Abstract
The phenomenon of spontaneous fMRI activity is increasingly being exploited to investigate the connectivity of functional networks in human brain with high spatial-resolution. Although mounting evidence points towards a neuronal contribution to this activity, its functional role and dependence on behavioral state remain unclear. In this work, we used BOLD fMRI at 7 T to study the modulation of spontaneous activity in occipital areas by various behavioral conditions, including resting with eyes closed, eyes open with visual fixation, and eyes open with fixation and focal visual stimulation. Spontaneous activity was separated from evoked activity and from signal fluctuations related to cardiac and respiratory cycles. We found that spontaneous activity in visual areas was substantially reduced (amplitude (44%) and coherence (25%)) with the fixation conditions relative to the eyes-closed condition. No significant further modulation was observed when the visual stimulus was added. The observed dependence on behavioral condition suggests that part of spontaneous fMRI signal fluctuations represents neuronal activity. Possible mechanisms for the modulation of spontaneous activity by behavioral state are discussed. The observed linear superposition of spontaneous fMRI activity with focal evoked activity related to visual processing has important implications for fMRI studies, which ideally should take into account the effect of spontaneous activity to properly define brain activations during task conditions.
Keywords: 7T BOLD fMRI, evoked activity, eyes open/closed, spontaneous activity, visual cortex
INTRODUCTION
Blood-oxygenation-level-dependent (BOLD) functional MRI (fMRI) allows the study of subtle changes in brain activity in response to sensory stimuli or the execution of cognitive tasks. Increasingly, this technique is used to study spontaneous brain activity as well. Several studies have demonstrated that much of the brain shows a substantial amount of spontaneous signal fluctuations with spatially correlating patterns that resemble the functional subdivision of the brain (Biswal et al. 1995; Lowe et al. 1998; Cordes et al. 2001, Hampson et al. 2002, Greicius et al. 2003, De Luca et al. 2006). These findings furthermore suggest that spontaneous BOLD signals might provide an opportunity to map brain functional connectivity without the need for carefully designed behavioral tasks. For instance, initial patient studies have indicated altered connectivity in neurological diseases (for a review see He et al. 2007) such as multiple sclerosis (Lowe et al. 2002) and Alzheimer’s disease (Greicius et al. 2004).
Despite this potential functional significance of spontaneous fMRI activity, little is know about its origin and role. While it might partly result from purely physiologic processes, including variations in respiratory and cardiac cycles (Birn et al. 2006; Shmueli et al. 2007), there is increasing evidence of a substantial neuro-electric and metabolic correlate (Laufs et al. 2003; Mantini et al. 2007; Horovitz et al. 2008; Shmuel and Leopold 2008; Fukunaga et al. 2008). Thus, spontaneous fMRI activity may report on ongoing and spatially correlated fluctuations in neuronal activity. Importantly, evidence for the existence of spatio-temporal patterns of spontaneous neuronal activity has come from other modalities as well, including optical imaging based on intrinsic hemodynamic signals (Arieli et al. 1996) and intra-cortical electrical recordings (Steriade, 2001; Leopold et al. 2003; Fiser et al. 2004; Buzsaki and Draghun, 2004). Studies of intra-cortical electrical activity have also indicated that spontaneous and evoked activity can coexist and mutually interact under certain conditions (Fiser et al. 2004; Hasenstaub et al. 2007).
The large majority of fMRI studies of spontaneous activity have looked at resting conditions, i.e. without the administration of sensory stimuli or cognitive task, and for this reason it is generally referred to as “resting-state activity”. However, it is unclear to what extent spontaneous activity is specific to rest and to what extent it is modulated by behavioral state or evoked activity. For instance, an inverse relationship between the index of wakefulness and the amplitude (or coherence) of spontaneous fMRI activity in the visual cortex has been found in human studies of sedation (Kiviniemi, et al. 2005) and sleep (Fukunaga et al. 2006; Horovitz et al. 2008). Nevertheless, the number of reports concerned with the modulation of spontaneous fMRI activity by simple tasks, such as visual fixation or the viewing of visual stimuli, is still limited. Studies of spontaneous fMRI activity during visual stimulation have found that BOLD signal excursions depend on task condition, but did not separate the contributions of spontaneous and evoked activity (Hampson et al. 2004; Nir et al. 2006). Two recent studies in the visual (de Zwart et al. 2008) and the motor system (Fox et al. 2006) found persistent spontaneous activity during sensory tasks but did not compare the amplitude or coherence of fMRI signal fluctuations between conditions. The precise characterization of this relationship is important for understanding the origin of spontaneous activity and its impact on the interpretation of evoked activity. Therefore, the goal of the current work was to quantify the modulation of spontaneous activity by behavioral state and evoked activity. For this purpose, we studied BOLD fMRI activity in the visual cortex of a group of healthy subjects (n=14) across a range of conditions. Part of this work was previously published in abbreviated abstract form (Bianciardi et al., 2008).
MATERIALS AND METHODS
Paradigm
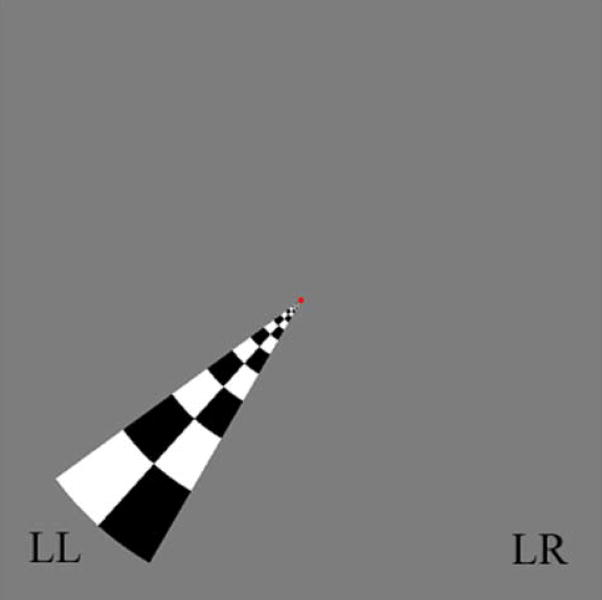
In order to quantify the modulation of spontaneous activity in the visual cortex by behavioral state and evoked activity, the following conditions were examined in separate fMRI runs: 1) “EC”= rest with eyes closed, a control condition with minimal attentional demands and visual input. 2) “F”= visual fixation onto a small dot in the center of a grey field, a condition with moderate attentional demands and minimal visual input. 3) “F+S”= fixation as under 2) plus visual stimulation using a contrast reversing, wedge-shaped checkerboard in the lower-left (LL) quadrant of the visual field, (Figure 1). The latter was presented in a blocked design with period of stimulation alternated with rest (grey field) at a relatively high frequency (0.083Hz, i.e. block design with 6s ON/6s OFF cycle) to allow separation of spontaneous from evoked activity (see Data Analysis). In a fourth fMRI run, a functional localizer scan was performed to allow selection of regions of interest (ROIs) in the visual cortex for analysis of spontaneous activity levels in the following locations: stimulated region (ROIST), its homologous contra-lateral cortex (ROIC-ST) and surrounding (ROIOTHER) visual areas (see Figure 2 and, for details, the paragraph “Localization of regions of interest”).
Figure 1. Employed stimulus and stimulation design.
The stimulus employed in the experiment is shown. It consists of a B/W wedge-shaped checkerboard contrast reversing at 7.5Hz. The wedge was used during: 1) fixation to a central dot concurrent with visual stimulation at 0.083Hz (block design with 6sON/6s OFF cycle, condition F+S); 2) in the functional localizer polar-angle mapping run, with the wedge performing a full clockwise rotation every 90s. During condition F+S, the stimulation frequency was carefully chosen so as to minimize the overlap in the spectral domain with spontaneous activity in the visual cortex (< 0.05Hz). The polar-angle mapping run was employed to define three regions of interest within the visual cortex (see Figure 2). Wedge positions LL (“lower left”) and LR (“lower right”) are at polar-angle equal to 228° and 132°, respectively (0° is at the upper vertical meridian).
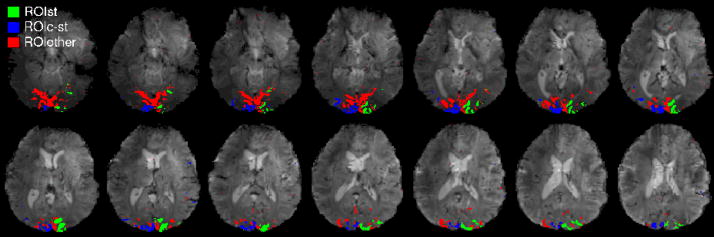
Figure 2. Characterization of functional regions of interest in the visual cortex.
By a functional localizer polar-angle mapping run (~8.5 min), employing a rotating wedge-shaped checkerboard (Figure 1), we defined (p < 0.0001, uncorrected for multiple comparisons) three functional regions of interest (ROIs): a stimulated region, ROIST (green), which comprises cortical areas responding to the wedge in position LL (Figure 1); a region contralateral to ROIST, ROIC-ST (blue), which responds to the same wedge, but in position LR (Figure 1); a region in the visual cortex responding to the rotating wedge in any position (excluding ROIST and ROIC-ST), ROIOTHER (red). ROIST and ROIC-ST correspond, respectively, to the stimulated area and to a task-unrelated area during the stimulation condition F+S; ROIOTHER comprises neighboring visual areas, and was used to extract the internal reference time-series for spontaneous fluctuations, used to define the spatial extent of spontaneous activity and its coherence. The three ROIs are displayed for a single subject (see Supplementary Material, Figure 1SM, for ROIs of another example data-set).
Run order was randomized across subjects, with pauses of few tens of seconds between functional scans.
Subjects
Fourteen healthy subjects (mean age 32, s.e. ± 2 years, 8 males) participated in the experiment. After receiving an explanation of the procedures, all subjects gave written informed consent. The human subject protocol was approved by the Institutional Review Board (IRB) of the National Institutes of Health (NIH).
Stimuli
Visual stimuli were presented using the software “Presentation v.11.0” (http://www.neurobs.com/) that ensured synchronization with the MR scanner. A Digital Light Processing (DLP) projector, located outside the MR scanner room, projected the stimuli on a translucent screen positioned on the head coil, viewed by the subject through a mirror and a prism (32° × 42° full field of view). Each stimulus used in the experiment was superimposed on a background grey image with a central red dot. Stimuli were isoluminant to the background.
The wedge-shaped checkerboard employed during both stimulation in condition F+S and during the functional localizer run, 16° long (i.e. eccentricity 0–16°) and 24° – polar angle - wide (Figure 1), reversed between light and dark at a rate of 7.5Hz. During condition F+S the polar angle (relative to the 12 o’clock position) of the wedge was equal to 228° (position LL, Figure 1). For the functional localizer (“polar angle mapping” run), the stimuli employed were as described previously (Sereno et al. 1995). The checkerboard was rotated clockwise using 3 s steps; during each 3 s step the wedge color was reversed from black to white, red to green and blue to yellow during the first, second, and third second, respectively. The polar-angle mapping was initiated with a 36 s rest (fixation to the central dot) period (note that the first 10 MR scans were not used for fMRI analysis, see below).
As a control of subject fixation, subjects were asked to report changes of dot color by button pressing, which was recorded with the fMRI data. For the functional localizer run, the dot color was changed after varying time intervals lasting on average 15s and ranging from 10–20 s. For the F+S and F conditions the dot color change was only executed at the beginning and the end of the run.
Data Acquisition
Imaging was carried out with a 7T General Electric Signa MRI scanner (GE Medical Systems, Milwaukee, WI) equipped with a highly sensitive 32-channel receive-only detector array (Nova Medical, Wilmington, MA). Due to the limited number of receiver channels available, only signals from 16 coils were acquired. BOLD contrast was obtained using gradient-recalled (GRE) echo planar T2*-weighted imaging (EPI). The acquisition of 36 2mm-thick slices was performed with repetition time (TR) of 3s, flip angle (FA) of 75°, echo-time (TE) of 32 ms and bandwidth of 250 kHz. Gap between slices was 0.2 mm and slices were acquired in interleaved order. The use of a SENSE acquisition rate of 3 provided an in plane isotropic resolution of 1.25 mm (FOV = 240×180mm; matrix = 192×144). The number of scans was equal to 115 for the each of the three investigated conditions, and 172 for the polar-angle mapping run. The first ten images were used as reference for coil sensitivity mapping and were discarded from further fMRI analysis. Head motion was minimized by the use of foam pads, placed in the space between the interior coating of the MRI detector array and the subject’s head. Additional functional runs and anatomical images were also acquired, but not employed in the analysis.
In order to improve data quality, compensation of respiration induced B0-field fluctuations was carried out by real-rime modulation of B0 shims (van Gelderen et al. 2007). For each subject, at the beginning of the experiment, a 2 minutes reference scan was acquired to measure the spatial distribution of B0 effects related to chest motion.
To account for movement-related fMRI signal fluctuations and BOLD effects due to cardiac pulsation and respiratory cycle, we also recorded the timing of physiological cycles by the use of a pulse-oximeter and respiratory bellow provided with the MR scanner. The recording was performed by a data acquisition card (National Instrument Corp., Austin, TX, USA) at a sampling rate of 250 Hz. The same card also recorded the MR scanner TTL pulses synchronized with the acquisition of each image volume. Finally, the stimulus computer recorded both the subjects’ button presses related to the task and MR scanner triggers, the latter being employed to synchronize stimulus delivery with image acquisition.
Data Analysis
Aim of data analysis was to characterize the correlation strength and amplitude of spontaneous fluctuations across ROIs and conditions.
MR images were reconstructed off-line by the use of phase-sensitive combination of individual coil data using separately acquired coil sensitivity reference maps. At this aim, a dedicated computer with home-written code using IDL 6.0 software (RSI Inc, Boulder, CO, USA) was employed. Several other pre-processing steps were carried out on each of the five data-sets per subject using FMRIB Software Library (FSL4.0, http://www.fmrib.ox.ac.uk/fsl/). Those steps included slice-timing to correct for slice acquisition delays, rigid body transformation to correct for head motion and co-registration between 4D-volumes of different runs in the same space. Retrospective physiological motion correction (RETROICOR, Glover et al, 2000) and removal of noise related to the respiration volume-per-unit-time (RVT, Birn et al. 2006) and to the cardiac rate (Shmueli et al. 2007) was also applied (home-written code using IDL 6.0) to data acquired during the three examined conditions EC, F, and F+S (not to data of the polar-angle-mapping run, where only evoked activity was investigated). This was performed to reduce any confound in the description of spontaneous activity, by constraining movement-related fMRI signal fluctuations and BOLD effects due to cardiac and respiratory cycles. RETROICOR correction, designed to deal with aliased motion and pulsation related effects due to physiological cycles, included two order Fourier series expanded in terms of cardiac and respiratory phases. RVT and cardiac-rate regressors consisted of respiration volume and cardiac rate time-courses sampled at each MR scanning time (TR = 3s), shifted by a delay equal to 6s. At this time lag, BOLD signals display maximum correlation with physiological regressors (Birn et al. 2006; Shmueli et al. 2007). For two subjects, we did not apply any physiological correction procedure, since, for technical problems, we could neither acquire good quality cardiac signals nor store physiological data.
Time-series were then converted to percentage signals, by computing the ratio of the signal in each voxel and each time point with the signal of a reference frame, and then multiplying by 100.
With the aim of assessing the statistical significance of effects of interest, for all runs and at the subject level, univariate regression analysis was performed with the Analysis of Functional NeuroImages tool (AFNI, http://afni.nimh.nih.gov/afni/) after data filtering (high-pass or low-pass filtering, or a combination of the two, as explained below). The covariates of interest for evoked responses resulted from the convolution of stimulus functions (based on the timing of stimulation events) with the SPM2 (http://www.fil.ion.ucl.ac.uk/spm/) standard hemodynamic response function. Then, linear contrasts were used to determine the amplitudes for effects of interest and tested for statistical significance taking into account the scan to scan within run residual error.
Localization of regions of interest
With the purpose of defining regions of functional interest for the present study in the visual cortex, we analyzed the functional localizer data-set as follows. Signal fluctuations occurring at very low frequencies were removed by high pass-filtering at 0.007Hz for polar-angle mapping data, the frequency cut-off being chosen in order to preserve all stimulus-related signals.
For the polar-angle mapping run, 30 covariates were included, each modeling the BOLD response to the wedge-shaped checkerboard in one of the 30 partially overlapping positions occupied during rotation. Separate contrast of the 12th and the 19th regressor led to the identification (t-test, p < 0.0001) of the stimulated area, ROIST, and of a task-unrelated area, ROIC-ST (“stimulated” and “task-unrelated” are referred to during condition F+S), respectively (see Figure 2); a third region of interest (including surrounding visual areas, other than ROIST and ROIC-ST) ROIOTHER (Figure 2) was identified by contrasting all the 30 covariates (F-test, p < 0.0001), after exclusion of common areas with ROIST and ROIC-ST.
Characterization of evoked and spontaneous activity
We characterized spontaneous activity in the three conditions and stimulus-related responses in the F+S condition by the following analysis.
The stimulation frequency of 0.083 Hz employed in condition F+S was carefully chosen in order to minimize its overlap in the frequency domain with spontaneous fluctuations, and preserve, at the same time, some detection efficiency for estimating BOLD response amplitudes (lower though than for the commonly employed block designs with longer epochs). Analyses of previous data (Fukunaga et al. 2006; Horovitz et al. 2008) acquired during visual stimulation, rest with eyes closed or open and during early sleep showed that most of the power of spontaneous activity in visual areas is below 0.05Hz, in agreement with previous findings in the occipital cortex during rest with eyes closed (Cordes et al. 2001) and anesthesia (Kiviniemi et al. 2000). In order to discriminate evoked from spontaneous signal fluctuations in the F+S condition, we employed a filtering strategy followed by analysis in different frequency ranges: high-pass filtered data with a cut-off frequency (fC) of 0.073 Hz were further analyzed to obtain evoked responses, and low-pass filtered data with the same cut-off were processed to extract spontaneous activity maps. Low-pass filtering (at about 0.08–0.1Hz), prior to correlations with the seed reference signal, is usually employed to define resting-state networks with optimized contrast-to-noise ratio (Biswal et al. 1995; Lowe et al. 1998; Hampson et al. 2002; Greicius et al. 2003): in our work, it was also crucial to remove evoked responses, during condition F+S, without taking out a large part of spontaneous fluctuations. At the same time, the chosen cut-off frequency used in high-pass filtering preserved all stimulus-related signals. In order to identify spontaneous activity with the same procedure, data low-pass filtering at fC was carried out during condition F and EC too. After low-pass filtering, we also corrected the data for low frequency drifts by modeling and removal of polynomials of degree three.
The spontaneous network (t-test, p < 0.005 Bonferroni corrected) in three conditions (Figure 3A–C) was obtained by correlating each voxel signal with the average signal in ROIOTHER (Figure 2), after low-pass filtering at fC. Bonferroni correction was applied with an average (± standard error, s.e.) number of brain voxels across subjects of 358390 ± 6951. To quantify the coherence of spontaneous signals within occipital areas (inter-regional correlation strength) in three conditions, we extracted the Pearson correlation coefficient (r) of each voxel in the stimulated and the contra-lateral area (ROIST and ROIC-ST, respectively, Figure 2); their group average is shown in Figure 4A (right panel). We also measured the amplitude of spontaneous fluctuations (Figure 4B) in terms of the temporal standard deviation of the average time-series within each of the three ROIs. Finally, single subject activation maps relative to significant stimulus evoked responses were identified by single regression analysis of the F+S high-pass filtered data-set (at fC) with a regressor for the block design stimulation (t-test, p < 0.0001).
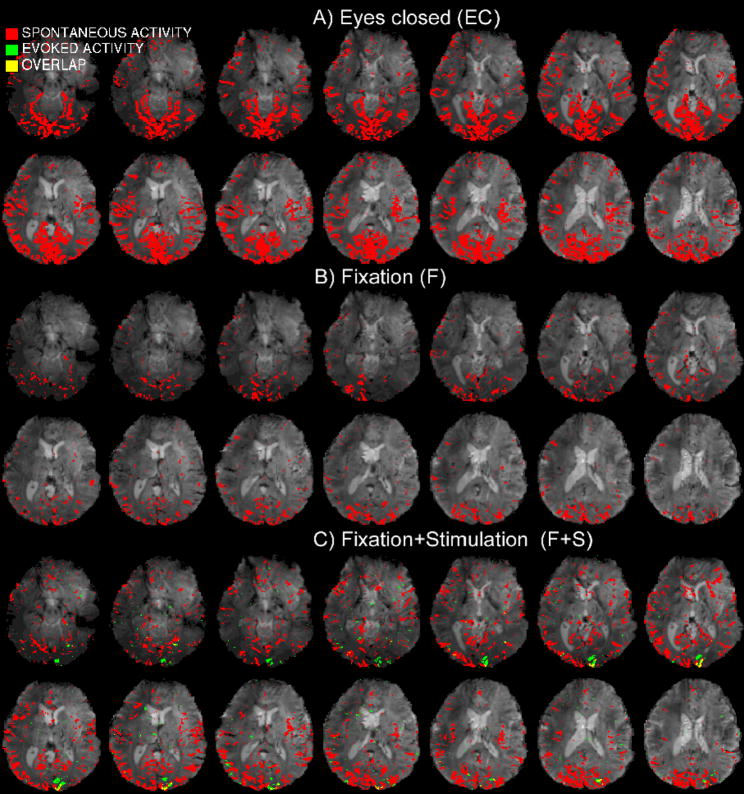
Figure 3. Activation maps of spontaneous and evoked signals.
Spontaneous activity patterns (red, p < 0.005 Bonferroni corrected, and same slices as in Figure 2), are displayed for the three investigated conditions (EC, F, and F+S) for an example data-set (results for another subject are shown in Supplementary Material, Figure 2SM). In general, correlation analysis of spontaneous signals with the internal reference time-series was performed after data low-pass filtering at 0.073Hz, which removed high frequency noise, as well as stimulus-related signals during condition F+S. Maps of spontaneous activity, evoked activity (green p < 0.0001) and their overlap (yellow) are displayed during condition F+S for the same subject.
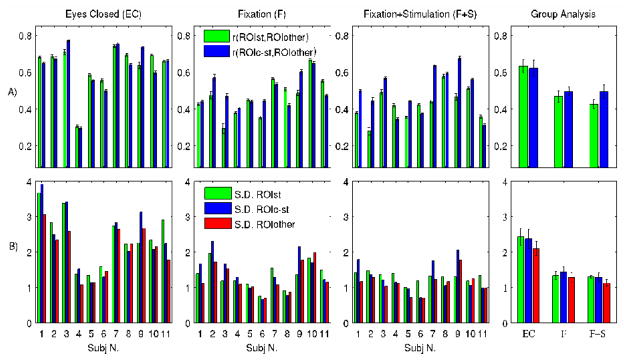
Figure 4. Coherence and amplitude of spontaneous activity in the visual cortex.
For the investigated conditions (F+S, F, EC) we show: A) the inter-regional coherence of spontaneous activity within the visual cortex at both the single subject (first three panels from the left, average ± s.e. across voxels,) and the group level (fourth panel, average ± s.e. across subjects, n = 11); for example r(ROIST, ROIOTHER) indicates the correlation strength of spontaneous signals in the stimulated region with the reference average signal extracted from neighboring visual areas; B) the amplitude of spontaneous fluctuations in three ROIs of the visual cortex (Figure 2). Their amplitude was measured in terms of the standard deviation of average signals within each of the three ROIs.
Spontaneous activity persisted during visual stimulation and was significantly reduced in terms of both coherence and amplitude during the fixation conditions with respect to the eyes closed condition.
Evaluation of the linear superposition or interaction between evoked and spontaneous activity
We tested if the effect of stimulation (amplitude of evoked activity, E) and spontaneous activity (S) was simply additive or if an interaction between E and S occurred during condition F+S. To this end, we implemented the following model:
| (1) |
The variable y (with sample values across subjects) was equal to the sum of the amplitude of spontaneous and evoked activity in ROIST during condition F+S; E was the amplitude of evoked activity in ROIST during condition F+S; S was equal to an estimate of spontaneous activity in absence of evoked activity (drawn either from ROIC-ST or ROIOTHER during condition F+S, or from any of the three defined ROIs during condition F); E*S was the interaction term (to leave unperturbed the parameter estimates of E and S, it was orthogonalized to both E and S); e was the error term; β0 an offset value (intercept); β1–3 the fitting parameters (slopes) of E, S, and of E*S, respectively. For each subject, S and E were estimated in the Fourier domain, as the square root of the mean power of the fMRI signal (averaged across each ROI) in the frequency range 0.01–0.073 Hz and at 0.083 Hz, respectively. To obtain E, the background activity at 0.083 Hz, estimated from linear interpolation of the activity at neighboring frequencies (0.0801 and 0.0865 Hz) in ROIST during condition F+S, was also subtracted.
We verified the presence of an interaction between the two activity types, i.e. if β3 in Eq. 1 differed significantly from zero (t-test for the interaction term, cI = [0 0 0 1]). We also tested if the amplitude of β1 plus β2 (t-test for the additive term, contrast cA = [0 1 1 0]) in Eq. 1 was significant (with β1 and β2 having the same sign), proving the validity of a linear superposition between evoked and spontaneous activity.
RESULTS
Localization of regions of interest
The three selected regions of interest (ROIs), employed to evaluate the amplitude and the coherence of spontaneous fluctuations in visual areas during conditions EC, F, and F+S are shown in Figure 2. These ROIs were identified by the use of an independent functional localizer run.
Spatial extent of spontaneous and evoked activity
All three behavioral conditions showed spontaneous activity that involved much of the visual cortex and some of the surrounding regions. An example is shown in Figure 3 (see also Figure 2SM of Supplementary Material). The highest correlation with the reference signal in the visual cortex were found in the bilateral primary and extra-striate visual cortices, including visual area V5/MT (Tootell et al. 1995); the spontaneous activity also extended, albeit with a lower coherence, to other sensory areas (e.g., in sensory-motor and temporal cortices), in frontal and parietal regions.
In the stimulation condition (F+S), stimulus-related activity was found in primary and neighboring extra-striate visual cortices (V1, V2, V3, and V4) of the right visual hemisphere, in agreement with stimulation displayed in the left hemi-field; moreover in nearly all subjects (8 out of 11) a clear bilateral activation of bilateral area MT/V5 was found, as expected for alternating checkerboard stimulation at 7.5Hz.
The spatial extent of spontaneous activity was generally largest during the EC condition (Figure 3A), and reduced during both the F and F+S conditions (Figure 3B–C). The spatial extent reduction was similar for the two fixation conditions, and equal on average to about 36% of the pattern obtained with the eyes closed for the pre-determined statistical threshold (p < 0.005 Bonferroni corrected, see Figure 3SM-A, Supplementary Materials).
During the F+S condition (Figure 3C), spontaneous activity overlapped the focally stimulated visual areas. The overlap of spontaneous BOLD fluctuations with the stimulated area (ROIST, Figure 2) at different statistical threshold (Figure 3SM-B, Supplementary Material) was similar for the homologous contra-lateral area (ROIC-ST, Figure 2) and for the visual cortex associated with nearly the entire visual field (ROIOTHER, Figure 2). The average overlap across ROIs was equal to about 45% for the chosen statistical thresholds (p < 0.005 Bonferroni corrected).
Strength of spontaneous activity in the visual cortex
Visual inspection of the spatial extent of spontaneous activity (Figure 3 and 3SM) in the visual cortex suggests a weakening with eyes opening that was similar for the F and F+S conditions. In order to quantify this finding, we evaluated the coherence (Figure 4A) and amplitude (Figure 4B) of spontaneous signals within three visual ROIs (Figure 2) defined by the use of a polar-angle mapping run as functional localizer. On average across the considered ROIs, this analysis (Figure 4) showed significant (p < 0.001) decrease in both the coherence (−24.7%) and amplitude (−43.8%), of spontaneous activity between the EC condition on one hand and the eyes open (F and F+S) conditions pooled together on the other. Nevertheless, spontaneous fluctuations still persisted in the visual cortex during the eyes open conditions, with their coherence significant at the group level (r > 0.42, p < 0.00001). No significant difference in the amplitude or the coherence of spontaneous activity was observed between the F and F+S conditions, or between ROIs in any condition (p < 0.05).
Spectral analysis of spontaneous and evoked signals in the visual cortex
In order to better visualize evoked and spontaneous fMRI signal components and convincingly show the similarity of the latter across the visual cortex, we extracted the signals from each ROI and performed a frequency analysis at the group level of the ROI average time-series (Figure 5) for the three employed conditions.
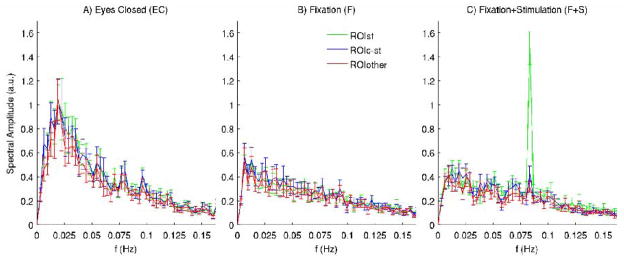
Figure 5. Characterization of spontaneous and evoked activity in the spectral domain.
For the three employed conditions A)-C), the spectral amplitude of average signals in three ROIs of the visual cortex (see Figure 2) are displayed. Error bars indicate mean ± s.e. across subjects (n = 11). Note the similarity of spontaneous fluctuations (predominant at lower frequencies) in different regions of the visual cortex and, for the F+S condition, the superposition of evoked responses at the stimulation frequency (0.083Hz) on spontaneous fluctuations in the stimulated area (ROIST) only. Moreover, spontaneous signal fluctuations are stronger during the eyes closed condition with enhanced signal amplitudes mostly below 0.05Hz, with a peak at about 0.02Hz.
Notably, in all conditions, we found (Figure 5) very similar spectral components between different ROIs, with the exception of a marked peak at the stimulation frequency (0.083Hz) in the stimulated area (ROIST, green) only, during the F+S condition. The occurrence of low-frequency (< 0.07 Hz) spontaneous oscillations was more prominent during rest with eyes closed, with a fluctuations peak at about 0.02 Hz, and with smaller oscillations amplitudes during the fixation conditions. On average across ROIs, signal amplitudes in the frequency range 0.013–0.054 Hz (except for frequencies 0.025Hz and 0.051Hz) were significantly reduced (p < 0.05, n = 11) during the fixation conditions (pooled together), see also Figure 6A.
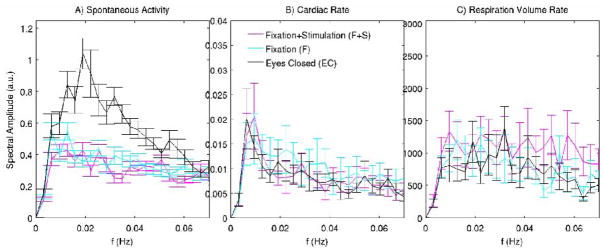
Figure 6. Characterization of spontaneous activity in the visual cortex and of possible confounds.
We display the spectral amplitude of group average spontaneous fluctuations (n = 11) in the visual cortex (A) and of confounding effects, i.e. cardiac (B) and respiration volume (C) rate (n = 9), during the three employed conditions (EC, F, F+S). Low-pass filtering at 0.073Hz was applied to spontaneous signals before averaging within the three defined ROIs in the visual cortex (Figure 2); for display purposes, the error bars in A) are across 33 repetitions (11 subject × 3 ROIs) per condition. The amplitudes of spontaneous BOLD signals in the frequency range 0.0127–0.054 Hz are reduced during the fixation conditions. This amplitude modulation is not associated to any systematic change in respiration and heartbeat rate across conditions at any frequency (range 0–0.16 Hz).
Evaluation of the linear superposition or interaction between evoked and spontaneous activity
The interaction between evoked and spontaneous activity was not significant (significance level p < 0.1, t-test for β3 in Eq. 1, β3 < 0.4). An additive model of evoked and spontaneous activity significantly (p < 0.0001, t-test for β1+β2 in Eq. 1, β1+β2 > 1.2; in each test, β1 and β2 were both positive) explained the fMRI signal changes in the stimulated area (ROIST) during the stimulation condition (F+S);
Physiological confounds
Significant BOLD fluctuations may arise from variations in respiration and heart rate (Birn et al. 2006; Shmueli et al. 2007). Furthermore, the amplitude of their fluctuation might be modulated by experimental condition, potentially confounding changes of spontaneous BOLD fluctuations resulting from neuronal activity. In the results presented here, these physiologic confounds were suppressed using regression analysis for each condition separately (see Materials and Methods, and the paragraph “Contribution of physiological noise to fMRI signal variance” in Supplementary Material). Nevertheless, this suppression might not be adequate, and therefore we tested if the higher amplitude of signal fluctuations during the EC condition might be related to an increased variability in heart and respiration rate. Comparison of the spectral amplitude of regressors representing cardiac and respiratory variations across the relevant frequency range (0–0.16 Hz) showed no significant changes between the eyes closed and the fixation conditions (Figure 6B–C). In addition, the autocorrelation of physiological signals was unperturbed by the condition, and it was different in structure and order with respect to that of spontaneous activity (Figure 4SM, Supplementary Material).
This finding suggests that potential physiological confounds (cardiac, respiratory related) are not responsible for the higher coherence and amplitude of spontaneous activity during the eyes closed condition.
DISCUSSION
Diminished spontaneous activity during eyes open conditions
The reduction of the amplitude (−44%) and coherence (−25%) of spontaneous fMRI activity in occipital areas with both eyes open conditions relative to the eyes closed condition suggests a modulation of spontaneous activity by behavioral state. Noticeably, the observed modulation of spontaneous activity during the fixation conditions (mainly between 0.01–0.05 Hz) was not associated with any change in the amplitude of possible physiological confounds (respiration, cardiac) across conditions. This finding adds to the growing evidence for a neuronal correlate (Laufs et al. 2003; Mantini et al. 2007; Horovitz et al. 2008; He et al. 2007; Fukunaga et al. 2008; Shmuel and Leopold 2008) to the phenomenon of spontaneous fMRI activity.
Many previous studies have characterized spontaneous fMRI activity in absence of stimulation, but few have quantified the dependence on behavioral condition. In the visual cortex, increased amplitude or coherence of spontaneous activity have been found during conditions of reduced alertness and light sleep (Kiviniemi, et al. 2005; Fukunaga et al. 2006; Horovitz et al. 2008). Also in the visual cortex, experiments by Yang (Yang et al. 2007) have demonstrated an increase in the amplitude of spontaneous activity with an eyes open condition relative to an eyes closed condition. This appears discordant with the findings of the current study. In part, this discrepancy might be attributable to methodological differences, most importantly a potential bias in the calculated amplitude of activity associated with the particular normalization method (i.e. scaling to the global mean, with few oblique slices centered on the visual cortex) used in the earlier study (Yang et al. 2007); moreover, the fixation task employed in the present work, requiring a moderate level of participation and attention, differed from the task used by Yang (Yang et al. 2007), in which a passive fixation task was used. Another study (Nir et al. 2006) found a similar amplitude of spontaneous activity in extra-striate (face and object) visual areas between the eyes closed and fixation condition without stimulation. During the revision of this manuscript, we became aware of recent work (McAvoy et al., 2008), in which the amplitude of spontaneous BOLD signal fluctuations in sensory and para-limbic areas was reduced by eyes opening or fixation, in agreement with our findings. Few studies have investigated the modulation of spontaneous fMRI activity by behavioral condition in other brain regions. In the motor cortex, a reduction of spontaneous activity has been found during deep anesthesia (Peltier et al. 2005); in the default mode network, the coherence of spontaneous fMRI activity was attenuated by a memory task (Fransson, 2006), but not by passive visual processing (Greicius et al. 2003). As opposed to Fransson’s results valid for the default mode network (Fransson, 2006), we didn’t find any change in the amplitude or coherence of signal fluctuations in the visual cortex between the visual fixation and the visual stimulation task. Two different cognitive processes might be responsible for spontaneous activity in the default mode network and in the visual cortex, respectively; alternatively, the visual stimulation task employed in our work did not engage as much attention and cognitive resources as a working memory task, and hence did not reduce spontaneous activity in the visual cortex. In summary, modulation of spontaneous activity by behavioral state has been observed in a number of brain regions, but has not been a consistent observation. This might in part be related to differences in experimental conditions, study design, and the brain region under study.
Absence of modulation by focal visual stimulation
Interestingly, the addition of visual stimulation to the fixation task did not further reduce the amplitude of spontaneous activity in the stimulated area, nor did it change coherence with other areas. Rather, spontaneous and stimulus-evoked activity were found to be additive. This persistence of spontaneous activity during sensory stimulus processing agrees with optical imaging and EEG studies (Arieli et al. 1996; Fiser et al. 2004), as well as with BOLD imaging at lower fields (Fox et al. 2006; Fox et al. 2007), which also found an approximately linear superposition of spontaneous and evoked fMRI signals in the brain. The absence of a modulation of spontaneous signals by stimulation might be related to the fact that spontaneous activity was already substantially suppressed by the eyes open condition, which included a fixation task and the presence of dim light. Nevertheless, this finding suggests that spontaneous BOLD activity in both stimulated areas and neighboring regions of the same network is not strongly affected by focal activity related to sensory processing. This has important implications for the general application of fMRI for the study of evoked activity, which ideally should take into account the effect of spontaneous activity during both resting and task conditions in order to properly define activations. This could be achieved by recently introduced methods that derive an estimate for the spontaneous activity from a reference region that is functionally connected to the stimulated area (Fox et al. 2006; de Zwart et al. 2008). The ongoing spontaneous activity during sensory stimulation, as found in this and previous work (Fox et al. 2006), might reflect fluctuations in alertness or other aspects of cognitive state, and as such be predictive of performance (Silver et al., 2007; Fox et al. 2007).
Comparison with studies of EEG oscillatory activity
Spontaneous fluctuations in electrophysiologocial measures of brain activity are well known but still poorly understood. In EEG studies, strong oscillatory signals are genrally seen during rest, sleep, anesthesia and epileptic states, and have reduced amplitude during conscious waking behavior (Buzsaki and Draguhn, 2004). Examples are the alpha rhythm in visual system and the mu rhythm in the motor system, both of which have been called “idling” or “inhibitory” rhythms. Alpha has been shown to be suppressed by activation of the oculo-motor system and visual input (Berger, 1929). In close analogy to our finding, EEG alpha band has a widespread reduction in activity of approximately 40% from eyes closed to eyes open conditions, with no topographic changes (Barry et al. 2007). Simultaneous EEG-fMRI studies of the alpha rhythm during the eyes closed condition have shown varying results with respect to the location of the correlation, in either the occipital lobe, extending to parietal regions (Goldman et al. 2002; Moosmann et al. 2003; Mantini et al. 2007), or within the attentional frontal-parietal system (Laufs et al. 2003). Moreover, comparing explicitly the eyes open and eyes closed conditions, alpha power negatively correlated with BOLD signals in visual areas (Feige et al. 2005), accounting for the different BOLD baseline levels in the two conditions (Fox and Raichle, 2007; Laufs et al. 2003) as a possible confound. Finally, recent investigations (Goncalves et al. 2006; Laufs et al. 2006) suggest that there is considerable individual variation in areas that correlate with alpha fluctuations, and each BOLD resting pattern can be related to a combination of different brain rhythms, or vice versa each brain rhythm is associated to several brain networks (Mantini et al. 2007). Interestingly, a continued or increased amplitude (or coherence) of BOLD activity in the visual cortex was found in sedation (Kiviniemi et al. 2005) and light sleep (Fukunaga et al. 2006, Horovitz et al. 2008), during which alpha activity is generally reduced with respect to resting conditions or absent (Lopes da Silva, 1991). Taken together, these studies indicate the relationship between EEG alpha and fMRI spontaneous activity in the visual cortex is complex and likely dependent on behavioral condition. In line with these studies, our result might not be exclusively linked to alpha activity, but rather relate to a redistribution of activity among the predominant electrophysiological rhythms.
Origin of spontaneous activity
The current findings invite speculation about possible origins for the observed modulation of spontaneous activity by behavioral state. The high amplitude and coherence of spontaneous activity during the eyes closed condition might originate from visual imagery during increased levels of relaxation. Alternatively, spontaneous activity could represent fluctuating levels of inhibition effectuated by central or top-down mechanisms. For example, different amplitudes of spontaneous activity might originate from varying levels of attention and oculo-motor control. During the eyes closed condition, alternations between inward and outward directed attention could lead to shifting amplitude and coherence of activity in functional networks (Fransson, 2006). During the eyes open conditions, attention was focused on a central fixation dot and was likely not affected by the focal stimulation. This could have allowed remaining fluctuations in (covert) attention in much of the extra-foveal visual cortex, including some of the area stimulated by the task. Finally, spontaneous activity might be an integral component of sensory processing (Fiser et al. 2004), with the modulation and triggering of spontaneous fluctuations representing the brain response to darkness and light. Therefore, further experiments will be needed to evaluate the contribution of these mechanisms to establish their role in the modulation of spontaneous activity. The topic is of particular interest also considering the possible use of spontaneous fMRI activity in the visual cortex as a tool for investigating neurological impairments (Panayiotopoulos, 1987).
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institutes of Neurological Disorders and Stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, Magee CA, Rushby JA. EEG differences between eyes-closed and eyes-open resting conditions. Clin Neurophysiol. 2007;118:2765–2773. doi: 10.1016/j.clinph.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Berger H. Über das Elektroenkephalogramm des Menschen. Berlin: Archiv für Psychiatrie und Nervenkrankheiten. 1929;87:527–570. [Google Scholar]
- Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart J, Duyn J. Spontaneous activity in the visual cortex persists during visual stimulation: a 7 T study; Proceedings of the 16th ISMRM Scientific Meeting; Toronto, Canada. 2008. p. 146. Book of Abstracts N. 747. [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- de Zwart JA, Gelderen P, Fukunaga M, Duyn JH. Reducing correlated noise in fMRI data. Magn Reson Med. 2008;59:939–945. doi: 10.1002/mrm.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige B, Scheffler K, Esposito F, Di Salle F, Hennig J, Seifritz E. Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. J Neurophysiol. 2005;93:2864–2872. doi: 10.1152/jn.00721.2004. [DOI] [PubMed] [Google Scholar]
- Fiser J, Chiu C, Weliky M. Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature. 2004;431:573–578. doi: 10.1038/nature02907. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Horovitz SG, de Zwart JA, van Gelderen P, Balkin TJ, Braun AR, Duyn JH. Metabolic origin of BOLD signal fluctuations in the absence of stimuli. J Cereb Blood Flow Metab. 2008 doi: 10.1038/jcbfm.2008.25. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Horovitz SG, van Gelderen P, de Zwart JA, Jansma JM, Ikonomidou VN, Chu R, Deckers RH, Leopold DA, Duyn JH. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging. 2006;24:979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J, Jr, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves SI, de Munck JC, Pouwels PJ, Schoonhoven R, Kuijer JP, Maurits NM, Hoogduin JM, Van Someren EJ, Heethaar RM, Lopes da Silva FH. Correlating the alpha rhythm to BOLD using simultaneous EEG/fMRI: inter-subject variability. Neuroimage. 2006;30:203–213. doi: 10.1016/j.neuroimage.2005.09.062. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Olson IR, Leung HC, Skudlarski P, Gore JC. Changes in functional connectivity of human MT/V5 with visual motion input. Neuroreport. 2004;15:1315–1319. doi: 10.1097/01.wnr.0000129997.95055.15. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci. 2007;27:9607–9622. doi: 10.1523/JNEUROSCI.2184-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Shulman GL, Snyder AZ, Corbetta M. The role of impaired neuronal communication in neurological disorders. Curr Opin Neurol. 2007;20:655–660. doi: 10.1097/WCO.0b013e3282f1c720. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH. Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG-fMRI study. Hum Brain Mapp. 2008;29:671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V, Jauhiainen J, Tervonen O, Paakko E, Oikarinen J, Vainionpaa V, Rantala H, Biswal B. Slow vasomotor fluctuation in fMRI of anesthetized child brain. Magn Reson Med. 2000;44:373–378. doi: 10.1002/1522-2594(200009)44:3<373::aid-mrm5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Kiviniemi VJ, Haanpaa H, Kantola JH, Jauhiainen J, Vainionpaa V, Alahuhta S, Tervonen O. Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Magn Reson Imaging. 2005;23:531–537. doi: 10.1016/j.mri.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Laufs H, Holt JL, Elfont R, Krams M, Paul JS, Krakow K, Kleinschmidt A. Where the BOLD signal goes when alpha EEG leaves. Neuroimage. 2006;31:1408–1418. doi: 10.1016/j.neuroimage.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, Krakow K. EEG-correlated fMRI of human alpha activity. Neuroimage. 2003;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex. 2003;13:422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva F. Neural mechanisms underlying brain waves: from neural membranes to networks. Electroencephalogr Clin Neurophysiol. 1991;79:81–93. doi: 10.1016/0013-4694(91)90044-5. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, Mathews VP. Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology. 2002;224:184–192. doi: 10.1148/radiol.2241011005. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy M, Larson-Prior L, Nolan TS, Vaishnavi SN, Raichle ME, d’Avossa G. Resting states affect spontaneous BOLD oscillations in sensory and paralimbic cortex. J Neurophysiol. 2008;100:922–931. doi: 10.1152/jn.90426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, Taskin B, Obrig H, Villringer A. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. Neuroimage. 2003;20:145–158. doi: 10.1016/s1053-8119(03)00344-6. [DOI] [PubMed] [Google Scholar]
- Nir Y, Hasson U, Levy I, Yeshurun Y, Malach R. Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. Neuroimage. 2006;30:1313–1324. doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos CP. Fixation-off-sensitive epilepsy in eyelid myoclonia with absence seizures. Ann Neurol. 1987;22:87–89. doi: 10.1002/ana.410220120. [DOI] [PubMed] [Google Scholar]
- Peltier SJ, Kerssens C, Hamann SB, Sebel PS, Byas-Smith M, Hu X. Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport. 2005;16:285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage. 2007;38:306–320. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Neural correlates of sustained spatial attention in human early visual cortex. J Neurophysiol. 2007;97:229–237. doi: 10.1152/jn.00677.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Impact of network activities on neuronal properties in corticothalamic systems. J Neurophysiol. 2001;86:1–39. doi: 10.1152/jn.2001.86.1.1. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Dale AM, Look RB, Sereno MI, Malach R, Brady TJ, Rosen BR. Visual motion aftereffect in human cortical area MT revealed by functional magnetic resonance imaging. Nature. 1995;375:139–141. doi: 10.1038/375139a0. [DOI] [PubMed] [Google Scholar]
- van Gelderen P, de Zwart JA, Starewicz P, Hinks RS, Duyn JH. Real-time shimming to compensate for respiration-induced B0 fluctuations. Magn Reson Med. 2007;57:362–368. doi: 10.1002/mrm.21136. [DOI] [PubMed] [Google Scholar]
- Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, Zang YF, Gong QY. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]