Abstract
Development of a routine detection assay for Campylobacter jejuni and Campylobacter coli in clinical specimens was undertaken by using the polymerase chain reaction (PCR). An oligonucleotide primer pair from a conserved 5' region of the flaA gene of C. coli VC167 was used to amplify a 450-bp region by PCR. The primer pair specifically detected 4 strains of C. coli and 47 strains of C. jejuni; but it did not detect strains of Campylobacter fetus, Campylobacter lari, Campylobacter upsaliensis, Campylobacter cryaerophila, Campylobacter butzleri, Campylobacter hyointestinalis, Wolinella recta, Helicobacter pylori, Escherichia coli, Shigella spp., Salmonella spp., Vibrio cholerae, Citrobacter freundii, or Aeromonas spp. By using a nonradioactively labeled probe internal to the PCR product, the assay could detect as little as 0.0062 pg of purified C. coli DNA, or the equivalent of four bacteria. In stools seeded with C. coli cells, the probe could detect between 30 and 60 bacteria per PCR assay. The assay was also successfully used to detect C. coli in rectal swab specimens from experimentally infected rabbits and C. jejuni in human stool samples.
Full text
PDF

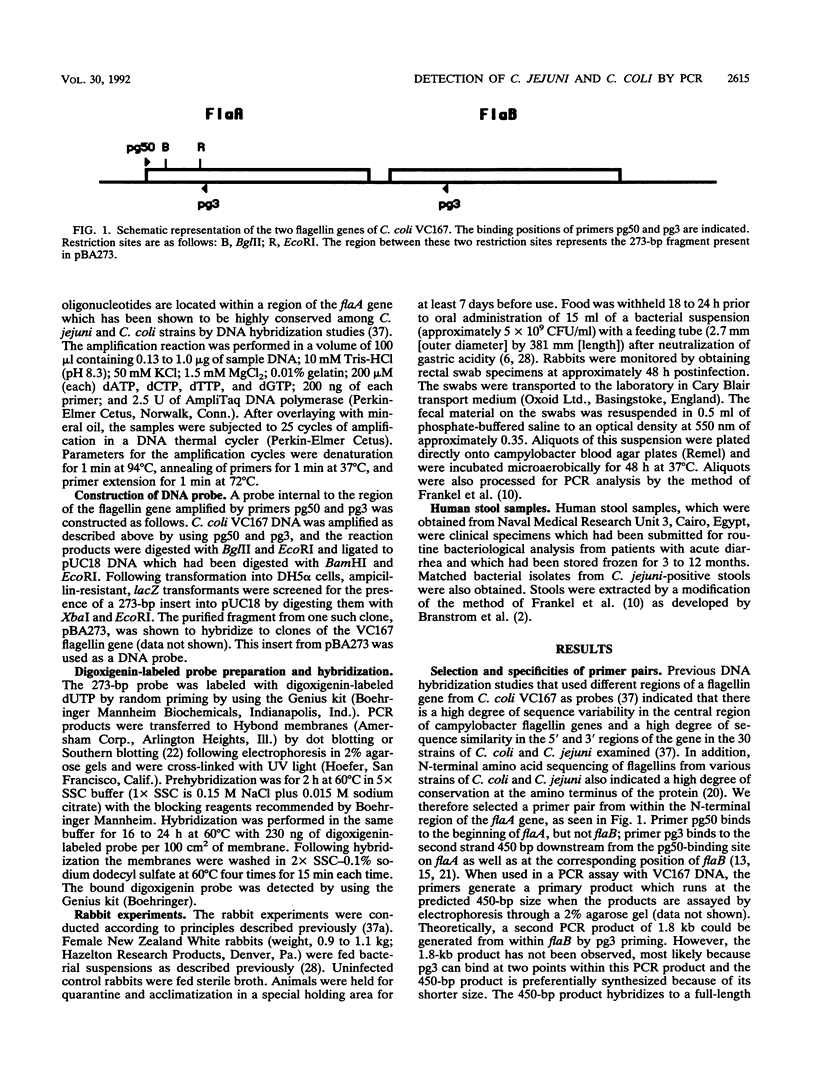




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstain J. M., Grimprel E., Lukehart S. A., Norgard M. V., Radolf J. D. Sensitive detection of Treponema pallidum by using the polymerase chain reaction. J Clin Microbiol. 1991 Jan;29(1):62–69. doi: 10.1128/jcm.29.1.62-69.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N., Taylor D. E. Use of pulsed-field agarose gel electrophoresis to size genomes of Campylobacter species and to construct a SalI map of Campylobacter jejuni UA580. J Bacteriol. 1990 Sep;172(9):5211–5217. doi: 10.1128/jb.172.9.5211-5217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll P., Phillips K., Tenover F. C. Evaluation of a rapid method of extracting DNA from stool samples for use in hybridization assays. J Clin Microbiol. 1989 Oct;27(10):2245–2248. doi: 10.1128/jcm.27.10.2245-2248.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cray W. C., Jr, Tokunaga E., Pierce N. F. Successful colonization and immunization of adult rabbits by oral inoculation with Vibrio cholerae O1. Infect Immun. 1983 Aug;41(2):735–741. doi: 10.1128/iai.41.2.735-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit D., Steyn L., Shoemaker S., Sogin M. Direct detection of Mycobacterium tuberculosis in clinical specimens by DNA amplification. J Clin Microbiol. 1990 Nov;28(11):2437–2441. doi: 10.1128/jcm.28.11.2437-2441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I. The polymerase chain reaction. A new method of using molecular genetics for medical diagnosis. N Engl J Med. 1990 Jan 18;322(3):178–183. doi: 10.1056/NEJM199001183220307. [DOI] [PubMed] [Google Scholar]
- Fischer S. H., Nachamkin I. Common and variable domains of the flagellin gene, flaA, in Campylobacter jejuni. Mol Microbiol. 1991 May;5(5):1151–1158. doi: 10.1111/j.1365-2958.1991.tb01888.x. [DOI] [PubMed] [Google Scholar]
- Frankel G., Giron J. A., Valmassoi J., Schoolnik G. K. Multi-gene amplification: simultaneous detection of three virulence genes in diarrhoeal stool. Mol Microbiol. 1989 Dec;3(12):1729–1734. doi: 10.1111/j.1365-2958.1989.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Guerry P., Alm R. A., Power M. E., Logan S. M., Trust T. J. Role of two flagellin genes in Campylobacter motility. J Bacteriol. 1991 Aug;173(15):4757–4764. doi: 10.1128/jb.173.15.4757-4764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., Logan S. M., Thornton S., Trust T. J. Genomic organization and expression of Campylobacter flagellin genes. J Bacteriol. 1990 Apr;172(4):1853–1860. doi: 10.1128/jb.172.4.1853-1860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyams K. C., Bourgeois A. L., Merrell B. R., Rozmajzl P., Escamilla J., Thornton S. A., Wasserman G. M., Burke A., Echeverria P., Green K. Y. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991 Nov 14;325(20):1423–1428. doi: 10.1056/NEJM199111143252006. [DOI] [PubMed] [Google Scholar]
- John G. H., Carlson J. O., Kimberling C. V., Ellis R. P. Polymerase chain reaction amplification of the constant and variable regions of the Bacteroides nodosus fimbrial gene. J Clin Microbiol. 1990 Nov;28(11):2456–2461. doi: 10.1128/jcm.28.11.2456-2461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Harris L. A., Trust T. J. Isolation and characterization of Campylobacter flagellins. J Bacteriol. 1987 Nov;169(11):5072–5077. doi: 10.1128/jb.169.11.5072-5077.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J., Guerry P. Evidence for posttranslational modification and gene duplication of Campylobacter flagellin. J Bacteriol. 1989 Jun;171(6):3031–3038. doi: 10.1128/jb.171.6.3031-3038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Nuijten P. J., van Asten F. J., Gaastra W., van der Zeijst B. A. Structural and functional analysis of two Campylobacter jejuni flagellin genes. J Biol Chem. 1990 Oct 15;265(29):17798–17804. [PubMed] [Google Scholar]
- Olive D. M. Detection of enterotoxigenic Escherichia coli after polymerase chain reaction amplification with a thermostable DNA polymerase. J Clin Microbiol. 1989 Feb;27(2):261–265. doi: 10.1128/jcm.27.2.261-265.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Rollins D. M., Haberberger R. L., Jr, Green A. E., Habash L., Strocko S., Walker R. I. Significance of flagella in colonization resistance of rabbits immunized with Campylobacter spp. Infect Immun. 1991 Jul;59(7):2259–2264. doi: 10.1128/iai.59.7.2259-2264.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk P. J., Zoltowska B., Trust T. J., Lane D. J., Olsen G. J., Pace N. R., Stahl D. A. Campylobacter pylori, the spiral bacterium associated with human gastritis, is not a true Campylobacter sp. J Bacteriol. 1987 May;169(5):2137–2141. doi: 10.1128/jb.169.5.2137-2141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop R. M., 2nd, Smibert R. M., Johnson J. L., Krieg N. R. Differential characteristics of catalase-positive campylobacters correlated with DNA homology groups. Can J Microbiol. 1984 Jul;30(7):938–951. doi: 10.1139/m84-147. [DOI] [PubMed] [Google Scholar]
- Shirai H., Nishibuchi M., Ramamurthy T., Bhattacharya S. K., Pal S. C., Takeda Y. Polymerase chain reaction for detection of the cholera enterotoxin operon of Vibrio cholerae. J Clin Microbiol. 1991 Nov;29(11):2517–2521. doi: 10.1128/jcm.29.11.2517-2521.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl Environ Microbiol. 1988 Sep;54(9):2185–2191. doi: 10.1128/aem.54.9.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Hiratsuka K. Use of non-radioactive DNA probes for detection of Campylobacter jejuni and Campylobacter coli in stool specimens. Mol Cell Probes. 1990 Aug;4(4):261–271. doi: 10.1016/0890-8508(90)90018-u. [DOI] [PubMed] [Google Scholar]
- Thornton S. A., Logan S. M., Trust T. J., Guerry P. Polynucleotide sequence relationships among flagellin genes of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1990 Aug;58(8):2686–2689. doi: 10.1128/iai.58.8.2686-2689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine J. L., Arthur R. R., Mobley H. L., Dick J. D. Detection of Helicobacter pylori by using the polymerase chain reaction. J Clin Microbiol. 1991 Apr;29(4):689–695. doi: 10.1128/jcm.29.4.689-695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eys G. J., Gravekamp C., Gerritsen M. J., Quint W., Cornelissen M. T., Schegget J. T., Terpstra W. J. Detection of leptospires in urine by polymerase chain reaction. J Clin Microbiol. 1989 Oct;27(10):2258–2262. doi: 10.1128/jcm.27.10.2258-2262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernars K., Heuvelman C. J., Chakraborty T., Notermans S. H. Use of the polymerase chain reaction for direct detection of Listeria monocytogenes in soft cheese. J Appl Bacteriol. 1991 Feb;70(2):121–126. doi: 10.1111/j.1365-2672.1991.tb04437.x. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Gillis T. P., Booth R. J., Looker D., Watson J. D. The use of a specific DNA probe and polymerase chain reaction for the detection of Mycobacterium leprae. J Infect Dis. 1990 Jul;162(1):193–200. doi: 10.1093/infdis/162.1.193. [DOI] [PubMed] [Google Scholar]
- Wise D. J., Weaver T. L. Detection of the Lyme disease bacterium, Borrelia burgdorferi, by using the polymerase chain reaction and a nonradioisotopic gene probe. J Clin Microbiol. 1991 Jul;29(7):1523–1526. doi: 10.1128/jcm.29.7.1523-1526.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]





