucose concentration, there was no significant change with Ex-4 (Fig. 6A & B) or with GLP-1 (data not shown), in accordance with the results from glucose oxidation experiments. Also, the adenylate cyclase activator forskolin (10 µM) did not affect oxygen consumption in mouse islets (data not shown).
Figure 6. Oxygen consumption of rodent islets in the absence or presence of Ex-4.
Single rat (A) and mouse (B) islets were adhered on glass coverslips inside a 35 mm dish using CellTak adhesive. After 30 min equilibration at 4 mM glucose, oxygen consumption was measured in response to 4, 7.5 and 16.6 mM glucose with and without 10 nM Ex-4 (10 nM). Data are means±SE of 3 experiments.
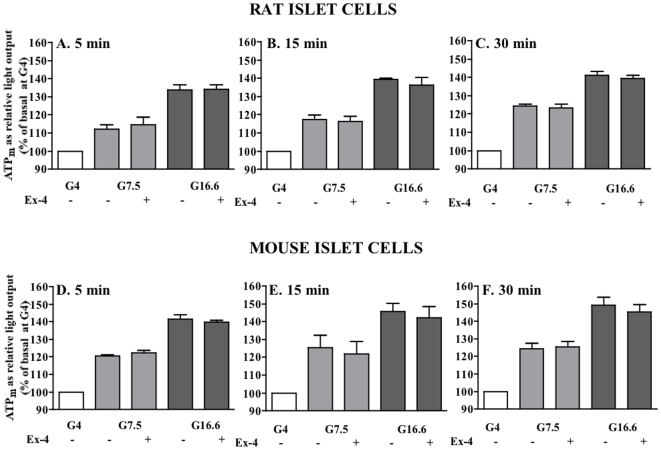
Enhanced mitochondrial metabolism and ATP production plays a central role in β-cell fuel signalling [55] and GLP-1 was shown to enhance mitochondrial ATP production in the tumoral β-cell line MIN6 [52]. We further measured mitochondrial ATP levels in isolated rat and mouse islet cells engineered to express mitochondrial-targeted luciferase, after incubation for 5 to 30 min, with different glucose concentrations and Ex-4. Glucose caused a marked and dose dependent increase in mitochondrial ATP, within 5 min of incubation, in both rat and mouse islets cells, but Ex-4 did not significantly change mitochondrial ATP at all tested glucose concentrations (Fig. 7A & B). Identical results were obtained using 20 nM GLP-1 (data not shown). We verified that the mitochondrial-luciferase-engineered islet cells respond normally to the respiratory substrates and inhibitors, by examining the effect of methylsuccinate (10 mM), a membrane permeable form of succinate and FCCP (10 µM), an uncoupler of oxidative phosphorylation. As expected, during a 15 min incubation, methylsuccinate enhanced ATP production above the basal (4 mM glucose) level, whereas, FCCP reduced ATP levels (data not shown).
Figure 7. Mitochondrial ATP levels of rodent islet cells in the absence or presence of Ex-4.
Dispersed rat (A,B,C) and mouse (D,E,F) islet cells were transduced with Ad-MtLuc-RFP. ATP levels in the presence of 4, 7.5 and 16.6 mM glucose with and without 10 nM Ex-4 were determined as photoemission resulting from luciferase-catalyzed oxidation of luciferin, in populations of approximately 250,000 single islet cells at 5, 15 and 30 min. Data are means±SE of 3 experiments.
Discussion
Fuel stimulated insulin secretion in the β-cells involves the production of metabolic coupling factors and an elevation in intracellular Ca2+ [1]. GLP-1 and Ex-4 enhance GSIS in the β-cells, cause a rise in cytosolic Ca2+, elevate cAMP and activate the PKA and PKB signaling pathways [3], [10], [19], [20], [21]. Because activation of Ca2+, cAMP and PKB signaling is known to modulate intermediary and energy metabolism in several cell types, we hypothesized that GLP-1 signaling to stimulate insulin secretion is in part linked to changes in β-cell metabolism and the production of metabolic coupling factors. The data in fact show that GLP-1 or Ex-4 barely affect β-cell metabolism at large, and therefore that GLP-1 induced insulin secretion may not involve metabolic signaling related to glucose, lipid and energy metabolism.
We [1], [5] and others [56] provided evidence that enhanced lipolysis in β-cells plays a role in GSIS. However, lipolysis does not appear to be involved in the acute amplification of GSIS by GLP-1 in normal islet tissue. Thus, in accordance with a previous study using isolated mouse islets [57], we observed that GLP-1 or Ex-4 do not affect rat islet lipolysis. Furthermore, islets from hormone sensitive lipase-KO mice exhibited GLP-1 stimulation of GSIS similar to that of control mouse islets [37]. The previously reported increase in lipolysis by acute treatment with GLP-1 in HIT (β) cells [40] is probably due to the inherent differences in established tumoral cell lines from normal islet β-cells.
Lipid amplification pathways of GSIS in the β-cell involve reduced fatty acid β-oxidation and concomitant increased esterification of fatty acids into glycerolipids [53], [54]. We have suggested that enhanced GL/FFA cycling is instrumental in the amplification of GSIS via the generation of lipid signalling molecules that act as metabolic coupling factors for insulin secretion [5], [35]. However, no effect of GLP-1 was noticed on either palmitate β-oxidation or its incorporation into glycerolipids, cholesterol esters or phospholipids. Thus, GLP-1 mediated enhancement of GSIS does not appear to be dependent on either lipolysis or alteration in GL/FFA cycling.
GLP-1 activates PKB in INS (β)-cells [27], [28], human [29] and rat islets (present study). PKB activation can lead to various metabolic effects in different cell types, including glucose transport [30] and glycogen synthesis [31] in muscle, and lipolysis in adipocytes [32]. In addition PKB activation inhibits AMPK [33], and activation of AMPK by AICAR or expression of a constitutively active AMPK mutant in β-cells curtail GSIS [34], [58]. However, despite GLP-1 activation of islet PKB, none of the studied parameters of β-cell metabolism were changed, suggesting that β-cell PKB activation is not linked to acute changes in β-cell metabolism.
Measurements of mitochondrial ATP, which rapidly increase within 5 min and respiration as oxygen consumption in rat and mouse islets revealed no change with Ex-4, in accordance with glucose oxidation measurements showing no effect of GLP-1. This indicates that GLP-1 does not amplify GSIS via changes in β-cell energy metabolism. Earlier work on the acute actions of GLP-1 on β-cell energy metabolism using the tumoral β-cell line MIN-6 documented that high concentrations (100 nM) of GLP-1 rapidly increased levels of ATP in both the cytosol and the mitochondrial matrix [52]. In contrast, we now report that activation of the GLP-1 receptor by 10 nM Ex-4 failed to alter levels of ATP in the mitochondrial matrix of both rat and mouse islet cells over a period of 5–30 min at different glucose concentrations. Even though in the present study and in [52], early changes in the mitochondrial ATP were measured (within few minutes of incubations), the different results obtained in [52] could be attributable to the use of excessively high GLP-1 concentration and also the tumoral cell line, MIN6. It is also possible that the tumoral β-cells appear to differ from normal β-cells as far as GLP-1 action on β-cell metabolism is concerned, both in terms of lipolysis and energy metabolism. It is possible that the interaction of the endoplasmic reticulum with the mitochondria, as described by Tsuboi and co-workers [52], occurs in MIN6 cells but not in normal rodent β−cells. Thus, Ca2+ released from the endoplasmic reticulum might not be a strong stimulus for mitochondrial ATP production in authentic β-cells.
Collectively the present results indicate that alterations of glucose, lipid and energy metabolism as well as ATP production are not involved in the mechanisms whereby GLP-1 augments GSIS. Perhaps of greater importance is the established ability of GLP-1 to stimulate cAMP production and to activate both PKA and Epac [19], [20]. These two cAMP-binding proteins regulate β-cell functions that are also under the control of glucose metabolism. Such functions include KATP channel activity, cytosolic Ca2+ handling, and insulin granule exocytosis. The present results also show that the higher glucose concentration-dependency of Ex-4 to stimulate insulin secretion is reflected in a similar high glucose concentration dependence for Ex-4-mediated [Ca2+]i increase in mouse β-cells. Ex-4-stimulated cAMP production, which precedes [Ca2+]i increase in islet cells [21], is not influenced by Ad-MtLuc-RFP viral infection indicating that the viral infection did not adversely affect the results of this study. Thus, the insulin secretagogue action of GLP-1 likely arises as a consequence of its ability to facilitate the action of glucose-derived metabolic coupling factors, rather than by directly stimulating metabolic signaling per se.
Another aspect of the present study is that it provides new information about β-cell activation of energy metabolism of this fuel sensing cells with respect to other tissues. In many cell types like muscle tissue, the activation of their primary biological function and energy demanding process drives mitochondrial metabolism via changes in redox, phosphorylation potential or Ca2+. In the present study, it came as a surprise that in spite of a very significant enhancement in GSIS by GLP-1 and Ex-4 at high glucose in both rat and mouse islets, amounting to approximately 20% of the total insulin content in 1 h (in the case of rat islets), no concomitant rise in fuel utilization, O2 consumption or ATP production was noticed. These results indicate that the energy consuming processes activated by GLP-1 and likely other glucoincretins, such as ion ATP-ases, the release of insulin from the ready-releasable pool of secretory granules, the ATP-dependent [59] refilling of this pool and recycling of excess plasma membrane via endocytosis following exocytosis, consume very little energy relatively to overall cell metabolism.
Recent studies in fact revealed that much of the energy needed for the secretory granule membrane fusion comes from the conformational changes of the proteins involved in this process. Thus, the energy needed for the fusion of membranes overcoming the repulsive forces, arises from the formation and folding of v- and t-SNARES [60]. In vitro experiments using artificial lipid bilayers demonstrated that the formation of a single SNARE complex (the v- and t-SNARE complex) provides sufficient energy for the fusion of the outer leaflets of the bilayers [61]. Release and re-cycling of v- and t-SNARES in high-energy form is accomplished by the involvement of the SNAP and NSF ATPase, with the hydrolysis of ATP, which is the only step where metabolic energy is invested [60]. Other proteins including Munc18–1 are also known to provide additional conformational energy to facilitate the fusion of SNARE complexes [60]. Thus, it is likely that the acute augmentation of GSIS at high glucose by GLP-1 is not dependent on elevated substrate oxidation and ATP production but may utilize the Ca2+-mediated and SNAREpin/Munc protein conformational energy dependent fusion of the docked secretory granules with plasma membrane to release their insulin content. Therefore, it seems that the insulin secretion process per se does not consume much of the β-cell metabolic energy even upon marked insulin release.
Finally, the data indicate that the β-cell is different than most cell types in terms of energy metabolism where activation of Ca2+, cAMP, PKB etc signaling promoted by a stimulus drives simultaneously a biological process (for example contraction), and cellular metabolism/ATP production to support it. Thus, β-cell metabolic activation appears to be primarily driven by substrate (fuel) availability, a “push” process [1], [62] rather than a “pull” mechanism secondary to enhanced insulin release. However the data do not discount the possibility that a marked rise in Ca2+ influx promoted by a potent secretagogue like glucose drives mitochondrial metabolism [63]. The present study also emphasizes the major differences that exist between normal and tumoral β-cell in term of metabolic activation.
Acknowledgments
We thank Andreas Wiederkehr (University of Geneva), Erik Joly (Montreal Diabetes Research Center, CR-CHUM) and Vincent Poitout (University of Montreal) for helpful discussions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: MP is funded by Merck-Frosst and the Canadian Institute of Health Research. MP is recipient of Canadian chair in diabetes and metabolism. GGH is funded by the National Institutes of Health (DK045817), EH by the American Diabetes Association (7-08-JF-18) and PJSS by the National Science Foundation (P41-RR-001395. JL is a recipient of a fellowship from Fonds de la Recherche en Sante du Quebec. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Prentki M. New insights into pancreatic beta-cell metabolic signaling in insulin secretion. Eur J Endocrinol. 1996;134:272–286. doi: 10.1530/eje.0.1340272. [DOI] [PubMed] [Google Scholar]
- 2.Prentki M, Joly E, El-Assaad W, Roduit R. Malonyl-CoA Signaling, Lipid Partitioning, and Glucolipotoxicity: Role in beta-Cell Adaptation and Failure in the Etiology of Diabetes. Diabetes. 2002;51(Suppl 3):S405–413. doi: 10.2337/diabetes.51.2007.s405. [DOI] [PubMed] [Google Scholar]
- 3.Wiederkehr A, Wollheim CB. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium. 2008;44:64–76. doi: 10.1016/j.ceca.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Nolan CJ, Prentki M. The islet beta-cell: fuel responsive and vulnerable. Trends Endocrinol Metab. 2008;19:285–291. doi: 10.1016/j.tem.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty Acid Signaling in the {beta}-Cell and Insulin Secretion. Diabetes. 2006;55(Suppl 2):S16–23. doi: 10.2337/db06-s003. [DOI] [PubMed] [Google Scholar]
- 6.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corkey BE, Glennon MC, Chen KS, Deeney JT, Matschinsky FM, et al. A role for malonyl-CoA in glucose-stimulated insulin secretion from clonal pancreatic beta-cells. J Biol Chem. 1989;264:21608–21612. [PubMed] [Google Scholar]
- 8.Wolf BA, Easom RA, McDaniel ML, Turk J. Diacylglycerol synthesis de novo from glucose by pancreatic islets isolated from rats and humans. J Clin Invest. 1990;85:482–490. doi: 10.1172/JCI114463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berne C. The metabolism of lipids in mouse pancreatic islets. The biosynthesis of triacylglycerols and phospholipids. Biochem J. 1975;152:667–673. doi: 10.1042/bj1520667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Hansotia T, Drucker DJ. GIP and GLP-1 as incretin hormones: lessons from single and double incretin receptor knockout mice. Regul Pept. 2005;128:125–134. doi: 10.1016/j.regpep.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, et al. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138:159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- 13.Girard J. The incretins: From the concept to their use in the treatment of type 2 diabetes. Part A: Incretins: Concept and physiological functions. Diabetes Metab. 2008;34:550–559. doi: 10.1016/j.diabet.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, et al. Protein kinase Czeta activation mediates glucagon-like peptide-1-induced pancreatic beta-cell proliferation. Diabetes. 2001;50:2237–2243. doi: 10.2337/diabetes.50.10.2237. [DOI] [PubMed] [Google Scholar]
- 16.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 17.Buteau J, Foisy S, Joly E, Prentki M. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52:124–132. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- 18.Dillon JS, Lu M, Bowen S, Homan LL. The recombinant rat glucagon-like peptide-1 receptor, expressed in an alpha-cell line, is coupled to adenylyl cyclase activation and intracellular calcium release. Exp Clin Endocrinol Diabetes. 2005;113:182–189. doi: 10.1055/s-2005-837526. [DOI] [PubMed] [Google Scholar]
- 19.Holz GG. New insights concerning the glucose-dependent insulin secretagogue action of glucagon-like peptide-1 in pancreatic beta-cells. Horm Metab Res. 2004;36:787–794. doi: 10.1055/s-2004-826165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holz GG. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes. 2004;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holz GG, Heart E, Leech CA. Synchronizing Ca2+ and cAMP oscillations in pancreatic beta-cells: a role for glucose metabolism and GLP-1 receptors? Focus on “regulation of cAMP dynamics by Ca2+ and G protein-coupled receptors in the pancreatic beta-cell: a computational approach”. Am J Physiol Cell Physiol. 2008;294:C4–6. doi: 10.1152/ajpcell.00522.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 23.Antinozzi PA, Ishihara H, Newgard CB, Wollheim CB. Mitochondrial metabolism sets the maximal limit of fuel-stimulated insulin secretion in a model pancreatic beta cell: a survey of four fuel secretagogues. J Biol Chem. 2002;277:11746–11755. doi: 10.1074/jbc.M108462200. [DOI] [PubMed] [Google Scholar]
- 24.Marynissen G, Leclercq-Meyer V, Sener A, Malaisse WJ. Perturbation of pancreatic islet function in glucose-infused rats. Metabolism. 1990;39:87–95. doi: 10.1016/0026-0495(90)90153-4. [DOI] [PubMed] [Google Scholar]
- 25.Roche E, Assimacopoulos-Jeannet F, Witters LA, Perruchoud B, Corkey BE, et al. Induction by glucose of genes coding for glycolytic enzymes in a pancreatic β-cell line (INS-1). J Biol Chem. 1997;272:3091–3098. doi: 10.1074/jbc.272.5.3091. [DOI] [PubMed] [Google Scholar]
- 26.Zhang TM, Maggetto C, Malaisse WJ. Hexose metabolism in pancreatic islets: glycogen synthase and glycogen phosphorylase activities. Biochem Med Metab Biol. 1994;51:129–139. doi: 10.1006/bmmb.1994.1017. [DOI] [PubMed] [Google Scholar]
- 27.Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, et al. Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia. 2004;47:806–815. doi: 10.1007/s00125-004-1379-6. [DOI] [PubMed] [Google Scholar]
- 28.Li L, El-Kholy W, Rhodes CJ, Brubaker PL. Glucagon-like peptide-1 protects beta cells from cytokine-induced apoptosis and necrosis: role of protein kinase B. Diabetologia. 2005;48:1339–1349. doi: 10.1007/s00125-005-1787-2. [DOI] [PubMed] [Google Scholar]
- 29.Park S, Dong X, Fisher TL, Dunn S, Omer AK, et al. Exendin-4 uses Irs2 signaling to mediate pancreatic beta cell growth and function. J Biol Chem. 2006;281:1159–1168. doi: 10.1074/jbc.M508307200. [DOI] [PubMed] [Google Scholar]
- 30.Ishiki M, Klip A. Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology. 2005;146:5071–5078. doi: 10.1210/en.2005-0850. [DOI] [PubMed] [Google Scholar]
- 31.Cazzolli R, Carpenter L, Biden TJ, Schmitz-Peiffer C. A role for protein phosphatase 2A-like activity, but not atypical protein kinase Czeta, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes. 2001;50:2210–2218. doi: 10.2337/diabetes.50.10.2210. [DOI] [PubMed] [Google Scholar]
- 32.Moule SK, Welsh GI, Edgell NJ, Foulstone EJ, Proud CG, et al. Regulation of protein kinase B and glycogen synthase kinase-3 by insulin and beta-adrenergic agonists in rat epididymal fat cells. Activation of protein kinase B by wortmannin-sensitive and -insensitive mechanisms. J Biol Chem. 1997;272:7713–7719. doi: 10.1074/jbc.272.12.7713. [DOI] [PubMed] [Google Scholar]
- 33.Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, et al. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 34.da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, et al. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J. 2003;371:761–774. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prentki M, Madiraju SR. Glycerolipid metabolism and signaling in health and disease. Endocr Rev. 2008;29:647–676. doi: 10.1210/er.2008-0007. [DOI] [PubMed] [Google Scholar]
- 36.Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, et al. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2006;290:E500–508. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- 37.Peyot ML, Nolan CJ, Soni K, Joly E, Lussier R, et al. Hormone-sensitive lipase has a role in lipid signaling for insulin secretion but is nonessential for the incretin action of glucagon-like peptide 1. Diabetes. 2004;53:1733–1742. doi: 10.2337/diabetes.53.7.1733. [DOI] [PubMed] [Google Scholar]
- 38.Nolan CJ, Leahy JL, Delghingaro-Augusto V, Moibi J, Soni K, et al. Beta cell compensation for insulin resistance in Zucker fatty rats: increased lipolysis and fatty acid signalling. Diabetologia. 2006;49:2120–2130. doi: 10.1007/s00125-006-0305-5. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham BA, Richard AM, Dillon JS, Daley JT, Civelek VN, et al. Glucagon-like peptide 1 and fatty acids amplify pulsatile insulin secretion from perifused rat islets. Biochem J. 2003;369:173–178. doi: 10.1042/BJ20021196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaney GC, Civelek VN, Richard AM, Dillon JS, Deeney JT, et al. Glucagon-like peptide 1 stimulates lipolysis in clonal pancreatic beta- cells (HIT).[In Process Citation]. Diabetes. 2001;50:56–62. doi: 10.2337/diabetes.50.1.56. [DOI] [PubMed] [Google Scholar]
- 41.Gotoh M, Maki T, Satomi S, Porter J, Bonner-Weir S, et al. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation. 1987;43:725–730. doi: 10.1097/00007890-198705000-00024. [DOI] [PubMed] [Google Scholar]
- 42.Heart E, Smith PJ. Rhythm of the beta-cell oscillator is not governed by a single regulator: multiple systems contribute to oscillatory behavior. Am J Physiol Endocrinol Metab. 2007;292:E1295–1300. doi: 10.1152/ajpendo.00648.2006. [DOI] [PubMed] [Google Scholar]
- 43.Massa ML, Borelli MI, Del Zotto H, Gagliardino JJ. Changes induced by sucrose administration on glucose metabolism in pancreatic islets in normal hamsters. J Endocrinol. 2001;171:551–556. doi: 10.1677/joe.0.1710551. [DOI] [PubMed] [Google Scholar]
- 44.Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, et al. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic beta cells. J Physiol. 2005;566:173–188. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chepurny OG, Holz GG. A novel cyclic adenosine monophosphate responsive luciferase reporter incorporating a nonpalindromic cyclic adenosine monophosphate response element provides optimal performance for use in G protein coupled receptor drug discovery efforts. J Biomol Screen. 2007;12:740–746. doi: 10.1177/1087057107301856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osbourn DM, Sanger RH, Smith PJ. Determination of single-cell oxygen consumption with impedance feedback for control of sample-probe separation. Anal Chem. 2005;77:6999–7004. doi: 10.1021/ac050326w. [DOI] [PubMed] [Google Scholar]
- 47.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ainscow EK, Rutter GA. Glucose-stimulated oscillations in free cytosolic ATP concentration imaged in single islet beta-cells: evidence for a Ca2+-dependent mechanism. Diabetes. 2002;51(Suppl 1):S162–170. doi: 10.2337/diabetes.51.2007.s162. [DOI] [PubMed] [Google Scholar]
- 49.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Z, Habener JF. Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic beta cell proliferation. J Biol Chem. 2008;283:8723–8735. doi: 10.1074/jbc.M706105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Widenmaier SB, Sampaio AV, Underhill TM, McIntosh CH. Non-canonical activation of Akt/PKB in beta -cells by the incretin hormone glucose-dependent insulinotropic polypeptide (GIP). J Biol Chem. 2009;284:10764–10773. doi: 10.1074/jbc.M809116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuboi T, da Silva Xavier G, Holz GG, Jouaville LS, Thomas AP, et al. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem J. 2003;369:287–299. doi: 10.1042/BJ20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roduit R, Nolan C, Alarcon C, Moore P, Barbeau A, et al. A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes. 2004;53:1007–1019. doi: 10.2337/diabetes.53.4.1007. [DOI] [PubMed] [Google Scholar]
- 54.Herrero L, Rubi B, Sebastian D, Serra D, Asins G, et al. Alteration of the malonyl-CoA/carnitine palmitoyltransferase I interaction in the beta-cell impairs glucose-induced insulin secretion. Diabetes. 2005;54:462–471. doi: 10.2337/diabetes.54.2.462. [DOI] [PubMed] [Google Scholar]
- 55.Maechler P. Mitochondria as the conductor of metabolic signals for insulin exocytosis in pancreatic beta-cells. Cell Mol Life Sci. 2002;59:1803–1818. doi: 10.1007/PL00012507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mulder H, Yang S, Winzell MS, Holm C, Ahren B. Inhibition of lipase activity and lipolysis in rat islets reduces insulin secretion. Diabetes. 2004;53:122–128. doi: 10.2337/diabetes.53.1.122. [DOI] [PubMed] [Google Scholar]
- 57.Sorhede Winzell M, Ahren B. Glucagon-like peptide-1 and islet lipolysis. Horm Metab Res. 2004;36:795–803. doi: 10.1055/s-2004-826166. [DOI] [PubMed] [Google Scholar]
- 58.Richards SK, Parton LE, Leclerc I, Rutter GA, Smith RM. Over-expression of AMP-activated protein kinase impairs pancreatic {beta}-cell function in vivo. J Endocrinol. 2005;187:225–235. doi: 10.1677/joe.1.06413. [DOI] [PubMed] [Google Scholar]
- 59.Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev. 2002;18:451–463. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 60.Malsam J, Kreye S, Sollner TH. Membrane fusion: SNAREs and regulation. Cell Mol Life Sci. 2008;65:2814–2832. doi: 10.1007/s00018-008-8352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li F, Pincet F, Perez E, Eng WS, Melia TJ, et al. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat Struct Mol Biol. 2007;14:890–896. doi: 10.1038/nsmb1310. [DOI] [PubMed] [Google Scholar]
- 62.Matschinsky FM. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 63.Sener A, Rasschaert J, Malaisse WJ. Hexose metabolism in pancreatic islets. Participation of Ca2(+)-sensitive 2-ketoglutarate dehydrogenase in the regulation of mitochondrial function. Biochim Biophys Acta. 1990;1019:42–50. doi: 10.1016/0005-2728(90)90122-k. [DOI] [PubMed] [Google Scholar]