Summary
The Hippo tumor-suppressor pathway controls tissue growth in Drosophila and mammals by regulating cell proliferation and apoptosis. The Hippo pathway includes the Fat cadherin, a transmembrane protein, which acts upstream of several other components that form a kinase cascade that culminates in the regulation of gene expression through the transcriptional coactivator Yorkie (Yki). Our previous work in Drosophila indicated that Merlin (Mer) and Expanded (Ex) are members of the Hippo pathway and act upstream of the Hippo kinase. In contrast to this model, it was suggested that Mer and Ex primarily regulate membrane dynamics and receptor trafficking, thereby affecting Hippo pathway activity only indirectly. Here, we examined the effects of Mer, Ex and the Hippo pathway on the size of the apical membrane and on apical-basal polarity complexes. We found that mer;ex double mutant imaginal disc cells have significantly increased levels of apical membrane determinants, such as Crb, aPKC and Patj. These phenotypes were shared with mutations in other Hippo pathway components and required Yki, indicating that Mer and Ex signal through the Hippo pathway. Interestingly, however, whereas Crb was required for the accumulation of other apical proteins and for the expansion of the apical domain observed in Hippo pathway mutants, its elimination did not significantly reverse the overgrowth phenotype of warts mutant cells. Therefore, Hippo signaling regulates cell polarity complexes in addition to and independently of its growth control function in imaginal disc cells.
Keywords: Cell polarity, Drosophila, Growth control, Hippo
Introduction
The conserved Hippo signal transduction pathway has emerged as a critical regulator of tissue size in Drosophila and mammals (Pan, 2007; Reddy and Irvine, 2008). In Drosophila, loss of Hippo signaling results in adults with severely overgrown tissues. These overgrowth phenotypes are produced because hippo (hpo) mutant cells show extra proliferation and are resistant to the apoptotic signals that would normally eliminate extra cells (Harvey et al., 2003; Jia et al., 2003; Pantalacci et al., 2003; Udan et al., 2003; Wu et al., 2003). The Hippo pathway thus regulates cell proliferation and apoptosis. Additionally, loss of Hippo signaling gives rise to cells with morphological defects. Here, we investigated the molecular basis of the effects on cell architecture and whether these effects are related to or independent of the growth control function of Hippo.
In the most common model of the Hippo pathway, the atypical cadherin Fat (Ft) acts as a receptor that transduces extracellular signals leading to the activation of the Hpo kinase (Bennett and Harvey, 2006; Silva et al., 2006; Willecke et al., 2006). Ft function requires the FERM domain adaptor protein Expanded (Ex) and the unconventional myosin Dachs (D) (Bennett and Harvey, 2006; Cho et al., 2006; Cho and Irvine, 2004; Mao et al., 2006; Silva et al., 2006; Willecke et al., 2006; Willecke et al., 2008). Ex acts, in part, redundantly to the related protein Merlin (Mer), the Drosophila homolog of the human neurofibromatosis type 2 (NF2) tumor suppressor gene. Although ex or mer single mutant cells have phenotypes that are weaker than those of hpo mutants, mer;ex double mutant cells phenocopy hpo mutants (Hamaratoglu et al., 2006). Genetic experiments indicate that Ft acts upstream of Ex and D (Reddy and Irvine, 2008), and that Mer acts in parallel to the Ft branch to regulate the Hippo pathway (Silva et al., 2006; Willecke et al., 2006). The molecular mechanisms of signal transduction upstream of Hpo, however, are not known and may involve interactions at several levels (Reddy and Irvine, 2008). Once activated, Hpo phosphorylates and activates Warts (Wts), a kinase that inhibits the activity of the transcriptional coactivator Yorkie (Yki) (Dong et al., 2007; Huang et al., 2005; Oh and Irvine, 2008; Wu et al., 2003). Yki is a positive regulator of growth, and overexpression of Yki causes severe overgrowths that resemble the loss-of-function phenotypes of the other pathway members, whereas cells mutant for yki grow poorly (Huang et al., 2005). Yki forms a complex with the Scalloped transcription factor and drives cell proliferation and cell survival through induction of target genes, such as the cell cycle regulator Cyclin E, the anti-apoptotic protein DIAP1, and the growth controlling microRNA bantam (Goulev et al., 2008; Nolo et al., 2006; Thompson and Cohen, 2006; Wu et al., 2008; Zhang et al., 2008). Hippo signaling thus suppresses cell proliferation by inhibiting the activity of Yki thereby suppressing the expression of its target genes.
In addition to their proliferative advantage and resistance to apoptosis, cells lacking hpo or wts have morphological defects (Justice et al., 1995; Wu et al., 2003; Xu et al., 1995). Clones of cells homozygous mutant for wts produce abnormal bristles and wts mutant cells in imaginal discs show apical hypertrophy (Justice et al., 1995). Here, we address how the Hippo pathway regulates apical membrane composition and whether this function contributes to the growth control function of the Hippo pathway. In relation to these questions, we have investigated the function of Mer and Ex in these processes, since it was found that the levels of some transmembrane receptors such as Ft and DER, the Drosophila EGF receptor, are elevated in mer;ex double mutant clones (Maitra et al., 2006). These findings led to the proposal that the primary role of Mer and Ex is in receptor endocytosis, raising the question of whether the effect on cell surface receptors is a Hippo-dependent or -independent function of Mer and Ex.
Results
Merlin and Expanded signaling requires Yorkie
In the model presented by Maitra et al. (Maitra et al., 2006) the effects of Mer and Ex on Hippo signaling may be indirect, for example by regulating the levels of a receptor of the Hippo pathway. This model is thus in contrast to the one presented above where Mer and Ex are integral components of the Hippo pathway, and predicts that the effects of Mer and Ex on receptor levels are independent of their effect on the Hippo pathway. To distinguish between these two models, we thus investigated whether the effects of Mer and Ex on receptor levels depend on the Hippo pathway.
First, we tested whether transmembrane receptors accumulate in mutants of members of the Hippo pathway. We found, as previously reported, that membrane levels of DER and Ft were elevated in mer;ex double mutant clones (Fig. 1A,B) (Maitra et al., 2006). The effects on DER levels were most conspicuous in eye discs posterior to the morphogenetic furrow, whereas significant upregulation of Ft was observed throughout clones in eye and wing discs. Importantly, we found that clones mutant for hpo or wts showed similar effects on DER and Ft levels (Fig. 1C,D; see also Fig. 8F; and data not shown). The upregulation of DER and Ft is thus not unique to mer;ex mutant cells but is shared with mutants in other Hippo pathway components. Second, we tested whether the accumulation of DER and Ft requires Yki function. To test this we generated mer;ex,yki triple mutant and yki;wts double mutant clones. We found that neither DER nor Ft levels were elevated in mer;ex,yki triple or in yki;wts double mutant clones (Fig. 1E,F, and data not shown). Notably, mer;ex,yki triple mutant clones and yki;wts double mutant clones were only occasionally observed, and these were always much smaller than their wild-type twin clones, indicating that Yki is essential for mer;ex and wts mutant cells to survive and proliferate (Fig. 1E,F, Fig. 2B) (Huang et al., 2005). In summary, the accumulation of signaling receptors in the absence of mer and ex is shared with other Hippo pathway mutants and requires Yki function, demonstrating that the regulation of receptor levels is a downstream effect of the Hippo pathway and supporting a model in which Mer and Ex act through the Hippo pathway to exert their effect.
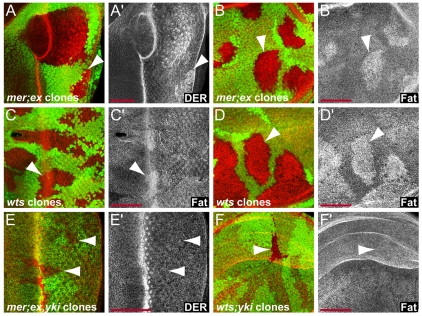
Fig. 1.
Merlin and Expanded signaling requires Yorkie. (A) Third instar eye imaginal disc containing mer;ex mutant clones marked by the absence of GFP expression (in green). Mutant clones had elevated membrane levels of DER (red in A, grayscale in A′) behind the morphogenetic furrow. Arrowheads point to clone borders in these and all subsequent panels. (B) mer;ex mutant clones in a wing disc showed elevated levels of Ft. (C,D) wts mutant clones in an eye (C) and a wing (D) disc accumulated Ft at the membrane. (E) mer;ex,yki triple mutant clones did not upregulate DER in an eye disc. (F) yki; wts double mutant clones did not upregulate Ft in a wing disc. Anterior is to the left and dorsal is up for all discs. Scale bars: 50 μm.
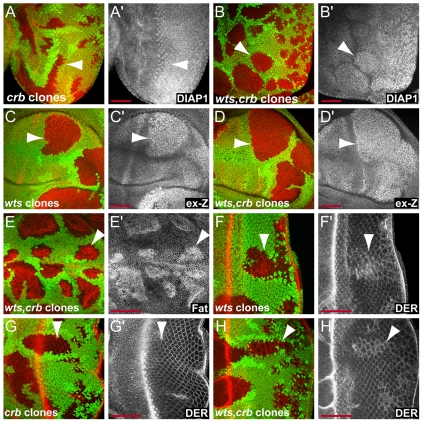
Fig. 8.
Crb is not required for Hippo target gene upregulation or receptor accumulation in warts mutant cells. (A,B) Eye imaginal discs containing crb and wts,crb double mutant clones marked by lack of GFP expression (green in A and B) stained for DIAP1 expression (red in A and B and grayscale in A′ and B′). (C,D) Wing imaginal discs containing wts and wts,crb double mutant clones marked by lack of GFP expression (green in C and D) stained for β-gal to detect the expression of an enhancer trap reporter insertion into the ex gene (red in C and D and grayscale in C′ and D′). (E-H) wts (F) or wts,crb double (E and H) mutant clones marked by lack of GFP expression (green in E, F and H) upregulate Fat and DER (red in E, F and H, grayscale in E′, F′ and H′). (G) Lack of Crb (marked by GFP expression in green) does not affect DER expression (red in G, grayscale in G′). Scale bars: 50 μm.
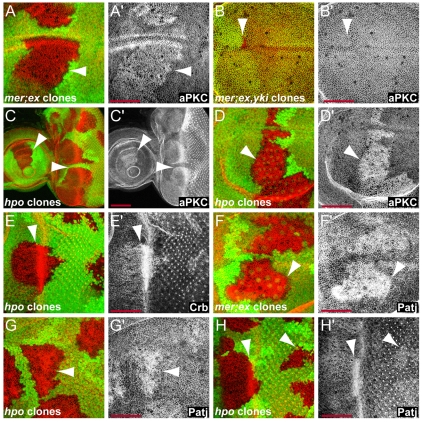
Fig. 2.
Hippo signaling regulates the levels of the apical membrane complex proteins aPKC, Crb and Patj in eye and wing discs. (A) Third instar wing discs containing mer;ex mutant clones marked by the absence of GFP expression (green) had increased membrane levels of aPKC (red in A and grayscale in A′). (B) mer;ex,yki triple mutant clones marked by the absence of Ex expression (green in B) had normal levels of aPKC (red in B and grayscale in B′). (C-H) mer;ex or hpo mutant clones had elevated levels of aPKC (C,D), Crb (E) and Patj (F-H) (red in overlay channels, grayscale in C′, D′,E′, F′, G′ and H′) in wing (D,F,G) and eye (C,E,H) discs. Anterior is to the left and dorsal is up for all discs. Scale bars: 50 μm.
Hippo signaling regulates the levels of the apical-basal cell polarity proteins aPKC, Crb and Patj in eye and wing discs
In addition to the accumulation of cell-surface receptors, wts mutant cells have expanded apical membranes (Justice et al., 1995). To gain insight into the molecular basis of these phenotypes, we examined the distribution of proteins that regulate apical-basal cell polarity and the amount of apical membrane in tissues mosaic for ft, mer;ex, hpo and wts. In particular, we focused on three protein complexes, the Atypical protein kinase C (aPKC) complex, the Crumbs (Crb) complex, and the Discs large (Dlg) complex. These highly conserved complexes are spatially localized along the apical-basal axis of epithelial cells in many different organisms and are essential to polarize cells along this axis. They are required to position cell-cell junctions and to divide the plasma membrane into the apical and basolateral domains (Assemat et al., 2008; Henrique and Schweisguth, 2003; Johnson and Wodarz, 2003; Suzuki and Ohno, 2006). The aPKC complex is the most apical complex and is composed of the Partition defective 6 (Par6), Bazooka (Par3) and aPKC proteins. The Crb complex also localizes apically and is composed of Patj, Stardust, and Crb. The Dlg complex, composed of Dlg, Scribble and Lethal giant larvae (Lgl), localizes to basolateral membranes. Work in embryonic epithelia showed that the aPKC and Crb complexes exclude the basal Dlg complex proteins from the apical membrane, whereas the Dlg complex excludes the apical proteins from the basolateral domain. These interactions then establish the proper balance between the different membrane domains (Bilder et al., 2003; Tanentzapf and Tepass, 2003).
When we examined the levels and distribution of the apical complex components Patj, Crb and aPKC, we found that their membrane levels were significantly increased in ft, mer;ex, hpo and wts mutant clones, as well as in cells that overexpress Yki (see Fig. 2 for mer;ex and hpo; Figs 4 and 5 for wts; supplementary material Fig. S1 for ft clones; and Fig. 4 for Yki overexpression). Notably, these increases were observed in many tissues, including the wing, antenna and eye imaginal discs. In eye disc mutant clones aPKC, Patj and Crb levels were affected anterior to the morphogenetic furrow, where cells are still uncommitted, as well as posterior to the furrow, where cells start to differentiate into photoreceptors and other cell types (Fig. 2C,E,H). This is in contrast to the effects on DER, which was significantly upregulated only in eye discs posterior to the furrow. The upregulation of apical complexes was thus much more pronounced and uniform than effects on DER and other transmembrane receptors. In all cases, the upregulation of aPKC, Patj and Crb was cell autonomous (Fig. 2). Similar to the regulation of DER and Ft, these effects required Yki, since the increase of aPKC in mer;ex mutant clones was no longer observed in mer;ex,yki triple mutant cells (Fig. 2A,B). Altogether, we conclude that the Hippo pathway regulates the membrane levels of apical complexes and that this effect of the Hippo pathway is a global effect, since upregulation of apical complexes was observed in many tissues and cell types.
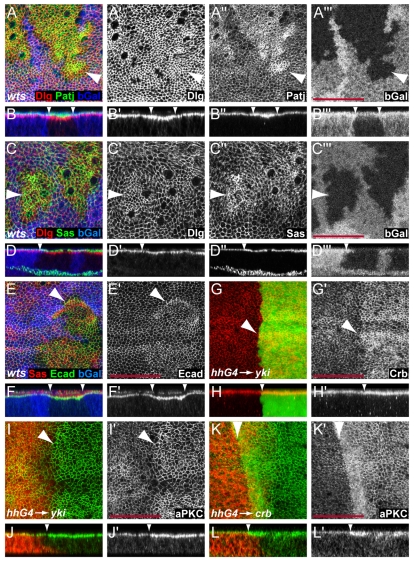
Fig. 4.
Accumulation of apical complexes in warts mutant cells leads to apical domain expansion. (A-F) Third instar wing imaginal discs containing wts mutant clones marked by the absence of β-gal expression (blue in A-F and gray in A′″-D′″). Discs were stained for the apical markers Patj (green in A,B and grey in A″,B″) and Sas (green in C,D, red in E,F and grey in C″,D″), the basolateral marker Dlg (red in A-D and grey in A′-D′), and the adherens junction marker E-cad (green in E,F and grey in E′,F′). (G-K) Crb (red in G,H and green in G′,H′) and aPKC levels (green in I-L and grey in I′-L′) were elevated in wing discs overexpressing Yki (G-J) and Crb (K,L) in their posterior compartments under the control of hh-Gal4. Posterior compartments are marked by coexpression of GFP (green in G,H). Anterior compartments are marked by Ci labeling (red in I-L). (B,D,F,H,J,L) Optical sections are shown through the wing pouch of the corresponding discs above them. Arrowheads mark clone borders. Scale bars: 50 μm.
Fig. 5.
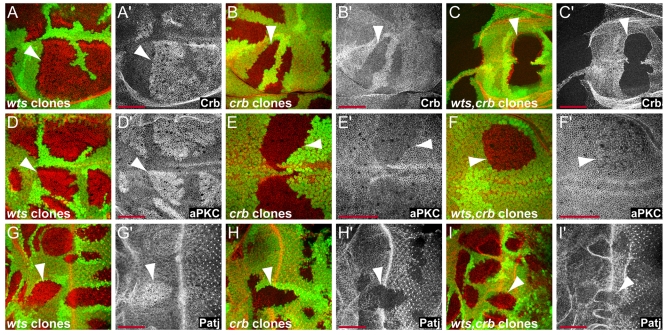
Crb is required for aPKC and Patj localization. (A-C) Crb, (D-F) aPKC, and (G-I) Patj expression in wts (left), crb (middle), and wts,crb (right) double mutant clones in wing (A-F) and eye (G-I) discs. Crb, aPKC and Patj levels were elevated in wts mutant clones, but the levels of Patj and aPKC in membranes were strongly reduced in crb and wts,crb mutant clones. Crb was lost in crb and wts,crb mutant clones as expected. Clones are marked by the absence of GFP expression (green) and Crb, aPKC, and Patj stainings are shown in red in the composite images and in grayscale to their right. Anterior is to the left and dorsal is up for all discs. Scale bars: 50 μm.
The regulation of apical complexes is a specific downstream effect of the Hippo pathway
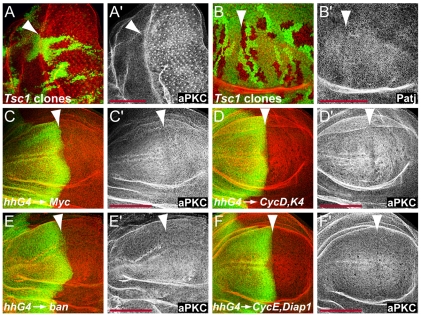
Next, we asked whether the accumulation of apical proteins was a specific effect of the Hippo pathway or a secondary consequence of the extra growth and proliferation induced in the absence of hpo. Therefore, we tested whether the levels of apical complexes were also controlled by other regulators of cell growth and proliferation. We assayed the expression of apical determinants in eye and wing imaginal discs containing Tsc1 mutant cell clones and in wing discs that overexpressed Cyclin D-Cdk4, Myc or activated Ras (RasV12) in the posterior compartment. We found no significant changes in aPKC, Crb or Patj membrane levels in these experiments (Fig. 3A-D, and data not shown). Promoting cell growth and accelerating cell division through overexpression of Cyclin D-Cdk4, Myc or activated Ras, or by loss of Tsc1, was thus not sufficient to induce changes in apical domain composition. Therefore, the regulation of apical complexes is a specific downstream effect of the Hpo pathway.
Fig. 3.
The regulation of apical complexes is a specific downstream effect of the Hippo pathway. (A,B) Tsc1 mutant clones marked by the absence of GFP expression (green in A and B) had normal levels of aPKC and Patj (red in A, B and grayscale in A′, B′) in eye (A) and wing (B) discs. (C-F) aPKC levels (red in the overlay channels, grayscale in C′, D′,E′ and F′) were not affected in wing discs overexpressing Myc (C), CycD and Cdk4 (D), the bantam miRNA (E), or CycE and DIAP1 (F) in their posterior compartments under the control of hh-Gal4. Anterior compartments are marked with Ci labeling (green in C, D, E and F). Anterior is to the left and dorsal is up for all discs. Arrowheads point to anterior-posterior compartment boundaries. Scale bars: 100 μm.
We then wanted to test whether the accumulation of apical complexes was in parallel to or downstream of the induction of other known Hippo target genes. We found that in wing discs, overexpressing the bantam miRNA or CycE and DIAP1 did not significantly affect the levels of aPKC (Fig. 3E,F). Another target gene of the Hippo pathway is ex, and Ex protein accumulates at the plasma membrane in hpo mutant cells (Hamaratoglu et al., 2006). However, the accumulation of Ex in hpo mutant cells is not responsible for the elevated membrane levels of apical complexes since aPKC and Patj still accumulate in mer;ex mutant clones, which no longer have Ex (Fig. 2A,F). We conclude that Hippo signaling regulates the apical domain complexes independently of its other known targets cycE, diap1, ex and bantam.
Accumulation of apical complexes in hippo mutant cells leads to apical domain expansion
The upregulation of apical domain complexes raised the questions of whether also the localization of apical-basal polarity complexes is affected and whether the observed accumulation is responsible for the morphological defects observed in wts mutant cells. We thus examined the localization of apical basal determinants and the adherens junction protein E-cadherin (E-cad) in optical sections through wing discs with wts mutant clones. We found that the increased amounts of Patj and aPKC were still localized at the apical ends of wts mutant cells, although their localization domains were expanded (Fig. 4A,B, and data not shown). Similarly, the apical marker Stranded at second (Sas) was upregulated and expanded in wts mutant clones (Fig. 4C,D). In contrast to the apical determinants and Sas, the levels and distribution of the basolateral determinant Dlg were not affected in wts mutant clones (Fig. 4A-D). In addition, the levels of E-cad were slightly elevated in wts mutant cells (Fig. 4E,F). Importantly, however, colabeling with apical and basolateral markers showed that the apical, junctional and basolateral domains were still separate in wts mutant clones with the apical domains expanded and the basolateral domains pushed more basally (Fig. 4B,D,F). The elevated levels of apical determinants such as Crb and aPKC are phenocopied in cells overexpressing Yki (Fig. 4G-J). We conclude that loss of Hippo signaling specifically affects the membrane levels of apical domain proteins but does not disrupt the mechanisms that establish and maintain cell polarity and cell junctions.
In early embryonic epithelia, overexpression of Crb affects the distribution of apical as well as basolateral markers and leads to an expansion of the apical domain (Izaddoost et al., 2002; Nam and Choi, 2003; Pellikka et al., 2002; Sotillos et al., 2004; Wodarz et al., 1995). Ectopic Crb recruits other Crb complex proteins as well as aPKC complex proteins, conferring apical identity (Izaddoost et al., 2002; Nam and Choi, 2003; Pellikka et al., 2002; Sotillos et al., 2004; Wodarz et al., 1995). We tested if this was also the case in imaginal disc epithelia. Indeed, overexpression of Crb recruited aPKC (Fig. 4K,L), similar to the upregulation of aPKC by Yki overexpression (Fig. 4I,J). Conversely, Crb is required for proper localization of Patj and aPKC since crb mutant imaginal disc cells had strongly reduced levels of aPKC and Patj at apical membranes, but the basolateral marker Dlg was not affected (Fig. 5B,E,H; supplementary material Fig. S2). These results are consistent with previous reports examining crb mutant embryos and clones in the pupal retina (Izaddoost et al., 2002; Nam and Choi, 2003; Pellikka et al., 2002; Sotillos et al., 2004; Wodarz et al., 1995). Loss of aPKC and Patj from apical membranes of crb mutant cells was strongest in eye discs and less severe in wing discs. As in the embryo, Crb is thus necessary and sufficient to recruit apical complexes in third instar imaginal disc cells.
The observation that Crb is required for proper localization of other apical determinants together with the fact that Crb overexpression causes phenotypes similar to loss of Wts raised the possibility that the accumulation of Crb in wts mutant cells is responsible for the increase in apical determinants. To test this hypothesis, we analyzed the phenotypes of wts,crb double mutant cells and found that wts,crb double mutant cells failed to localize aPKC and Patj at apical membranes and thus prevented the upregulation of not only Crb, but also of Patj and aPKC observed in wts mutant cells (Fig. 5).
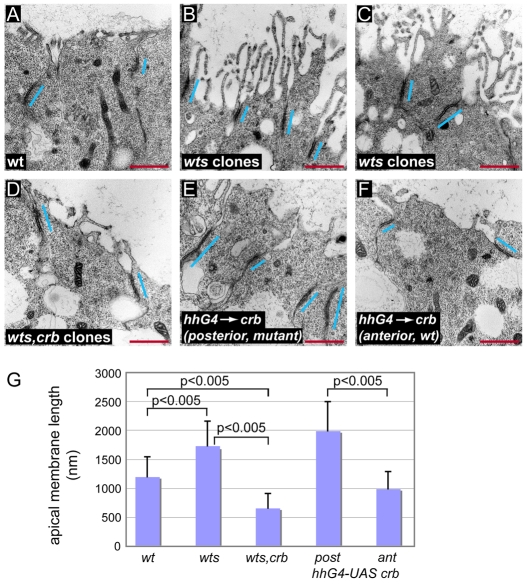
To gain more direct insights into the ultrastructure of wts and wts,crb mutant cells, we used transmission electron microscopy to examine cross sections through the wing pouch of imaginal discs that were composed nearly entirely of mutant cells (see the Materials and Methods). To determine the size of the apical domains, we measured the distance from a line connecting the apical ends of the adherens junctions to the apical tips of cells. We found that the average apical domain size in discs that are almost completely mutant for wts was over 1.5 times larger than that in wild-type discs (Fig. 6A-C,G) similar to the apical hypertrophy observed by Justice et al. (Justice et al., 1995). Measuring the lengths of the adherens junctions, we found that also the adherens junctions of wts mutant disc cells were about 1.5 times longer than those in wild-type discs (630±260 nm in wts discs versus 430±180 nm in wild-type discs). This elongation of the adherens junctions is consistent with the slight upregulation of the adherens junction component E-cad observed in the absence of various Hippo pathway components (Fig. 4E) (Jaiswal et al., 2006). Similarly to the loss of Wts, overexpression of Crb caused a 1.7-fold increase in the lengths of the apical domain and a 1.6-fold increase in the lengths of adherens junctions to 670±320 nm length (Fig. 6E,F,G). Notably, wts,crb double mutant cells had apical domains that were significantly smaller than those of wts mutant cells and were even smaller than those of wild-type cells (Fig. 6D,G). These results suggest that Crb is necessary and sufficient for apical membrane expansion and they are consistent with a model in which the accumulation of Crb is a major factor for the apical hypertrophy of wts mutant cells. Surprisingly, however, the adherens junctions were still longer in wts,crb double mutant cells (680±200 nm) compared with wild-type cells (430±180 nm). Thus, the effect of the Hippo pathway on the lengths of adherens junctions is at least partially independent of its effect on apical determinants. In summary, wts mutant cells show a marked expansion of the apical domain, which is largely prevented by simultaneous removal of Crb, while the basolateral domain remains unaffected.
Fig. 6.
Crb is required for the apical hypertrophy of wts mutant cells. (A-F) Representative transmission electron micrographs of third instar wing imaginal discs from a wild-type larva (A), larvae containing wts (B and C) or wts,crb mutant clones (D), a disc overexpressing Crb in its posterior compartment under the control of a hh-Gal4 driver (E), and the anterior wild-type half of the same disc (F). Blue bars indicate the position and size of adherens junctions. Red scale bars: 500 nm. (G) Average apical domain lengths for the different genotypes shown in A-F. P-values (as measured using the Student's t-test) between some of the genotypes are indicated. Values represent the means ± s.e.m. of 20-30 samples.
The regulation of growth and apical domain complexes are independent downstream effects of the Hippo pathway
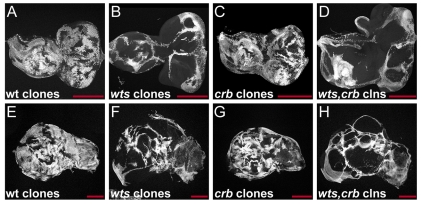
Cells mutant for hpo overproliferate and have increased amounts of apical complex proteins such as Crb and aPKC. Interestingly, overexpression of Crb and aPKC also causes overproliferation of imaginal disc cells (Eder et al., 2005; Lu and Bilder, 2005), raising the possibility that the accumulation of Crb and aPKC contribute to the overproliferation phenotype of hpo mutant cells. To test this possibility, we compared the growth of wts mutant clones with that of wts,crb double mutant clones since removal of crb from wts mutant cells prevents the accumulation of Crb, aPKC and Patj, and thus eliminates the effects that are caused by the accumulation of Crb or other apical determinants. When mitotic recombination was induced with high frequency throughout eye and wing discs by expressing Flipase with the eyFLP and ubxFLP drivers, we found that wts,crb double mutant clones overproliferated as much as wts mutant clones (Fig. 7A-H). Thus, wts and wts,crb mutant clones occupied similarly large portions of eye and wing discs, which were much larger than crb mutant or wild-type control clones (Fig. 7A-H). To more precisely determine the growth behavior of mutant cells we measured the size of mutant clones and compared it with that of the associated twin clones in discs in which clones were induced at low frequency using hsFLP. The founder cells that produce a mutant clone and its twin clone are sister cells produced by a mitotic recombination event and are thus born at the same time. Thus, comparison of the sizes of a mutant clone and its twin clone provides a relative measure of the growth behavior of mutant cells. We found that crb mutant cells behaved similarly to wild-type cells and produced clones that were about the same size as their twin clones (1.29±0.44 times larger than twin clones compared with 1.21±0.32 times larger for wild-type clones). By contrast, wts mutant clones were over five times larger than their twin clones (5.36±2.54 times larger), demonstrating the increased proliferation potential of wts mutant cells. Importantly, wts,crb double mutant clones were as large as wts mutant clones (5.57±2.75 times larger than their twin clones), demonstrating that removal of Crb from wts mutant cells did not impair their overgrowth phenotype. Thus, upregulation of Crb, aPKC and Patj are not required for the overgrowth phenotypes of wts mutant cells. Consistent with these results, we found that the Hippo target genes diap1 and ex were still upregulated in wts,crb double mutant cells, similarly to their induction in wts mutant cells (Fig. 8A-D). We conclude that the upregulation of apical complexes is not required for the overgrowth phenotype of wts mutant cells. The Hippo pathway, therefore, regulates the amount of apical complexes in addition to and independently of its growth control function.
Fig. 7.
Crb is not required for warts mutant overgrowth phenotypes. (A-D) Eye imaginal discs from third instar larvae containing wt (A), wts (B), crb (C), and wts,crb (D) mutant clones that are marked by the absence of GFP expression (gray). Clones were induced using eyFLP. (E-H) Wing imaginal discs from third instar larvae containing wt (E), wts (F), crb (G) and wts,crb (H) mutant clones marked by the absence of GFP expression (gray). Clones were induced using ubxFLP. Anterior is to the left and dorsal is up for all discs. Scale bars: 200 μm.
Finally, we asked whether the increased levels of apical complex proteins in wts mutant cells could account for the observed buildup of transmembrane receptors at the apical plasma membrane. Interestingly, the accumulation of DER and Ft was also observed in wts,crb double mutant cells (Fig. 8E-H), indicating that the effect of the Hippo pathway on the membrane levels of DER and Ft are also independent of the accumulation of apical complexes and the expansion of the apical membrane.
Discussion
Our results show that the Hippo pathway regulates the amount of apical protein complexes and thereby the size of the apical domain and that this effect is independent of its growth control function. Importantly, the regulation of apical complexes is a specific effect of the Hippo pathway, since other growth control pathways do not regulate apical complexes. In addition, this effect of the Hippo pathway is a general effect, since upregulation of apical complexes was observed in multiple tissues and cell types. Although overexpression of Crb and aPKC are sufficient to drive extra growth, our results show that the upregulation of apical complexes is not required for the overgrowth phenotype and for the induction of Hippo target genes in wts mutant cells. We thus conclude that the Hippo pathway regulates the amount of apical complexes in Drosophila imaginal disc cells in addition to and independently of its growth control function.
It was previously suggested that Mer and Ex regulate the levels of membrane receptors independently of the Hippo pathway. However, our results show that the upregulation of DER, Ft and apical complexes was similar in hpo and wts mutant cells and mer;ex double mutant cells, and that this effect requires Yki. These results thus indicate that Mer and Ex act through the Hippo pathway to exert their effect and that they are bona fide members of the Hippo pathway. Similar conclusions were drawn by Feng et al. based on their observation that overexpression of wts suppressed the lethality and overgrowth phenotypes of ex mutants (Feng and Irvine, 2007).
How does the Hippo pathway regulate the size of the apical domain and the amount of the apical complexes? Our observation that Yki is required and sufficient for the effect on the apical domain indicates that this effect of the Hippo pathway is mediated by transcriptional regulation. However, although the upregulation of Crb is necessary and sufficient for the expansion of the apical domain and for the accumulation of the other apical polarity complex proteins, it is not required for the upregulation of DER and Ft, which still accumulate in wts,crb double mutant cells. We thus favor a model in which the Hippo pathway regulates the turnover of several apical membrane components, for example through regulation of endocytosis. Notably, Maitra et al. (Maitra et al., 2006) found that mer;ex mutant cells in wing imaginal discs have defects in Notch (N) endocytosis, which leads to accumulation of N. Moreover, the endosomal protein HRS accumulates in hpo mutant follicle cells in Drosophila ovaries (Yu et al., 2008), and we observed a similar accumulation of HRS in wts mutant clones in imaginal discs (F.H. and G.H., unpublished data). These observations thus support the hypothesis that Hippo signaling regulates the amount of endocytosis and membrane turnover, thereby affecting the amount of apical membrane proteins. The target of Yki that mediates these effects, however, is currently not known.
Several other studies also demonstrated roles for the Hippo pathway beyond its function in growth control. For example, the Hippo pathway is required for the proper selection of photoreceptor subtypes in the Drosophila eye (Mikeladze-Dvali et al., 2005), and it is required in follicle cells to generate a signal that polarizes the underlying oocyte (Meignin et al., 2007; Polesello and Tapon, 2007; Yu et al., 2008). For both of these functions, Hippo signals through Yki, but Yki may regulate different sets of target genes, since the phenotypic effects are different. In addition, the Hippo pathway regulates cellular behavior through pathways that may not require Yki and thus may not involve the regulation of gene expression. For example, Yki-independent functions of the Hippo pathway may regulate dendritic tiling of larval neurons (Emoto et al., 2006) and the death of salivary gland cells during metamorphosis (Dutta and Baehrecke, 2008). Our finding that Hippo regulates apical polarity complexes in addition to and independently of its growth control function in imaginal discs cells thus further reveals the complex function of this pathway in the regulation of cellular behavior.
Materials and Methods
Mutant clones were induced using the FLP-FRT system and ubiGFP marked FRT chromosomes (Xu and Rubin, 1993). Generation of mer;ex and yki;wts double mutant clones was described previously (Hamaratoglu et al., 2006; Huang et al., 2005). mer;ex,yki triple mutant clones were induced in flies with the genotype: y w mer4 FRT19A/Y; exBQ FRT40A ykiB5/p{w+,yki+} p{w+,mer+} FRT40A ykiB5; hsFLP MKRS/+ and clones were marked by the absence of Ex expression. Other alleles used are null alleles: hpo42-47 (Wu et al., 2003), wtsx1 (Xu and Rubin, 1993), Tsc1IQ69 (Udan et al., 2003) and crb11A2 (Bilder et al., 2003). The UAS-Gal4 system (Brand and Perrimon, 1993) was used for overexpression with the following stocks: hh-Gal4 [from Laura A. Johnston (Columbia University, New York, NY)], UAS-crbintra [from Kwang-Wook Choi (Baylor College of Medicine, Houston, TX)], UAS-yki (Huang et al., 2005), UAS-myc (Johnston et al., 1999), UAS-cycD, UAS-cdk4 (Datar et al., 2000), UAS-bantam (Hipfner et al., 2002), UAS-diap1 [from Bruce Hay (California Institute of Technology, Pasadena, CA)], and UAS-cycE (Richardson et al., 1995).
Antibody stainings
Antibody stainings of imaginal discs were done as described previously (Kango-Singh et al., 2002). The following antibodies were used (dilutions and source in parentheses): mouse anti-Dlg (1:300; DSHB), guinea-pig anti-Ex [1:2000; from Richard G. Fehon (University of Chicago, Chicago, IL)], rat anti-Ft [1:2000; from Michael A. Simon (Stanford University, Stanford, CA)], rabbit anti-aPKC (1:500; Santa Cruz Biotechnology), mouse anti-Crb (1:200; from K. Choi,), mouse anti-Patj [1:500; from Hugo Bellen (Baylor College of Medicine, Houston, TX)], rat anti-Ci [1:150; from Robert A. Holmgren (Northwestern University, Evanston, IL)], mouse anti-DIAP1 (1:200; from Bruce Hay,), rat anti-DER [1:1000; Pernille Rorth (The National University of Singapore, Singapore)], rabbit-β-gal (1:600; Cappel, Aurora, OH), mouse-β-gal (1:2000; Promega, Madison, WI), rabbit-Sas [1:2000; from Deborah J. Andrew (The Johns Hopkins University School of Medicine, Baltimore, MD)].
Transmission electron microscopy on imaginal discs
Wing discs nearly entirely mutant for wts and wts,crb were produced by mitotic recombination of FRT82B wts and FRT82B wts,crb chromosomes using ubx-FLP and ubi-GFP marked homologous FRT82B chromosomes. Wing discs were dissected from third instar larvae and discs that showed almost entirely mutant pouches, as detected by the absence of GFP expression, were selected under a fluorescence stereoscope. Discs were then fixed overnight in modified Trump's fixative (1.4% cacodylic acid, 4% paraformaldehyde, 2% glutaraldehyde). The discs were then washed briefly in distilled water (two to three times) and fixed for 2 hours in 2% osmium tetroxide. After three more brief rinses in distilled water, the discs were dehydrated through an ethanol series (50%, 70%, 80%, 90% and 95%, two times each for 5 minutes, and 100%, three times, 10 minutes each). The discs were next dehydrated with two 30-minute washes in propylene oxide. The resin used was from the EMBED 812 resin kit (Electron Microscopy Sciences, Hatfield, PA). The discs were infiltrated with resin through a wash series of three parts propylene oxide:one part resin, one part propylene oxide:one part resin, and one part propylene oxide:three parts resin, for at least 30 minutes. The discs were incubated in pure resin overnight, and transferred to silicone molds filled with resin the next day. Samples were cured at 60°C for 48 hours and discs were sectioned perpendicular to the anterior-posterior axis through the pouch region. Sections were stained with uranyl acetate and lead citrate prior to transmission electron microscopy (TEM) analysis.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/14/2351/DC1
We thank Deborah Andrew, Hugo Bellen, David Bilder, Seth Blair, Peter Bryant, Kwang-Wook Choi, Richard G. Fehon, Bruce Hay, Allen Laughon, Graeme Mardon, Marek Mlodzik, Duojia Pan, Michael A. Simon, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Bank (University of Iowa) for fly stocks and antibodies. We thank Kenn Dunner, Jr for help with EM imaging. Special thanks to Markus Affolter in whose laboratory part of this work was done. We thank Vincent Dion and Oguz Kanca for critical reading of the manuscript. This work was supported by an NIH grant to G.H., and non-overlapping Roche Research Foundation and MC-IEF postdoctoral fellowships to F.H. The M. D. Anderson EM Core Facility is supported by NIH grant CA 16672. Deposited in PMC for release after 12 months.
References
- Assemat, E., Bazellieres, E., Pallesi-Pocachard, E., Le Bivic, A. and Massey-Harroche, D. (2008). Polarity complex proteins. Biochim. Biophys. Acta 1778, 614-630. [DOI] [PubMed] [Google Scholar]
- Bennett, F. C. and Harvey, K. F. (2006). Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr. Biol. 16, 2101-2110. [DOI] [PubMed] [Google Scholar]
- Bilder, D., Schober, M. and Perrimon, N. (2003). Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat. Cell Biol. 5, 53-58. [DOI] [PubMed] [Google Scholar]
- Brand, A. H. and Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. [DOI] [PubMed] [Google Scholar]
- Cho, E. and Irvine, K. D. (2004). Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development 131, 4489-4500. [DOI] [PubMed] [Google Scholar]
- Cho, E., Feng, Y., Rauskolb, C., Maitra, S., Fehon, R. and Irvine, K. D. (2006). Delineation of a Fat tumor suppressor pathway. Nat. Genet. 38, 1142-1150. [DOI] [PubMed] [Google Scholar]
- Datar, S. A., Jacobs, H. W., de la Cruz, A. F., Lehner, C. F. and Edgar, B. A. (2000). The Drosophila cyclin D-Cdk4 complex promotes cellular growth. EMBO J. 19, 4543-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, J., Feldmann, G., Huang, J., Wu, S., Zhang, N., Comerford, S. A., Gayyed, M. F., Anders, R. A., Maitra, A. and Pan, D. (2007). Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, S. and Baehrecke, E. H. (2008). Warts is required for PI3K-regulated growth arrest, autophagy, and autophagic cell death in Drosophila. Curr. Biol. 18, 1466-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder, A. M., Sui, X., Rosen, D. G., Nolden, L. K., Cheng, K. W., Lahad, J. P., Kango-Singh, M., Lu, K. H., Warneke, C. L., Atkinson, E. N. et al. (2005). Atypical PKCiota contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc. Natl. Acad. Sci. USA 102, 12519-12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto, K., Parrish, J. Z., Jan, L. Y. and Jan, Y. N. (2006). The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature 443, 210-213. [DOI] [PubMed] [Google Scholar]
- Feng, Y. and Irvine, K. D. (2007). Fat and expanded act in parallel to regulate growth through warts. Proc. Natl. Acad. Sci. USA 104, 20362-20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulev, Y., Fauny, J. D., Gonzalez-Marti, B., Flagiello, D., Silber, J. and Zider, A. (2008). SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr. Biol. 18, 435-441. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu, F., Willecke, M., Kango-Singh, M., Nolo, R., Hyun, E., Tao, C., Jafar-Nejad, H. and Halder, G. (2006). The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol. 8, 27-36. [DOI] [PubMed] [Google Scholar]
- Harvey, K. F., Pfleger, C. M. and Hariharan, I. K. (2003). The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114, 457-467. [DOI] [PubMed] [Google Scholar]
- Henrique, D. and Schweisguth, F. (2003). Cell polarity: the ups and downs of the Par6/aPKC complex. Curr. Opin. Genet. Dev. 13, 341-350. [DOI] [PubMed] [Google Scholar]
- Hipfner, D. R., Weigmann, K. and Cohen, S. M. (2002). The bantam gene regulates Drosophila growth. Genetics 161, 1527-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J., Wu, S., Barrera, J., Matthews, K. and Pan, D. (2005). The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122, 421-434. [DOI] [PubMed] [Google Scholar]
- Izaddoost, S., Nam, S. C., Bhat, M. A., Bellen, H. J. and Choi, K. W. (2002). Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature 416, 178-183. [DOI] [PubMed] [Google Scholar]
- Jaiswal, M., Agrawal, N. and Sinha, P. (2006). Fat and Wingless signaling oppositely regulate epithelial cell-cell adhesion and distal wing development in Drosophila. Development 133, 925-935. [DOI] [PubMed] [Google Scholar]
- Jia, J., Zhang, W., Wang, B., Trinko, R. and Jiang, J. (2003). The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 17, 2514-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K. and Wodarz, A. (2003). A genetic hierarchy controlling cell polarity. Nat. Cell Biol. 5, 12-14. [DOI] [PubMed] [Google Scholar]
- Johnston, L. A., Prober, D. A., Edgar, B. A., Eisenman, R. N. and Gallant, P. (1999). Drosophila myc regulates cellular growth during development. Cell 98, 779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice, R. W., Zilian, O., Woods, D. F., Noll, M. and Bryant, P. J. (1995). The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9, 534-546. [DOI] [PubMed] [Google Scholar]
- Kango-Singh, M., Nolo, R., Tao, C., Verstreken, P., Hiesinger, P. R., Bellen, H. J. and Halder, G. (2002). Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129, 5719-5730. [DOI] [PubMed] [Google Scholar]
- Lu, H. and Bilder, D. (2005). Endocytic control of epithelial polarity and proliferation in Drosophila. Nat. Cell Biol. 7, 1232-1239. [DOI] [PubMed] [Google Scholar]
- Maitra, S., Kulikauskas, R. M., Gavilan, H. and Fehon, R. G. (2006). The tumor suppressors Merlin and expanded function cooperatively to modulate receptor endocytosis and signaling. Curr. Biol. 16, 702-709. [DOI] [PubMed] [Google Scholar]
- Mao, Y., Rauskolb, C., Cho, E., Hu, W. L., Hayter, H., Minihan, G., Katz, F. N. and Irvine, K. D. (2006). Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development 133, 2539-2551. [DOI] [PubMed] [Google Scholar]
- Meignin, C., Alvarez-Garcia, I., Davis, I. and Palacios, I. M. (2007). The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr. Biol. 17, 1871-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikeladze-Dvali, T., Wernet, M. F., Pistillo, D., Mazzoni, E. O., Teleman, A. A., Chen, Y. W., Cohen, S. and Desplan, C. (2005). The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell 122, 775-787. [DOI] [PubMed] [Google Scholar]
- Nam, S. C. and Choi, K. W. (2003). Interaction of Par-6 and Crumbs complexes is essential for photoreceptor morphogenesis in Drosophila. Development 130, 4363-4372. [DOI] [PubMed] [Google Scholar]
- Nolo, R., Morrison, C. M., Tao, C., Zhang, X. and Halder, G. (2006). The bantam microRNA is a target of the hippo tumor-suppressor pathway. Curr. Biol. 16, 1895-1904. [DOI] [PubMed] [Google Scholar]
- Oh, H. and Irvine, K. D. (2008). In vivo regulation of Yorkie phosphorylation and localization. Development 135, 1081-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, D. (2007). Hippo signaling in organ size control. Genes Dev. 21, 886-897. [DOI] [PubMed] [Google Scholar]
- Pantalacci, S., Tapon, N. and Leopold, P. (2003). The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 5, 921-927. [DOI] [PubMed] [Google Scholar]
- Pellikka, M., Tanentzapf, G., Pinto, M., Smith, C., McGlade, C. J., Ready, D. F. and Tepass, U. (2002). Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature 416, 143-149. [DOI] [PubMed] [Google Scholar]
- Polesello, C. and Tapon, N. (2007). Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr. Biol. 17, 1864-1870. [DOI] [PubMed] [Google Scholar]
- Reddy, B. V. and Irvine, K. D. (2008). The Fat and Warts signaling pathways: new insights into their regulation, mechanism and conservation. Development 135, 2827-2838. [DOI] [PubMed] [Google Scholar]
- Richardson, H., O'Keefe, L. V., Marty, T. and Saint, R. (1995). Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development 121, 3371-3379. [DOI] [PubMed] [Google Scholar]
- Silva, E., Tsatskis, Y., Gardano, L., Tapon, N. and McNeill, H. (2006). The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr. Biol. 16, 2081-2089. [DOI] [PubMed] [Google Scholar]
- Sotillos, S., Diaz-Meco, M. T., Caminero, E., Moscat, J. and Campuzano, S. (2004). DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J. Cell Biol. 166, 549-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, A. and Ohno, S. (2006). The PAR-aPKC system: lessons in polarity. J. Cell Sci. 119, 979-987. [DOI] [PubMed] [Google Scholar]
- Tanentzapf, G. and Tepass, U. (2003). Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat. Cell Biol. 5, 46-52. [DOI] [PubMed] [Google Scholar]
- Thompson, B. J. and Cohen, S. M. (2006). The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell 126, 767-774. [DOI] [PubMed] [Google Scholar]
- Udan, R. S., Kango-Singh, M., Nolo, R., Tao, C. and Halder, G. (2003). Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 5, 914-920. [DOI] [PubMed] [Google Scholar]
- Willecke, M., Hamaratoglu, F., Kango-Singh, M., Udan, R., Chen, C. L., Tao, C., Zhang, X. and Halder, G. (2006). The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr. Biol. 16, 2090-2100. [DOI] [PubMed] [Google Scholar]
- Willecke, M., Hamaratoglu, F., Sansores-Garcia, L., Tao, C. and Halder, G. (2008). Boundaries of Dachsous Cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc. Natl. Acad. Sci. USA 105, 14897-14902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz, A., Hinz, U., Engelbert, M. and Knust, E. (1995). Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell 82, 67-76. [DOI] [PubMed] [Google Scholar]
- Wu, S., Huang, J., Dong, J. and Pan, D. (2003). hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114, 445-456. [DOI] [PubMed] [Google Scholar]
- Wu, S., Liu, Y., Zheng, Y., Dong, J. and Pan, D. (2008). The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev. Cell 14, 388-398. [DOI] [PubMed] [Google Scholar]
- Xu, T. and Rubin, G. M. (1993). Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223-1237. [DOI] [PubMed] [Google Scholar]
- Xu, T., Wang, W., Zhang, S., Stewart, R. A. and Yu, W. (1995). Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121, 1053-1063. [DOI] [PubMed] [Google Scholar]
- Yu, J., Poulton, J., Huang, Y. C. and Deng, W. M. (2008). The hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity. PLoS ONE 3, e1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L., Ren, F., Zhang, Q., Chen, Y., Wang, B. and Jiang, J. (2008). The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev. Cell 14, 377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.