Abstract
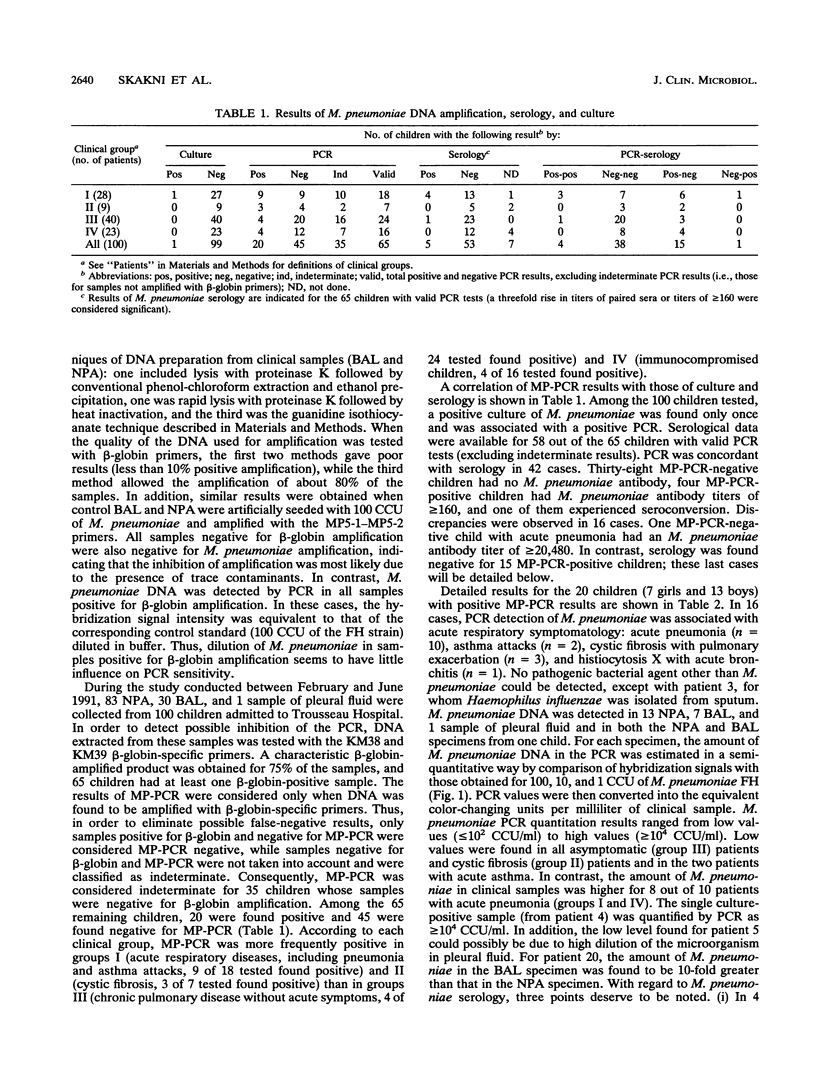
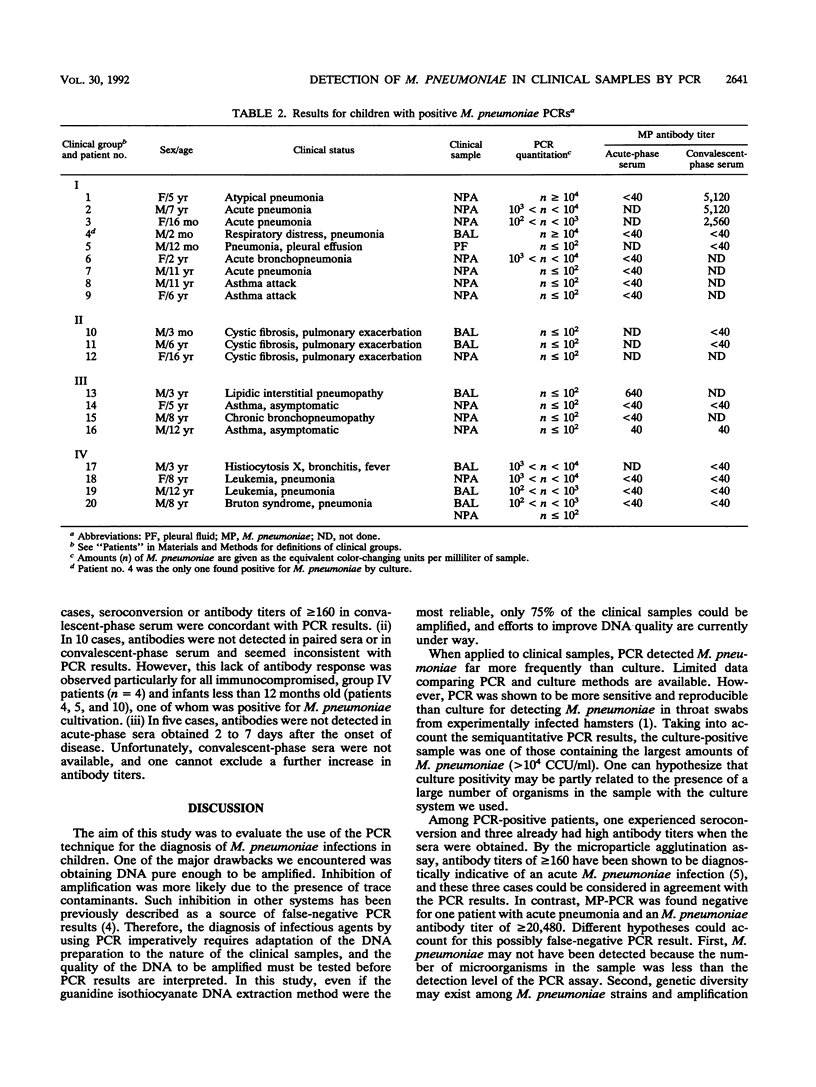
The polymerase chain reaction (PCR) technique was used to detect Mycoplasma pneumoniae DNA in clinical samples (nasopharyngeal aspirations or bronchoalveolar lavages) obtained from 100 children, 1 month to 16 years old. PCR allowed the detection of M. pneumoniae DNA from 20 out of the 100 patients studied. In 16 cases, PCR positivity was associated with acute respiratory symptomatology. For five PCR-positive patients, a positive culture or a serological response evidenced acute M. pneumoniae infections. A lack of antibody response was observed particularly with immunocompromised children and infants less than 12 months old. The amount of M. pneumoniae DNA in the PCR was estimated in a semiquantitative way by comparison of its hybridization signal with those obtained for 100, 10, and 1 color-changing unit (CCU) of the M. pneumoniae FH strain. Small amounts (less than or equal to 10(2) CCU/ml) of M. pneumoniae were found in samples from asymptomatic patients, while larger amounts (greater than or equal to 10(2) to greater than or equal to 10(4) CCU/ml) were found for 8 out of 10 patients with acute pneumonia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernet C., Garret M., de Barbeyrac B., Bebear C., Bonnet J. Detection of Mycoplasma pneumoniae by using the polymerase chain reaction. J Clin Microbiol. 1989 Nov;27(11):2492–2496. doi: 10.1128/jcm.27.11.2492-2496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom R., Sol C. J., Salimans M. M., Jansen C. L., Wertheim-van Dillen P. M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990 Mar;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia W. K., Spence L., Dunkley L., Bradbury W. Development of urease conjugated enzyme-linked immunosorbent assays (ELISA) for the detection of IgM and IgG antibodies against Mycoplasma pneumoniae in human sera. Diagn Microbiol Infect Dis. 1988 Oct;11(2):101–107. doi: 10.1016/0732-8893(88)90078-8. [DOI] [PubMed] [Google Scholar]
- Clewley J. P. The polymerase chain reaction, a review of the practical limitations for human immunodeficiency virus diagnosis. J Virol Methods. 1989 Aug;25(2):179–187. doi: 10.1016/0166-0934(89)90031-1. [DOI] [PubMed] [Google Scholar]
- Echevarría J. M., León P., Balfagón P., López J. A., Fernández M. V. Diagnosis of Mycoplasma pneumoniae infection by microparticle agglutination and antibody-capture enzyme-immunoassay. Eur J Clin Microbiol Infect Dis. 1990 Mar;9(3):217–220. doi: 10.1007/BF01963842. [DOI] [PubMed] [Google Scholar]
- Hyman H. C., Yogev D., Razin S. DNA probes for detection and identification of Mycoplasma pneumoniae and Mycoplasma genitalium. J Clin Microbiol. 1987 Apr;25(4):726–728. doi: 10.1128/jcm.25.4.726-728.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E., Bennewitz A., Bredt W. Reaction pattern of human anti-Mycoplasma pneumoniae antibodies in enzyme-linked immunosorbent assays and immunoblotting. J Clin Microbiol. 1986 Mar;23(3):517–522. doi: 10.1128/jcm.23.3.517-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. S., Søndergård-Andersen J., Uldum S. A., Lind K. Detection of Mycoplasma pneumoniae in simulated clinical samples by polymerase chain reaction. Brief report. APMIS. 1989 Nov;97(11):1046–1048. doi: 10.1111/j.1699-0463.1989.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Kleemola S. R., Karjalainen J. E., Räty R. K. Rapid diagnosis of Mycoplasma pneumoniae infection: clinical evaluation of a commercial probe test. J Infect Dis. 1990 Jul;162(1):70–75. doi: 10.1093/infdis/162.1.70. [DOI] [PubMed] [Google Scholar]
- Kok T. W., Marmion B. P., Varkanis G., Worswick D. A., Martin J. Laboratory diagnosis of Mycoplasma pneumoniae infection. 3. Detection of IgM antibodies to M. pneumoniae by a modified indirect haemagglutination test. Epidemiol Infect. 1989 Dec;103(3):613–623. doi: 10.1017/s0950268800031010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok T. W., Varkanis G., Marmion B. P., Martin J., Esterman A. Laboratory diagnosis of Mycoplasma pneumoniae infection. 1. Direct detection of antigen in respiratory exudates by enzyme immunoassay. Epidemiol Infect. 1988 Dec;101(3):669–684. doi: 10.1017/s0950268800029551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind K., Lindhardt B. O., Schütten H. J., Blom J., Christiansen C. Serological cross-reactions between Mycoplasma genitalium and Mycoplasma pneumoniae. J Clin Microbiol. 1984 Dec;20(6):1036–1043. doi: 10.1128/jcm.20.6.1036-1043.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Tilton R. C., Dias F., Kidd H., Ryan R. W. DNA probe versus culture for detection of Mycoplasma pneumoniae in clinical specimens. Diagn Microbiol Infect Dis. 1988 Jun;10(2):109–112. doi: 10.1016/0732-8893(88)90048-x. [DOI] [PubMed] [Google Scholar]
- Uldum S. A., Søndergård-Andersen J., Skov Jensen J., Lind K. Evaluation of a commercial enzyme immunoassay for detection of Mycoplasma pneumoniae specific immunoglobulin G antibodies. Eur J Clin Microbiol Infect Dis. 1990 Mar;9(3):221–223. doi: 10.1007/BF01963843. [DOI] [PubMed] [Google Scholar]