Abstract
Spo11 and the Rad50-Mre11 complex have been indirectly implicated in processes associated with DNA replication. These proteins also have been shown to have early meiotic roles essential for the formation of a programmed DNA double-strand break known in Saccharomyces cerevisiae to initiate meiotic recombination. In both S. cerevisiae and the basidiomycete Coprinus cinereus, spo11 and rad50 mutants are defective in chromosome synapsis during meiosis. Here we demonstrate that a partial restoration of synapsis occurs in C. cinereus spo11 and rad50 mutants if premeiotic DNA replication is prevented. Double mutants were constructed with spo11–1 or rad50–4 and another mutant, spo22–1, which does not undergo premeiotic DNA replication. In both cases, we observed an increase in the percentage of nuclei containing synaptonemal complex (SC) structures, with concomitant decreases in the percentage of nuclei containing axial elements (AE) only or no structures. Both types of double mutants demonstrated significant increases in the average numbers of AE and SC, although SC-containing nuclei did not on average contain more AE than did nuclei showing no synapsis. Our results show that Spo11-induced recombination is not absolutely required for synapsis in C. cinereus, and that the early meiotic role of both Spo11 and Rad50 in SC formation partially depends on premeiotic S phase. This dependency likely reflects either a requirement for these proteins imposed by the premeiotic replication process itself or a requirement for these proteins in synapsis when a sister chromatid (the outcome of DNA replication) is present.
Meiosis consists of genomic DNA replication followed by a reductional and then an equational separation of chromosomes. In most organisms examined, the reductional segregation uses both crossing-over between nonsister chromatids and the presence of intact sister chromatid cohesion to facilitate proper homolog disjunction (1). Additionally, the alignment of homologous chromosomes culminates in the formation of the synaptonemal complex (SC). In Saccharomyces cerevisiae, the formation of this structure is known to require the initiation of meiotic recombination. “Early” meiotic recombination mutants (2, 3), which fail to form the programmed double-strand breaks (DSBs) used for recombination initiation, are unable to form SC.
At least 11 gene products are required for the initiation of meiotic recombination (4), and several have been well characterized. Spo11 is a type II topoisomerase-like protein that has been shown in S. cerevisiae to create the programmed DSBs that initiate meiotic recombination (5). However, it recently has been demonstrated that Spo11 function in meiosis is not limited to this transesterase function. A tyrosine to phenylalanine mutation, which prevents DSB formation, does not abolish the function of the Spo11 protein in the DSB-independent homologous chromosome pairing that precedes synapsis (6). In contrast, a spo11 null mutation causes a severe reduction in homolog pairing (7).
The action of Rad50 in DSB formation and, therefore, recombination initiation is less clear. However, it is likely that this function occurs in conjunction with the Mre11 and Xrs2 proteins in S. cerevisiae (8, 9). Although the full extent to which Spo11 and Rad50 contribute to recombination initiation and synapsis is not certain, it is likely that these proteins function in processes concomitant with or immediately after DNA replication (6, 10, 11). For example, all members of the S. cerevisiae RAD52 epistasis group tested are hypersensitive to hydroxyurea (HU), an indirect inhibitor of DNA replication (10). In addition, rad50, rad51, and rad53 mutants exhibit irreversible HU sensitivity; they decrease in viability after exposure to and removal from the drug (10). This inability to survive after HU treatment can be suppressed by pretreatment with α-factor to cause G1 arrest and, therefore, to prevent entry into an HU-impaired replication program (10). Taken together, the HU irreversibility and rescue by α-factor indicate that the rad50 mutant lacks the S phase arrest normally triggered by replication inhibition. Thus, although rad50 null mutants are viable in S. cerevisiae, Rad50 may contribute to a checkpoint function necessary under certain conditions (e.g., when replication is inhibited).
Rad50 may also function in the processing of stalled replication forks or in the repair of replication-induced damage (12). These functions may be important events in triggering checkpoint arrest during S phase and may account for the suspected role of Rad50 in that process. In S. cerevisiae, Rad50 recently has been demonstrated to be involved in one of two Rad52-dependent pathways of telomere maintenance in the absence of telomerase (13). It is likely that this form of telomere maintenance depends on break-induced replication (BIR; ref. 14). The role of Rad50 in BIR may be identical to its role in replication, because this type of repair has been proposed as a possible mechanism for restarting broken replication forks (14).
There is further evidence that the S phase function of Rad50 may not be limited to a possible checkpoint role. In S. cerevisiae, a rad50 deletion mutation shows allele-specific synthetic lethality with pol30 (proliferating cell nuclear antigen) mutant strains exhibiting the cell-cycle defect of an accumulation of G2 cells (11). The lethality likely reflects a replication deficiency because the pol30 mutants that exhibit synthetic lethality with rad50 accumulate small single-stranded DNA fragments, identified by sedimentation of genomic DNA through alkaline sucrose gradients, during DNA synthesis. These molecules presumably arise from the incomplete processing of Okazaki fragments during lagging-strand synthesis (11).
Although Rad50 has been suggested to have possible overlapping roles in an S phase checkpoint, Okazaki fragment processing, and replication restart, less is known about the interplay between Spo11 function and DNA replication. However, there does seem to be some level of cross-talk between premeiotic DNA replication and the function of Spo11, as a spo11 deletion mutation leads to a shortened premeiotic S phase (6). The specific functions that Spo11 and Rad50 may have during replication also may be relevant to their requirement in meiotic recombination initiation and chromosome synapsis.
Because Spo11 and Rad50 have been implicated in processes relevant to DNA replication, we were interested in whether the early meiotic role of these proteins is related to premeiotic S phase. In the basidiomycete fungus Coprinus cinereus, as in S. cerevisiae (15–17), mutations in the spo11 and rad50 genes can result in the inability of these strains to form the SC normally associated with pachytene chromosomes (18, 19). If the observed SC defects in spo11 and rad50 mutants stem from an earlier defect during or immediately after premeiotic S phase, then a bypass of S phase might obviate the requirement for the proteins in synapsis. That is, their role in synapsis may reflect one or more replication-dependent requirements for these proteins.
To test the validity of this proposed relationship, we have taken advantage of a unique meiosis-specific mutant of C. cinereus. The spo22–1 mutant fails to undergo premeiotic DNA replication (20). Pukkila et al. (21) showed that triple synapsis, which is extensive in wild-type triploids of C. cinereus, is absent in spo22–1, thus confirming that these chromosomes are largely, if not completely, unreplicated. However, despite the inability of this mutant to make a sister chromatid, homologous chromosomes align perfectly and form SC with no obvious morphological defects (20, 21). We investigated axial element (AE) formation and synapsis in double mutants of spo22–1 and either spo11–1 or rad50–4 (18, 19, 22) to ask whether spo22–1 can suppress the synapsis defects observed in the two single mutants. We observed a striking suppression of the synapsis defects of spo11–1 and rad50–4 mutants by the spo22–1 mutation. This result suggests that Spo11 and Rad50 function in the establishment of a class of nuclei that are competent for synapsis, and that the requirement for this function depends on the passage of cells through premeiotic S phase.
Materials and Methods
Strains and Culture Conditions.
Double mutants were derived from C. cinereus strain spo22–1 (PJP; ref. 20) and either spo11–1 (19, 22) or rad50–4 (18). spo22–1 was mated to spo11–1 or to rad50–4, and ≈150 spores were picked from each cross. spo11–1 mutants were identified on the basis of growth on hygromycin B (22), and spo11–1;spo22–1 double mutants were identified as fruiting white when crossed with spo22–1 (meiotic mutants fruit white as a result of a meiotic defect, whereas wild-type strains fruit black as a result of the production of black spores). rad50–4 mutants were identified based on sensitivity to ionizing radiation and rad50–4;spo22–1 double mutants were identified by crossing radiation-sensitive isolates to spo22–1 and looking for white fruit bodies. Crosses of monokaryotic progeny of a spo11–1 X spo22–1 cross were used in the production of the following dikaryotic strains (isolate numbers are given after the genotypes): TSM1 (wild type, 4 × 34), TSM2 (spo22–1, 12 × 26), TSM5 (spo11–1;spo22–1, 8 × 34), TSM6 (spo11–1;spo22–1, 8 × 52), and TSM7 (spo11–1;spo22–1, 8 × 41). TSM8 (rad50–4;spo22–1, 55 × 16) and TSM9 (rad50–4;spo22–1, 55 × 17) represent crosses of progeny from a rad50–4;spo22–1 (isolate 55) × Java-6 (23) cross with the same rad50;spo22–1 parent strain (isolate 55). Strains TSM3 (spo11–1; refs. 19, 22) and TSM4 (rad50–4; ref. 18) are the parental single-mutant strains used in the creation of the double mutants. The data set analyzed for TSM3 is the same as that presented in ref. 19. TSM1 is a cross between a spo11–1;spo22–1 strain and a wild-type sibling; all other dikaryons used were homokaryotic. In addition, control crosses were done between sibling strains to verify phenotypes. A spo11–1;spo22–1 double mutant (isolate 34) was crossed with a sibling spo22–1 strain (isolate 26) and also with a sibling spo11–1 strain (isolate 7) to create the control strains TSM10 (spo22–1 × spo11–1;spo22–1; 26 × 34) and TSM11 (spo11–1 × spo11–1;spo22–1; 7 × 34).
Culture conditions, matings, and fruiting conditions were as described (24). Basidiospore viabilities were determined as described in ref. 18 with the following results: TSM1, 78%; TSM2, 2.7%; TSM3, 1.6%; TSM4, 0% (this measurement is from ref. 18); TSM5, 0%; TSM8, 0.2%; and TSM9, 1%.
Microscopy.
Chromosome spreads were silver-stained according to methods described in Pukkila et al. (25). Spreads were viewed and photographed by using a JEOL transmission electron microscope. Photographs then were examined to identify AE and SC formation in each of the strains. Spreads were scored as follows: having distinct SC; having no SC but visible AE formation; and having no SC or AE formation. The SC-containing class always contained two or more SC segments; the minimum SC length was 0.3 mm.
Measurements.
Photographs were scanned by using PHOTOSHOP 5.0 (Adobe Systems, Mountain View, CA), and analysis was performed on a Macintosh computer by using the public domain nih image program (developed at the U.S. National Institutes of Health and available on the internet at http://rsb.info.nih.gov/nih-image/); we measured total SC and AE length per nucleus. Each AE was traced by using nih image, and SC length measurements were obtained by measuring between AE. Statistical differences between strains were evaluated by using a t test to compare the differences between the mean values of the two samples in question.
Results
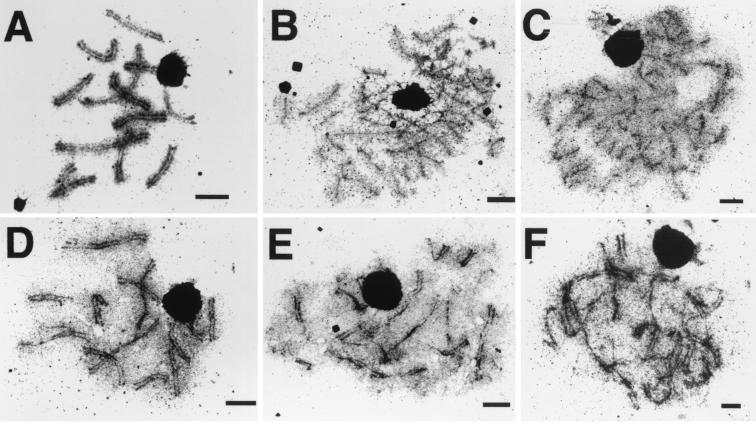
We investigated AE formation and synapsis in double mutants of spo22–1 and either spo11–1 (19, 22) or rad50–4 (ref. 18; the C. cinereus rad12 gene is orthologous to the S. cerevisiae gene RAD50 and is referred to as rad50 in this paper; S.N.A. and M.E.Z., unpublished data). As single mutants, little SC was formed in strains homozygous for spo11–1 or rad50–4 mutations [0% and 23% of cells, respectively, formed SC, (ref. 19; Figs. 1 B and C and 2)]; instead, these strains principally produced AE. In contrast, 100% of meiotic nuclei in wild-type and spo22–1 strains formed SC (Figs. 1 A and D and 2).
Figure 1.
SC formation in wild-type and mutant strains. (A) TSM1, wild type; (B) TSM3, spo11–1; (C) TSM4, rad50–4; (D) TSM2, spo22–1; (E) TSM5, spo11–1;spo22–1; and (F) TSM8, rad50–4;spo22–1. [Scale bars = 2 μm.]
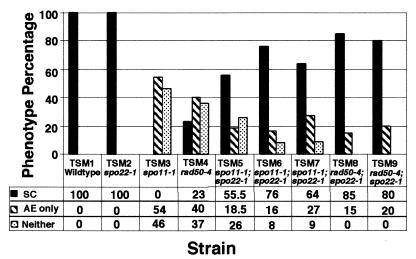
Figure 2.
Comparison of wild-type, single-, and double-mutant strains. Chromosome spreads were examined and categorized according to the presence or absence of AE and SC. For each strain, at least two mushrooms were sampled. Total numbers of spreads were: TSM1, 22; TSM2, 19; TSM3, 60; TSM4, 30; TSM5, 27; TSM6, 45; TSM7, 38; TSM8, 40; and TSM9, 27.
For the three homozygous spo11–1;spo22–1 strains, an average of 65% of nuclei formed SC structures (Fig. 2, strains TSM5, TSM6, and TSM7), a dramatic increase relative to the spo11–1 single mutant. In the double-mutant strains, the average percentage of nuclei containing AE decreased 2.6-fold, and the average percentage of nuclei with unstructured chromosomes decreased 3.2-fold relative to the spo11–1 single mutant. Thus, the AE-only and unstructured classes of nuclei were comparably affected by the spo22–1 mutation. In control experiments, crosses between sibling strains of types spo22–1 × spo22–1 (TSM2) and spo22–1 × spo11–1;spo22–1 (TSM10) showed full SC formation and thus exhibited the phenotype of the spo22–1 mutant (Fig. 1D and data not shown). A cross between a spo11–1 single mutant and its spo11–1;spo22–1 double-mutant sibling (TSM11) had a phenotype similar to the homozygous spo11–1 single mutant (TSM3) shown in Figs. 1B and 2. Of the 30 nuclei that were examined, 6% had SC, 64% had AE only, and 30% were unstructured. A wild-type strain (spo11+;spo22+) crossed to its spo11–1;spo22–1 double-mutant sibling showed complete SC formation (Fig. 1A), demonstrating the recessive nature of both of these mutations.
The SC-containing class of nuclei in homozygous rad50–4;spo22–1 strains was an average of 3.6-fold larger than that in the single rad50–4 mutant; SC was formed in 80% and 85% of the cells in two separate crosses (Fig. 1 C and F and Fig. 2). Notably, we were unable to detect any unstructured nuclei in the rad50–4;spo22–1 double mutants. Furthermore, the average number of AE-only nuclei was 2.3-fold lower in the rad50–4;spo22–1 double mutant than in the rad50–4 single mutant (Fig. 2). Therefore, as for spo11–1, the enhancement of synapsis in the rad50–4 background by spo22–1 reflects decreases in both the unstructured and AE-only classes of nuclei. For TSM4, the rad50–4 × rad50–4 single mutant cross, we analyzed two sets of data, one that we generated (Figs. 1C, 2, and 3) and that of Ramesh and Zolan (17). Similar results were obtained for both data sets.
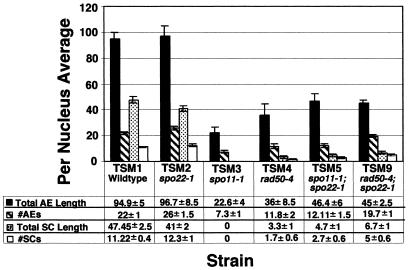
We wanted to address whether the increase in the SC-containing class of nuclei observed in the double mutants correlated with an overall increase in the average number and/or length of AE and SC per nucleus, or whether the increase in this class represents a regulatory shift or structural change that leads to competence for synapsis. We already knew that spo11–1 could demonstrate essentially complete AE formation without the production of any SC (ref. 19; Fig. 1B), and therefore it seemed unlikely that an increase in AE formation per se would trigger SC formation. However, it did seem possible that, on average, a spo22–1 double mutant with either spo11–1 or rad50–4 might display more SC precursor structures (i.e., AE) than the single mutants did. We therefore tabulated AE and SC number and length for both single and double mutants. For each analysis, we included the number of nuclei with no AE or SC structures as part of the total number of nuclei used to calculate mean values. The spo22–1 mutant exhibits numbers and lengths of both AE and SC comparable to those in wild-type strains (Fig. 3); values for the average length of both AE and SC in spo22–1 strains were indistinguishable from those of wild type at a 95% confidence level. The spo22–1 strain exhibited an average of 26 AE, as expected for normal, complete AE formation. In this experiment, the number of AE per nucleus (22 AE per nucleus) was significantly different from that of wild type at a 95% but not a 98% confidence level; this observation undoubtedly reflects technical problems in observing all structures in wild-type cells. Thus, for either spo22–1 or wild type, a given data set may show a slight deviation from the expected value. In contrast, spo11–1 and rad50–4 mutants had severe defects in both the numbers and lengths of these chromosomal structures (Fig. 3).
Figure 3.
Comparison of SC and AE length vs. the number of SC and AE segments. Total SC and AE lengths were measured and the sum was divided by the total number of nuclei to determine the average length per nucleus for each strain listed in the table. For each chromosome spread, AE segments were counted as one segment for each continuous piece regardless of length. A single SC segment represents continuous association of two AE. Data are presented as ± indicates the SEM. SC and AE lengths are in micrometers (μm).
The spo11–1;spo22–1 double mutant exhibited increases in the average length of both AE and SC relative to the spo11–1 strain. The average number of AE structures per nucleus increased by 1.7-fold (Fig. 3); this number is significantly different from that of the spo11–1 mutant at a 95% confidence level. The spo11–1;spo22–1 double mutant exhibited a 2.1-fold increase in the average length of AE per nucleus. The average value for the double mutant is significantly different from that of the spo11–1 single mutant at a 99.8% confidence level. The average number and length of SC structures per nucleus increased from 0 to 2.7 structures and 0 to 4.7 μm, respectively (Fig. 3), and the spo11–1;spo22–1 double mutant is significantly different from spo11–1 in both the number and length of SC structures with 99.9% confidence levels. These differences in the SC data are a reflection of the complete absence of any SC in the spo11–1 single mutant (Figs. 1B, 2, and 3).
For rad50–4, the average number and length of AE per nucleus increased by 1.7- and 1.3-fold, respectively, for the rad50–4;spo22–1 double mutant relative to the rad50–4 single mutant (Fig. 3). The average number of SC structures per nucleus increased by 2.9-fold and the average length of SC structures per nucleus increased by 2-fold. Although increases in both the average number and the average lengths of AE and SC were observed for the rad50–4;spo22–1 double-mutant strain, only the increases in the average number of AE and SC per nucleus are significantly different from the wild-type values (99.5% and 99.9% confidence levels, respectively). The mean values of the lengths of these structures do not demonstrate significant differences between the single- and double-mutant strains at a 95% confidence level.
Given the relative increase in both number and length of AE and SC structures in the spo11;spo22–1 double mutant and the increase in the number of AE and SC elements in the rad50–4;spo22–1 double mutant, we wanted to address whether the increase in synapsis seen in these strains was coupled with an elevation of AE formation. Specifically, we asked whether those double-mutant nuclei that made SC had, on average, more or longer AE than did those that had AE structures only. In the spo11–1;spo22–1 strain, no significant differences at 95% confidence levels were observed in the number or length of AE structures between nuclei that contained SC and those that did not. Similarly, there was no statistical difference in the average numbers of AE structures per nucleus in SC-containing and SC-lacking rad50–4;spo22–1 nuclei. Additionally, there was no statistical difference in the average lengths of AE per nucleus in the SC-containing and SC-lacking classes of the double mutant.
Discussion
Spo11 and Rad50 have been implicated in S phase-related events. The involvement of these two proteins in premeiotic DNA replication-associated activities therefore may explain, at least in part, their requirement early in meiosis. Defects in Spo11 and Rad50 function severely impair the synapsis of homologous chromosomes. To test whether the replication-associated function of these proteins is relevant to synapsis, we asked whether the mutant spo22–1, which fails to undergo premeiotic DNA replication, can suppress the synapsis defects observed in the spo11–1 and rad50–4 mutants. The phenotype of the spo22–1 mutant is similar to that of the cdc4, clb5, and clb6 mutants of S. cerevisiae, which fail to undergo premeiotic DNA replication (26–29). These strains proceed through prophase I, and cdc4 strains have been demonstrated to produce SC (26–29). However, the spo22–1 mutant does differ from these mutants in that its defect is limited to meiosis; the others exhibit mitotic defects as well.
We observed a suppression of the synapsis defects in spo11–1 and rad50–4 mutants by the spo22–1 mutation. We also have found that the prophase I DNA content of spo11–1;spo22–1 double-mutant nuclei is approximately half the amount in spo11–1 and wild-type nuclei (W.J.C., S.T.M., M. Celerin, C. Johnson, E. Sierra, and M.E.Z., unpublished work). Assuming that the rad50–4;spo22–1 double mutant is similarly affected for premeiotic DNA replication, our results suggest that the requirement for both Spo11 and Rad50 in synapsis at least partially depends on the passage of cells through premeiotic S phase. More specifically, this result demonstrates that these two proteins function in S phase-related processes that are important for synapsis. The S phase-dependent function of Spo11 contributes to both the number and length of AE and SC, whereas the S phase-related role of Rad50 seems to be principally a contribution to the number of these structures. It is uncertain what the S phase-dependent effect of these proteins on the numbers of AE and SC structures actually means. However, one interpretation of this effect is that the loss of activity of Spo11 and Rad50 prevents the establishment of a class of nuclei that are competent for AE formation and/or synapsis. It is unclear whether the loss of activity of these proteins triggers a checkpoint that prevents the progression to pachytene stage, or whether the absence of a structural contribution made by these proteins precludes the initiation of synapsis on a region-by-region basis.
In addition to the establishment of competence for the formation of AE and SC structures, the S phase-dependent function of Spo11, unlike that of Rad50, serves as a determinant of the length of these structures, once their formation has initiated. Interestingly, the increase in SC formation in both the spo11–1;spo22–1 and rad50–4;spo22–1 double-mutant strains does not correlate with an obvious increase in the length and number of AE structures per nucleus. Therefore, the S phase-associated function of Spo11 and Rad50 in SC formation is probably not a direct extension of their S phase-related contribution to AE formation.
There are two basic ways of thinking about an S phase-dependent requirement for these proteins. First, they may perform essential functions during premeiotic DNA replication. When these requirements are left unfulfilled, prophase I events (such as synapsis) are abnormal. Second, the presence of a sister chromatid, the result of premeiotic DNA replication, could be inhibitory to synapsis in the absence of either Spo11 or Rad50 function.
In support of a replication role for these proteins, numerous potential functions, such as contributions to a replication blockage checkpoint, a pathway for processing Okazaki fragments, and a pathway for restarting stalled replication forks, have been suggested for Rad50 (as discussed in the Introduction). It is unclear whether Spo11 plays a direct role in premeiotic DNA replication. However, Cha et al. (6) have demonstrated that the length of the premeiotic replication program of the nucleus is shortened in the absence of Spo11 function. These authors suggest that in wild-type cells, Spo11-mediated activities contribute to a kinetic barrier(s) that hinders the progression of premeiotic S phase. Presumably, a feedback mechanism is in place such that nucleus-wide replication is slowed to accommodate the regional formation of Spo11-dependent chromosome structures. Our observed suppression of the synapsis defects in spo11–1 and rad50–4 mutants by the prevention of premeiotic S phase may indicate a direct role of Spo11 and Rad50 in replication that is important for synapsis, or their requirement during premeiotic S phase to form chromosomal features that are required for synapsis. If DNA replication is bypassed, as in a spo22–1 background, then these structures become unnecessary for synapsis.
Our work also raises the possibility that the failure of our spo11–1 and rad50–4 mutants to form SC is directly related to the presence of a sister-chromatid. These proteins could mediate, or modify, sister-chromatid associations that are important for synapsis. The notion that these proteins may have a role in sister chromatid associations necessary for DNA repair is supported by the mitotic hyperrecombination phenotype observed in some rad50, mre11, and xrs2 mutants (30–33). This phenotype can be attributed to the channeling of mitotic DNA lesions to a homologous chromosome for repair in G2, when normally the sister chromatid would be used. Like rad50, mre11, and xrs2 mutants in yeast, the spo11 mutant mei-W68 of Drosophila melanogaster also exhibits a hyperrecombination phenotype (34, 35), a feature consistent with a defect in associative processes important for sister-chromatid-mediated repair. However, it is possible that these hyperrecombination phenotypes are a result of defects in replication, as this phenotype also is associated with mutations in genes that have direct roles in replication (36, 37). Therefore, the precise meaning of the hyperrecombination phenotype as it relates to defects in sister-chromatid associations is unclear.
γ-Radiation survival curves in S. cerevisiae demonstrate that rad50, mre11, and xrs2 null mutant strains are defective in their ability to repair during the G2 phase of the cell cycle. However, these mutant strains exhibit diploid-specific increases in resistance to ionizing radiation (30–32, 38). Additionally, Moore and Haber (39) observed a G2-specific form of end-joining repair that required the function of Rad50. These observations suggest that this complex of proteins functions in DNA repair processes that require a sister chromatid but is not essential for repair events that use a homologous chromosome. Other observations, however, indicate that the requirement for Mre11, and by extension for Rad50, in DNA repair is not limited to the G2 stage of the cell cycle (40). Therefore, any function that Rad50 may have in sister-chromatid cohesion is unlikely to represent the full extent of its contribution to DNA repair. Support for a sister-chromatid association role for Rad50 is found in the inclusion of this protein in the structural maintenance of chromosomes (SMC) family (41). Hirano (42) proposed that the basic unifying role of SMC proteins is to serve as ATP-modulated DNA cross-linkers. Although AE form in the rad50–4 mutant (Figs. 1C and 2), sister-chromatid associations may be deficient or inappropriate such that synapsis is inhibited. For example, unlike during mitosis, when chromatin loops of sister chromatids face in opposite directions, in meiosis I sister chromatids are oriented asymmetrically, such that the chromatin loops of both are positioned on the same side of the AE (43–45). Therefore, one possibility is that Rad50 is required to cross-link sister-chromatid DNA to create and/or stabilize this orientation. The loss of Rad50 function might result in the inability to form an AE that is competent for synapsis, as inappropriate interactions between sisters might effectively block the availability of a chromatid for synapsis with the homolog. In a spo22–1 mutant, the absence of a sister chromatid creates an asymmetric distribution of chromatin loops and, therefore, liberates single chromatids so that each is available for synapsis with the homologous (nonsister) chromatid.
The notion that Spo11 and Rad50 may function in some aspect of sister-chromatid associations is not mutually exclusive with the possibility that they also operate in processes associated with replication. The proteins that are responsible for sister-chromatid cohesion act either before or during replication to set up the chromosomal structural determinants necessary for proper sister-chromatid interactions. The sister-chromatid adherin protein Mis4, from Schizosaccharomyces pombe (46), remains associated with chromosomes throughout the cell cycle, and its inactivation during G1 results in lethality during the subsequent S phase. The meiosis-specific cohesin Rec8 has been demonstrated, in both S. cerevisiae (47) and S. pombe (48), to localize with chromosomes before DNA replication. Furthermore, Cha et al. (6) observed a lengthening of premeiotic S phase relative to wild type in an S. cerevisiae rec8 null mutant. This observation suggests that Rec8 is a positive modulator of premeiotic DNA S phase and that the process of replication probably is influenced by the initial steps in establishing proper sister-chromatid interactions. Thus, our observations of a DNA replication-induced requirement for Spo11 and Rad50 could mean that these proteins serve a replication-associated role in setting up meiotic chromatin structures (e.g., sister-chromatid associations) necessary for synapsis.
We observed a dramatic increase in the number of nuclei that exhibit synapsis in spo11–1;spo22–1 and rad50–4;spo22–1 double mutants relative to the spo11–1 and rad50–4 single mutants, respectively. However, synapsis is not complete in the double mutants, and we do not know whether it is principally homologous or nonhomologous. It may be possible to use recently developed procedures (49) for the sequential observation of chromosome spreads with fluorescence and electron microscopy to determine whether observed regions of synapsis are between homologs. Because the spo11–1 mutant undergoes a high level of homolog pairing (19), it is likely that, for this mutant at least, some if not most synapsis is homologous. One explanation for only partial suppression of the asynaptic phenotypes is that the spo22–1 mutation may be leaky, and a small fraction of chromosomal regions may still undergo replication such that it is not possible to achieve complete rescue. A more likely explanation, however, is that Spo11 and Rad50 have additional functions that are not made dispensable by the absence of premeiotic replication and probably are important for both the number and length of AE and SC structures that form. Although we have demonstrated that Spo11-induced recombination is not required for synapsis in a spo22–1 mutant, recombination might facilitate synapsis, even in the absence of a sister chromatid.
Although the spo22–1 mutation prevents significant premeiotic DNA replication, it is not known whether replication fails to initiate or initiates and is then unable to proceed. It is possible that replication is initiated in the spo22–1 mutant and impeded such that replication-fork stalling occurs. Blocked replication forks have been associated with an elevation of spontaneous DSB formation (50). Therefore, it is possible that the suppression of the synapsis defects in the spo11–1;spo22–1 and rad50–4;spo22–1 double mutants results from the partial functional replacement of Spo11 and Rad50 with spontaneous DSBs caused by arrested replication forks throughout the genome. Such a model must include the stable maintenance of replication-associated DSBs for an extended period, through karogamy and the entry into meiosis in C. cinereus. This is an unproven, but testable, idea. It has been shown that DSBs can be maintained for many hours (51) and this possibility, which has not been ruled out in this study, deserves further attention.
Whether the impediment to synapsis in the spo11–1 and rad50–4 mutants stems from a regulatory or a structural barrier is not clear. However, Spo22 does contribute, at least partially, to the barrier, because the level of AE and SC formation increases in the spo11–1;spo22–1 and rad50–4;spo22–1 double mutants relative to the spo11–1 and rad50–4 single mutants. Additionally, Spo11 and Rad50 normally are required (i.e., in a spo22+ strain) to overcome the Spo22-dependent barrier to synapsis. For example, there could be a Spo22-dependent checkpoint that blocks SC assembly in the absence of Spo11 and Rad50 function. An obvious question is whether the known roles of Spo11 and Rad50 in DSB formation and recombination initiation account for their requirement in overcoming the barrier. The DSB-forming function of Spo11 does not completely account for its role in meiosis; for example, it is not required for homologous chromosome pairing (6). The spo11–1 mutant gene encodes a predicted protein that likely fails to make meiotic DSBs (19), but this protein, if stable, may retain other functions. Similarly, the rad50–4 mutation may not be a null allele (S.N.A. and M.E.Z., unpublished data).
If Spo11 is universally responsible for the initiation of meiotic recombination, then recombination is not required for synapsis in C. cinereus, because the spo11–1;spo22–1 double mutant is able to form SC. A striking dichotomy exists between the animals Caenorhabditis elegans and D. melanogaster and the fungi C. cinereus and S. cerevisiae in the ability of spo11 mutants to form SC. Although these animal spo11 mutants apparently synapse normally (52–54), spo11 mutants in the fungi are asynaptic (refs. 14 and 19; Figs. 1B and 2). Our results indicate that this difference cannot be attributed simply to differences in the role of recombination in SC formation. Therefore, organisms may differ in whether replication-associated roles of Spo11 and Rad50 are required for synapsis.
Acknowledgments
We thank P. Pukkila for the spo22–1 strain, R. Turner for electron microscopy, M. Celerin for the spore viability data, and K. Young for assistance with the manuscript. We thank P. Foster for help with the statistical analysis and N. Kleckner, P. Foster, F. Stahl, B. Sly, A. Many, M. Celerin, and E. Gerecke for helpful discussions and comments on the manuscript. This work was supported by National Institutes of Health Grant GM43930 (to M.E.Z.). S.T.M. was supported by National Science Foundation Minority Postdoctoral Research Fellowship DBI-9628899. W.J.C. was supported by National Institutes of Health Training Grant 2T32GM07757, by a McCormick Science Fellowship (College of Arts and Sciences, Indiana University), and by a J. Stewart and Dagmar K. Riley Fellowship (College of Arts and Sciences, Indiana University). S.N.A. was supported by National Institutes of Health Training Grant 2T32GM07757.
Abbreviations
- AE
axial element(s)
- SC
synaptonemal complex(es)
- DSB
double-strand break
- HU
hydroxyurea
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.190346097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.190346097
References
- 1.Moore D P, Orr-Weaver T L. Curr Top Dev Biol. 1998;37:263–299. doi: 10.1016/s0070-2153(08)60177-5. [DOI] [PubMed] [Google Scholar]
- 2.Malone R E. Mol Gen Genet. 1983;189:405–412. doi: 10.1007/BF00325902. [DOI] [PubMed] [Google Scholar]
- 3.Petes T D, Malone R E, Symington L S. In: The Molecular and Cellular Biology of the Yeast Saccharomyces, Genome Dynamics, Protein Synthesis, and Energetics. Broach J R, Pringle J R, Jones E W, editors. New York: Cold Spring Harbor Lab. Press; 1991. pp. 407–521. [Google Scholar]
- 4.Paques F, Haber J E. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keeney S, Giroux C N, Kleckner N. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 6.Cha R S, Weiner B M, Keeney S, Dekker J, Kleckner N. Genes Dev. 2000;14:493–503. [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner B M, Kleckner N. Cell. 1994;77:977–991. doi: 10.1016/0092-8674(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 8.Johzuka K, Ogawa H. Genetics. 1995;139:1521–1532. doi: 10.1093/genetics/139.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Usui T, Ohta T, Oshiumi H, Tomizawa J, Ogawa H, Ogawa T. Cell. 1998;95:705–716. doi: 10.1016/s0092-8674(00)81640-2. [DOI] [PubMed] [Google Scholar]
- 10.Allen J B, Zhou Z, Siede W, Friedberg E C, Elledge S J. Genes Dev. 1994;8:2401–2415. doi: 10.1101/gad.8.20.2401. [DOI] [PubMed] [Google Scholar]
- 11.Merrill B J, Holm C. Genetics. 1998;148:611–624. doi: 10.1093/genetics/148.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haber J E. Cell. 1998;95:583–586. doi: 10.1016/s0092-8674(00)81626-8. [DOI] [PubMed] [Google Scholar]
- 13.Le S, Moore J K, Haber J E, Greider C W. Genetics. 1999;152:143–152. doi: 10.1093/genetics/152.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haber J E. Trends Biochem Sci. 1999;24:271–275. doi: 10.1016/s0968-0004(99)01413-9. [DOI] [PubMed] [Google Scholar]
- 15.Giroux C N, Dresser M E, Tiano H F. Genome. 1989;31:88–94. doi: 10.1139/g89-017. [DOI] [PubMed] [Google Scholar]
- 16.Alani E, Padmore R, Kleckner N. Cell. 1990;61:419–436. doi: 10.1016/0092-8674(90)90524-i. [DOI] [PubMed] [Google Scholar]
- 17.Nairz K, Klein F. Genes Dev. 1997;11:2272–2290. doi: 10.1101/gad.11.17.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramesh M A, Zolan M E. Chromosoma. 1995;104:189–202. doi: 10.1007/BF00352184. [DOI] [PubMed] [Google Scholar]
- 19.Celerin M, Merino T S, Stone J E, Menzie A M, Zolan M E. EMBO J. 2000;19:2739–2750. doi: 10.1093/emboj/19.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanda T, Arakawa H, Yasuda Y, Takemaru T. Exp Mycol. 1990;14:218–226. [Google Scholar]
- 21.Pukkila P J, Shannon K B, Skrzynia C. Can J Bot. 1995;73, Suppl. 1:S215–S220. [Google Scholar]
- 22.Cummings W J, Celerin M, Crodian J, Brunick L K, Zolan M E. Curr Genet. 1999;36:371–382. doi: 10.1007/s002940050512. [DOI] [PubMed] [Google Scholar]
- 23.Binninger D M, Skrzynia C, Pukkila P J, Casselton L A. EMBO J. 1987;6:835–840. doi: 10.1002/j.1460-2075.1987.tb04828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zolan M E, Tremel C J, Pukkila P J. Genetics. 1988;120:379–387. doi: 10.1093/genetics/120.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pukkila P J, Skrzynia S, Lu B C. Dev Genet (Amsterdam) 1992;14:403–410. [Google Scholar]
- 26.Byers B, Goetsch L. Proc Natl Acad Sci USA. 1975;72:5056–5060. doi: 10.1073/pnas.72.12.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simchen G, Hirschberg J. Genetics. 1977;86:57–72. doi: 10.1093/genetics/86.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horesh O, Simchen G, Friedmann A. Chromosoma. 1979;75:101–115. doi: 10.1007/BF00330628. [DOI] [PubMed] [Google Scholar]
- 29.Stuart D, Wittenberg C. Genes Dev. 1998;12:2698–2710. doi: 10.1101/gad.12.17.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanov E L, Korolev V G, Fabre F. Genetics. 1992;132:651–664. doi: 10.1093/genetics/132.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bressan D A, Olivares H A, Nelms B E, Petrini J H. Genetics. 1998;150:591–600. doi: 10.1093/genetics/150.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreau S, Ferguson J R, Symington L S. Mol Cell Biol. 1999;19:556–566. doi: 10.1128/mcb.19.1.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malone R E, Ward T, Lin S, Waring J. Curr Genet. 1990;18:111–116. doi: 10.1007/BF00312598. [DOI] [PubMed] [Google Scholar]
- 34.Baker B S, Carpenter A T C. Genetics. 1972;71:255–286. doi: 10.1093/genetics/71.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker B S, Carpenter A T C, Ripoll P. Genetics. 1978;90:531–578. doi: 10.1093/genetics/90.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartwell L H, Smith D. Genetics. 1985;110:381–395. doi: 10.1093/genetics/110.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tishkoff D X, Filosi N, Gaida G M, Kolodner R D. Cell. 1997;88:253–263. doi: 10.1016/s0092-8674(00)81846-2. [DOI] [PubMed] [Google Scholar]
- 38.Fabre F, Boulet A, Roman B. Mol Gen Genet. 1985;195:139–143. doi: 10.1007/BF00332736. [DOI] [PubMed] [Google Scholar]
- 39.Moore J K, Haber J E. Mol Cell Biol. 1996;16:2164–2173. doi: 10.1128/mcb.16.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bressan D A, Baxter B K, Petrini J H. Mol Cell Biol. 1999;19:7681–7687. doi: 10.1128/mcb.19.11.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirano T, Mitchison T J, Swedlow J R. Curr Opin Cell Biol. 1995;7:329–336. doi: 10.1016/0955-0674(95)80087-5. [DOI] [PubMed] [Google Scholar]
- 42.Hirano T. Genes Dev. 1999;13:11–19. doi: 10.1101/gad.13.1.11. [DOI] [PubMed] [Google Scholar]
- 43.von Wettstein D, Rasmussen S W, Holm P B. Annu Rev Genet. 1984;18:331–413. doi: 10.1146/annurev.ge.18.120184.001555. [DOI] [PubMed] [Google Scholar]
- 44.Gimenez-Abian J F, Clarke D J, Mullinger A M, Downes C S, Johnson R T. J Cell Biol. 1995;131:7–17. doi: 10.1083/jcb.131.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleckner N. Proc Natl Acad Sci USA. 1996;93:8167–8174. doi: 10.1073/pnas.93.16.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furuya K, Takahashi K, Yanagida M. Genes Dev. 1998;12:3408–3418. doi: 10.1101/gad.12.21.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein F, Mahr P, Galova M, Buonomo S B C, Michaelis C, Nairz K, Nasmyth K. Cell. 1999;98:91–103. doi: 10.1016/S0092-8674(00)80609-1. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe Y, Nurse P. Nature (London) 1999;400:461–464. doi: 10.1038/22774. [DOI] [PubMed] [Google Scholar]
- 49.Barlow A L, Hulten M A. Chromosoma. 1997;106:293–303. doi: 10.1007/s004120050250. [DOI] [PubMed] [Google Scholar]
- 50.Michel B, Ehrlich S D, Uzest M. EMBO J. 1997;2:430–438. doi: 10.1093/emboj/16.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelms B E, Maser R S, Mackay J F, Lagally M G, Petrini J H J. Science. 1998;280:590–592. doi: 10.1126/science.280.5363.590. [DOI] [PubMed] [Google Scholar]
- 52.Dernburg A F, McDonald K, Moulder G, Barstead R, Dresser M, Villeneuve A M. Cell. 1998;94:387–398. doi: 10.1016/s0092-8674(00)81481-6. [DOI] [PubMed] [Google Scholar]
- 53.McKim K S, Green-Marroquin B L, Sekelsky J J, Chin G, Steinberg C, Khodosh R, Hawley R S. Science. 1998;279:876–878. doi: 10.1126/science.279.5352.876. [DOI] [PubMed] [Google Scholar]
- 54.McKim K S, Hayashi-Hagihara A. Genes Dev. 1998;12:2932–2942. doi: 10.1101/gad.12.18.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]