SYNOPSIS
In patients with heart failure, treatment and survival are directly related to the etiology. Clinically, as a practical first step, patients are classified as having either ischemic or nonischemic cardiomyopathy and this delineation is usually based on the presence or absence of epicardial coronary artery disease. However, this approach does not account for patients with nonischemic cardiomyopathy who also have coronary artery disease, which may be either incidental or partly contributing to myocardial dysfunction (mixed cardiomyopathy). By allowing direct assessment of the myocardium, delayed enhancement cardiovascular magnetic resonance (DE-CMR) may aid in addressing these conundrums. In this article we explore how DE-CMR may be helpful in identifying ischemic and nonischemic myopathic processes and detail a systematic approach using this technique to determine the etiology of cardiomyopathy.
Keywords: Heart failure, cardiomyopathy, ischemic, nonischemic, cardiovascular magnetic resonance, delayed enhancement imaging
INTRODUCTION
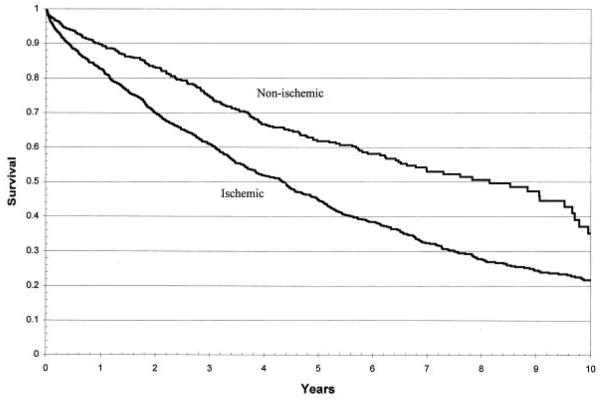
Several classification schemes have been proposed for the various cardiomyopathies. Some are based on the disease phenotype, while others focus on the genetic aspects of disease1, 2. Although these detailed classification methods are important in the research arena, in the clinical setting, a practical first step is to categorize patients as having ischemic or nonischemic cardiomyopathy (CM). This simple classification is important because it can directly impact patient management. If ischemic CM is identified, there are clearly defined treatment strategies that have proven survival benefit, such as coronary artery bypass grafting in patients with left main or 3-vessel disease3. This classification also provides insight into patient prognosis; ischemic CM is associated with reduced survival compared with nonischemic CM (Figure 1)4. As a second step, within the broad category of nonischemic CM, determining the specific etiology is undertaken. Again the importance of determining the etiology is related to patient treatment and survival. For instance, beta-blockers are beneficial in some forms of nonischemic CM (eg. idiopathic dilated CM) but not others (eg. cardiac amyloidosis)5, and the prognosis is quite variable depending on the underlying cause of nonischemic cardiomyopathy. Patients with peripartum CM have significantly better survival than those with idiopathic dilated CM, who in turn have better survival than those with infiltrative myocardial diseases6.
Figure 1.
Unadjusted Kaplan-Meier survival curves for ischemic versus nonischemic cardiomyopathy. Reproduced with permission from Felker GM, Shaw LK, O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39(2):210–218.
Clinically, identifying the presence or absence of obstructive epicardial coronary artery disease (CAD), via coronary angiography or stress testing, has been the cornerstone in differentiating ischemic from nonischemic CM. Delayed enhancement cardiovascular magnetic resonance (DE-CMR) may provide a novel approach to determining the etiology by allowing a direct assessment of myopathic processes. Comparative studies with histopathology have shown that the presence, extent, and location of hyperenhancement (HE) by DE-CMR is a precise indicator of nonviable myocardium in both ischemic7 and nonischemic heart disease8, 9. Although nonviable myocardium can be identified by several imaging modalities, DE-CMR may be particularly suited to evaluating the myocardial processes in cardiomyopathy. DE-CMR offers significantly higher spatial resolution (40-fold greater than radionuclide imaging10), can systematically detect subendocardial infarcts that are missed by SPECT11, and may even identify micro scars that cannot be detected by other imaging techniques12(Figure 2).
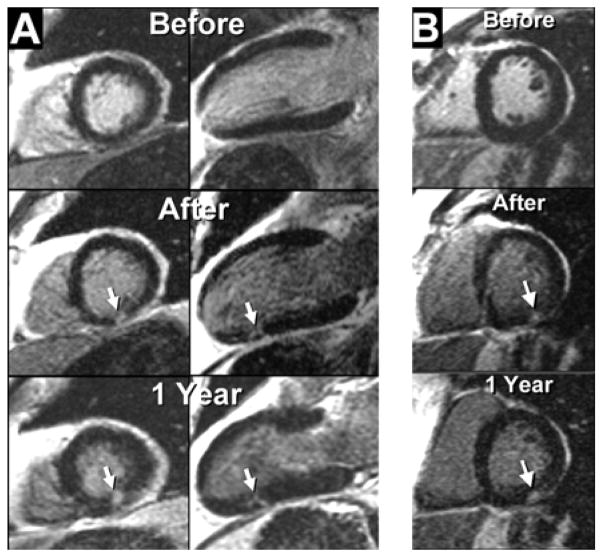
Figure 2.
Images before and after coronary stenting demonstrate the ability of delayed enhancement cardiac magnetic resonance to identify micro-infarcts. Arrows point to new discrete regions of hyperenhancement in the inferior wall related to the procedure. Adapted with permission from Ricciardi MJ, Wu E, Davidson CJ, Choi KM, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103(23):2780–2783.
In this chapter we will present an overall imaging approach for the diagnosis of cardiomyopathy, which is fundamentally based on DE-CMR. We will describe how this approach is based on the underlying myocardial pathophysiology that is present in ischemic and nonischemic cardiomyopathy. Additionally, we will discuss how a DE-CMR based approach may provided insight into the conundrums that can occur when coronary artery disease coexists with nonischemic cardiomyopathy.
TRADITIONAL APPROACH TO ETIOLOGY
Ischemic CM is usually diagnosed by identifying the presence of obstructive CAD. The coronary anatomy can be assessed either directly, i.e. x-ray coronary angiography, or indirectly via stress-testing depending on the pretest probability of disease, which is derived from a combination of historical, clinical, electrocardiographic, and laboratory data. An individual with few risk factors and atypical symptoms may undergo stress testing (or simple reassurance if the risk is deemed particularly low), whereas a different individual with multiple risk factors and classic symptoms of angina may proceed directly to the catheterization laboratory. Likewise, the broad diagnosis of nonischemic CM is usually also made by assessing the coronary anatomy, either directly or indirectly. Once significant CAD has been excluded, a comprehensive evaluation including possibly endomyocardial biopsy may allow a specific etiology to be determined.
However, there are several drawbacks to the traditional approach. For one, it appears that many patients do not undergo definitive testing (invasive coronary angiography), and the accuracy of diagnosing CAD indirectly is only moderate. Autopsy data from patients enrolled in the ATLAS (Assessment of Treatment with Lisinopril and Survival) Trial13, a large multicenter randomized trial of patients with moderate to severe heart failure, underscores this concern. Of the 171 patients who had autopsy, 70% (n=120) were diagnosed in life as having ischemic CM and 30% (n=51) as having nonischemic CM. Autopsy documented that 17% of those diagnosed with ischemic CM did not have CAD whereas 31% of those diagnosed with nonischemic CM had significant CAD. Overall, 21% had an incorrect clinical diagnosis.
One possible reason for the high rate of misdiagnosis may be an over reliance on certain findings from noninvasive imaging. Initially, it was thought that ventricular dysfunction due to nonischemic CM was primarily global rather than segmental as in ischemic CM, and that this characteristic could be used to distinguish these disorders by echocardiography14. However, it is now recognized that segmental wall motion abnormalities are evident in up to 60 percent of patients with non-ischemic dilated CM even when patients with left bundle branch block are excluded15. Furthermore, it is known that radionuclide scintigraphy with either dipyridamole or exercise testing is unreliable in differentiating ischemic heart disease from nonischemic CM since both groups of patients may have evidence of reversible and fixed perfusion abnormalities16.
Additionally, in cases where a specific cause of nonischemic CM is being explored, classic imaging features such as “granular sparkling” on echocardiography in cardiac amyloidosis are limited to a few rare diseases and may be less accurate than first reported. In fact, in a recent study of 196 patients clinically suspected to have cardiac amyloidosis, this finding had a sensitivity of only 26%17. Endomyocardial biopsy, the presumed gold standard, has limitations as well. Because of sampling error, test sensitivity may be low18, 19, and especially in the chronic setting, many patients with cardiomyopathy may show only nonspecific changes on biopsy (e.g. cell loss and fibrosis). Not surprisingly, Felker et al.6 reported that among 1230 patients that underwent endomyocardial biopsy as part of an evaluation for unexplained cardiomyopathy, a specific histologic diagnosis was provided by the biopsy in only 15% of patients.
Finally, simple distinctions between cardiomyopathies based on the presence or absence of CAD can be problematic. For instance, it is possible that a patient with nonischemic CM may also have CAD, but which is incidental and is not contributing to contractile dysfunction. The quandary is that coronary atherosclerosis is common, especially in the elderly and those with diabetes mellitus, even if patients are asymptomatic and do not have myocardial dysfunction20. A recent study in 1,921 patients with symptomatic heart failure and LV dysfunction (LVEF <40%) undergoing diagnostic coronary angiography highlights this issue4. Among patients with LV dysfunction “out of proportion” to their degree of CAD (i.e. those with single vessel disease), prognosis was more similar to those with nonischemic CM than the remaining group with ischemic CM. The authors concluded that even though obstructive CAD is present, those with single-vessel disease should be reclassified as nonischemic for prognostic purposes.
Another problem with the traditional approach is the inherent assumption that ischemic and nonischemic cardiomyopathy cannot coexist. However, it is plausible that both ischemic and nonischemic myopathic processes, can be present simultaneously and may be contributing independently or synergistically towards systolic dysfunction (mixed cardiomyopathy). Thus, these limitations indicate that the presence of obstructive coronary atherosclerosis is not synonymous with an ischemic etiology, and that even with objective information such as from invasive coronary angiography, the appropriate classification for a given patient is not always clear.
PATHOPHYSIOLOGICAL BASIS OF A NEW APPROACH USING DE-CMR
Since the cardiomyopathies represent diseases of the myocardium with attendant ventricular dysfunction, one might expect that focusing on the myocardium as opposed to the coronary arteries may provide added diagnostic insight. The importance of assessing myocardial viability is readily evident in patients with ischemic heart disease who may undergo coronary revascularization21. However, the assessment of viability, or conversely, the detection of necrosis or scarring, may also be important in many patients in whom coronary revascularization is not an issue, such as those with nonischemic CM. The utility of DE-CMR in the setting of cardiomyopathy is based on the understanding that rather than simply measuring viability, the presence and pattern of hyperenhancement (nonviable myocardium) holds additional information. In this section, we will first explore the mechanism of myocardial hyperenhancement in the setting of acute and chronic myocardial infarction, and review the spatial progression of cell death that occurs over time following coronary occlusion. These will set the basis for understanding the DE-CMR findings that can occur in the setting of ischemic CM. Next, we will review the initial studies comparing the presence and pattern of hyperenhancement in ischemic CM to that found in idiopathic dilated CM. Then, a brief description of findings in other forms of nonischemic CM will follow, along with a summary of the current understanding of the pathophysiological basis of hyperenhancement in nonischemic CM.
Mechanism of Hyperenhancement in Myocardial Infarction
Both acute and chronic myocardial infarction are accurately depicted by DE-CMR as hyperenhanced regions, independent of wall motion and reperfusion status7. However, the gadolinium chelates that are used for DE-CMR are biologically inert and are not actively transported by any cells or tissues. Moreover, the tissue composition of acute MI (necrotic myocytes and acute inflammation) is completely different than that of chronic MI (dense collagenous scar). Thus, it is difficult to understand how a “non-specific” contrast agent can distinguish between viable and infarcted myocardium, especially across the wide range of tissue environments that occur during infarct healing.
Although the mechanism of myocardial hyperenhancement in the setting of infarction has not been fully elucidated, one has been proposed that accounts for the variety of tissues that can characterize nonviable myocardium22. The proposed mechanism is based on two simple facts. First, gadolinium chelates are extra cellular contrast agents that cannot cross myocyte cell membranes23. Secondly, in normal myocardium, myocytes are densely packed and thus myocyte intracellular space forms the majority (78%) of the volume24. Conceptually, it then follows that the volume of distribution of gadolinium in a hypothetical voxel of normal myocardium is small, and the overall number of gadolinium molecules is low (Figure 3). In acute MI, there is myocyte membrane rupture, which allows additional gadolinium to diffuse into what was previously intracellular space. This in turn results in increased gadolinium concentration25, shortened T1 relaxation times, and therefore hyperenhancement. Loss of sarcolemmal membrane integrity is thought to be very tightly related to cell death, and the idea that an event specific to cell death is related to hyperenhancement would explain the nearly one-to-one relationship of hyperenhancement to necrotic tissue found in acute MI7.
Figure 3.
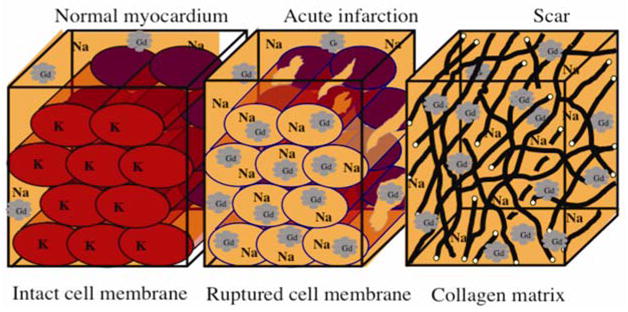
Mechanisms of hyperenhancement in myocardial infarction: Scar may be the result of chronic infarction or nonischemic damage (see text for details). Adapted from Kim RJ, Elliott MD, Judd RM. Assessment of Myocardial Viability by Contrast Enhancement. In: Higgins CB, de Roos A, eds. MRI and CT of the Cardiovascular System. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
In chronic MI, myocytes have been replaced with collagenous scar tissue. In this situation, the interstitial space is expanded, which again leads to increased gadolinium concentration and hyperenhancement25. In both acute and chronic MI (and all stages in-between) one can consider viable myocytes as actively excluding gadolinium contrast media. Thus, the unifying mechanism for the hyperenhancement of nonviable myocardium may then be the absence of viable myocytes rather than any inherent properties that are specific for acutely necrotic tissue, collagenous scar, or other forms of nonviable tissue.
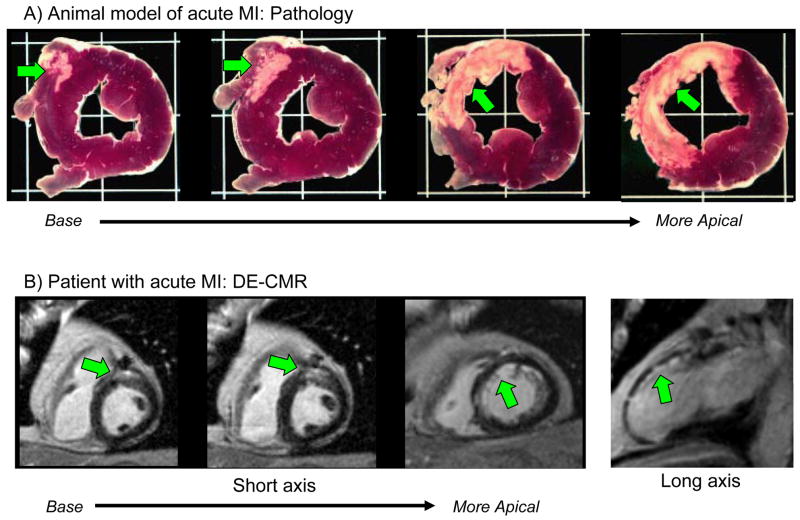
The “Wavefront” of Ischemic Necrosis
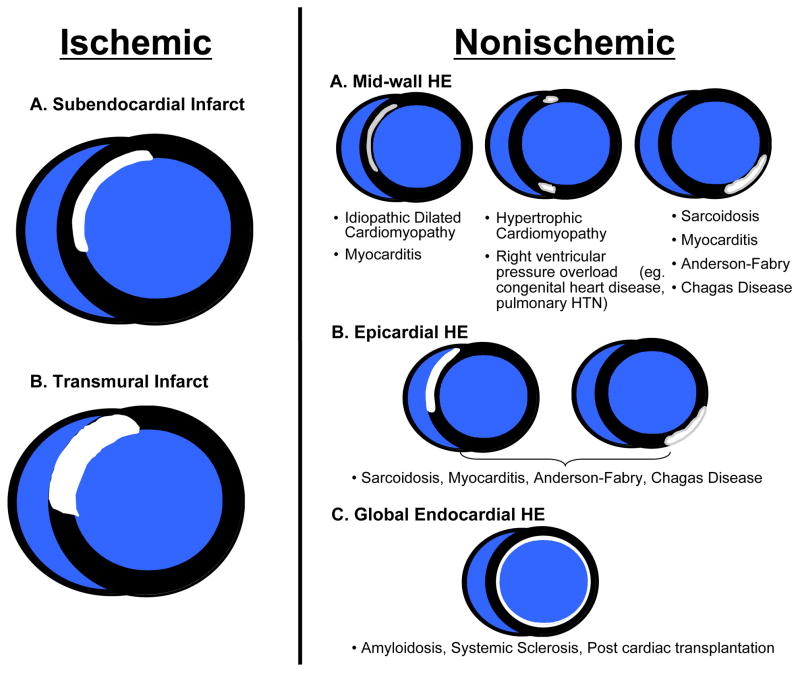
The typical pattern of hyperenhancement that occurs in patients with prior MI and thus in those with ischemic CM can be explained by the pathophysiology of ischemia. Following a coronary occlusion, myocardial contractility falls within seconds throughout the “area at risk”, which is the ischemic zone with reduced perfusion. Although blood begins to flow by way of preexisting collaterals—vascular channels that interconnect ordinary arteries—collateral flow is lowest and myocardial oxygen consumption highest in the subendocardium. As a consequence, ischemia is most severe and necrosis begins first in the subendocardium starting about 15–20 minutes after total occlusion26. Necrosis then progresses as a “wavefront” toward the epicardium over the next few hours. During this period the size of the “area at risk” remains the same, but the size of the infarcted region within the “area at risk” increases continuously towards a transmural infarction (Figure 4). Another facet of the wavefront phenomenon is that increasing the time or severity of ischemic injury generally increases the transmurality of infarction, whereas the circumferential extent of infarction does not increase appreciably since the lateral margins are established relatively early in the ischemic period26. Therefore, an “ischemic-type” or “CAD-type” pattern of hyperenhancement should always involve the subendocardium (i.e. subendocardial or transmural) and be located in a region, which is consistent with the perfusion territory of an epicardial coronary artery. Conversely, a “non-CAD-type” pattern of hyperenhancement is one that spares the subendocardium and is secondary to nonischemic disorders.
Figure 4.
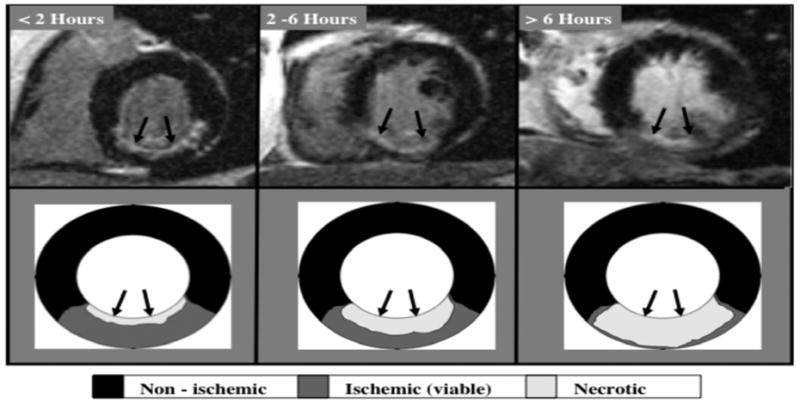
The typical hyperenhancement pattern of myocardial infarction can be explained by the pathophysiology of ischemia. Little or no cellular necrosis is found until about 15 min after occlusion. Over the next few hours a wavefront of necrosis begins in the subendocardium and moves progressively towards the epicardium. During this period, the infarcted region within the ischemic zone increases continuously and can ultimately become transmural. Reproduced with permission from Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26(15):1461–1474.
Ischemic Versus Idiopathic Dilated Cardiomyopathy
Several studies have compared the presence and pattern of hyperenhancement on DE-CMR among patients with various forms of cardiomyopathy. Wu et al.10 were the first to report that DE-CMR may hold promise in differentiating ischemic from nonischemic, idiopathic dilated CM. In this study, nearly all patients with CAD and prior MI had myocardial hyperenhancement, whereas none of the patients with idiopathic dilated CM or the normal volunteers had hyperenhancement. In another study, Bello et al.27 evaluated a cohort of patients with severe LV systolic dysfunction (mean EF was 26±11%); hyperenhancement was found in 100% of patients with ischemic CM, but in only 12% with idiopathic dilated CM. In a group of ambulatory patients seen at a heart failure clinic, Casolo et al.28, noted myocardial hyperenhancement in 98% of patients with CAD as compared with 16% in those without CAD. Other recent studies showed similar findings29. From these data we can conclude that myocardial hyperenhancement consistent with gross myocardial scarring is virtually always present in patients with chronic ischemic CM, whereas it is relatively rare in patients with idiopathic dilated CM. This conclusion is consistent with previous pathology studies30, 31. Schuster and Bulkley30 demonstrated that virtually all patients with congestive heart failure and significant CAD have gross myocardial scarring at autopsy, even in those without clinical history of myocardial infarction, angina, or Q-waves. Conversely, Roberts et al.32 found visible scars at necropsy in only 14% of patients with idiopathic dilated CM.
Recently, McCrohon et al.33 performed DE-CMR in 90 patients diagnosed with ischemic (n=27) or idiopathic dilated CM (n=63) on the basis of coronary angiography. Whereas all patients with ischemic CM had myocardial hyperenhancement, 41% of patients with idiopathic dilated CM also had hyperenhancement. Although the prevalence of hyperenhancement in patients with idiopathic dilated CM in this study appears to be significantly higher than that reported by Wu et al.10 and Bello et al.27, it is important to note that there were differences on how hyperenhancement was defined in these studies. For instance in the earlier studies, only hyperenhancement patterns consistent with regions of prior myocardial infarction were included in the analysis. Taking into account the pattern of hyperenhancement (CAD-type versus non-CAD-type), McCrohon et al. found that 13% of patients with idiopathic dilated CM had CAD-type hyperenhancement, whereas 28% had non-CAD-type. These findings are now consistent with those of Bello et al.27 in that among patients with idiopathic dilated CM the rate of CAD-type hyperenhancement was quite low in both studies (13% vs. 12%). Additionally, these rates are nearly identical with the rate of chronic infarcts found at autopsy in patients with idiopathic dilated CM by Roberts et al.32 and in the ATLAS substudy by Uretsky et al.13 (14% and 12%, respectively).
The clinical interpretation of CAD-type hyperenhancement in patients with idiopathic dilated CM is unclear. Certainly the occurrence of recanalization after an occlusive coronary event or embolization from minimally stenotic but unstable plaque is well documented34, 35. Therefore, McCrohon et al. believed that these patients were incorrectly diagnosed by coronary angiography as patients with idiopathic dilated CM. On the other hand, 28% of patients in the study by McCrohon et al. had patchy or linear striae of hyperenhancement limited to the mid-myocardium of the ventricular wall, and were classified as having non-CAD-type scar. In our experience, linear midwall hyperenhancement is usually found in the basal interventricular septum. It is observed more frequently in patients with longstanding LV dysfunction and in those with possible prior history of myocarditis. Current studies in patients with idiopathic dilated CM suggest that the presence of midwall hyperenhancement is associated with worsened prognosis, even after adjustment for left ventricular ejection fraction36.
Hyperenhancement Patterns in Nonischemic CM
In the studies above, only patients with ischemic or idiopathic dilated CM were evaluated. Patients with hypertrophic cardiomyopathy, myocarditis, infiltrative disorders, or other forms of nonischemic CM were excluded. Some of these diseases will be addressed in detail in other chapters, but a brief summary of DE-CMR findings in several cardiomyopathies are listed in Table 1.
Table 1.
Scar Characteristics in Nonischemic Cardiomyopathies by DE-CMR
| Disease | Hyperenhancement patterns | Reported prevalence | References |
|---|---|---|---|
| Idiopathic dilated CM | Linear mid-wall, typically in the basal septum | 10% to 35% | 29, 33, 36, 60 |
| Hypertrophic CM |
|
48% to 89%; nearly 100% in patients with severe hypertrophy (wall thickness >30 mm) | 37, 53–63 |
| Infiltrative | |||
| Amyloidosis |
|
69%–80% | 64–67 |
| Sarcoidosis |
|
33%–50%; almost 100% in known cardiac sarcoidosis | 52, 68–70 |
| Infectious/Inflammatory | |||
| Myocarditis |
|
75%–100% | 8, 40, 71–73 |
| Chagas disease |
|
20% in seropositive asymptomatic patients; 86% in clinical Chagas disease; 100% in those with VT | 39 |
| Genetic/Miscellaneous | |||
| Anderson–Fabry disease |
|
31%–50% | 38, 74, 75 |
| Muscular dystrophy |
|
32%–75% | 76–78 |
| Pulmonary hypertension | Right ventricular insertion sites of the interventricular septum | 97% | 79 |
| Systemic sclerosis |
|
15%–66% | 80–82 |
| Takotsubo CM | HE typically absent | 83, 84 | |
| Uremic CM |
|
14% and 14% (in patients with ESRD) | 85 |
| Limited data from single reports with few patients | |||
| Aortic stenosis | Focal, patchy HE in patients with severe hypertrophy | 27% (6 of 22 patients) | 86 |
| Churg-Strauss syndrome | Narrow rim of subendocardial HE, not confined to a single coronary territory | 82% (9 of 11 patients with suspected cardiac involvement) | 87 |
| HIV CM |
|
33% (4 of 12 in patients with elevated BNP) | 88 |
| Lamin A/C CM | Mid-myocardial fibrosis in basal interventricular septum | 45% (5 of 11 patients) | 89 |
| Peripartum CM | HE absent | 8 patients studied | 90 |
| Post chemotherapy (trastuzumab) CM | Epicardial pattern often involving the lateral wall | 100% (in 10 patients with LV ejection fraction <40%) | 91 |
| Primary desminopathies | Mid-wall | 36% (4 of 11 patients) | 92 |
| SLE | Inferolateral segment with subendocardial sparing | 38% (3 of 8 patients) | 93 |
CAD=coronary artery disease; CM=cardiomyopathy; ESRD=end stage renal disease; HE=hyperenhancement; LV=left ventricle; MI=myocardial infarction; RV=right ventricle; SLE=systemic lupus erythematosus; VT=ventricular tachycardia
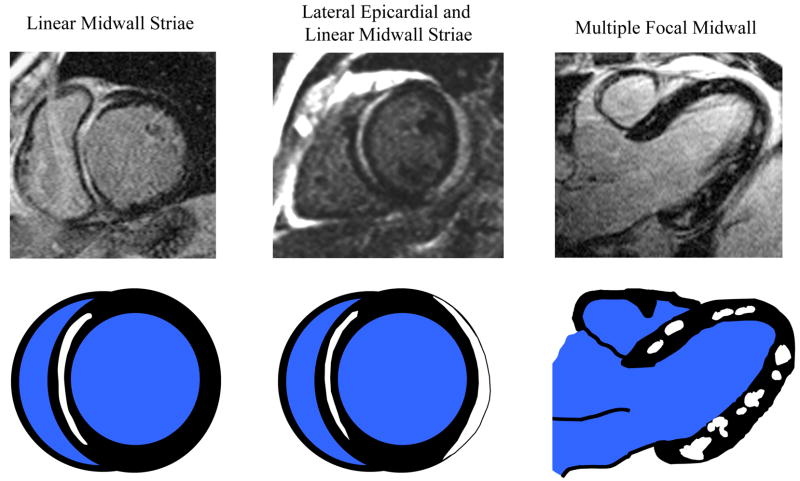
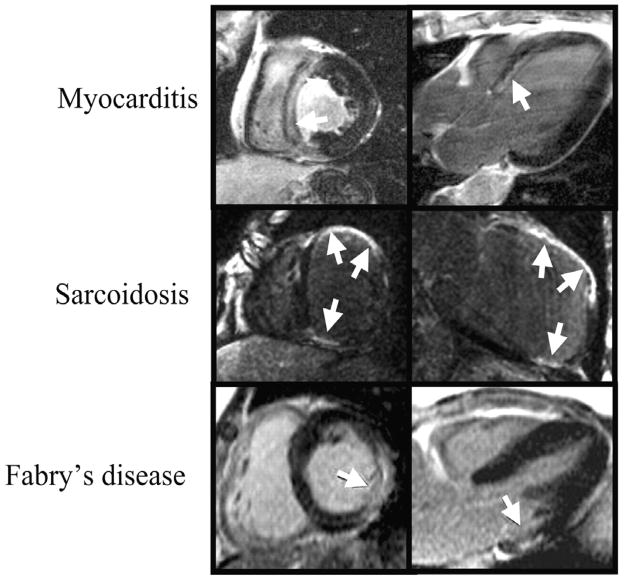
The location and pattern of scarring on DE-CMR are often quite distinct, and a pattern recognition approach based on the visualization of hyperenhancement will provide useful diagnostic information as to the likely etiology. Figure 5 illustrates potential hyperenhancement patterns that may be encountered in clinical practice with a partial list of their differential diagnoses. Importantly, there is emerging data that suggest that certain nonischemic cardiomyopathies have a predilection for specific scar patterns. For instance, in the setting of LV hypertrophy, the presence of mid-wall hyperenhancement in one or both junctions of the interventricular septum and right ventricular free wall is highly suggestive of hypertrophic cardiomyopathy37, whereas mid-wall or epicardial hyperenhancement in the inferolateral wall is consistent with Anderson-Fabry disease38. Likewise, in a patient with the appropriate demographic profile, involvement of both the LV basal lateral wall and apex appears to be relatively specific for Chagas disease39. Moreover, instead of an infinite variety of patterns, it appears that general classification is possible into a limited number of common hyperenhancement phenotypes. It should be noted, however, that in some disorders more than one pattern could occur or coexist in the same patient. As an example, there are three commonly encountered patterns of hyperenhancement in patients with myocarditis (Figure 6). Interestingly, it appears that certain patterns of hyperenhancement are related to the type of virus causing myocarditis, and importantly, to the clinical course 40.
Figure 5.
Hyperenhancement patterns that one may encounter in clinical practice. If hyperenhancement is present, the endocardium should be involved in patients with ischemic disease. Isolated midwall or epicardial hyperenhancement strongly suggests a nonischemic etiology. Reproduced with permission from Shah DJ, Judd RM, Kim RJ. Myocardial Viability. In: Edelman RR, Hesselink JR, Zlatkin MB, Crues JV, eds. Clinical Magnetic Resonance Imaging. 3rd ed. New York, NY: Elsevier; 2006.
Figure 6.
The top row displays delayed enhancement images of three commonly encountered patterns of hyperenhancement in patients with myocarditis. The bottom row is a schematic representation of the respective hyperenhancement patterns.
Pathophysiological Basis of Hyperenhancement in Nonischemic Cardiomyopathy
Currently, there are two lines of evidence indicating that areas of gadolinium hyperenhancement represent regions of nonviable myocardium. First, the locations and patterns of hyperenhancement seen in vivo in patients with various forms of nonischemic CM appear to match the locations and patterns of scarred or necrotic myocardium seen in gross pathology specimens of autopsy studies. In patients with hypertrophic cardiomyopathy scarring is often prominent at the RV insertion sites into the ventricular septum, which neatly matches the pattern of hyperenhancement41. In patients with fatal myocarditis a common location for necrosis with inflammatory infiltrates is the epicardial portion of the LV free wall42, which again matches in vivo DE-CMR observations. Second, there is now data, although limited, comparing in vivo hyperenhancement patterns with gross pathology findings in the same patient. In a case report of a patient with hypertrophic cardiomyopathy, there was a direct relationship between the presence and location of hyperenhancement on DE-CMR and the presence and location of replacement scarring on pathology performed 49 days later following cardiac transplantation9. Similarly, in a patient with Anderson-Fabry disease, nearly exact concordance was seen between in vivo DE-CMR and whole-heart histological validation performed 22 months later after witnessed sudden cardiac death43.
Grossly visible scarring, however, represents only one type of myocardial fibrosis. Diffuse fine interstitial fibrosis is common in many cardiomyopathies, including idiopathic dilated CM32, and one important question would be whether this form of fibrosis also leads to focal hyperenhancement on delayed-enhancement imaging. This is unlikely for two reasons. First, DE-CMR is sensitive to regional differences in gadolinium accumulation rather than to an overall increase because the technique depends on the ability to “null” signal from “remote” (presumably normal) myocardium44, 45. Therefore, cardiac disorders that lead to focal regions of fibrosis will enhance, whereas disorders that lead to global changes will not. Second, one should remember that the voxel resolution of DE-CMR is approximately 1.8 mm × 1.3 mm × 6 mm. A region of hyperenhancement that is visible on DE-CMR will comprise several voxels, and the pathology analogue would be a macroscopic scar that is visible to the naked eye. Thus, DE-CMR depicts regions of replacement scarring in vivo that previously could only be detected at autopsy, but currently, it is unable to identify the presence of diffuse reticular interstitial fibrosis.
A STEPWISE APPROACH INCORPORATING DE-CMR
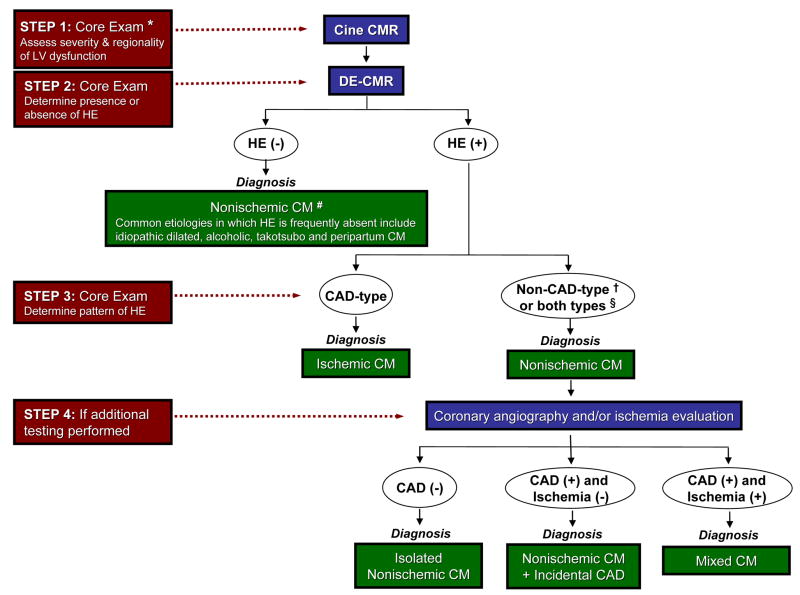
Recently, a systematic approach to interpreting DE-CMR images in patients with heart failure or cardiomyopathy (Figure 7) has been proposed22, 46. In clinical practice, DE-CMR is almost never performed or interpreted in isolation, and a slightly modified approach to determining the etiology of cardiomyopathy is shown in Figure 8. Cine imaging is nearly always part of an initial evaluation of heart failure and is part of the core CMR examination. As discussed elsewhere in other chapters, cine CMR is arguably the “gold standard” technique for the evaluation of myocardial structure and function. Small changes in ejection fraction and cardiac mass are more readily detected (and with greater precision) using cine CMR than echocardiography47, 48, and therapeutic interventions for patients with heart failure may be best assessed using CMR. Thus, Step 1 is to assess the severity and regionality of LV dysfunction, along with an assessment of chamber sizes, wall thickness, and valvular function, using cine CMR.
Figure 7.
A proposed stepwise approach for evaluating patients with heart failure incorporating delayed enhancement cardiovascular magnetic resonance. CAD=coronary artery disease; CM=cardiomyopathy; CMR=cardiac magnetic resonance; DE=delayed enhancement; HE=hyperenhancement; LV=left ventricle. * core exam typically consists of cine followed by delayed-enhancement imaging. # presumes long-standing, chronic cardiomyopathy and severe ventricular dysfunction. † see Table 1 and Figure 5 for details of non-CAD-type patterns of HE in various nonischemic cardiomyopathies. § often patients with nonischemic CM can have both non-CAD and CAD type HE (e.g. sarcoidosis)
Figure 8.
Hyperenhancement (arrows) in nonischemic cardiomyopathies can sometimes mimic that of coronary artery disease. The patient in the upper row had biopsy proven HHV6 myocarditis and no coronary artery disease by angiography. Cardiac sarcoidosis can cause transmural scarring resulting in wall thinning just as myocardial infarction (middle row). The lesions of Anderson-Fabry disease are usually intramural, except for few cases where also the subendocardium can be affected (bottom row). Bottom row, modified with permission from Moon JC, Sachdev B, Elkington AG, McKenna WJ, Mehta A, Pennell DJ, Leed PJ, Elliott PM. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur Heart J. 2003;24(23):2151–2155.
Step 2 is to determine the presence or absence of hyperenhancement on delayed-enhancement CMR. In patients with longstanding ischemic CM, virtually all patients have evidence of prior MI (as discussed above). The implication is that in patients with severe cardiomyopathy but without hyperenhancement, the diagnosis of nonischemic CM should be strongly considered. Common etiologies in which hyperenhancement is frequently absent include idiopathic dilated, alcoholic, Takotsubo, and peripartum cardiomyopathy (Table 1).
If hyperenhancement is present, Step 3 is to classify the location and distribution of hyperenhancement as CAD-type or non-CAD-type. For this determination, the concept that ischemic injury progresses as a “wavefront” from the subendocardium to the epicardium is crucial. Correspondingly, hyperenhancement patterns that spare the subendocardium and are limited to the middle or epicardial portion of the LV wall are clearly non-CAD-type. For a given patient, however, it is important to note that both CAD-type and non-CAD-type hyperenhancement may be present. This does not necessarily indicate a mixed cardiomyopathy, since both patterns are commonly present in certain nonischemic cardiomyopathies (e.g. cardiac sarcoidosis). As a second part of this step, further classification should be considered if hyperenhancement is present in a non-CAD pattern. Depending on the clinical history and associated data a specific etiology may be entertained as described in Table 1 and Figure 5.
In some patients, additional testing might aid in the interpretation of DE-CMR findings (Step 4). These may be acquired during the same CMR imaging session (e.g. coronary MRA, stress perfusion CMR), or via other modalities prior or subsequent to CMR. In a patient with an ischemic myopathic process (CAD-type scarring) knowledge of the coronary anatomy and/or presence of ischemia will be valuable for decisions regarding treatment including revascularization. However, in a patient with a nonischemic myopathic process (non-CAD-type scarring) additional testing may hone the precise etiology or etiologies of cardiomyopathy. If coronary angiography demonstrates no atherosclerosis, the ventricular dysfunction is solely due to nonischemic CM. If there is anatomic evidence of coronary atherosclerosis, but no evidence of ischemia on functional testing, this suggests the presence of nonischemic CM with incidential CAD. On the other hand, if there are coronary lesions and inducible ischemia, this patient is likely to have a mixed disorder and have ischemic as well as nonischemic cardiomyopathy concomitantly.
Potential Complexities
Several issues may complicate the clinical interpretation of DE-CMR. Although the absence of hyperenhancement suggests a nonischemic process, this presumes long-standing, chronic cardiomyopathy and severe ventricular dysfunction. If the LV ejection fraction is only mildly reduced (e.g. LVEF > 40%) or if the cardiomyopathy is of recent onset, then an ischemic process may be operative and dysfunctional regions without hyperenhancement may represent areas of stunned or hibernating myocardium.
Occasionally CAD-type hyperenhancement may be present along with normal appearing coronary arteries on x-ray angiography. There are several possible explanations for this scenario: (1) Coronary artery disease is present but was missed by angiography. In our experience this is rare but can occur when there is a flush, ostial occlusion of a secondary coronary branch. The infarct-related-artery is usually small and correspondingly, the size of hyperenhancement is also small. (2) Coronary artery disease is present but represents a dynamic process and a ruptured plaque with acute thrombotic occlusion—while leading to acute MI—may have healed without residual stenosis49. In this case intravascular ultrasound may demonstrate substantial coronary atherosclerosis with remodeling and preserved luminal patency. (3) A nonischemic cardiomyopathy is present but coronary vasospasm or emboli may have lead to an MI. Tests for abnormal coronary reactivity or the appropriate clinical scenario (e.g. the presence of intracardiac thrombus) may be helpful in this situation. (4) A nonischemic cardiomyopathy is present but the hyperenhancement pattern mimics that of myocardial infarction. Examples are shown in Figure 8, and it is worthwhile to remember that certain nonischemic disorders (ie. infiltrative CM) are more likely to present with CAD-type hyperenhancement than others.
Finally, when discerning the pattern of hyperenhancement, some caveats should be mentioned. Novices should be cautioned against a quick decision for non-CAD-type hyperenhancement. Additionally, readers should make a distinction between patterns that are clearly non-CAD-type (such as often found in myocarditis, see Figure 6), and one or two patchy lesions that vaguely appear to spare the subendocardium. For the latter (and when in doubt), one should opt for CAD-type hyperenhancement. In this regard, it is important to bear in mind that the etiology of heart failure is ischemic heart disease in nearly 70% of patients50. Moreover, it is in those with ischemic heart disease that effective therapy including revascularization may substantially change patient outcome. Other common sources of error in interpreting hyperenhancement patterns are listed in Table 2 and shown on Figures 9 and 10.
Table 2.
Common Sources of Error in Interpreting Hyperenhancement Patterns
| Pitfalls | Comments/Teaching points |
|---|---|
| Patients with ischemic CM who appear to have non-CAD-type HE | |
|
|
|
|
| Patients with nonischemic CM who appear to have CAD-type HE | |
|
|
| Miscellaneous | |
|
|
|
|
CAD=coronary artery disease; CM=cardiomyopathy; DE-CMR=delayed enhancement cardiac magnetic resonance; HE=hyperenhancement; LV=left ventricle; RV=right ventricle; TI=inversion time
Figure 9.
The most basal aspect of a myocardial infarction can spare the subendocardium as illustrated by gross pathology in an canine heart with acute MI (top row; modified with permission from Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100(19):1992–2002) and in vivo delayed enhancement images from a patient with acute infarction (bottom row); arrows point to infarcted myocardium. Examining the more distal contiguous slices and orthogonal views demonstrates the subendocardial involvement by the infarction. Thus, before deciding that hyperenhancement is non-CAD-type, e.g. “spares the subendocardium”, note that subendocardial sparing refers to the entire extent of hyperenhancement and not merely a small portion.
Figure 10.
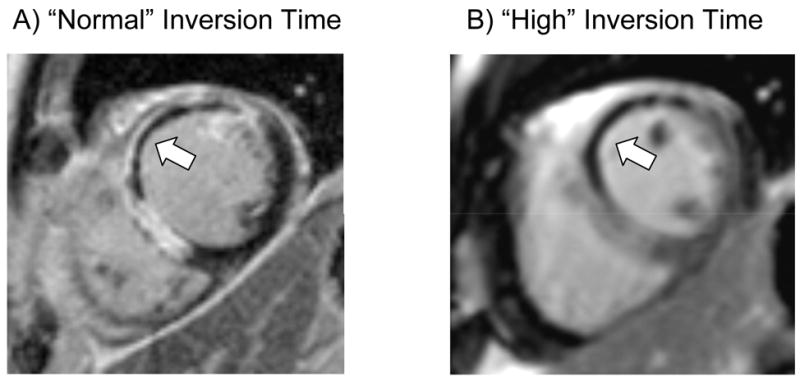
It may be difficult to differentiate normal myocardium from no-reflow zones within the core of an acute MI (arrow, Panel A) using inversion times to null the normal myocardium since both regions appear black. However, with higher inversion times (~600 ms), the no-reflow region which remains black, may be reliably differentiated from normal myocardium, which is now gray in appearance (Panel B).
SUMMARY
In conclusion, DE-CMR may be a valuable tool in evaluating the etiology of cardiomyopathy. As it provides direct assessment of myopathic processes, it represents a fundamentally different approach then traditionally undertaken, which consists of tests to determine the presence or absence of coronary artery disease. A step-wise algorithm to interpreting CMR images is presented, which accounts for differences in the presence, location, and pattern of myocardial hyperenhancement among cardiomyopathies. Additionally, a combination of DE-CMR with assessment of coronary anatomy and/or functional testing for ischemia may allow delineation of patients with nonischemic CM and incidental CAD and patients with mixed cardiomyopathy. In the future, this approach should be evaluated in larger populations for diagnostic accuracy, cost effectiveness, and ultimately to determine if patient outcome can be improved.
Acknowledgments
Funding support: This work was supported in part by National Institutes of Health grant RO1-HL64726 (RJK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93(5):841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 3.Alderman EL, Fisher LD, Litwin P, Kaiser GC, Myers WO, Maynard C, Levine F, Schloss M. Results of coronary artery surgery in patients with poor left ventricular function (CASS) Circulation. 1983;68(4):785–795. doi: 10.1161/01.cir.68.4.785. [DOI] [PubMed] [Google Scholar]
- 4.Felker GM, Shaw LK, O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39(2):210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 5.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 6.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342(15):1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 7.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100(19):1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 8.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109(10):1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 9.Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;43(12):2260–2264. doi: 10.1016/j.jacc.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 10.Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet. 2001;357(9249):21–28. doi: 10.1016/S0140-6736(00)03567-4. [DOI] [PubMed] [Google Scholar]
- 11.Wagner A, Mahrholdt H, Thomson L, Hager S, Meinhardt G, Rehwald W, Parker M, Shah D, Sechtem U, Kim RJ, Judd RM. Effects of time, dose, and inversion time for acute myocardial infarct size measurements based on magnetic resonance imaging-delayed contrast enhancement. J Am Coll Cardiol. 2006;47(10):2027–2033. doi: 10.1016/j.jacc.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 12.Ricciardi MJ, Wu E, Davidson CJ, Choi KM, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103(23):2780–2783. doi: 10.1161/hc2301.092121. [DOI] [PubMed] [Google Scholar]
- 13.Uretsky BF, Thygesen K, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Packer M, Poole-Wilson PA, Ryden L. Acute coronary findings at autopsy in heart failure patients with sudden death: results from the assessment of treatment with lisinopril and survival (ATLAS) trial. Circulation. 2000;102(6):611–616. doi: 10.1161/01.cir.102.6.611. [DOI] [PubMed] [Google Scholar]
- 14.Corya B, Feigenbaum H, Rasmussen S, Black MJ. Echocardiographic features of congestive cardiomyopathy compared with normal subjects and patients with coronary artery disease. Circulation. 1974;49(6):1153–1159. doi: 10.1161/01.cir.49.6.1153. [DOI] [PubMed] [Google Scholar]
- 15.Wallis DE, O’Connell JB, Henkin RE, Costanzo-Nordin MR, Scanlon PJ. Segmental wall motion abnormalities in dilated cardiomyopathy: a common finding and good prognostic sign. J Am Coll Cardiol. 1984;4(4):674–679. doi: 10.1016/s0735-1097(84)80392-7. [DOI] [PubMed] [Google Scholar]
- 16.Glamann DB, Lange RA, Corbett JR, Hillis LD. Utility of various radionuclide techniques for distinguishing ischemic from nonischemic dilated cardiomyopathy. Arch Intern Med. 1992;152(4):769–772. [PubMed] [Google Scholar]
- 17.Rahman JE, Helou EF, Gelzer-Bell R, Thompson RE, Kuo C, Rodriguez ER, Hare JM, Baughman KL, Kasper EK. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43(3):410–415. doi: 10.1016/j.jacc.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 18.Kubo N, Morimoto S, Hiramitsu S, Uemura A, Kimura K, Shimizu K, Hishida H. Feasibility of diagnosing chronic myocarditis by endomyocardial biopsy. Heart Vessels. 1997;12(4):167–170. doi: 10.1007/BF02767044. [DOI] [PubMed] [Google Scholar]
- 19.Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64(10):1235–1245. doi: 10.1016/s0025-6196(12)61286-5. [DOI] [PubMed] [Google Scholar]
- 20.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115(21):2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 21.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343(20):1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 22.Shah DJ, Judd RM, Kim RJ. Myocardial Viability. In: Edelman RR, Hesselink JR, Zlatkin MB, Crues JV, editors. Clinical Magnetic Resonance Imaging. 3. New York, NY: Elsevier; 2006. [Google Scholar]
- 23.Koenig SH, Spiller M, Brown RD, 3rd, Wolf GL. Relaxation of water protons in the intra- and extracellular regions of blood containing Gd(DTPA) Magn Reson Med. 1986;3(5):791–795. doi: 10.1002/mrm.1910030514. [DOI] [PubMed] [Google Scholar]
- 24.Polimeni PI. Extracellular space and ionic distribution in rat ventricle. Am J Physiol. 1974;227(3):676–683. doi: 10.1152/ajplegacy.1974.227.3.676. [DOI] [PubMed] [Google Scholar]
- 25.Rehwald WG, Fieno DS, Chen EL, Kim RJ, Judd RM. Myocardial magnetic resonance imaging contrast agent concentrations after reversible and irreversible ischemic injury. Circulation. 2002;105(2):224–229. doi: 10.1161/hc0202.102016. [DOI] [PubMed] [Google Scholar]
- 26.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56(5):786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 27.Bello D, Shah DJ, Farah GM, Di Luzio S, Parker M, Johnson MR, Cotts WG, Klocke FJ, Bonow RO, Judd RM, Gheorghiade M, Kim RJ. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation. 2003;108(16):1945–1953. doi: 10.1161/01.CIR.0000095029.57483.60. [DOI] [PubMed] [Google Scholar]
- 28.Casolo G, Minneci S, Manta R, Sulla A, Del Meglio J, Rega L, Gensini G. Identification of the ischemic etiology of heart failure by cardiovascular magnetic resonance imaging: diagnostic accuracy of late gadolinium enhancement. Am Heart J. 2006;151(1):101–108. doi: 10.1016/j.ahj.2005.03.068. [DOI] [PubMed] [Google Scholar]
- 29.Soriano CJ, Ridocci F, Estornell J, Jimenez J, Martinez V, De Velasco JA. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol. 2005;45(5):743–748. doi: 10.1016/j.jacc.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 30.Schuster EH, Bulkley BH. Ischemic cardiomyopathy: a clinicopathologic study of fourteen patients. Am Heart J. 1980;100(4):506–512. doi: 10.1016/0002-8703(80)90663-8. [DOI] [PubMed] [Google Scholar]
- 31.Boucher CA, Fallon JT, Johnson RA, Yurchak PM. Cardiomyopathic syndrome caused by coronary artery disease. III: Prospective clinicopathological study of its prevalence among patients with clinically unexplained chronic heart failure. Br Heart J. 1979;41(5):613–620. doi: 10.1136/hrt.41.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts WC, Siegel RJ, McManus BM. Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients. Am J Cardiol. 1987;60(16):1340–1355. doi: 10.1016/0002-9149(87)90618-7. [DOI] [PubMed] [Google Scholar]
- 33.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108(1):54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 34.Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation. 2000;101(5):570–580. doi: 10.1161/01.cir.101.5.570. [DOI] [PubMed] [Google Scholar]
- 35.Arroyo LH, Lee RT. Mechanisms of plaque rupture: mechanical and biologic interactions. Cardiovasc Res. 1999;41(2):369–375. doi: 10.1016/s0008-6363(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 36.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48(10):1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 37.Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40(12):2156–2164. doi: 10.1016/s0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
- 38.Moon JC, Sachdev B, Elkington AG, McKenna WJ, Mehta A, Pennell DJ, Leed PJ, Elliott PM. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur Heart J. 2003;24(23):2151–2155. doi: 10.1016/j.ehj.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Rochitte CE, Oliveira PF, Andrade JM, Ianni BM, Parga JR, Avila LF, Kalil-Filho R, Mady C, Meneghetti JC, Lima JA, Ramires JA. Myocardial delayed enhancement by magnetic resonance imaging in patients with Chagas’ disease: a marker of disease severity. J Am Coll Cardiol. 2005;46(8):1553–1558. doi: 10.1016/j.jacc.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 40.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114(15):1581–1590. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 41.Kim RJ, Judd RM. Gadolinium-enhanced magnetic resonance imaging in hypertrophic cardiomyopathy: in vivo imaging of the pathologic substrate for premature cardiac death? J Am Coll Cardiol. 2003;41(9):1568–1572. doi: 10.1016/s0735-1097(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 42.Shirani J, Freant LJ, Roberts WC. Gross and semiquantitative histologic findings in mononuclear cell myocarditis causing sudden death, and implications for endomyocardial biopsy. Am J Cardiol. 1993;72(12):952–957. doi: 10.1016/0002-9149(93)91113-v. [DOI] [PubMed] [Google Scholar]
- 43.Moon JC, Sheppard M, Reed E, Lee P, Elliott PM, Pennell DJ. The histological basis of late gadolinium enhancement cardiovascular magnetic resonance in a patient with Anderson-Fabry disease. J Cardiovasc Magn Reson. 2006;8(3):479–482. doi: 10.1080/10976640600605002. [DOI] [PubMed] [Google Scholar]
- 44.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218(1):215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 45.Kim RJ, Shah DJ, Judd RM. How we perform delayed enhancement imaging. J Cardiovasc Magn Reson. 2003;5(3):505–514. doi: 10.1081/jcmr-120022267. [DOI] [PubMed] [Google Scholar]
- 46.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26(15):1461–1474. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 47.Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21(16):1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 48.Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2(4):271–278. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
- 49.Kotani J, Mintz GS, Castagna MT, Pinnow E, Berzingi CO, Bui AB, Pichard AD, Satler LF, Suddath WO, Waksman R, Laird JR, Jr, Kent KM, Weissman NJ. Intravascular ultrasound analysis of infarct-related and non-infarct-related arteries in patients who presented with an acute myocardial infarction. Circulation. 2003;107(23):2889–2893. doi: 10.1161/01.CIR.0000072768.80031.74. [DOI] [PubMed] [Google Scholar]
- 50.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 51.Kim RJ, Elliott MD, Judd RM. Assessment of Myocardial Viability by Contrast Enhancement. In: Higgins CB, de Roos A, editors. MRI and CT of the Cardiovascular System. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 52.Matoh F, Satoh H, Shiraki K, Odagiri K, Saitoh T, Urushida T, Katoh H, Takehara Y, Sakahara H, Hayashi H. The usefulness of delayed enhancement magnetic resonance imaging for diagnosis and evaluation of cardiac function in patients with cardiac sarcoidosis. J Cardiol. 2008;51(3):179–188. doi: 10.1016/j.jjcc.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Wilson JM, Villareal RP, Hariharan R, Massumi A, Muthupillai R, Flamm SD. Magnetic resonance imaging of myocardial fibrosis in hypertrophic cardiomyopathy. Tex Heart Inst J. 2002;29(3):176–180. [PMC free article] [PubMed] [Google Scholar]
- 54.Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41(9):1561–1567. doi: 10.1016/s0735-1097(03)00189-x. [DOI] [PubMed] [Google Scholar]
- 55.Amano Y, Takayama M, Takahama K, Kumazaki T. Delayed hyper-enhancement of myocardium in hypertrophic cardiomyopathy with asymmetrical septal hypertrophy: comparison with global and regional cardiac MR imaging appearances. J Magn Reson Imaging. 2004;20(4):595–600. doi: 10.1002/jmri.20172. [DOI] [PubMed] [Google Scholar]
- 56.Teraoka K, Hirano M, Ookubo H, Sasaki K, Katsuyama H, Amino M, Abe Y, Yamashina A. Delayed contrast enhancement of MRI in hypertrophic cardiomyopathy. Magn Reson Imaging. 2004;22(2):155–161. doi: 10.1016/j.mri.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Moon JC, Mogensen J, Elliott PM, Smith GC, Elkington AG, Prasad SK, Pennell DJ, McKenna WJ. Myocardial late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy caused by mutations in troponin I. Heart. 2005;91(8):1036–1040. doi: 10.1136/hrt.2004.041384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soler R, Rodriguez E, Monserrat L, Mendez C, Martinez C. Magnetic resonance imaging of delayed enhancement in hypertrophic cardiomyopathy: relationship with left ventricular perfusion and contractile function. J Comput Assist Tomogr. 2006;30(3):412–420. doi: 10.1097/00004728-200605000-00011. [DOI] [PubMed] [Google Scholar]
- 59.Dumont CA, Monserrat L, Soler R, Rodriguez E, Fernandez X, Peteiro J, Bouzas B, Pinon P, Castro-Beiras A. [Clinical significance of late gadolinium enhancement on cardiovascular magnetic resonance in patients with hypertrophic cardiomyopathy] Rev Esp Cardiol. 2007;60(1):15–23. [PubMed] [Google Scholar]
- 60.Matoh F, Satoh H, Shiraki K, Saitoh T, Urushida T, Katoh H, Takehara Y, Sakahara H, Hayashi H. Usefulness of delayed enhancement magnetic resonance imaging to differentiate dilated phase of hypertrophic cardiomyopathy and dilated cardiomyopathy. J Card Fail. 2007;13(5):372–379. doi: 10.1016/j.cardfail.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Melacini P, Corbetti F, Calore C, Pescatore V, Smaniotto G, Pavei A, Bobbo F, Cacciavillani L, Iliceto S. Cardiovascular magnetic resonance signs of ischemia in hypertrophic cardiomyopathy. Int J Cardiol. 2008;128(3):364–373. doi: 10.1016/j.ijcard.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 62.Paya E, Marin F, Gonzalez J, Gimeno JR, Feliu E, Romero A, Ruiz-Espejo F, Roldan V, Climent V, de la Morena G, Valdes M. Variables associated with contrast-enhanced cardiovascular magnetic resonance in hypertrophic cardiomyopathy: clinical implications. J Card Fail. 2008;14(5):414–419. doi: 10.1016/j.cardfail.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 63.Suk T, Edwards C, Hart H, Christiansen JP. Myocardial scar detected by contrast-enhanced cardiac magnetic resonance imaging is associated with ventricular tachycardia in hypertrophic cardiomyopathy patients. Heart Lung Circ. 2008;17(5):370–374. doi: 10.1016/j.hlc.2008.03.080. [DOI] [PubMed] [Google Scholar]
- 64.Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole-Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111(2):186–193. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 65.Perugini E, Rapezzi C, Piva T, Leone O, Bacchi-Reggiani L, Riva L, Salvi F, Lovato L, Branzi A, Fattori R. Non-invasive evaluation of the myocardial substrate of cardiac amyloidosis by gadolinium cardiac magnetic resonance. Heart. 2006;92(3):343–349. doi: 10.1136/hrt.2005.061911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hosch W, Libicher M, Ley S, Heye T, Schnabel P, Dengler TJ, Katus HA, Kauffmann GW, Kauczor HU, Kristen AV. [MR imaging in cardiac amyloidosis--morphology, function and late enhancement] Rofo. 2008;180(7):639–645. doi: 10.1055/s-2008-1027337. [DOI] [PubMed] [Google Scholar]
- 67.Vogelsberg H, Mahrholdt H, Deluigi CC, Yilmaz A, Kispert EM, Greulich S, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008;51(10):1022–1030. doi: 10.1016/j.jacc.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 68.Shimada T, Shimada K, Sakane T, Ochiai K, Tsukihashi H, Fukui M, Inoue S, Katoh H, Murakami Y, Ishibashi Y, Maruyama R. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium-DTPA-enhanced magnetic resonance imaging. Am J Med. 2001;110(7):520–527. doi: 10.1016/s0002-9343(01)00677-5. [DOI] [PubMed] [Google Scholar]
- 69.Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Dassen WR, Gorgels AP, Crijns HJ. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45(10):1683–1690. doi: 10.1016/j.jacc.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 70.Tadamura E, Yamamuro M, Kubo S, Kanao S, Saga T, Harada M, Ohba M, Hosokawa R, Kimura T, Kita T, Togashi K. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. AJR Am J Roentgenol. 2005;185(1):110–115. doi: 10.2214/ajr.185.1.01850110. [DOI] [PubMed] [Google Scholar]
- 71.Laissy JP, Hyafil F, Feldman LJ, Juliard JM, Schouman-Claeys E, Steg PG, Faraggi M. Differentiating acute myocardial infarction from myocarditis: diagnostic value of early- and delayed-perfusion cardiac MR imaging. Radiology. 2005;237(1):75–82. doi: 10.1148/radiol.2371041322. [DOI] [PubMed] [Google Scholar]
- 72.Yelgec NS, Dymarkowski S, Ganame J, Bogaert J. Value of MRI in patients with a clinical suspicion of acute myocarditis. Eur Radiol. 2007;17(9):2211–2217. doi: 10.1007/s00330-007-0612-3. [DOI] [PubMed] [Google Scholar]
- 73.Gutberlet M, Spors B, Thoma T, Bertram H, Denecke T, Felix R, Noutsias M, Schultheiss HP, Kuhl U. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology. 2008;246(2):401–409. doi: 10.1148/radiol.2461062179. [DOI] [PubMed] [Google Scholar]
- 74.Weidemann F, Breunig F, Beer M, Sandstede J, Stork S, Voelker W, Ertl G, Knoll A, Wanner C, Strotmann JM. The variation of morphological and functional cardiac manifestation in Fabry disease: potential implications for the time course of the disease. Eur Heart J. 2005;26(12):1221–1227. doi: 10.1093/eurheartj/ehi143. [DOI] [PubMed] [Google Scholar]
- 75.Beer M, Weidemann F, Breunig F, Knoll A, Koeppe S, Machann W, Hahn D, Wanner C, Strotmann J, Sandstede J. Impact of enzyme replacement therapy on cardiac morphology and function and late enhancement in Fabry’s cardiomyopathy. Am J Cardiol. 2006;97(10):1515–1518. doi: 10.1016/j.amjcard.2005.11.087. [DOI] [PubMed] [Google Scholar]
- 76.Silva MC, Meira ZM, Gurgel Giannetti J, da Silva MM, Campos AF, Barbosa Mde M, Starling Filho GM, Ferreira Rde A, Zatz M, Rochitte CE. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J Am Coll Cardiol. 2007;49(18):1874–1879. doi: 10.1016/j.jacc.2006.10.078. [DOI] [PubMed] [Google Scholar]
- 77.Yilmaz A, Gdynia HJ, Baccouche H, Mahrholdt H, Meinhardt G, Basso C, Thiene G, Sperfeld AD, Ludolph AC, Sechtem U. Cardiac involvement in patients with Becker muscular dystrophy: new diagnostic and pathophysiological insights by a CMR approach. J Cardiovasc Magn Reson. 2008;10(1):50. doi: 10.1186/1532-429X-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Puchalski MD, Williams RV, Askovich B, Sower CT, Hor KH, Su JT, Pack N, Dibella E, Gottliebson WM. Late gadolinium enhancement: precursor to cardiomyopathy in Duchenne muscular dystrophy? Int J Cardiovasc Imaging. 2009;25(1):57–63. doi: 10.1007/s10554-008-9352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanz J, Dellegrottaglie S, Kariisa M, Sulica R, Poon M, O’Donnell TP, Mehta D, Fuster V, Rajagopalan S. Prevalence and correlates of septal delayed contrast enhancement in patients with pulmonary hypertension. Am J Cardiol. 2007;100(4):731–735. doi: 10.1016/j.amjcard.2007.03.094. [DOI] [PubMed] [Google Scholar]
- 80.Tzelepis GE, Kelekis NL, Plastiras SC, Mitseas P, Economopoulos N, Kampolis C, Gialafos EJ, Moyssakis I, Moutsopoulos HM. Pattern and distribution of myocardial fibrosis in systemic sclerosis: a delayed enhanced magnetic resonance imaging study. Arthritis Rheum. 2007;56(11):3827–3836. doi: 10.1002/art.22971. [DOI] [PubMed] [Google Scholar]
- 81.Kobayashi H, Yokoe I, Hirano M, Nakamura T, Nakajima Y, Fontaine KR, Giles JT, Kobayashi Y. Cardiac Magnetic Resonance Imaging with Pharmacological Stress Perfusion and Delayed Enhancement in Asymptomatic Patients with Systemic Sclerosis. J Rheumatol. 2008 doi: 10.3899/jrheum.080377. [DOI] [PubMed] [Google Scholar]
- 82.Nassenstein K, Breuckmann F, Huger M, Ladd SC, Schlosser T, Kreuter A, Barkhausen J. Detection of Myocardial Fibrosis in Systemic Sclerosis by Contrast-Enhanced Magnetic Resonance Imaging. Rofo. 2008 doi: 10.1055/s-2008-1027864. [DOI] [PubMed] [Google Scholar]
- 83.Mitchell JH, Hadden TB, Wilson JM, Achari A, Muthupillai R, Flamm SD. Clinical features and usefulness of cardiac magnetic resonance imaging in assessing myocardial viability and prognosis in Takotsubo cardiomyopathy (transient left ventricular apical ballooning syndrome) Am J Cardiol. 2007;100(2):296–301. doi: 10.1016/j.amjcard.2007.02.091. [DOI] [PubMed] [Google Scholar]
- 84.Eitel I, Behrendt F, Schindler K, Kivelitz D, Gutberlet M, Schuler G, Thiele H. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur Heart J. 2008;29(21):2651–2659. doi: 10.1093/eurheartj/ehn433. [DOI] [PubMed] [Google Scholar]
- 85.Mark PB, Johnston N, Groenning BA, Foster JE, Blyth KG, Martin TN, Steedman T, Dargie HJ, Jardine AG. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006;69(10):1839–1845. doi: 10.1038/sj.ki.5000249. [DOI] [PubMed] [Google Scholar]
- 86.Debl K, Djavidani B, Buchner S, Lipke C, Nitz W, Feuerbach S, Riegger G, Luchner A. Delayed hyperenhancement in magnetic resonance imaging of left ventricular hypertrophy caused by aortic stenosis and hypertrophic cardiomyopathy: visualisation of focal fibrosis. Heart. 2006;92(10):1447–1451. doi: 10.1136/hrt.2005.079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wassmuth R, Gobel U, Natusch A, Schneider W, Kettritz R, Dietz R, Luft FC, Schulz-Menger J. Cardiovascular magnetic resonance imaging detects cardiac involvement in Churg-Strauss syndrome. J Card Fail. 2008;14(10):856–860. doi: 10.1016/j.cardfail.2008.07.227. [DOI] [PubMed] [Google Scholar]
- 88.Breuckmann F, Nassenstein K, Kondratieva J, Esser S, Erbel R, Potthoff A, Brockmeyer NH, Neumann T, Barkhausen J. MR characterization of cardiac abnormalities in HIV+ individuals with increased BNP levels. Eur J Med Res. 2007;12(5):185–190. [PubMed] [Google Scholar]
- 89.Raman SV, Sparks EA, Baker PM, McCarthy B, Wooley CF. Mid-myocardial fibrosis by cardiac magnetic resonance in patients with lamin A/C cardiomyopathy: possible substrate for diastolic dysfunction. J Cardiovasc Magn Reson. 2007;9(6):907–913. doi: 10.1080/10976640701693733. [DOI] [PubMed] [Google Scholar]
- 90.Mouquet F, Lions C, de Groote P, Bouabdallaoui N, Willoteaux S, Dagorn J, Deruelle P, Lamblin N, Bauters C, Beregi JP. Characterisation of peripartum cardiomyopathy by cardiac magnetic resonance imaging. Eur Radiol. 2008;18(12):2765–2769. doi: 10.1007/s00330-008-1067-x. [DOI] [PubMed] [Google Scholar]
- 91.Fallah-Rad N, Lytwyn M, Fang T, Kirkpatrick I, Jassal DS. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J Cardiovasc Magn Reson. 2008;10(1):5. doi: 10.1186/1532-429X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Strach K, Sommer T, Grohe C, Meyer C, Fischer D, Walter MC, Vorgerd M, Reilich P, Bar H, Reimann J, Reuner U, Germing A, Goebel HH, Lochmuller H, Wintersperger B, Schroder R. Clinical, genetic, and cardiac magnetic resonance imaging findings in primary desminopathies. Neuromuscul Disord. 2008;18(6):475–482. doi: 10.1016/j.nmd.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 93.Abdel-Aty H, Siegle N, Natusch A, Gromnica-Ihle E, Wassmuth R, Dietz R, Schulz-Menger J. Myocardial tissue characterization in systemic lupus erythematosus: value of a comprehensive cardiovascular magnetic resonance approach. Lupus. 2008;17(6):561–567. doi: 10.1177/0961203308089401. [DOI] [PubMed] [Google Scholar]