Abstract
Circulating glycosyltransferases including xylosyltransferases I (XylT1) and II (XylT2) are potential serum biomarkers for various diseases. Understanding what influences the serum activity of these enzymes as well as the sources of these enzymes is important to interpreting the significance of alterations in enzyme activity during disease. This article demonstrates that in the mouse and human the predominant XylT in serum is XylT2. Furthermore, that total XylT levels in human serum are approximately 200% higher than those in plasma due in part to XylT released by platelets during blood clotting in vitro. In addition, the data from Xylt2 knock-out mice and mice with liver neoplasia show that liver is a significant source of serum XylT2 activity. The data presented suggest that serum XylT levels may be an informative biomarker in patients who suffer from diseases affecting platelet and/or liver homeostasis.
Keywords: glycosyltransferase, liver, platelets, proteoglycans, serum
Introduction
Xylosyltransferases (EC 2.4.2.26), one of the earliest glycosyltransferases described, initiate the assembly of glycosaminoglycan chains to the core proteins of proteoglycans by catalyzing the transfer of xylose from the nucleoside diphosphate donor (UDP-xylose) to designated serine residues in the protein acceptor substrate (reviewed in Wilson (2004)). In mammals, two active isoenzymes exist (Götting et al. 2000; Cuellar et al. 2007; Pönighaus et al. 2007; Voglmeir et al. 2007) each consisting of a type II transmembrane protein thought to reside in the endoplasmic reticulum and/or cis-Golgi (reviewed in Wilson (2004)). Subsequent to xylosylation, a tetrasaccharide linker is made on which the assembly of chondroitin sulfate, heparan sulfate, dermatan sulfate, and heparin occurs. Both xylosyltransferases I and II (XylT1 and XylT2, respectively) have stem regions like other glycosyltransferases (Paulson and Colley 1989; Kleene and Berger 1993) that are susceptible to proteolytic cleavage allowing the enzymes to exit the cell's Golgi apparatus and ultimately to enter the circulation (Götting et al. 1999).
Altered serum XylT activity has been proposed as a biomarker of altered proteoglycan metabolism in various diseases ranging from diabetes to systemic sclerosis (Götting et al. 2007). Increased fibrosis or/and accumulation of extracellular matrix components including proteoglycans in affected tissues occurs in these conditions leading to the speculation that increased serum XylT activity represents increased proteoglycan biosynthesis (Götting et al. 1999; 2007). Similarly, decreased serum XylT activity has also been found in diabetic patients suggesting that decreased XylT activity (Götting et al. 2008) may contribute to the decrease in proteoglycans in diabetic nephropathy (Raats et al. 2000). It is uncertain which isoenzyme(s) are responsible for the steady-state level of XylT activity in normal serum and which are responsible for the increased serum activities in various disease states. Higher serum XylT activity is reported in pseudoxanthoma elasticum (PXE) patients and attributed to XylT1. However, no proof that this isoenzyme is responsible for the increased XylT activity is given (Götting et al. 2005). Polymorphisms in XYLT1 are associated with PXE and diabetic nephropathy (Schön, Prante, et al. 2006; Schön, Schulz, et al. 2006). An XYLT1 coding region G > T polymorphism is associated with increased serum XylT levels in PXE patients (Schön, Schulz, et al. 2006). In contrast, it is also associated with decreased serum glycosaminoglycans and normal serum XylT levels in normal subjects (Ambrosius et al. 2008). Confusingly, polymorphisms in XYLT2 have also been reported to occur in a subset of PXE patients with greater organ involvement (Schön, Schulz, et al. 2006). Therefore, which isoenzyme predominates in the serum of healthy individuals and patients and from which tissue it arises is unclear. To better understand the potential utility of serum XylT activity as a biomarker, we investigated which isoenzymes were present in normal serum and what may be source(s) of alteration of XylT activity during disease. Using the recently published Xylt2 knock-out mouse (Xylt2−/−) (Condac et al. 2007) and known differences in nucleotide acceptor affinities (Götting et al. 1998; Casanova et al. 2008), we show for the first time that the predominant circulating isoenzyme in mice and humans is XylT2 and that human platelets during clot formation in vitro significantly contribute to total serum XylT activity levels. Our previous studies have shown that the liver is primarily dependent on XylT2 for proteoglycan biosynthesis (Condac et al. 2007). This report demonstrates that in a mouse model of liver neoplasia serum XylT activity decreases implicating the liver as a significant contributor to total serum XylT activity. This report is the first identification of in vivo sources of serum XylT activity and the first experimental demonstration of a disease impacting a specific organ leading to changes in serum XylT activity levels.
Results and discussion
XylT2 is the predominant isoenzyme in serum
To investigate the relative contribution of XylT2 and XylT1 to serum XylT activity levels, serum total XylT activity was determined in Xylt2−/− mice (Table I). XylT assays using the well-known bikunin nuclear acceptor peptide (BIKp)(QEEEGSGGGQKK) (Weilke et al. 1997) showed in Xylt2−/− mice that the total XylT activity is decreased ≈99% as compared to Xylt2+/+ mice. This strongly suggests that XylT2 is the predominant isoenzyme in mouse serum and that XylT2 may also be the predominant XylT isoenzyme in human serum. The relative contribution of each human isoenzyme to serum XylT activity was examined by exploiting known differences in substrate affinities between human XylT1 and XylT2. Assays using the BIKp or similar peptides show comparable KM values for human XylT1 and XylT2. Human XylT1 from JAR cells and Pichia pastoris conditioned media have KM values of 22 μM (Götting et al. 1998) and 2.5 μM (Casanova et al. 2009), respectively. Human XylT2 from P. pastoris conditioned media has KM values of 1.9 μM (Casanova et al. 2008) and 6.1 μM (Casanova et al. 2009). Therefore, the BIKp is not a differential acceptor substrate for the human XylT isoenzymes but is a good acceptor substrate for total XylT activity measurements. However, 3-A4-amyloid protein precursor protein peptide (l-APPp) (TENEGSGLTNIK) is reported to be a much better acceptor substrate for human XylT1 (KM of 20.1 μM) than for human XylT2 (KM > 10,000 μM) (Götting et al. 1998; Casanova et al. 2008). Thus, measurements using the BIKp acceptor would detect total activity due to both human isoenzymes and those with l-APPp would detect only XylT1. Interestingly, the results showed that XylT1 activity in human serum using l-APPp is 6% of serum total XylT activity as assayed with BIKp (Table I). Similarly in Xylt2+/+ mice sera, assays with l-APPp suggest that XylT1 constitutes approximately 4% of the serum total XylT activity (Table I). Taken together, these data indicate that XylT activity in normal human and mouse serum is due primarily to the XylT2.
Table I.
Serum XylT activity
| Xylt2+/+ | Xylt2−/− | Human | |
|---|---|---|---|
| BIKp | 227.71 ± 37.48 | 1.10 ± 0.30a,c | 75.14 ± 4.74 |
| l-APPp | 9.13 ± 1.59 | 0.29 ± 0.14b,c | 4.32 ± 0.38 |
mU/L ± standard deviation; Xylt2+/+mice, n = 5; Xylt2−/− mice, n = 5; Human, n = 4.
aP < 0.0001 as compared to Xylt2+/+ using BIKp as a substrate.
bP < 0.0001 as compared to Xylt2+/+ using L-APPp as a substrate.
cAverage dpm readings were greater than 2-fold above background.
Mouse XylT1 and XylT2 kinetics
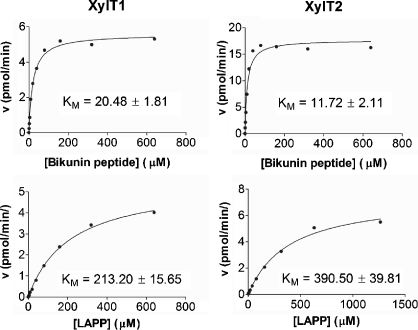
Mouse XylT assays using the BIKp and l-APPp assume conserved kinetics between the mouse and human isoenzymes for these substrates. Intriguingly, using l-APPp on Xylt2+/+ and Xylt2−/− sera showed higher XylT1 values in Xylt2+/+ mice than in Xylt2−/− mice (Table I). This suggests that indeed XylT1 activity levels differ between wild-type and mutant mice or that our assumptions regarding similar affinities between the mouse and human isoenzymes for BIKp and l-APPp are incorrect. Therefore, we determined the kinetics of the mouse isoenzymes for the BIKp and l-APPp (Figure 1). Kinetics of recombinant forms of mouse XylT1 and XylT2 (rmXylT1 and rmXylT2) showed these isoenzymes had KM values of 20.48 μM and 11.72 μM, respectively, for the BIKp. These values are comparable to those reported for human XylT1 and XylT2 as described above using BIKp and similar bikunin peptides. However, the KM values for rmXylT1 and rmXylT2 using l-APPp as the acceptor substrate showed that rmXylT1 and rmXylT2 had similar KM values of 213.2 μM and 390.5 μM, respectively, in contrast to previous studies of the human isoenzymes showing that human XylT1 has a much greater affinity for l-APPp than human XylT2. For both mouse isoenzymes, these results indicate that the BIKp is a better acceptor substrate than l-APPp, and that despite 93.4% homology in amino acid sequences between mouse and human XylT2 (Pönighaus et al. 2007) some species differences in substrate specificities may exist.
Fig. 1.
Mouse XylT1 and XylT2 have similar KM for BIKp and l-APP. Left panels show plots for XylT1 and right panels for XylT2. The reaction velocity was measured using increasing concentrations of acceptor substrate and saturating levels of UDP-xylose (see Materials and Methods).
Total XylT activity is higher in serum than plasma
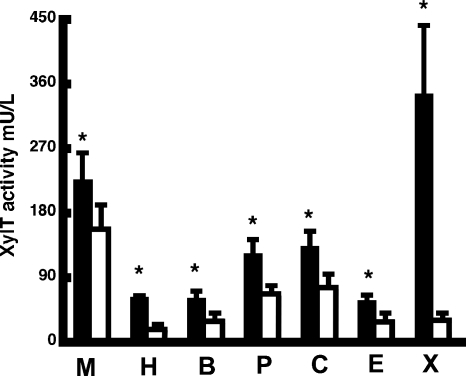
Given the potential utility of serum XylT activity as a biomarker in several diseases, understanding the influences on and the source(s) of serum XylT activity is important in clarifying the significance of serum XylT levels in predicting disease severity and in determining the etiologic role of XylT1 and XylT2 in these diseases. Because serum and plasma differ considerably biochemically, there is likelihood that these differences could augment XylT activity, and depending on how blood samples are collected and processed, these differences may affect XylT measurements. As shown in Figure 2, serum and plasma total XylT activities do differ. In Xylt2+/+ mice, total serum XylT activity was found to be 42% higher than plasma. Moreover, Figure 2 shows increased serum total XylT activity as compared to plasma levels in multiple species including humans where values are 200% higher in serum. Interestingly, in the Xylt2−/− mice, no significant difference between serum and plasma total XylT activity was found (1.10 mU/L, n = 5, versus 0.84 mU/L, n = 5, P = 0.23). Although these values are at the lower limits of detection possibly leading to undistinguishable differences, the loss of XylT2 in the Xylt2−/− mice results in the loss of most if not all of the detectable differences between serum and plasma. These results show that in all species checked total XylT levels are higher in serum than plasma and that in mice this difference is most likely due to XylT2.
Fig. 2.
XylT activity is greater in serum than plasma. Using the BIKp, serum (black bars) and plasma (white bars) XylT activity measured in males of multiple species including amphibians consistently shows higher levels in serum. In all cases the difference was statistically significant as indicated by the asterisk. (M) Mouse P = 0.015, n = 5; (H) Human P = 0.0000007, n = 4; (B) Baboon P = 0.0025, n = 4; (P) Porcine P = 0.002, n = 4; (C) Canine P = 0.02, n = 4; (E) Equine P = 0.015, n = 3; (X) Xenopus P = 0.00008, n = 5.
Platelets are a significant contributor to serum total XylT activity
During in vitro clot formation, activated platelets release factors from their α-granules leading to the formation of serum (Harrison and Cramer 1993). Higher total XylT activity in serum as compared to plasma suggests that platelets may be an important source of XylT activity. To examine this, gel-filtered human platelets were collected from normal volunteers and the XylT activity released from these cells upon activation was measured and compared to XylT activity present in unactivated platelets (Table II). These experiments found that unactivated platelets harbor significant XylT activity that is released upon activation with thrombin. Therefore, XylT released from platelets is one reason why serum total XylT activity is higher than plasma total XylT levels. This also suggests that diseases affecting platelet activation may alter serum total XylT activity.
Table II.
Human platelet XylT activity
| Treatment | Supernatant | Pellet |
|---|---|---|
| No thrombin | None detected | 42.9 ± 9.21 |
| Thrombin | 33.73a ± 5.96 | 5.51 ± 0.99 |
mU/1012 platelets ± standard deviation, n = 4.
aP < 0.0001 as compared to thrombin activated pellet.
BIKp is the acceptor substrate.
Liver is one contributor to serum total XylT activity
Human HepG2 liver cells secrete XylT activity (Götting et al. 1998) and these cells predominately express XYLT2 (Cuellar et al. 2007) suggesting that XylT activity in these cells arises from XylT2. Using the BIKp, total XylT activity of 23.71 ± 8.89 μU/mg protein in HepG2-conditioned media was found to exceed that present in the cells at 1.45 ± 0.30 μU/mg protein. Therefore, HepG2 cells are very efficient at releasing XylT suggesting that in vivo liver cells may contribute significant XylT activity to circulating levels. Notably, previous observations in the Xylt2−/− mice demonstrate that XylT2 is the predominate liver isoenzyme for hepatocellular proteoglycan biosynthesis (Condac et al. 2007) and as shown above, these mice have essentially no serum total XylT activity. These findings further suggest that the liver is a major source of serum XylT2 activity. However, since the Xylt2−/− mice lack XylT2 activity in all tissues, these studies do not address the contribution of nonhepatic tissues to serum XylT2 activity. If the liver is an important source of circulating XylT2 activity, alterations in liver homeostasis may significantly impact serum total XylT measurements. To examine this question, we measured serum total XylT activity in mice with liver neoplasia. In the mouse model used, the mice have transgenes for liver-directed expression of c-Myc and metallothionein promoter-driven expression of TGFα leading to the development of hepatocellular carcinomas (Murakami et al. 1993). Interestingly, we found reduced total XylT activity in sera from mice possessing visible tumors (77.68 ± 30.89 mU/L, n = 3) as compared to controls (159.95 ± 15.24 mU/L, n = 3) (average ± SD, P = 0.01). These experiments further suggest that the liver is a significant source of circulating XylT2 activity and that under conditions of liver disease, serum XylT2 activity can be reduced likely due to decreased secretion by the dysfunctional liver.
Conclusions and perspectives
There is a growing literature describing alterations in serum XylT levels associated with diseases that have significant fibrosis and/or turnover of the extracellular matrix. Secreted XylT has no currently known function and likely does not include proteoglycan biosynthesis since donor UDP-monosaccharides are known not to be present in significant amounts outside the Golgi apparatus (Varki et al. 2009). Although highly speculative, released XylT may represent a byproduct of increased secretory activity by certain cells types. Although a byproduct, it may still be clinically important. Determining the clinical utility of serum XylT levels with respect to disease will come from knowing its source(s) and what may affect its activity. This report shows first that the predominant human and mouse circulating XylT isoenzyme is XylT2. Second we show that serum total XylT activity levels are higher than plasma levels in part due to the XylT released during platelet activation which in the mouse is XylT2, and third we show that the liver is a significant source for circulating XylT2 levels. These results indicate that factors and disease conditions that affect platelet activation and/or liver function could affect total serum XylT activity. Notably, given the tissue distribution of XylT2 gene expression (Condac et al. 2007; Pönighaus et al. 2007), additional sources other than the liver may contribute to circulating XylT2 activity. Furthermore, XylT1 activity may contribute to total XylT serum activity in those diseases that affect specific tissues known to express Xylt1 (e.g., kidney and spleen) (Condac et al. 2007; Pönighaus et al. 2007). Therefore, serum XylT activity in patients with specific diseases needs to be examined using isoenzyme-specific tools, e.g., XylT isoenzyme-specific antibodies and/or differential acceptor substrates. Correlation of XylT isoenzyme changes with tissue-specific changes for each disease would give insight into the cell type and biochemical mechanism(s) responsible for alterations in total serum XylT activity in disease.
Material and methods
Materials
The Xylt2−/− mice were generated as previously described (Condac et al. 2007) and the c-Myc/TGFα doubly transgenic mice were generated as previously described (Murakami et al. 1993). All mice were housed in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The Institutional Animal Care and Use Committee approved all animal procedures and experiments. All reagents were obtained from Sigma-Aldrich (St Louis, MO) unless otherwise specified.
Serum and plasma collection
Using isoflurane, mouse blood was collected by heart puncture. For plasma, blood was collected into sodium citrate or K3 EDTA and centrifuged at 8000 × g. For serum, blood was incubated for 30 min at room temperature then centrifuged at 8000 × g for 15 min. Frogs (Xenopus laevis) were anesthetized by 0.05% benzocaine followed by blood collection from the heart. Plasma and serum samples were prepared similar to those for the mouse. Human blood from normal male volunteers 25–55 years of age was collected in BD Vacutainer SST for serum and BD Vacutainer K3 EDTA for plasma. Blood samples from baboons, dogs, pigs, and horses were collected into sodium citrate or K3 EDTA for plasma and no anticoagulant for serum. All animal samples were obtained from nongonadectomized males except for the horses which were geldings. Plasma and serum samples were stored at −80°C until assayed. Multiple freezing and thawing were avoided by thawing the samples and immediately performing assays. The University of Oklahoma Health Sciences Center Institutional Review Board approved all procedures for human experiments.
XylT assays
To measure XylT activity, cell lysates (50 μL), conditioned media (50 μL), serum (10–25 μL), or plasma (10–25 μL), platelet supernatant (10–30 μL), and platelet pellet (10–30 μL) were incubated with 1.13 μM UDP-[14C]-d-xylose 150–250 mCi/mol (Perkin Elmer, Waltham, WA), 7.46 μM UDP-d-xylose (CarboSource, University of Georgia, Atlanta, GA) and 160 μM acceptor peptide (BIKp or L-APPp) (Bio-Synthesis, Lewisville, TX) in a total reaction volume of 100 μL containing 25 mM 2-(4-morpholino)-ethane sulfonic acid, pH 6.5, 25 mM KCl, 5 mM KF, 5 mM MgCl2, 5 mM MnCl2 for 1 h at 37°C. The reaction was stopped by placing reactions on ice. BSA, 1.5 mg, was added as a carrier and the reaction product was precipitated with 10% trichloroacetic acid/4% phosphotungstic acid (Pfeil and Wenzel 2000). After 30 min incubation on ice, tubes were centrifuged and the precipitates were washed twice with 750 μL trichloroacetic acid 5%, incubated 15 min at 4°C, and centrifuged 15 min at 12,000 × g. Radioactivity was measured in the final precipitates after resuspension in 400 μL of 1N NaOH. One unit of enzymatic activity represents the incorporation of 1 μmol xylose/min into the acceptor peptide. For each sample, the reactions were done in duplicate or triplicate. Michaelis–Menten constants were determined with the purified rmXylT protein from Chinese hamster ovary cells and saturating levels of UDP-xylose. Recombinant mouse XylT was incubated with various concentrations of acceptor peptides indicated, 1.13 μM UDP-[14C]-d-xylose, and 7.46 μM cold UDP-d-xylose. All values were determined in duplicate. A nonlinear regression fit of the Michaelis–Menten plot was used to calculate the KM using GraphPad Prism version 5.01, GraphPad Software, San Diego, CA.
Human platelet XylT activity
Blood was drawn immediately into citrate dextrose (38.1 mM citric acid, 74.8 mM Na3 citrate, 136 mM glucose), and gel-filtered platelets were prepared as described (Alberio et al. 2000). Platelets were counted and equal numbers for each donor were activated with thrombin 0.5 U/mL for 15 min at 37°C followed by centrifugation at 9000 × g for 10 min. Supernatants were transferred to new tubes and the cellular pellets were washed with 500 μL Tris-HCl 20 mM, pH 7.5, and centrifuged again. Final pellets were homogenized in 50 μL of 2-(4-morpholino)-ethane sulfonic acid 25 mM, pH 6.5, 25 mM KCl, 5 mM KF, 5 mM MgCl2, 5 mM MnCl2, 0.1% Triton-X 100. Samples of 10–30 μL of supernatants and homogenized pellets were used for enzyme assays.
Recombinant mouse XylT
The Xylt2 expression construct was a modification of the minigene construct previously described (Cuellar et al. 2007). The modified minigene used produced a protein lacking the 45 N-terminal amino acids including the transmembrane domain as predicted by comparison with the human XylT2-predicted transmembrane domain (Wilson 2004) and from Kyte–Doolittle hydrophobicity predictions. The Xylt1 expression cassette produced a protein that was lacking 94 N-terminal amino acids including the transmembrane domain which was predicted as for XylT2. Both Xylt1 and Xylt2 expression cassettes were cloned into a modified pcDNA3.1 vector (Invitrogen, Carlsbad, CA) containing a transferrin signal peptide and an HPC4 epitope tag in frame on the N-terminus that was used for immunopurification. Reading frames of both constructs were verified by sequencing (see supplementary data for more detail).
Supplementary Data
Supplementary data for this article is available online at http://glycob.oxfordjournals.org/.
Funding
This work was partially funded by an applied research grant from the Oklahoma Center for the Advancement of Science and Technology [HR08-046 to MEH] and National Institutes of Health Grant [P20-RR018758 to MEH].
Acknowledgments
We are grateful to Paul Friese for platelet isolation and Diane McFarlane for supplying horse sera. TGFα and c-Myc heterozygous mice were kindly provided by Snorri S. Thorgeirsson at the National Cancer Institute, Bethesda, MD. We thank Debra Saunders for indispensable technical assistance.
Conflict of interest statement
None declared.
References
- Alberio L, Safa O, Clemetson KJ, Esmon CT, Dale GL. Surface expression and functional characterization of alpha-granule factor V in human platelets: Effects of ionophore A23187, thrombin, collagen, and convulxin. Blood. 2000;95:1694–1702. [PubMed] [Google Scholar]
- Ambrosius M, Kleesiek K, Götting C. The xylosyltransferase I gene polymorphism c.343G>T (p.A115S) is associated with decreased serum glycosaminoglycan levels. Clin Biochem. 2008;42:1–4. doi: 10.1016/j.clinbiochem.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Casanova JC, Kuhn J, Kleesiek K, Götting C. Heterologous expression and biochemical characterization of soluble human xylosyltransferase II. Biochem Biophys Res Commun. 2008;365:678–684. doi: 10.1016/j.bbrc.2007.10.206. [DOI] [PubMed] [Google Scholar]
- Casanova JC, Roch C, Kuhn J, Kleesiek K, Götting C. First in-gel detection and purification of human xylosyltransferase II. Biochem Biophys Res Commun. 2009;379:243–248. doi: 10.1016/j.bbrc.2008.12.033. [DOI] [PubMed] [Google Scholar]
- Condac E, Silasi-Mansat R, Kosanke S, Schoeb T, Towner R, Lupu F, Cummings RD, Hinsdale ME. Polycystic disease caused by deficiency in xylosyltransferase 2, an initiating enzyme of glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA. 2007;104:9416–9421. doi: 10.1073/pnas.0700908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar K, Chuong H, Hubbell SM, Hinsdale ME. Biosynthesis of chondroitin and heparan sulfate in chinese hamster ovary cells depends on xylosyltransferase II. J Biol Chem. 2007;282:5195–5200. doi: 10.1074/jbc.M611048200. [DOI] [PubMed] [Google Scholar]
- Götting C, Hendig D, Adam A, Schön S, Schulz V, Szliska C, Kuhn J, Kleesiek K. Elevated xylosyltransferase I activities in pseudoxanthoma elasticum (PXE) patients as a marker of stimulated proteoglycan biosynthesis. J Mol Med. 2005;83:984–992. doi: 10.1007/s00109-005-0693-x. [DOI] [PubMed] [Google Scholar]
- Götting C, Kuhn J, Brinkmann T, Kleesiek K. Xylosylation of alternatively spliced isoforms of Alzheimer APP by xylosyltransferase. J Protein Chem. 1998;17:295–302. doi: 10.1023/a:1022549121672. [DOI] [PubMed] [Google Scholar]
- Götting C, Kuhn J, Kleesiek K. Human xylosyltransferases in health and disease. Cell Mol Life Sci. 2007;64:1498–1517. doi: 10.1007/s00018-007-7069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götting C, Kuhn J, Kleesiek K. Serum xylosyltransferase activity in diabetic patients as a possible marker of reduced proteoglycan biosynthesis. Diabetes Care. 2008;31:2018–2019. doi: 10.2337/dc08-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götting C, Kuhn J, Zahn R, Brinkmann T, Kleesiek K. Molecular cloning and expression of human UDP-d-xylose:Proteoglycan core protein beta-d-xylosyltransferase and its first isoform XT-II. J Mol Biol. 2000;304:517–528. doi: 10.1006/jmbi.2000.4261. [DOI] [PubMed] [Google Scholar]
- Götting C, Sollberg S, Kuhn J, Weilke C, Huerkamp C, Brinkmann T, Krieg T, Kleesiek K. Serum xylosyltransferase: A new biochemical marker of the sclerotic process in systemic sclerosis. J Invest Dermatol. 1999;112:919–924. doi: 10.1046/j.1523-1747.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- Harrison P, Cramer EM. Platelet alpha-granules. Blood Rev. 1993;7:52–62. doi: 10.1016/0268-960x(93)90024-x. [DOI] [PubMed] [Google Scholar]
- Kleene R, Berger EG. The molecular and cell biology of glycosyltransferases. Biochim Biophys Acta. 1993;1154:283–325. doi: 10.1016/0304-4157(93)90003-7. [DOI] [PubMed] [Google Scholar]
- Murakami H, Sanderson ND, Nagy P, Marino PA, Merlino G, Thorgeirsson SS. Transgenic mouse model for synergistic effects of nuclear oncogenes and growth factors in tumorigenesis: Interaction of c-myc and transforming growth factor alpha in hepatic oncogenesis. Cancer Res. 1993;53:1719–1723. [PubMed] [Google Scholar]
- Paulson JC, Colley KJ. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989;264:17615–17618. [PubMed] [Google Scholar]
- Pfeil U, Wenzel KW. Purification and some properties of UDP-xylosyltransferase of rat ear cartilage. Glycobiology. 2000;10:803–807. doi: 10.1093/glycob/10.8.803. [DOI] [PubMed] [Google Scholar]
- Pönighaus C, Ambrosius M, Casanova JC, Prante C, Kuhn J, Esko JD, Kleesiek K, Götting C. Human xylosyltransferase II is involved in the biosynthesis of the uniform tetrasaccharide linkage region in chondroitin sulfate and heparan sulfate proteoglycans. J Biol Chem. 2007;282:5201–5206. doi: 10.1074/jbc.M611665200. [DOI] [PubMed] [Google Scholar]
- Raats CJ, Van Den Born J, Berden JH. Glomerular heparan sulfate alterations: Mechanisms and relevance for proteinuria. Kidney Int. 2000;57:385–400. doi: 10.1046/j.1523-1755.2000.00858.x. [DOI] [PubMed] [Google Scholar]
- Schön S, Prante C, Bahr C, Tarnow L, Kuhn J, Kleesiek K, Götting C. The xylosyltransferase I gene polymorphism c.343G>T (p.A125S) is a risk factor for diabetic nephropathy in type 1 diabetes. Diabetes Care. 2006;29:2295–2299. doi: 10.2337/dc06-0344. [DOI] [PubMed] [Google Scholar]
- Schön S, Schulz V, Prante C, Hendig D, Szliska C, Kuhn J, Kleesiek K, Götting C. Polymorphisms in the xylosyltransferase genes cause higher serum XT-I activity in patients with pseudoxanthoma elasticum (PXE) and are involved in a severe disease course. J Med Genet. 2006;43:745–749. doi: 10.1136/jmg.2006.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Esko J, Colley KJ. Cellular organization of glycosylation. In: Varki A, et al., editors. Essentials of Glycobiology. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009. p. xxix. 784. [PubMed] [Google Scholar]
- Voglmeir J, Voglauer R, Wilson IB. XT-II, the second isoform of human peptide-O-xylosyltransferase, displays enzymatic activity. J Biol Chem. 2007;282:5984–5990. doi: 10.1074/jbc.M608087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilke C, Brinkmann T, Kleesiek K. Determination of xylosyltransferase activity in serum with recombinant human bikunin as acceptor. Clin Chem. 1997;43:45–51. [PubMed] [Google Scholar]
- Wilson IB. The never-ending story of peptide O-xylosyltransferase. Cell Mol Life Sci. 2004;61:794–809. doi: 10.1007/s00018-003-3278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.