Abstract
IL-32 is a newly discovered protein found in human and certain primates, but absent in rodent. Various reports suggest its role as a proinflammatory mediator. Since vascular endothelium is critical in inflammation, we investigate IL-32 in endothelial cells. We found that the gene is expressed in human endothelial cells and Akt strongly induces its expression. Sequence analysis indicates IL-32β as the major isoform in endothelial cells. Surprisingly, we did not detect any secretion of IL-32β in human endothelial cells; instead we observed co-localization of IL-32β with endoplasmic reticulum, suggesting IL-32β is an intracellular protein in these cells. Promoter analysis identified a minimum required region for IL-32 transcription at −0.1 kb to +0.5 kb around the initially identified transcription start site. We also defined a transcriptional suppressor-binding site at −2.0 kb to −1.5 kb. Importantly, RNA ligase mediated rapid amplification of cDNA ends in endothelial cells determined the transcription start site at the 328 bp downstream from the original identified site. Finally, we found a positive correlation of IL-32 levels with human breast cancer and glioblastoma multiforme (GBM). These findings improve our understanding of IL-32 in vascular endothelium. IL-32 expression might be valuable as a biomarker for cancer.
Keywords: IL-32, blood vessel, promoter analysis, RACE, cancer
Introduction
IL-32 (a.k.a NK4) was originally isolated from activated human natural killer cells upon stimulation with IL-2 or activation of human T cells by mitogens (1). Recently, this gene was rediscovered in human lymphocytes (2). Although IL-32 does not share sequence homology with any known cytokine families, IL-32 induces expression of various cytokines, such as TNFα and IL-8, in lymphocytes and monocytic cells (2).
The full length IL-32 gene is composed of 705 base pair. IL-32 exists in four splice variants in blood cells, named IL-32α, β, γ and δ, with IL-32α as the major isoform in hematopoietic cells (2). Interestingly, computer genomic analysis indicates that IL-32 is only present in human. The highest homology to human IL-32 is found in equine tissue only at 31.8%, and no homologue to this gene is found in rodent (2).
Since IL-32 expression is regulated by inflammatory cytokines in human peripheral lymphocyte cells, it has been implicated that it may play a role in inflammatory/autoimmune diseases (2). Further analysis indeed shows an elevation of IL-32 in human inflammatory diseases, such as rheumatoid arthritis (3–5), ulcerative colitis and Crohn’s disease (6, 7), as well as an elevation of IL-32 in 41% of human stomach cancer and 71% of human lung cancer (8), consistent with the notion that inflammation contributes to cancer progression (9).
Vascular endothelium separates blood from tissues and plays an important role in inflammation. Therefore, we investigated IL-32 in vascular endothelium. We show here that IL-32 is expressed in human endothelial cells. IL-32β, a major isoform in endothelial cells, is an intracellular protein located in the ER. We identified a major transcription initiation site in endothelial cells, as well as mapped the IL-32 promoter. Consistently, we observed an elevation of IL-32 expression in human breast cancer and human brain cancer.
Material and Methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) (Clonetics, San Diego, CA) and bovine aortic vascular endothelial cells (BAVECs) provided by Dr. Douglas Vaughan at Vanderbilt University were grown on 0.1 % gelatin-coated plates in endothelial growth medium (EGM, Clonetics). Adenoviral vectors directing the expression of β-galactosidase (Ad β-gal), GFP (AdGFP), and Akt (AdAkt) were used. Viral vectors were propagated in 293 cells and purified by CsCl column (10).
IL-32 cDNA synthesis, cloning and construction of adenovirus
IL-32β cDNA was isolated from HUVECs by RT-PCR, and cloned into the pEGFP-N3 expression vector for intracellular imaging (BD Biosciences, Mountain View, CA). IL-32β fused with 6His and V5 tags at the C-terminus was cloned into an adenoviral vector and adenovirus directing the expression of IL-32β (AdIL-32 β) was developed as described (10).
Northern blot analysis and RT-PCR
For analysis of IL-32 expression, HUVECs were infected with adenoviral vectors for 48 hour. Total RNAs were isolated using RNeasy kit (Qiagen, Valencia, CA) and subjected to Northern blot analysis. 32P labeled cDNA probes for IL-32 mRNA were hybridized using Express Hyb (BD Biosciences). Tissue distribution of IL-32 was examined using multiple tissue cDNA panels (Clontech, Mountain View, CA). IL-32 was amplified using specific primer sets: ATGTGCTTCCCGAAGGTCCTCTCTGA (forward) and TCATTTTGAGGATTGGGGTTCAGAGC (reverse). Glyceraldehyde 3-phosphate dehydrogenase (G3PDH) was used as an internal control.
Real time qRT-PCR was performed using cDNA from paired human breast cancer and adjacent normal tissues acquired from a large epidemiological study on breast cancer (11). Human brain cancer tissues and normal brain sample were obtained from the tissue bank at the Vanderbilt-Ingram Cancer Center. Total RNA (1 μg) was used for the first-strand cDNA synthesis using iScript ™ cDNA synthesis kit (Bio Rad, Hercules, CA). IQ™ SYBR® Green supermix (Bio Rad) was used on iCycler (Bio Rad) using IL-32 primers; 5′-CGACTTCAAAGAGGGCTACC (forward) and 5′-GAGTGAGCTCTGGGTGCTG (reverse). 18S rRNA was used as a loading control.
ELISA and Western blot
Cell lysate and the medium were collected following infection of HUVECs with AdGFP or AdIL-32β, respectively, for 48 hours. The culture media were concentrated using Centriplus ® YM-10 (Millipore Corporation, Bedford, MA). ELISA was performed using Ni-NTA HisSorb™ Strips (Qiagen) and an anti V5 antibody (Invitrogen, Carlsbad, CA) according to the manufacture’s protocol.
For Western blot, cell lysate and concentrated media were immunoprecipitated with a V5 antibody and the samples were run in SDS-PAGE. Immunoblotting of the samples was performed with anti-V5 antibody and a horseradish peroxidase conjugated anti-mouse secondary antibody (Promega, Madison, WI).
Cell imaging
BAVECs were transfected with either pEGFP-IL32β or an empty vector using Lipofectamin 2000 (Invitrogen). The following day, the cells were incubated with ER-TrackerTM Red (Molecular Probes, Inc. Eugene, OR) at 100 nM for 15 min prior to fixation with 4 % formaldehyde. The cells were analyzed by LSM 510 Confocal microscopy at the VUMC Cell Imaging Shared Resource.
Promoter analysis and RLM-RACE
A 2.5kb fragment of IL-32 promoter region was generated from HUVECs by PCR. Deletion mutants were generated from the 2.5kb fragment by PCR. These DNA fragments were then cloned into pGL3 expression vector (Promega). The plasmids were transfected into 3T3 cells or HUVECs with an internal control plasmid, phRL-SV40 (Promega) that expresses renilla luciferase. Relative luciferase unit was measured using the Dual-Luciferase® Reporter (DLR) assay kit (Promega) at 48 hours after transfection. Transfection efficiency was normalized against the internal control, renilla luciferase. Promoter activity was calculated over the vector control, pGL3.
RNA ligase mediated rapid amplification of cDNA ends (RLM-RACE) using FirstChoice® RLM-RACE kit (Ambion, Foster City, CA) was performed in HUVECs stimulated with IL-1β (R & D systems, Minneapolis, MN) at 100 pg/ml for 4 hours according to the manufacture’s protocol. 5′ RACE adapter was ligated to decapped mRNA, reverse transcribed, and amplified by PCR with primer targeting RACE adapter and specific primer within the IL32 gene (5′-GAGTGAGCTCTGGGTGCTG). PCR products were cloned into pGEM®-T Easy vector (Promega), and sequenced at Vanderbilt DNA sequencing core.
Statistics
Results are reported as mean ± SEM. Statistical analysis was done using a two-tailed Student’s t-test. Differences were considered statistically significant at p<0.05.
Results
IL-32 is present in human endothelial cells and Akt induces its expression
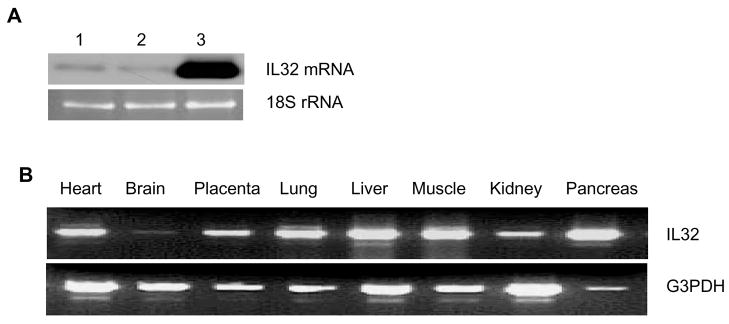
Based on the potential role of IL-32 in inflammation and vascular endothelium as a major mediator in inflammation, we first examined expression of IL-32 in human endothelial cells. We found that IL-32 is expressed in these cells, and Akt, a protein kinase B downstream of growth factors and inflammatory cytokines, strongly induces its expression (Figure 1A). Further analysis on a panel of human samples indicates a ubiquitous expression of IL-32 in all the organs tested, with minimum expression in the brain (Figure 1B), consistent with published results (2, 3).
Figure 1. IL-32 is present in human endothelial cells and Akt induces its expression.
HUVECs were infected with control Ad β-gal (lane 2) or AdAkt (lane 3) adenoviral vector (MOI=10), respectively, for 48 hours. Uninfected cells were used as a control (lane 1). IL-32 mRNA was analyzed by Northern blot. 18S rRNA, visualized with ethidium bromide and UV, was used as a loading control (Panel A). IL-32 was amplified using a human tissue cDNA panel by PCR, and G3PDH was used as an internal control (Panel B). Each experiment was repeated at least twice. Representative images were shown.
IL32β is not secreted by vascular endothelial cells
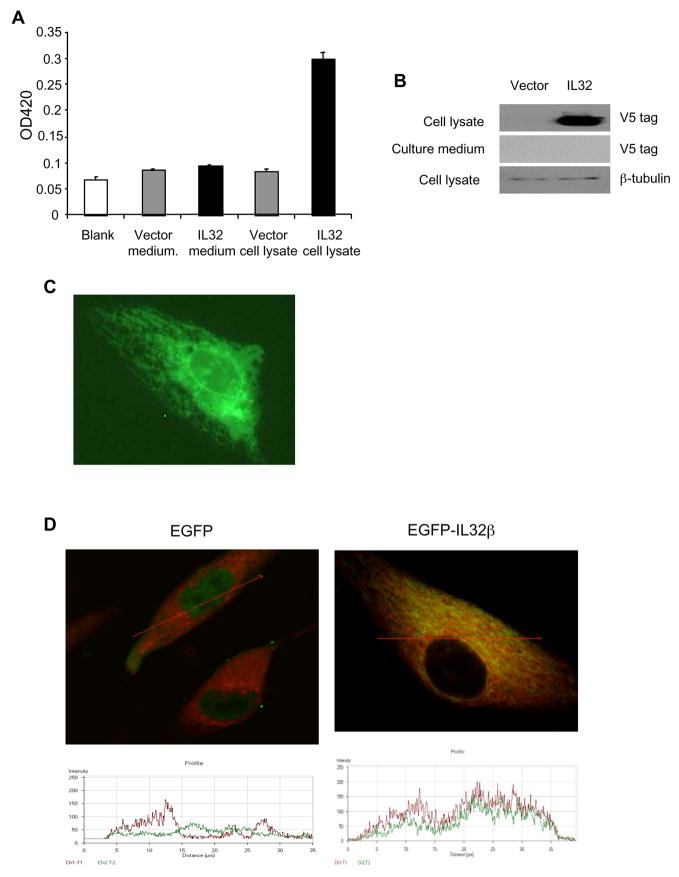
We cloned IL-32 from HUVECs stimulated with IL-1β and sequenced. Among three isoforms, IL-32α, IL-32β, and IL-32ε, of IL32 detected in endothelial cells, IL-32β is the major isoform in human endothelial cells. Thus, we focused on IL-32β in subsequent studies. Conflicting data have been reported regarding whether IL-32 is a secreted or intracellular protein (2, 8, 12). To address this issue, we developed an adenoviral vector directing the expression of IL-32β tagged with His and V5 epitopes at C-termini (AdIL-32β) for efficient transduction and high levels of transgene expression in endothelial cells. Cell lysate and concentrated conditioned media were analyzed by both ELISA and Western blot using antibodies against different epitope tags. Despite a clear demonstration of high levels of IL-32β in cell lysate infected with AdIL-32β, we failed to detect any IL-32β in the cell culture media (Figure 2A and 2B), which suggests IL-32β is likely an intracellular protein in vascular endothelial cells.
Figure 2. IL-32 β is an intracellular protein in human endothelial cells.
HUVECs were infected with control AdGFP or AdIL-32β adenoviral vector (MOI=10), respectively, for 48 hours. Cell lysate and concentrated conditioned media (10-fold concentration) were analyzed for IL-32 levels using a sandwich ELISA assay with antibodies against His and V5 tags (Panel A), and Western blot probed with an anti V5 antibody (Panel B). BAVECs were transfected with a c-terminal EGFP tagged IL-32β expression vector (pEGFP-IL-32β) and visualized under fluorescent microscopy (Panel C). BAVECs were transfected with an EGFP expression vector or pEGFP-IL-32β vector, respectively, for 24 hours. Cells were co-stained with an ER marker, ER-TrackerTM Red, and analyzed by confocal microscopy (D, upper panel). EGFP (green) and ER-TrackerTM Red distribution was analyzed by optical cross dissection of the cell (red line) (Panel D, bottom panel). **p<0.01.
To determine the intracellular localization of IL-32β, we constructed a plasmid expression vector fusing EGFP at the C-terminus of IL-32β (IL-32β-pEGFP). We transfected the construct into bovine endothelial cells (BAVEC) over HUVECs for easy transfection. IL-32β distribution visualized by EGFP expression showed that IL-32β is mainly in the cytosol but not in the nucleus or at plasma membrane (Figure 2C). In addition, we stained the IL-32β-pEGFP transfected cells with an ER specific tracking dye (red). We found that IL-32β is co-localized with the ER specific marker under confocal microscopy and spectral analysis (Figure 2D). These findings suggest that IL-32β, the major isoform in human endothelial cells, is an intracellular protein that localizes in the ER.
IL-32 promoter analysis
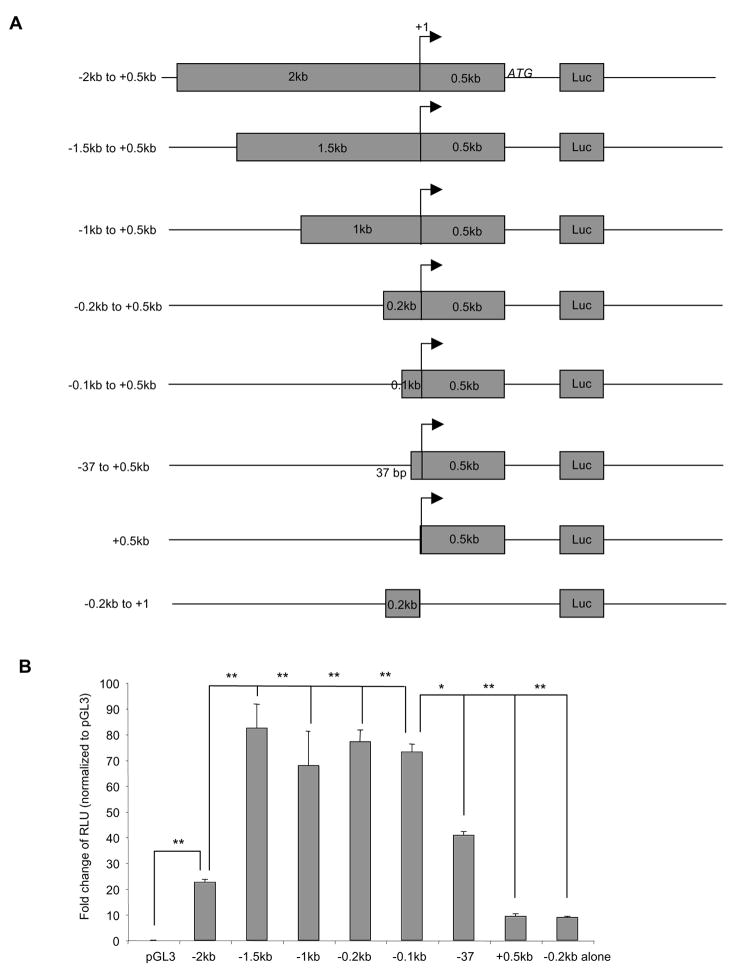
To investigate transcriptional regulation of IL-32 gene, we analyzed the promoter region. We cloned a 2.5 kb DNA fragment ranging from −2 kb upstream and 0.5 kb down stream from the original published transcriptional start site (18). A variety of truncation mutants were generated by PCR and then cloned into the pGL3 luciferase vector. Luciferase activity was measured as a readout for the promoter activity using NIH3T3 cells transfected with the vectors (Figure 3A).
Figure 3. IL-32 promoter analysis.
Various IL-32 promoter constructs were cloned into a pGL3 vector in front of a luciferase (Luc) reporter (Panel A). +1 is the original reported transcription start site. These constructs were transfected with an internal control plasmid expressing renilla luciferase into NIH3T3 cells for 24 hours, followed by measuring luciferase activities. Transfection efficiency was normalized using renilla luciferase unit. Promoter activity as calculated as fold changes over empty vector pGL3 transfected cells (Panel B). *p<0.05, **p<0.01. Each experiment was repeated at least three times.
Of the various constructs tested, we identified a transcriptional suppressor binding site between −2.0kb and −1.5kb upstream the transcription start site. Deletion of this fragment (500bp) significantly increased promoter activity (Figure 3A). We also identified a minimum required region (600bp) for the promoter activity, which covers −100bp upstream and +500bp downstream around the reported transcription start site. Deletion of the promoter region up to −0.1kb upstream of the transcription start site had no effects on promoter activity (Figure 3B). In contrast, complete deletion of upstream fragment or deletion of the 0.5kb downstream fragment around the transcription start site dramatically reduced the promoter activity (Figure 3B). Addition of the extra 37bp upstream of the predicted initiation site (−37) that contains a putative NF-κB binding site partially restored the promoter activity (Figure 3B). These data suggest that the region between −0.1kb to +0.5kb is necessary for IL-32 transcription.
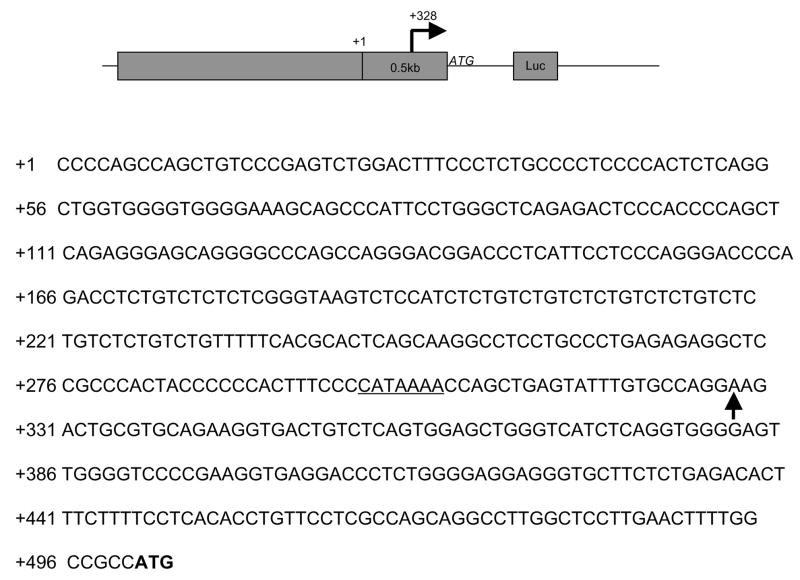
Since we observed minimum promoter activity with the fragment containing only 100 bp upstream of predicted transcription start site and majority of the promoter activity is in the downstream from the start site, we suspected whether the reported transcription site is correct. Therefore, we used 5′ RACE with RNA isolated from HUVECs to determine the transcription start site. PCR was performed on RACE products using a forward RACE-specific primer and a reverse IL-32 specific primer. Subsequent sequencing analysis of these PCR products confirmed our prediction and identified the major transcription start site resides at 328bp downstream from the original reported site. There is also a putative TATA box at 25 bp upstream of the transcription start site identified by 5′ RACE. (Figure 4). We did not find any clone showing a transcription start site at the originally reported site. There is also no TATA box around the originally reported transcription start site. This finding reveals a true transcription start site for IL-32 in vascular endothelial cells.
Figure 4. Determination of IL-32 transcription start site in endothelial cells.
HUVECs were treated with IL-1β at 100 pg/ml for 4 hours to induce IL-32 transcription. Total RNA was isolated and subjected to the RACE reaction. The PCR products were cloned and sequenced. +1 is the original reported transcription start site. Arrows indicate the identified transcription start site, which is 328bp downstream from the original reported transcription start site. The underlined sequence represents a TATA binding site. Bold letters represent the translational initiation site.
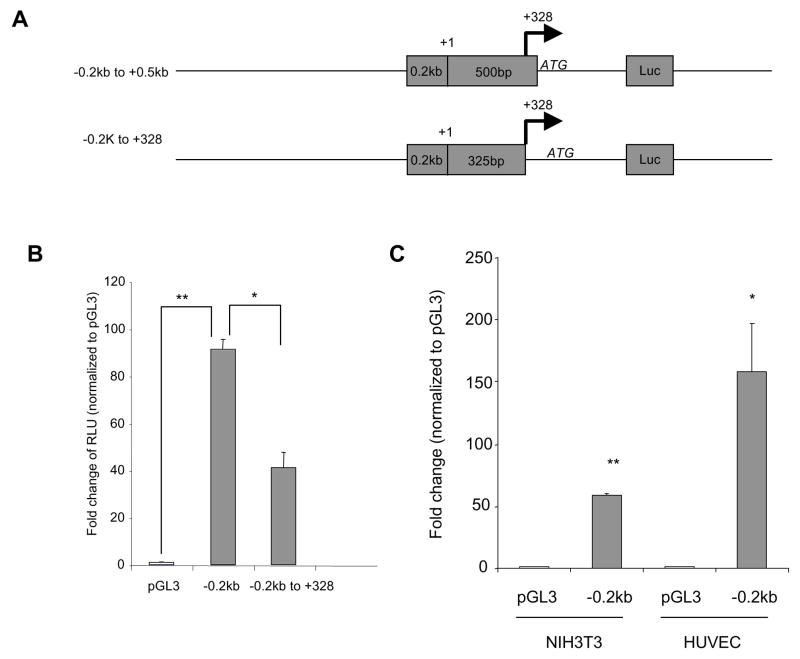
As the major transcription start site in endothelial cells is at 328 downstream from the original reported start site, it means the minimum IL-32 promoter (600bp) covers actually 428bp upstream and 172bp downstream of the transcription start site. To determine the role of this downstream region, we deleted this 172bp fragment from a parental vector (−0.2kb to 0.5kb) that retains full promoter activity (Figure 5A), Surprisingly, deletion of this 172bp region significantly decreased promoter activity compared to controls (Figure 5B); thus, the 172 bp downstream of the newly identified transcription start site is also required for the full activity of IL-32 promoter. In addition, we compared promoter activity of the −0.2kb to 0.5kb fragment in HUVECs and NIH3T3 cells. We found that this fragment displays increased promoter activity in endothelial cells compared to fibroblast (Figure 5C), which is consistent with our observation of higher levels of IL-32 in endothelial cells than non-endothelial cells (unpublished data). Thus, our data suggest that the fragment containing not only 428bp upstream but also 172bp downstream of the identified transcription start site are required to maintain IL-32 promoter activity.
Figure 5. Identification of a downstream sequence from the transcription start site important for promoter activity.
A 172bp deletion downstream from the identified transcription site was generated based on the minimum promoter construct (−0.2kb to +0.5kb) (Panel A). +1 is the original reported transcription start site. Arrows indicate the newly identified transcription start site. These constructs were transfected into NIH3T3 cells, and promoter activity was analyzed as described in Figure 3 (Panel B). The minimum promoter construct (−0.2kb to +0.5kb) was transfected into NIH3T3 cells or HUVECs and the promoter activity was compared between the two cell types (Panel C). *p<0.05, **p<0.01. Each experiment was repeated at least three times.
IL-32 levels is increased in breast and brain cancer tissues
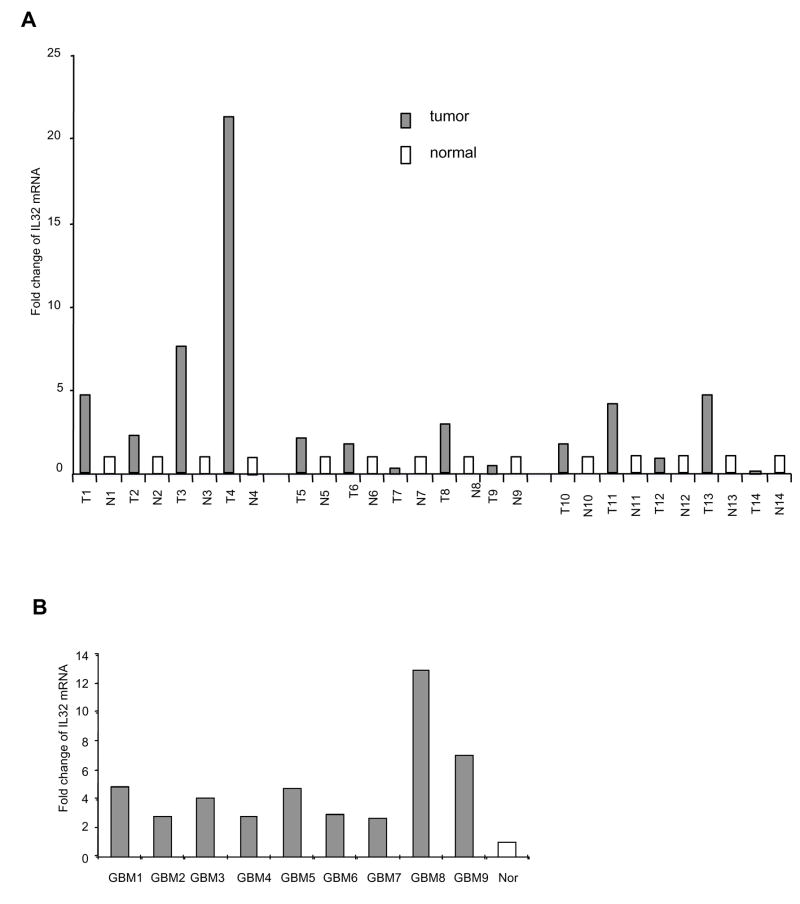
Inflammation is a risk factor in cancer (9) and IL-32 has been implicated in inflammation (2). In a screen of IL-32 expression in variety types of human cells, we found that IL-32 is highly expressed in human endothelial cells compared to non-endothelial cells including epithelial cells, fibroblast, smooth muscle cells and leukocytes (unpublished data). Thus, we compared IL-32 expression in human tumor tissues vs normal controls. We acquired a panel of paired breast tumor samples and adjacent normal breast tissues from the same patients. Among 14 paired sample analyzed, we found elevation of IL-32 levels in 10 tumor samples compared to the corresponding adjacent normal tissues (Figure 6A). In addition, we also observed a consistent increase of IL-32 levels in all 9 human brain GBM tumor samples compared to a non-pathological brain sample (Figure 6B). These data are consistent with published results showing a positive correlation of IL-32 expression with human stomach cancer and human lung cancer (8), supporting a role of IL-32 in inflammation and cancer progression, which warrant further study.
Figure 6. IL-32 expression is elevated in human cancer tissues.
IL-32 mRNA was measured by real time RT-PCR using specific primer sets in paired human breast tumor tissues (T) and adjacent normal breast tissues (N) (Panel A), as well as human GBM tissues and normal brain tissue (Nor) (Panel B). 18S rRNA was used to normalize the IL-32 expression.
Discussion
The field of IL-32 is rapidly growing. It started from its role as an inducer of TNFα (2), and now its function is extended to inflammatory disease such as rheumatoid arthritis (3–5), ulcerative colitis and Crohn’s disease (6, 7), and viral infection(13–15), bacterial infection (15, 16), and cancer (8, 17). As vascular endothelium is an important mediator in inflammation, we characterized IL-32 in vascular endothelial cells. We found that IL-32 is present in human endothelial cells and Akt activation upregulates its expression. To investigate gene regulation, we mapped the promoter region of IL-32. We identified the major transcription initiation site in endothelial cells, a suppressor binding region, as well as a minimum promoter region of IL-32. Finally, we showed an increase of IL-32 in human breast cancer and brain tumor samples. These findings improve our understanding of IL-32 in vascular endothelium.
Transcription is directed by promoter regions. These define the transcription start site, and also the set of cellular conditions under which the promoter is active. Proper transcription initiation is one of the essential steps for efficient mRNA synthesis. Using RNA ligase mediated rapid amplification of cDNA ends (RLM-RACE), we found that the major transcription start site is located 328 bp downstream of the originally reported site in human endothelial cells (18). There is a TATA box approximately 25 bp upstream from this site. In contrast, we did not detect any transcription initiation from the originally identified site. There is also no TATA box present in the previously defined site. What causes this difference is currently unknown. One of the potential limitations of the current study is that most of the promoter analysis was done in fibroblast instead of endothelial cells. Since in more complex species, it is common for genes to have several different transcription start sites under different conditions. Therefore, it is conceivable to believe that different type of cells may utilize different initiation site for transcription as we report the new transcription start site in vascular endothelial cells, and the original observation was made in RNA isolated from tissues (18).
Interleukins and cyokines are reported to induce IL-32 expression (2). We found that inflammatory cytokines regulates IL-32 expression in endothelial cells (unpublished data). To understand the mechanism of gene regulation, we mapped the promoter region of IL-32. We identified a minimum promoter region of IL-32 in approximately 600bp fragment. This fragment covers 428bp upstream and 172bp downstream of the transcription start site. Deletion of this downstream 172bp region partially reduced promoter activity. Interestingly, we also identified a transcription suppressor-binding region in the promoter, which is located between 2kb and 1.5kb upstream of the originally identified transcription initiation site.
Contradictive data have been reported regarding whether IL-32 is a secreted protein as other interleukin does. Kim et al reported IL-32 as a secreted protein in NK cells and human peripheral blood cells in response to cytokine stimulation as well as IL-32α and IL-32β overexpressing Cos7 cells by transiently transfection (2). Using ELISA, both IL-32α and IL-32β were detected in the culture media in stably transfected K-562 lymphoblast cells (8). However, despite overexpression of tagged IL-32β in endothelial cells using both adenoviral and plasmid vectors in combination with extensive analysis using Western blot and ELISA, we failed to detect any secreted IL-32β in cultured endothelial cells. We subsequently showed that the protein is an intracellular protein localized to the ER. This result is in agreement with a report suggesting that IL-32β is not a secreted protein in T cells (12). These authors suggest that the secretion detected by former investigators might have been due to cytoplasmic protein spilled out from apoptotic cells induced by IL-32 (12). In addition, there is also a possibility that the addition of c-terminal tag on IL-32 might have altered the secretion pattern.
IL-32 is implicated in inflammation. As chronic inflammation is linked to various human cancers (9), an involvement of IL-32 in inflammation suggests a potential role in cancer. Furthermore, elevated NF-κB signaling pathway in tumors may lead to an increase of IL-32 expression as IL-32 is induced in a NF-κB dependent manner (unpublished data). Consistent with published data showing an increase of IL-32 in stomach cancer and lung cancer (8), we found an increase of IL-32 in 71% of breast cancer tissues and 100% of GBM compared to normal tissues. These findings indicate a role of IL-32 in tumor development, and IL-32 expression could be further developed as a potential biomarker for cancer.
In summary, this study characterizes IL-32 gene in human endothelial cells. IL-32β is the major isoform in endothelial cells and Akt induces its expression. IL-32β does not appear to be secreted from endothelial cells but rather co-localized in ER. We further identify the transcription start site, as well as map the transcription activating and suppressing region. Finally, our result suggests a role of IL-32 in tumor development.
Acknowledgments
We thank Drs. Tony Weil, Michael Freeman, and Doug Mortlock at Vanderbilt University Medical Center for simulative discussion. We thank Vanderbilt Cell Imaging Shared Resources for technical assistance. We thank Drs. Wei Zheng and Reid Thompson for providing tumor samples. This work is supported in part by grants from NIH (CA108856, NS45888 and AR053718) to PCL and a training grant to HK (T32CA093240).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dahl CA, Schall RP, He HL, et al. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol. 1992;148:597–603. [PubMed] [Google Scholar]
- 2.Kim SH, Han SY, Azam T, et al. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Shoda H, Fujio K, Yamaguchi Y, et al. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res Ther. 2006;8:R166. doi: 10.1186/ar2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joosten LA, Netea MG, Kim SH, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2006;103:3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cagnard N, Letourneur F, Essabbani A, et al. Interleukin-32, CCL2, PF4F1 and GFD10 are the only cytokine/chemokine genes differentially expressed by in vitro cultured rheumatoid and osteoarthritis fibroblast-like synoviocytes. Eur Cytokine Netw. 2005;16:289–292. [PubMed] [Google Scholar]
- 6.Netea MG, Azam T, Ferwerda G, et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2005;102:16309–16314. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shioya M, Nishida A, Yagi Y, et al. Epithelial overexpression of interleukin-32alpha in inflammatory bowel disease. Clin Exp Immunol. 2007;149:480–486. doi: 10.1111/j.1365-2249.2007.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim KH, Shim JH, Seo EH, et al. Interleukin-32 monoclonal antibodies for immunohistochemistry, Western blotting, and ELISA. J Immunol Methods. 2008;333:38–50. doi: 10.1016/j.jim.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4:641–648. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 10.Lin P, Buxton JA, Acheson A, et al. Antiangiogenic gene therapy targeting the endothelium-specific receptor tyrosine kinase Tie2. Proc Natl Acad Sci U S A. 1998;95:8829–8834. doi: 10.1073/pnas.95.15.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu H, Shu XO, Cui Y, et al. Association of genetic polymorphisms in the VEGF gene with breast cancer survival. Cancer Res. 2005;65:5015–5019. doi: 10.1158/0008-5472.CAN-04-2786. [DOI] [PubMed] [Google Scholar]
- 12.Goda C, Kanaji T, Kanaji S, et al. Involvement of IL-32 in activation-induced cell death in T cells. Int Immunol. 2006;18:233–240. doi: 10.1093/intimm/dxh339. [DOI] [PubMed] [Google Scholar]
- 13.Carter KL, Cahir-McFarland E, Kieff E. Epstein-barr virus-induced changes in B-lymphocyte gene expression. J Virol. 2002;76:10427–10436. doi: 10.1128/JVI.76.20.10427-10436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nold MF, Nold-Petry CA, Pott GB, et al. Endogenous IL-32 controls cytokine and HIV-1 production. J Immunol. 2008;181:557–565. doi: 10.4049/jimmunol.181.1.557. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Liu Y, Mukhtar MM, et al. Activation of interleukin-32 pro-inflammatory pathway in response to influenza A virus infection. PLoS ONE. 2008;3:e1985. doi: 10.1371/journal.pone.0001985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Netea MG, Azam T, Lewis EC, et al. Mycobacterium tuberculosis Induces Interleukin-32 Production through a Caspase- 1/IL-18/Interferon-gamma-Dependent Mechanism. PLoS Med. 2006:3. doi: 10.1371/journal.pmed.0030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcondes AM, Mhyre AJ, Stirewalt DL, et al. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc Natl Acad Sci U S A. 2008;105:2865–2870. doi: 10.1073/pnas.0712391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strausberg RL, Feingold EA, Grouse LH, et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A. 2002;99:16899–16903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]