Abstract
Current models of affective disorders implicate alterations in norepinephrine, serotonin, dopamine, and CRF/cortisol; however treatments targeted at these neurotransmitters or hormones have led to imperfect resolution of symptoms, suggesting that the neurobiology of affective disorders is incompletely understood. Until now retinoids have not been considered as possible contributors to affective disorders. Retinoids represent a family of compounds derived from Vitamin A that perform a large number of functions, many via the vitamin A product, retinoic acid. This signaling molecule binds to specific retinoic acid receptors in the brain which, like the glucocorticoid and thyroid hormone receptors, are part of the nuclear receptor superfamily and regulate gene transcription. Research in the field of retinoic acid in the CNS has focused on the developing brain, in part stimulated by the observation that isotretinoin (13-cis retinoic acid), an isomer of retinoic acid used in the treatment of acne, is highly teratogenic for the CNS. More recent work has suggested that retinoic acid may influence the adult brain; animal studies indicated that the administration of isotretinoin is associated with alterations in behavior as well as inhibition of neurogenesis in the hippocampus. Clinical evidence for an association between retinoids and depression includes case reports in the literature, studies of health care databases, and other sources. A preliminary PET study in human subjects showed that isotretinoin was associated with a decrease in orbitofrontal metabolism. Several studies have shown that the molecular components required for retinoic acid signaling are expressed in the adult brain ; the overlap of brain areas implicated in retinoic acid function and stress and depression suggest that retinoids could play a role in affective disorders. This report reviews the evidence in this area and describes several systems that may be targets of retinoic acid and which contribute to the pathophysiology of depression.
1. Introduction
Retinoids are a family of compounds derived from vitamin A that are necessary for the function of many tissues in the developing and adult vertebrate. The majority of these functions are performed by the vitamin A metabolite retinoic acid (RA), which binds to receptors of the nuclear receptor superfamily, and regulates gene expression. In the adult, RA influences the function of the urogenital system, epithelia of the gut and respiratory tracts, as well as skin, bone and cells of the immune system. In the embryo, RA is essential to guide growth, development, and patterning in many organ systems, while exposure to abnormal concentrations of RA, for instance through treatment of acne with isotretinoin (13-cis RA), results in multiple malformations, including abnormalities in the developing brain (Coberly et al 1996; Lammer and Armstrong 1992; McCaffery et al 2003).
The role of RA in regulating CNS development has been extensively investigated (as reviewed by Maden (Maden 2001)) but, until recently, it was scarcely considered that RA may also influence neuronal function in the adult brain. Accumulating evidence has shown, however, that RA signaling occurs in the brain of the adult (Krezel et al 1999; Luo et al 2004a; Sakai et al 2004; Thompson Haskell et al 2002; Zetterstrom et al 1999; Zetterstrom et al 1994), suggesting that the adult brain, like the embryo, may be sensitive to exposure to excess RA. This emerging research is relevant to reports of depression and suicide in acne patients treated with 13-cis-RA. When examined together, the evidence suggests a possible under-recognized neural signaling pathway that could explain, in part, symptoms of affective disorders, as well as shed light on the incomplete explanation of mood disturbances using the current neurochemical systems of serotonin, dopamine and norepinephrine.
Retinoids are known to bind to retinoid receptors in the brain and to exert effects on gene transcription. Retinoid receptors are concentrated in limbic areas that have been associated with depression, including the amygdala, prefrontal cortex, and hippocampus. Retinoids also influence neurochemical systems that have been implicated in depression, in particular dopamine but to some extent serotonin and norepinephrine. According to the concepts proposed in this paper these effects can translate into behavioral effects, including symptoms of affective disorders. As we describe below, retinoids like all-trans-RA (the active form of vitamin A), an isomeric variant of 13-cis-RA, have psychiatric effects at high doses, and when deficient can lead to impairment of learning and memory. Studies in both humans and animals show that 13-cis-RA can induce depressive behaviors. Clinical evidence for an association between 13-cis-RA and depression in humans comes from similar neuropsychiatric effects with other retinoids in its class (e.g., Vitamin A), case reports in the literature, temporal association, challenge-rechallenge, and dose response cases. This report proposes RA as a previously under-recognized neurochemical system that is involved in the pathophysiology of depression. Section 2 of this review describes the general neurobiology of RA, while section 3 discusses the behavioral effects of exposure to retinoids in animal studies as well as the clinical literature on 13-cis-RA and depression. Finally, in section 4, retinoids are described as novel neurosignalling molecules that can modulate, at least in part, symptoms of affective disorders.
2. Neurobiology of the Retinoids
2.1 Retinoid Structure and Metabolism
Vitamin A is defined as all the C20-beta-ionone derivatives that exhibit the biological activity of all-trans retinol (International Union of Nutritional Sciences) (1978). The structure of retinol is given as the first structure in figure 1, consisting of a cyclohexenyl ring with a side chain containing 4 trans double bonds and an alcohol end group. In this review we use the term retinoids to describe the biological family of compounds derived from retinol. The different retinoids are often modified on the end group of the side chain, which can be oxidized for instance to form retinaldehyde (Fig. 1, structure 2) and then further oxidized to form RA (Fig. 1, structure 3). Vitamin A is obtained in the diet either from meat in the form of retinyl esters (Fig. 1, structure 4) that are hydrolyzed to retinol, or from plants as beta-carotene (Fig. 1, structure 5), which can be cleaved to retinaldehyde and then reduced to retinol. The enterocytes of the intestinal lumen convert the retinol to retinyl esters, which are inserted into chylomicrons for transport to the liver, the main storage organ for retinoids (although other tissues may make use of retinyl esters transported by the chylomicrons). The stored retinyl esters in the liver can be hydrolyzed back to retinol when required, delivered systemically to all cells that require these compounds bound in the plasma to retinol binding protein (RBP). A useful overview of these steps can be found in the Introduction to “Vitamin A in Health and Disease”, by Rune Blomhoff (Blomhoff 1994).
Figure 1.
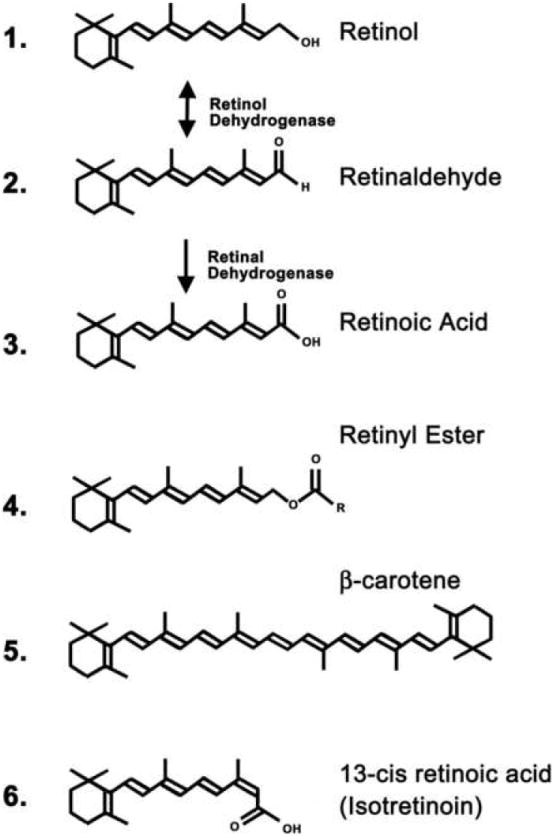
Chemical structures of retinoid family members. The double headed arrow between retinol (structure 1) and retinaldehyde (structure 2) indicates the interconversion between the two retinoids catalyzed by retinol dehydrogenase, either oxidising retinol to retinaldehyde or catalyzing the reverse reductive reaction. The single arrow between retinaldehyde (structure 2) and retinoic acid (structure 3) indicates the irreversible oxidation catalyzed by retinaldehyde dehydrogenase. Common retinyl esters (structure 4) include retinyl acetate (R = CH3) and retinyl palmitate (R = C15H31)
A number of retinol products are essential for certain tissue functions. For example, retinaldehyde is the chromophore of visual pigment (Wolf 2004), and the retro-retinoids are required for immune function (O’Connell et al 1996). It is accepted, however, that the majority of retinoid functions are mediated via its control of transcription through RA and its activation of specific RA receptors. A general role for RA is the regulation of cell proliferation and differentiation; for instance in the embryo this is a key function for RA in the control of growth of many organ systems, including the CNS (McCaffery et al 2003), and such functions are carried over into the adult where RA controls proliferation and differentiation of dividing cells of the respiratory, urinary, and intestinal tracts, as well as in bone and skin (Sporn et al 1994). In these cells RA is generated locally from retinol obtained from the plasma, and thus it is essential that these cells express the appropriate synthetic enzymes. Retinol in the plasma enters the cell where it is first oxidized to retinaldehyde by a retinol dehydrogenase, and then further oxidized to RA by a retinaldehyde dehydrogenase (Fig 1, structures 1 to 3). Whereas the retinol dehydrogensases are very widespread the retinaldehyde dehydrogenases (RALDHs) are highly localized and are the key enzymes that determine where RA is synthesized (Duester 2000). RA then enters the nucleus and binds to the RA receptors to activate gene transcription. Other retinoids are also known to bind to the RA receptors, including 4-oxo-retinoic acid and other oxidative derivatives (Idres et al 2002), 4-oxo-retinol (Ross and De Luca 1996) and didehydroretinol (Sani et al 1997). At the present time, however, RA is the only retinoid detected in the adult brain that activates the RAR class of receptors (Kane et al 2005).
2.2 Retinoic Acid Signaling via Nuclear Receptors
The RA receptors (RARs) are members of the nuclear receptor superfamily that includes the receptors for estrogen (ER), androgen (AR), mineralocorticoids (MR), glucocorticoids (GR) and thyroid hormone (THR). All of these receptors function in a similar manner (Bastien and Rochette-Egly 2004) — the receptor, either present in the nucleus, or moving from the cytoplasm into the nucleus, binds to a response element in the promoter of responsive genes. In the presence of ligand the receptor switches in conformation and releases corepressors that would otherwise keep the gene repressed in the absence of ligand. This is followed by recruitment of a chromatin remodeling complex that decompacts the chromatin, allowing the transcriptional machinery to gain access to the promoter and transcription to proceed. The RA response element (RARE), like most nuclear receptor response elements, binds to receptor dimers. Most frequently the RAR is dimerized to a closely related nuclear receptor, RXR. Although the RXRs can bind 9-cis RA, it is now considered unlikely that this is an endogenous ligand for the RXRs in most tissues. The RXRs can act independently of ligand and ligand activation may not be necessary for the function of this nuclear receptor (Rowe 1997). However, a potential group of ligands exists in the form of polyunsaturated fatty acids, including the omega-3 fatty acid docosahexanoic acid (DHA), and its affinity for RXR may account for some of the actions of DHA in the brain (Lengqvist et al 2004). It is of interest that omega-3 fatty acids have been shown to have efficacy in the treatment of depression (Stoll et al 1999); however this action may not necessarily be via the activation and interaction of RXR with RAR, but may be the result of RXR’s binding to the many other nuclear receptors to which RXR can heterodimerize including thyroid hormone, vitamin D and peroxisome proliferator-activated receptors (Rowe 1997).
Three genes exist each for RAR (alpha, beta and gamma) and RXR (alpha, beta and gamma) and gene splicing significantly increases the number of variants (Chambon 1996). Although marginal differences exist in the affinity of the RAR receptors for RA isomers and the other transcriptionally active retinoids (Kastner et al 1994), it would seem that they perform overlapping functions. Certainly the phenotype of the vitamin A deficient animal can only be replicated by double null mutations of the receptors (Lohnes et al 1994; Mendelsohn et al 1994).
2.3 Retinoic Acid Signaling in the Brain
The RA receptors are widely distributed in the adult brain (Krezel et al 1999; Zetterstrom et al 1999; Zetterström et al 1994) which would suggest that RA is necessary for tasks throughout the brain. However, RA itself is much less widely distributed (Mey and McCaffery 2004) and, if the RA receptors are bound to RAREs without ligand, as described in section 2.2, then they may act to repress transcription. There are regions, though, where RA acts as a positive transcriptional regulator, as indicated using transgenic RA reporter mice. Several such transgenic lines exist and use either 1) a transgene consisting of a RARE driving a lacZ reporter gene (Colbert et al 1993; Rossant et al 1991) to report RA induced gene transcription or 2) a fusion of the Gal-4 DNA-binding domain linked with the ligand-binding domain of RAR alpha —when these mice are crossed with reporter mice with lacZ controlled by an upstream activating sequence then these report RA binding to the RA receptor (Mata De Urquiza et al 1999). In the embryo, these reporter mice show RA signaling in a variety of regions in the CNS (Colbert et al 1993; Luo et al 2004b; Mata De Urquiza et al 1999; Zhang et al 2003); however, as the CNS matures, RA signaling is restricted to progressively limited regions (Thompson Haskell et al 2002). This deactivation of the RA signaling system is not due to restricted amounts of RA however, since injections of RA into the reporter mouse do not activate the reporter in regions in which it is not already expressed (Luo et al 2004b), thus this loss of responsiveness is presumably at a genomic level. Regions of the brain that exhibit RA signaling as determined using RA reporter mice include the limbic system, in particular the hippocampus (section 2.4) (Krezel et al 1999; Luo et al 2004b; Misner et al 2001; Sakai et al 2004; Zetterstrom et al 1999) as well as the medial prefrontal cortex including prefrontal and cingulate cortex and retrosplenial area together with subregions of the thalamus and hypothalamus (Luo et al 2004b). One suggested common feature of several of the regions of RA signaling is that these are areas of high neuronal plasticity (McCaffery et al 2006; Thompson Haskell et al 2002). Plasticity is the capacity of the brain to rearrange the pattern of neuronal connections and is essential for learning and memory as well as damage repair; neuronal plasticity also plays a role in the stress response, which may be altered in affective disorders. These rearrangements include changes in synaptic strength, as measured by long-term potentiation (LTP) and long-term depression (LTD), synaptic turnover and neuritic remodeling (of dendrites) and neurogenesis. Such dynamic processes have parallels to the developmental events that generate the brain in the embryo and it is not surprising that several of these events, LTP and LTD (Chiang et al 1998), as well as neurite outgrowth (Corcoran and Maden 1999; Hunter et al 1991; Wu et al 1998) and neurogenesis (Crandall et al 2004; Misner et al 2001; Sakai et al 2004) are regulated by RA. The hippocampus (Sapolsky 2003) and olfactory system (Wilson et al 2004) are two regions in which neural plasticity has been extensively described and are regions of focus of RA signaling from studies of RA reporter animals. In the olfactory system RA signaling has been described in the population of GABAergic interneurons in the olfactory bulb (Thompson Haskell et al 2002), cells which undergo turnover throughout adult life through neurogenesis. RA may also regulate cell proliferation in the olfactory epithelium (Asson-Batres et al 2003). The hippocampus though is the region most consistently identified in the analysis of different RA reporter mice (McCaffery et al 2006; Misner et al 2001; Sakai et al 2004) and, as an area in which plasticity is crucial to its function in memory (Silva 2003), this would be predicted to be a key site of RA signaling.
2.4 Retinoic Acid Signaling in the Hippocampus
The hippocampus is the main region of the adult brain in which the influence of RA and vitamin A has been examined to a significant degree (McCaffery et al 2006). Synaptic plasticity in the hippocampus is evident in the phenomenon of LTP and LTD, which lead to long-lasting modifications of synaptic efficiency. Null mutation of RAR beta results in loss of LTP and LTD as well as deficits in a spatial learning task (Chiang et al 1998). Confounding the interpretation of this result though is the lack of expression of RAR beta in the mouse hippocampus (Krezel et al 1999; Zetterstrom et al 1999). Since these null mutants are not conditional, the effect of receptor loss may be during development of the hippocampus. That vitamin A, and presumably RA, influence LTP and LTD is implied, however, from experiments in which vitamin A deficiency in rats results in loss of long-term synaptic plasticity (Misner et al 2001). Such vitamin A deficiency experiments also lead to defective performance in spatial learning and memory tasks (Cocco et al 2002). Further, in aged mice, where RA signaling in the brain may be reduced, supplemental RA can help reverse an age-related decrease in hippocampal LTP (Etchamendy et al 2001).
A second aspect of plasticity in the hippocampus is the phenomenon of neurogenesis — the hippocampus is one of the few regions of the adult brain in which new neurons are constantly born (Alvarez-Buylla and Lim 2004). Given that RA is a regulator of neuronal differentiation in the embryonic CNS it is no surprise that RA also influences this process in the adult brain (Crandall et al 2004; Jacobs et al 2006; Wang et al 2005). New neurons replenish granule cells within the dentate gyrus and it is the granule neurons that are the site of strongest RA signaling in the hippocampus (Misner et al 2001; Sakai et al 2004). It has been suggested that RA in the hippocampus is generated by the RA synthetic enzyme RALDH2 in the adjacent meninges and that this regulates hippocampal neurogenesis (Sakai et al 2004). When the mouse is exposed to excess RA, either the trans or the 13-cis isomers, then this may interfere with this endogenous signaling system, resulting in a decrease in neurogenesis (Crandall et al 2004).
2.5 Retinoic Acid Signaling in the Corpus Striatum and Nucleus Accumbens
The transgenic RA reporter lines point to regions of plasticity in the brain as predominant regions of RA signaling. Do these reporter lines faithfully report all regions of RA signaling however? It is possible, for instance, that epigenetic mechanisms may exist to silence the RARE-reporter transgene, while allowing RAREs associated with other genes to function. An example in the embryo of a region where RA signaling occurs, but no reporter response is evident, is the ganglionic eminence, which is responsive to RA (Toresson et al 1999a; Waclaw et al 2004) and is a region of RA synthesis via the RALDH3 enzyme but shows little expression of the RA reporter (Luo et al 2004b). Thus, it is possible that RA signaling may prove to be more extensive in the brain. One such region in the adult brain is the corpus striatum, neurons of which derive from the ganglionic eminence. Several lines of evidence point to the importance of RA in the function of the corpus striatum, as well as the nucleus accumbens, which is adjacent to the medial and ventral striatum and is part of the functional circuit of these components of the basal ganglia. Both the striatum and nucleus accumbens strongly express RARbeta and the retinoid binding protein CRBPI (cellular retinol binding protein I) and CRABPI and II (cellular retinoic acid binding protein I and II). Recent quantitation of RA concentrations in the adult brain found that the striatum showed the highest levels in the brain (Kane et al 2005). The source of RA synthesis for the dorsal striatum and nucleus accumbens is presumed to be the synthetic enzyme RALDH1, which is present in the dopaminergic terminals that innervate the striatum from the ventral tegmental area (McCaffery and Drager 1994; Wagner et al 2002). Thus, the striatum and nucleus accumbens are regions where RA may be supplied in a similar fashion to that of dopamine — by transfer of the synthetic enzyme along terminals from the ventral tegmental area that innervate the striatum and nucleus accumbens. Interestingly, RA may also modulate the action of dopamine by regulating the D2 dopamine receptor. The D2 dopamine receptor gene is RA inducible in both rat and mouse (Samad et al 1997; Wolf 1998), and in the rat RA has been described as a key inducer of this receptor in the ganglionic eminence (Valdenaire et al 1998). Two candidate genes regulated by vitamin A in the striatum are neurogranin and GAP43, both of which are involved in synaptic plasticity. Deficiency of vitamin A in rats results in a decline in these genes in the striatum but does not effect their expression in the hippocampus or cerebral cortex (Husson et al 2004). However, whereas application of RA to the vitamin A deficient rats restored GAP43 levels, neurogranin did not return to control amounts suggesting that the latter protein may be regulated by a vitamin A derivative other than RA (Husson et al 2004). Indications of a behavioral phenotype resulting from inhibition of RA signaling in the striatum come from studies of double null mutation of RARbeta with either RXRbeta or RXRgamma. Such mutations result in impaired locomotion typical of abnormal striatal function and possibly related to a decrease in the dopamine D1 and D2 type receptors in striatal neurons (Krezel et al 1998). Although it is possible that the phenotype of these RA receptor null mutations is due to a developmental effect the high expression of RA signaling components in the adult striatum suggests that an influence on adult function may be a significant component.
3. Behavioral Effects of 13-cis RA (Isotretinoin) in Animal and Human Models
3.1 Behavioral Effects in Animal Models
Preliminary evidence that changes in RA signaling may promote depression has come from behavioral studies on animals. In a recent study in mice, that closely paralleled the clinical situation of 1 mg/kg/day isotretinoin over 6 weeks, depression-like behaviors in the animals were revealed, including decreased swimming in a forced swim test as well as poorer performance in tail suspension tests (O’Reilly et al 2006). Rats administered doses of 13-cis RA, which gave similar plasma levels to isotretinoin used clinically, did not show severe depression-like behaviors although the animals exhibited several altered responses in behavioral tests. Whereas the rats showed no change in a forced swim test, 91 day old animals (corresponding to young adults) given 7.5 mg/day for 28 days, showed a decrease in open field activity, used as an animal model for depression (Ferguson et al 2005b). No effects were seen in older rats and because the majority of their behavioral tests for depression did not show an effect with drug treatment, the authors concluded “chronic 13-cis-RA treatment did not severely affect depression-like behaviors in rats.” Although not altering neurotransmitter or neurotransmitter metabolites generally throughout the brain, interestingly the 7.5 mg/day 13-cis RA dose resulted in a rise in dopamine and serotonin metabolites in the striatum (Ferguson et al 2005a), a region that has been implicated in mood disorders. Overall, these results using rodent models provide evidence that: 1) 13-cis RA can affect brain function and behavior; 2) younger individuals may be more vulnerable to effects of 13-cis RA, and; 3) mice are more sensitive to 13-cis RA than rats, which show few behavioral effects.
3.2 Behavioral Effects in Humans
Studies in human subjects also support a role for an association between retinoids and affective disorders. These primarily come from studies of isotretinoin (Accutane), or 13-cis-RA, a drug that inhibits sebaceous gland function, keratinization, and inflammatory responses, and is approved by the Food and Drug Administration (FDA) for the treatment of cystic and nodular acne (Cunliff et al 1997; Goulden et al 1995; Orfanos et al 1997). Isotretinoin is identical to the predominant physiological form of RA, all-trans RA, differing only in molecular geometry, having its double bond at C13 in cis conformation (Fig. 1, structure 6). As reviewed by William Blaner (Blaner 2001) there is very strong evidence that 13-cis RA is produced endogenously, and 13-cis RA is present in the plasma at higher or similar concentrations as the all-trans isomer (Eckhoff and Nau 1990; Tang and Russell 1991). Although 13-cis RA only weakly binds to the RA receptors, evidence indicates that 13-cis RA is isomerized to all-trans RA in tissues (Shih et al 1986; Tsukada et al 2000) and thus 13-cis RA acts like all-trans RA to regulate transcription via the RA receptors. An important physiological difference between the isomers is that 13-cis RA remains in the circulation much longer than its all-trans counterpart in humans, with an elimination half-life of 20 hours, versus 0.9 hours for all-trans RA while peak plasma concentrations are reached at 2-4 hours after an oral dosage (Weigan and Chou 1998).
Because of its teratogenic effects isotretinoin was originally approved only for severe acne, today however only about 8% of patients treated with isotretinoin have severe acne (Goulden et al 1995).
Case reports of neuropsychiatric side effects have also been associated with other retinoids used for therapeutic purposes. Below we review the epidemiology of depression, general mechanisms of drug-induced depression, and the factors used to assess the potential relationship between a drug and a particular side effect.
3.2.1 Epidemiology of Depression
Major depression is a common disorder that is associated with substantial loss of productivity. Major depression is defined by the Diagnostic and Statistical Manual–IV (DSM-IV) as depressed mood most of the day, nearly every day, with markedly diminished interest or pleasure in activities most of the day. This is associated with contradictory symptoms of significant weight loss or weight gain, decrease or increase in appetite, insomnia or hypersomnia, psychomotor agitation or retardation, fatigue or loss of energy, feelings of worthlessness or excessive or inappropriate guilt, diminished ability to think or concentrate, recurrent thoughts of death, and recurrent suicidal ideation or suicide attempts. About 30,000 people in the US kill themselves every year, with depression being a common cause of suicide. The National Comorbidity Survey Replication Study looked at 9090 individuals over 18 and found a lifetime prevalence of major depression of 16% (Kessler et al 2003). These figures refer to the number of people who have depression at any time in their lives, however, not those who have depression at any given time point.
More relevant to the question of isotretinoin and depression is the question of incidence of depression. This is the number of individuals who will develop the new onset of depression during a given time frame, e.g. during the four months of a typical isotretinoin trial. Data from the original National Comorbidity Study show the one year incidence of depression is 1.6%. There were no appreciable differences between young people age 18-29 (1.7%) and older individuals 45-64 (1.4%) (Eaton et al 1989). Although few studies have been conducted in children under 18, the suicide rate in teenagers is half of what it is in any age group except for young children (CDC 2006).
3.2.2 Drug Induced Depression
Drug-induced depression is defined as a depression that occurs in the context of administration of a medication or substance, can likely be attributed to the use of that medication, and which resolves with discontinuation of the medication (APA 2000). Several drugs have been shown to cause symptoms of depression including corticosteroids. These are in a similar class as retinoids, binding to members of the nuclear receptor superfamily, and promoting gene transcription through activation of specific promoters. Calcium channel blockers have been associated with an increase in the suicide rate in Sweden (Lindberg et al 1998). Reserpine, a drug used for hypertension, acting through a known mechanism of depleting monoamines, has also been associated with depression. Other drugs that have been implicated include interferon, lipid lowering drugs, angiotensin converting enzyme inhibitors, digoxin, sedative hypnotics, and psychostimulants (Patten and Love 1994). As reviewed below, isotretinoin is the only non-psychotropic drug in the top 10 list of drugs most commonly reported to be associated with depression.
3.2.3 Evidence on an Association between Isotretinoin, Depression and Suicidality
Factors that assess the causative effects of a drug on an adverse event such as depression include: 1) class effect; 2) evidence from case reports and a review of the literature; 3) temporal association of drug administration and the event; 4) challenge/dechallenge (and rechallenge) cases; 5) dose response; and; 6) biological plausibility (2000a; Strom 2005). These factors as they relate to isotretinoin and depression and suicide are reviewed below.
3.2.3.1 Class Effect: Neuropsychiatric Effects of Vitamin A
If a drug in the same chemical class as isotretinoin had a similar side effect to the latter then this would provide evidence that isotretinoin was associated with this adverse event. Vitamin A is such a chemical, which is in the same retinoid class as isotretinoin. Because Vitamin A is the parent compound of RA increased amounts of this substrate would be expected to result in elevated levels of RA. Thus it would be predicted that many of the adverse side effects of isotretinoin will be similar to those of patients taking high doses of Vitamin A although, since it requires several metabolic steps for vitamin A to be converted to RA (Fig. 1), and several alternative pathways also exist into which vitamin A may be channeled, vitamin A would not be expected to be as potent as RA. Indeed, large doses of vitamin A can have a number of other neurological and mental effects including nausea, vomiting, weakness, fatigue, irritability, drowsiness, loss of appetite, ataxia, decreased interest, headache, and diplopia (double vision) (Bass 1959; Bendich and Langseth 1989; Fishbane et al 1995; Gerber et al 1954; Restak 1972; Rodahl and Moore 1943; Shaw and Niccoli 1953; Stimson 1961). These effects appear to be more common in children and adolescents (Lombaert and Carton 1976; Pasquariello et al 1977; Stimson 1961).
There have been multiple cases of psychiatric consequences of Vitamin A intoxication reported in the literature. These include cases of aggression and depression (Restak 1972), depression (McCance-Katz and Price 1992) and psychosis (Fishbane et al 1995; Haupt 1977).
Landy (Landy 1985) described the syndrome of pibloktoq, characterized by explosive outbursts in Arctic peoples. He attributed it to high exposure to Vitamin A through the diet of polar bear liver and the internal organs of other Arctic animals, which contain poisonous amounts of Vitamin A. Arctic explorers who ate polar bear liver developed symptoms of drowsiness, irritability, headaches, nausea, irrational and bizarre behavior, tingling, and in some cases delirium, within hours of ingestion. Repeated exposure to ingestion caused a repeat of the symptoms, showing a challenge/dechallenge effect (Landy 1985), although the rapidity of the effect implies that this is distinct from isotretinoin’s possible influence on depression.
Multiple case reports found benign intracranial hypertension, or pseudotumor cerebri, characterized by headache, papilledema, and normal CT/MRI and CSF, in children and adults exposed to high levels of vitamin A (Alemayehu 1995; Gerber et al 1954; Gomber and Chellani 1996; Lombaert and Carton 1976; Marie and See 1954; Oliver and Havener 1958; Seigel and Spackman 1972; Selhorst et al 1984; Spector and Carlisle 1984). Selhorst (Selhorst et al 1984) reported that 5/50 patients with “idiopathic” pseudotumor cerebri were found by dietary history to be ingesting large amounts of Vitamin A in their diet. These behavioral effects of vitamin A provide further hints that the retinoids may promote affective disorders.
Further evidence of class effect comes from other retinoids that are used therapeutically. Three cases of depression with the retinoid etretinate have been reported, one with a positive rechallenge (Henderson and Highet 1989). In addition, there is one report of suicidality associated with acitretin (Arican et al 2006).
3.2.3.2 Case Reports and Review of the Literature Related to Suicide and Depression with Isotretinoin
A significant number of case reports exist in the literature that describe depression, suicidality, psychosis, violence and aggression developing in conjunction with isotretinoin treatment (Bigby and Stern 1988; Bravard et al 1993; Byrne et al 1998; Byrne and Hnatko 1995a; Duke and Guenther 1993; Gatti and Serri 1991; Hazen et al 1983; Hull and D’Arcy 2003; Hull and D’Arcy 2004; Hull and D’Arcy 2005; Hull and Demkiw-Bartel 2000; La Placa 2005; Meyskens 1982; Middelkoop 1999; Robusto 2002; Scheinman et al 1990; Villalobos et al 1989). Some of the cases used the Hamilton Depression Scale (Ham-D), a clinician administered rating scale that quantitates the severity of depression. A score of greater than 15 is considered clinically significant depression. Most of the cases had a dose of 0.5-1 mg/kg/day, which for the typical 70 kg person translates into 40 or 80 mg per day, respectively, based on the available doses of isotretinoin. Byrne and colleagues (Byrne et al 1998; Byrne and Hnatko 1995a) reported on 3 cases of depression associated with isotretinoin use, none of whom had a family or personal prior psychiatric history. All were evaluated with the Hamilton Depression Scale and found to have clinically significant increases in depressive symptoms. Bravard, Krug, & Rzeznick (Bravard et al 1993) reported three cases in which isotretinoin administration was associated with the development of depression. One patient attempted suicide. Duke and Guenther (Duke and Guenther 1993) reported two cases of depression associated with isotretinoin administration.
Isotretinoin administration has also been associated with psychosis. Villalobos, Ellis, & Snodgrass (Villalobos et al 1989) reported on a case of a 16 year old boy treated with 40 mg of isotretinoin who developed hallucinations, paranoia, and incoherent speech. With discontinuation and treatment with antipsychotic medication the symptoms improved over a 1-month period. Barak and colleagues (Barak et al 2005) reported five cases of manic psychosis that developed following treatment with isotretinoin out of a group of 500 soldiers treated in a military dermatology clinic for acne.
These case reports provide cumulative evidence of an association between isotretinoin use and depression, suicidality, psychosis, violence and aggression (including impulsivity and irritability). Although no single case report in isolation is conclusive, in sum they provide accumulating evidence of an association, especially when interpreted in conjunction with the other causal factors outlined below.
Data from Case Series and Uncontrolled Clinical Trials
Five studies have assessed depression rates in clinical samples of isotretinoin treated patients. Hull and Demkiw-Bartel (Hull and Demkiw-Bartel 2000) studied 121 patients treated with isotretinoin and found evidence of depression in 5 (4%) that persisted throughout the course of treatment. Hazen et al (Hazen et al 1983) reported that 6 out of 110 (5.5%) patients with acne or related disorders treated with 1-2 mg/kg/day isotretinoin developed symptoms of depression, including depressed mood, crying spells, malaise, or forgetfulness, within two weeks of initiation of the medication. Bruno (Bruno et al 1984) reported on 94 patients treated with variable doses of isotretinoin and ten subjects with acne but not in treatment. Eleven percent of patients reported depression. Scheinman et al (Scheinman et al 1990) reported on 7 cases of depression associated with isotretinoin administration. These patients were part of a larger clinical trial of isotretinoin in 700 patients treated for cystic acne and other skin disorders. Ferahbas et al reported on 23 patients treated for acne with isotretinoin for 16 weeks at .5-1 mg/kg. There were no reports of depression or suicide and no change in mean score on the Montgomery-Asberg Depression Rating Scale (MADRS) (Ferahbas et al 2004).
Two uncontrolled clinical trials have been performed in patients treated with isotretinoin or antibiotics. Chia et al (Chia et al 2005) studied 132 subjects aged 12-19 years with moderate to severe acne who were treated with isotretinoin or antibiotic in a non-randomized non-blinded fashion; subjects were assessed for depression before and after treatment with the Center for Epidemiological Studies Depression Scale (CES-D). No differences in the CES-D scores were found after treatment between groups.
Ng et al (Ng and Schweitzer 2003) studied 215 patients treated in a non-randomized unblinded fashion with either 1 mg/kg/day of isotretinoin or antibiotic for acne. Although the authors reported no differences in self-reported depression, as measured by the Beck Depression Inventory, 5/174 accutane patients (versus 0/41 antibiotic) developed depression by self report, and dropped out of the study. This study suggests a rate of isotretinoin induced depression of about 3% versus none for antibiotic treatment.
Friedman et al (Friedman et al 2005) reported on mental health utilization in members of the Israel Defense Forces. Subjects were 18-21 years old and included 1,419 with acne treated with isotretinoin and 1102 with psoriasis who were not treated with the drug. Mental health services utilization over a 5 year period in isotretinoin versus psoriasis patients was 17.2% v 12.5% (p=.0003). There was also a significant increase in suicidal thoughts and suicide attempts (p=0.04). These findings were consistent with psychiatric side effects of isotretinoin.
Overall, these studies suggest a relationship between isotretinoin and depression. Estimates of the incidence of depression following treatment with isotretinoin in the four largest series range from 1% (Scheinman et al 1990) to 4% (Hull and Demkiw-Bartel 2000) to 6% (Hazen et al 1983) to 11% (Bruno et al 1984). Lower frequencies are probably found when studies are based only on self-reporting since it has been noted that patients report behavioral changes (impulsivity, decreased performance in school or work) less frequently than family members (Meyskens 1982). Nevertheless, the frequency of depression resulting from isotretinoin is low enough where epidemiological studies are required to be carefully performed with a large patient pool in a non-retrospective manner.
Retrospective Analyses of Databases
Four studies have used clinical databases to retrospectively assess the relationship between isotretinoin and depression. Hersom et al (Hersom et al 2003) conducted a prescription symmetry analysis in 2,281 patients identified retrospectively in a database as having taken isotretinoin or an antidepressant. They did not find that patients were more likely to receive an antidepressant after receiving isotretinoin. Jick and colleagues (Jick et al 2000) looked at 7,195 patients treated with isotretinoin and 13,700 oral antibiotic users with acne from the Saskatchewan Health Database and 340 isotretinoin and 676 antibiotic users from the United Kingdom General Practice Research Database. Prevalence of suicide, attempted suicide, and “neurotic and psychotic disorders” based on computer-recorded histories at 0.5 to 5 years before and more than 5 years after medication use were compared between the groups. The authors reported no increase in relative risk of newly diagnosed depression or psychosis (1.0) or suicide/attempted suicide (0.9) with isotretinoin administration. The United Health Care Study (Neary et al 2001) found a statistically significant increase in depression in isotretinoin users when depression was defined as the coding for diagnosis of depression and/or antidepressant prescription. In a study from Quebec of 18,183 subjects who received isotretinoin therapy, treatment with antidepressant and diagnosis of depression or hospitalization were assessed. Exposure to isotretinoin in the five months before the development of depression was compared to two previous five month control periods in the same subjects. Isotretinoin resulted in a statistically significant three fold increased adjusted risk for depression. Minocycline, an antibiotic used to treat acne, was not found to increase the risk of depression (Azoulay et al 2006).
In summary, two out of four studies based on databases found a relationship between isotretinoin use and depression. Studies that found a positive relationship are distinguished from the negative studies by using a definition of depression that included both antidepressant prescriptions as well as a diagnosis and/or hospitalization for depression. This more specific definition is more likely to prevent non-depressed patients from being included in the depressed group, thereby diluting the sample. Future studies will require the use of randomized controlled trials with adequate sample sizes and specific behavioral measures as outcome variables to assess the relationship between isotretinoin and depression as well as factors that could put individuals at risk for the development of depression with isotretinoin treatment.
Data from Public Governmental Agency Reports
Another potential source of information regarding the possible relationship between isotretinoin and depression and suicide is the reports of drug-related adverse events that are submitted to government agencies such as the FDA and the WHO. Such reports of drug-related adverse events are likely to underestimate the true frequency of these occurrences and are estimated to represent only 1-10% of the true incidence of the event (2000a). However, if the number of reports of adverse events is above a certain minimal frequency, it may provide evidence of a potential relationship, providing impetuous for further investigation. Also, one can compare the frequency of reporting of adverse events for a particular medication relative to other medications used for the treatment of the same disorder. For instance, since isotretinoin’s introduction in 1982, depression has been the 6th most common side effect of isotretinoin reported to the FDA (2000b). Isotretinoin ranked fourth in the top 10 of all drugs in the FDA database that were associated with risk of depression as a side effect (Wysowski et al 2001a).
A review of the adverse drug reactions (ADR) reported to the WHO, United Kingdom Medicines Control Agency (MCA) (1982-1998), and the manufacturer Roche was described by Middelkoop (Middelkoop 1999). Among patients treated for acne, isotretinoin was found to be associated with a much greater number of psychiatric adverse events and suicides than antibiotics, accounting for 60% of all acne treatment-related adverse psychiatric events in spite of the fact that antibiotics are prescribed more commonly than isotretinoin. The authors found 47 cases of suicide, 67 suicide attempts, and 56 reports of suicidal ideation reported to the WHO in the 1982-1998 time period (Middelkoop 1999). Psychiatric symptoms reported included depression, amnesia, anxiety, mood swings, insomnia and suicide.
Wysowski, Pitts, and Beitz (Wysowski et al 2001a) published a review of cases of isotretinoin-related depression and suicide that had been reported to the FDA between 1982 and 2000. During that time period, the FDA received a total of 431 ADRs: 37 reports of isotretinoin-treated patients who had committed suicide; 110 reports of isotretinoin-treated patients who had been hospitalized for depression, suicidal ideation, or suicide attempts; and 284 reports of patients with depression who had not been hospitalized. In the Adverse Events Reporting System (AERS) database isotretinoin ranked 4th and 5thof all drugs in the number of reports of depression and serious depression, respectively, and ranked 10th for suicide attempt, being the only nonpsychiatric drug in the top 10 for suicide attempt. The cases also showed evidence of a temporal relationship between administration of isotretinoin and the development of depression, i.e. when the medication was stopped and restarted, there was a remission of depression followed by an increase in depressive symptoms. The authors concluded that there were several lines of evidence to support a relationship between isotretinoin and depression.
From 1983-1999 the Canadian Adverse Drug Reaction Monitoring Program (CADRMP) received 16 reports describing depression and other reactions of a putative psychiatric nature associated with the use of isotretinoin (1999). Of 222 adverse events reported to Health Canada from 1983-2002, 56 (25%) involved psychiatric events including depression and suicidal ideation (Wooltorton 2003). From 1985 to 1998 the Australian Adverse Drug Reactions Committee received 129 adverse reactions, of which 12 involved depression (1998a). The Australian ADR Committee received 21 reports of suicidal attempts or ideation through June 2005, one of which was the subject of a Coronial investigation (2005). From 1983-1998 the Irish Medicine Boards received six reports of depression with isotretinoin, including one fatal suicide (1998b). From 1997-2001 the Danish Medicines Agency reported 34 psychic adverse reactions with isotretinoin, 23 with depression (2002).
In summary there are consistent reports of depression, suicide, psychosis and aggression associated with isotretinoin use from all countries that have made their adverse event reporting public. In addition, psychiatric adverse effects are consistently higher than for other treatments for acne, and tend to be higher than for most other medications, with the exception of psychotropics, which are now acknowledged to be associated with an increased risk of suicidality.
Improvement in Self-Image Resulting from Acne Treatment
Some have argued that treatment of acne with isotretinoin leads to an improvement in self image and therefore a reduction in depression. Studies have reported improvement in feelings of general well-being or self image (Cassileth et al 1982; Gupta et al 1990; Shuster et al 1978), or feelings of anxiety in patients with cystic acne following isotretinoin administration (Garrie and Garrie 1978; Gupta et al 1990; Jacobs et al 2001; Kellett and Gawkrodger 1999; Marqueling and Zane 2005; Medansky et al 1981; Rubinow et al 1987; Van der Meeren et al 1985). In general, these findings support the action of isotretinoin to improve the patient’s self-image and satisfaction with treatment, however these are not indicators of an absence of clinical symptoms of depression.
3.2.3.3 Temporal Association Between Isotretinoin Treatment and Depression and Suicide
One of the factors used to judge the relationship between a drug and its putative side effects is the temporal association between the administration of the drug and the presumed adverse effect. In most of the cases reported, patients were on isotretinoin for several weeks before the development of depression and suicidality. This suggests that the effects of isotretinoin are long term, rather than related to acute plasma concentrations. Many authors reported the onset of depression at one month after start of treatment (Bigby and Stern 1988; Bravard et al 1993; Bruno et al 1984; Byrne and Hnatko 1995a; Cott and Wisner 1999; Duke and Guenther 1993; Gatti and Serri 1991; Hazen et al 1983; Hull and D’Arcy 2003; Hull and D’Arcy 2004; Hull and D’Arcy 2005; Hull and Demkiw-Bartel 2000; La Placa 2005; Meyskens 1982; Middelkoop 1999; Ng et al 2001; Robusto 2002; Scheinman et al 1990; Villalobos et al 1989; Wysowski et al 2001b). Of the case series, Hazen (Hazen et al 1983) reported onset of depression at 2 weeks in the six patients in their paper, while all three of the other case series reported onset at one month (Bruno et al 1984; Hull and Demkiw-Bartel 2000; Scheinman et al 1990). Other authors reported an onset of symptoms at two months after start of treatment (Bigby and Stern 1988; Burket and Storrs 1987; Hepburn 1990; Lindemayr 1986). Most of the other cases developed depression or committed suicide at some point during treatment (2-4 months after onset of treatment) although specific details are not always given (Barak et al 2005; Bravard et al 1993; Byrne and Hnatko 1995a; Cotterill and Cunliff 1997; Ng et al 2002; Scheinman et al 1990; Wysowski et al 2001b). Of course it is always possible (especially in the suicide cases) that symptoms were not reported at the time they actually started, or that patients denied depression.
Overall, these cases imply a temporal relation between isotretinoin treatment and the development of depression and suicide. Symptoms usually begin after several weeks of treatment, with in some cases a longer delay. This delay in symptoms is similar to the time course of antidepressant response. Although antidepressant drugs are known to have an immediate effect on serotonin and norepinephrine concentrations in the brain, they take up to 1-2 months to work, implying that antidepressant action is an indirect effect on a long-term event such as an adaptive change in a neurotransmitter system or an increase in hippocampal neurogenesis. If isotretinoin promotes depression by acting in the opposite direction of the same mechanism then it would be expected to require an extended period of time to induce depression.
3.2.3.4 Cases of Challenge/Dechallenge Related to Isotretinoin and Depression and Suicide
Another factor important in assessing causality is the relationship of challenge/dechallenge. These are cases where a patient develops depression after starting the drug, and has a remission of symptoms when the drug is stopped. In some cases the drug is given again, or rechallenged, in which case the symptoms return. Challenge/dechallenge cases are considered to be adequate in and of themselves as evidence of a drug causing a particular adverse outcome (Strom 2005). Scheinman et al (Scheinman et al 1990) reported on 7 cases of depression associated with isotretinoin administration. One of the patients was rechallenged with isotretinoin and experienced a recurrence of symptoms 3 months after re-initiation of treatment. Several other authors have reported individual cases of abatement of symptoms of depression and/or suicidality after discontinuation of isotretinoin (Bigby and Stern 1988; Bravard et al 1993; Burket and Storrs 1987; Byrne and Hnatko 1995b; Duke and Guenther 1993; Ng et al 2002). For instance, Barak reported that 4/5 patients who developed psychosis after receiving isotretinoin had an improvement in symptoms with cessation of isotretinoin and treatment with antipsychotics (Barak et al 2005). Ng et al (Ng et al 2001) reported a case of a 17 year old man who developed symptoms of depression two weeks after starting isotretinoin therapy. The symptoms improved with reduction of isotretinoin dose and treatment with sertraline. Villalobos, Ellis, & Snodgrass (Villalobos et al 1989) reported a case of isotretinoin administration associated with hallucinations, paranoia, and incoherence. The behavior stopped with discontinuation and re-started with re-administration of the medication. Wysowski, Pitts, and Beitz (Wysowski et al 2001a) published a review of cases of isotretinoin-related depression and suicide that had been reported to the FDA between 1982 and 2000. They described several cases of positive rechallenged with re-administration of isotretinoin. Pitts reported (2000a) 41 cases that had been reported to the FDA of positive challenge/dechallenge/rechallenge. Seventy six percent were without psychiatric history, the median recovery time was 4.5 days, and the time to onset of depression was shorter for the second course of treatment.
In summary the challenge/dechallenge cases are perhaps the most compelling evidence for an association between isotretinoin and psychiatric side effects. In the cases of repeated on-off cycles of medication the fact that this is due to chance alone is highly unlikely.
3.2.3.5 Dose Response between Isotretinoin and Depression and Suicide
Dose response, another factor used in the assessment of causality, refers to the concept that higher doses of the drug will lead to a greater frequency of the adverse event. As evidence of dose response, Meyskens (Meyskens 1982) reported that 25% of his patients receiving doses of 3 mg/kg/day of isotretinoin (3-6 time higher than the dose used for acne) developed symptoms of depression (i.e. much higher than the 3-4% reported in most other series). Evidence also comes from cases where symptoms of depression recede when the dose of isotretinoin is reduced. Pitts reported six cases where symptoms of depression resolved when the dose of isotretinoin was reduced, leading her to conclude that a “possible dose response” was observed in these patients (2000a). Ng et al (Ng et al 2001) reported a case in which symptoms of depression with isotretinoin improved with reduction of isotretinoin dose and treatment with sertraline. Bruno compared acne patients taking high dose isotretinoin (0.75-1.21 mg/kg/day) to low dose (0.75-1.21 mg/kg/day). Symptoms of depression were higher in high dose versus low dose.
4. Mechanisms By Which Retinoids May Mediate Affective Disorders
If retinoids modulate symptoms of affective disorders, what is the mechanism? As described in section 3.2, there is evidence that isotretinoin has neurological side effects and is associated with depression. RA has a variety of effects on brain neurochemical systems believed to be involved in depression, in particular dopamine (section 4.2) but also serotonin via induction of the serotonin 1A receptor (Charest et al 1993) and norepinephrine through the transporter for this neurotransmitter (Matsuoka et al 1997).
The regions of RA activity; limbic brain areas, including the hippocampus, prefrontal cortex, and striatum, are all areas that been hypothesized to play a role in depression (Bremner 2002; George et al 1994). Modulation by RA of the key neurotransmitters involved in depression in those brain regions associated with this syndrome means that RA has the potential to influence depression. These mechanisms are reviewed below.
4.1 Neural Circuitry of Depression
The neural circuitry of depression, as established through brain imaging studies, has a high degree of overlap with the neural circuitry of RA. As discussed in sections 2.3-2.5, this circuitry includes the striatum (Austin et al 1992; Baxter et al 1985; Biver et al 1994; Buchsbaum et al 1984; Drevets and Raichle 1992; Hurwitz et al 1990; Mayberg 1994; Mayberg et al 1994), hippocampus (Bremner et al 2004; Sheline et al 1996) and prefrontal cortex. The medial prefrontal cortex (including orbitofrontal cortex and anterior cingulate) has been shown through functional imaging studies to be involved in depression (Bench et al 1990; Dolan and Friston 1989; George et al 1994). Studies have shown that the medial prefrontal cortex plays an important role in modulating emotion in normal human subjects as demonstrated by a number of paradigms for induction of emotional states (Benkelfat et al 1995; Drevets et al 1997; George et al 1995; Lane et al 1997; Reiman et al 1997). Multiple PET and SPECT studies have found decreased metabolism or blood flow at baseline in depressed patients in medial prefrontal cortex (including orbitofrontal cortex, inferior frontal cortex and anterior cingulate) (Austin et al 1992; Bench et al 1992; Buchsbaum et al 1986; Drevets et al 1997; Hurwitz et al 1990; Martinot et al 1990; Mayberg 1994; Mayberg et al 1997; Sackeim et al 1990; Scott et al 1994). PET studies in patients with Huntington’s or Parkinson’s disease and comorbid depression have also shown decreased metabolism and/or blood flow in the prefrontal cortex, including orbitofrontal cortex and anterior cingulate (Mayberg et al 1992; Mayberg et al 1990; Ring et al 1994). Induction of depressive relapse in patients with treated depression in remission results in decreased function in the orbitofrontal cortex (Bremner et al 1997; Bremner et al 2003), while improvement in depression has been associated with an increase in medial prefrontal metabolism (Bench et al 1995).
4.2 Relationship between RA and Dopamine
The dopaminergic system is under particular influence of RA and has been hypothesized to play a role in dysregulation of mood and emotion (Jentsch et al 2000). The regulation of the dopaminergic system by RA has been studied in the embryo and in progenitor cell lines. From such analysis RA is able to induce a series of proteins that transduce the dopamine signal including DARPP-32, the D1 dopamine receptor, the Golf olfactory G protein, and adenylyl cyclase type V (Toresson et al 1999b; Wang and Liu 2005). Clearly RA can promote dopaminergic signaling in the embryo, but the influence of RA on the adult brain is uncertain. Null mutation of RA receptors results in a decrease in dopamine signaling however this effect may result from lack of embryonic development of the dopamine signaling system (Krezel et al 1998). If the effects of RA in the adult are similar to that in the embryo then this indicates that RA influences a system associated with depression; however the direction of its effect on gene expression within the dopaminergic system is the opposite to what would be expected for an agent that promotes depression. The dopamine hypothesis of depression proposes that a decline in dopaminergic activity is central to the etiology of depression (Diehl and Gershon 1992) whereas RA increases expression of genes involved in dopamine signal transduction. The more general monoamine hypothesis proposes that depletion of one or more neurotransmitters in this class (dopamine, norepinephrine or serotonin) is a key factor in the pathophysiology of depression (Delgado 2000). RA could decrease norepinephrine in the short term by virtue of its induction of the norepinephrine receptor (Matsuoka et al 1997) which terminates noradrenergic neurotransmission through reuptake of the neurotransmitter. RA may also potentially regulate the human serotonin transporter gene, acting in a region that includes variable number tandem repeats (VNTR) whose polymorphism has been associated with a predisposition to affective disorders (Fiskerstrand et al 1999). The VNTR contains motifs that are potential RA response elements although this paper did not show a direct interaction between the RA receptor and the VNTR.
The crucial aspect to note, however, is that the effects of 13-cis RA on depression are not immediate but require at least several weeks (see section 3.2.3.3). It may be concluded from this that any effect of 13-cis RA is not via a direct influence on gene expression but must be a secondary effect that accumulates over time. Such an effect may be complex and may involve, for instance, initial induction of the dopaminergic system resulting, over time, in negative feedback and a long-term decline in some elements of dopaminergic transmission. It is of interest that in post-mortem brains from victims of antidepressant-treated suicides, the D2 dopamine receptor, rather than declining as might be expected from the “dopamine hypothesis” of depression, instead is higher in number compared to matched controls (Bowden et al 1997)). The D2 dopamine receptors however are lower in ligand affinity. The D2 dopamine receptor gene is a central target of RA given that it contains a RA response element within its promoter (Samad et al 1997) and is inducible in both the rat and the mouse (Valdenaire et al 1998; Wolf 1998). It is possible in the adult that induction of D2 dopamine receptor by RA may also have a longer-term effect of expression of greater number of receptors with lower affinity. Such results suggests that long-term manipulation of the dopaminergic (as well as other neurotransmitter) systems may have unexpected and unintended effects.
4.3 RA and the Hippocampus
A second mechanism by which RA may contribute to depression is through inhibition of neurogenesis in the hippocampus. As noted above, the treatment of mice with 13-cis RA results in a decrease in hippocampal neurogenesis (Crandall et al 2004; Misner et al 2001; Sakai et al 2004) and decreased hippocampal neurogenesis has been proposed to contribute to the development of depression (Duman 2004). Exposure to stress (which is used as an animal model of depression) is associated with a decrease in neurogenesis, while a deprived environment, which is used as a model of childhood neglect and is linked to depression, also results in a decrease in neurogenesis (Kempermann et al 1997; Kempermann et al 1998). This contrasts with antidepressant treatment or exercise (which has an anti-depressant effect (Blumenthal et al 1999)), which lead to an improvement in depression-like behaviors and an increase in neurogenesis (Chen et al 2000; Coyle and Duman 2003; D’Sa and Duman 2002; Duman 2004; Garcia 2002; Jacobs 2002; Kempermann 2002; Malberg et al 2000; Santarelli et al 2003; van Praag et al 1999). If 13-cis RA has the reverse effect to these antidepressants, reducing neurogenesis, this provides a plausible mechanism by which isotretinoin may contribute to depression. However, it is not clear whether neurogenesis plays a primary or secondary role in mediating depression, which continues to be a source of debate (Duman 2004; Henn and Vollmayr 2004).
A second irregularity in the hippocampus associated with depression is a reduction in hippocampal volume, with negative correlations between smaller volume and clinically relevant outcomes such as number of hospitalization for depression and number of lifetime days with depression (Bremner 2002; Bremner et al 2000; Mervaala et al 2000; Sheline et al 1999; Sheline et al 1996; Steffens et al 2000; Steffens and Krishnan 1998; Vakili et al 2000). Although an association has been suggested between a reduction in hippocampal volume and a decline in neurogenesis (Sheline 2003) the small number of neurons generated in the hippocampus is very unlikely to be able to influence the total volume of the hippocampus and thus an alternative mechanism is likely to be involved. However, 13-cis RA may well impact human hippocampal volume via such an alternative mechanism because, in the mouse, 13-cis RA has been demonstrated to reduce hippocampal volume after 3 weeks of treatment (McCaffery et al 2006).
4.4 RA and the Frontal Cortex
The orbitofrontal cortex is another brain region that may mediate the effects of RA on symptoms of depression and the mammalian frontal cortex is one of the few regions of the adult brain where RA is synthesized (Wagner et al 2006). Human subjects with lesions of the medial prefrontal cortex (including orbitofrontal cortex) such as the famous case of Phineas Gage, who had a railroad spike pass through these areas of medial prefrontal cortex, show dysfunction of normal emotions and an inability to relate in social situations that require correct interpretation of the emotional expressions of others, while speech and cognition are intact (Damasio et al 1994). Patients with orbitofrontal damage also have impairments in impulse control with increased violent aggression and impairments in emotional regulation (Bechara et al 1994; Berlin et al 2004). Animals with lesions of the medial prefrontal cortex have an impaired ability to regulate emotional responding (Morgan and LeDoux 1995; Morgan et al 1993). Patients with depression were found to have a loss of glia and/or neurons in the orbitofrontal cortex (Rajkowska et al 1999) and other related parts of the medial prefrontal cortex, including subgenual cortex (Ongur et al 1998). MRI brain imaging studies showed smaller volume of the orbitofrontal cortex in patients with depression (Bremner et al 2002; Lacerda et al 2004; Lai et al 2000; Lee et al 2003) in addition to decreased orbitofrontal function with depressive relapse (Bremner et al 1997; Bremner et al 2003).
It is probable that the orbitofrontal cortex, which has both input and output connections to the hippocampus, plays a critical role in the effects of RA on the brain. This cortical region is another area in which dopamine has been tied to depression (specifically mesolimbic pathways) (Jentsch et al 2000) and D2 dopamine receptor expression in the prefrontal cortex may be a target for RA (Vincent et al 1995). The hippocampus modulates dopaminergic function in the medial prefrontal cortex (Peleg-Raibstein et al 2005) and deficits in hippocampal function lead to a downstream effect on orbitofrontal function (Jay et al 2004; Rocher et al 2004). Based on these findings alterations in hippocampal-medial prefrontal function have been hypothesized to play a role in neuropsychiatric symptoms (Peleg-Raibstein et al 2005). Retinoids may lead to a decrease in orbitofrontal function via their effect on the hippocampus. Clinically, many patients on isotretinoin exhibit signs of behavioral dysinhibition and affective lability that suggest orbitofrontal dysfunction. Brain imaging studies described below are in fact consistent with decreased orbitofrontal function with isotretinoin administration. Therefore retinoids may mediate dysregulation in hippocampal-orbitofrontal function that contributes to symptoms of depression.
The effect of isotretinoin on brain function was assessed in acne patients. Twenty-eight subjects completed four months of treatment with 13-cis RA or antibiotic with PET FDG imaging of brain metabolism and assessment of depression with the Hamilton Depression Scale (Ham-D) before and after treatment. Thirteen subjects were treated with 13-cis RA and 15 with antibiotics. All subjects suffered from acne. Administration of isotretinoin (but not antibiotic) was associated with a 16% decrease in brain metabolism in the orbitofrontal cortex after four months of treatment (Bremner et al 2005).
Five patients treated with isotretinoin had symptoms of headache. These patients also had subtle changes in irritability and/or mood as assessed by self, family, or the research staff. These subjects all had decreases in orbitofrontal brain metabolism with isotretinoin administration. A representative subject is shown in Fig. 2. One subject in the isotretinoin group and one in the antibiotic group had a clinically significant increase in depression as measured by the Ham-D score (greater than 9 point increase); however there were no significant increases in Ham-D scores in the groups as a whole or differences between groups. There were no correlations between the Ham-D and brain function. Thus, although as expected from a study of a small population of isotretinoin treated patients there was no overall rise in depression, there was a significant change in rate in metabolism in a brain region (orbitofrontal cortex) that is a component in the neural circuitry that contributes to depression. It may be conjectured that isotretinoin may interfere with function in this and perhaps other regions leading to increased susceptibility to depression in some individuals.
Figure 2.
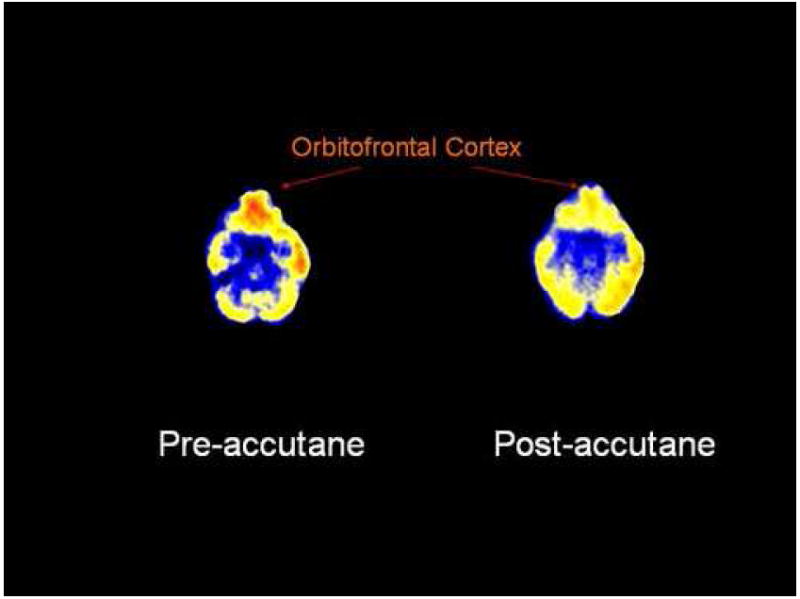
PET FDG measurement of brain metabolism before and after treatment with isotretinoin. There is a visible decrease in function of the orbitofrontal cortex in this representative patient after four months of treatment with isotretinoin. This patient developed headache and subtle behavioral changes, but not clinical depression.
5. Discussion
This paper has reviewed the evidence for a link between retinoids and affective disorders. A number of lines of evidence are consistent with such a relationship. This evidence is as follows:
Preclinical and clinical results suggest that either an excess or deficiency of RA is associated with behavioral changes that include symptoms of depression.
Studies in animal models, in particular the mouse, indicate that exposure to 13-cis RA results in depressive-like behavior and well as a decline in learning and memory.
RA can enter the brain and directly modulate neurotransmitter systems hypothesized to mediate symptoms of depression, in particular the dopaminergic pathways but also, to a limited extent, serotonin and norepinephrine pathways. Further, RA can act on neurotransmitters in brain regions associated with depression including the hippocampus and striatum.
The prefrontal cortex is a third region influenced by RA, in which isotretinoin treatment in the human results in a decline in metabolism.
These results strongly suggest a link between 13-cis RA and depression; however the association is likely to be highly complex and the results are not yet sufficiently detailed to provide a precise model of how 13-cis RA may engender depression. Nevertheless, they provide an initial guide to the systems that may be affected. 13-cis RA may not act on a single system but have multiple depressogenic actions, including a dysregulation of neurotransmitters in striatum and hippocampus (in particular the dopaminergic system), suppression of hippocampal neurogenesis and interference with prefrontal cortex function. The exact action of RA in these systems will require further research and it is probable that there will be an interrelationship between several of these actions. One likely example of this cross-talk is in the pathway between the hippocampus and prefrontal cortex that has been proposed to be impaired in depression — this impairment occurring through loss of dopamine or stress, degrading plasticity of the hippocampal-prefrontal synapses (Jay et al 2004). The possible importance of RA in regulating neural plasticity as well as dopamine signaling suggests an action of RA on this integrative pathway.
13-cis RA operates directly on the brain because it acts on an endogenous system of RA signaling. As already described, 13-cis RA acts via the RA receptors by virtue of its isomerization to all-trans RA in tissues (Shih et al 1986; Tsukada et al 2000) and the detrimental effects of 13-cis RA occur because of its presence in the brain at an inappropriate concentration, time or place. If exposure to 13-cis RA results in depression through its interference with the set of neurotransmitters and molecular networks that protect against depression, then this implies that endogenous RA may normally regulate these same neural processes. The corollary of this is that, just as an excess of RA may promote depression, a decline in RA signaling may advance this condition. Such a fall in RA signaling may occur as a result of vitamin A deficiency, mutation in genes required for the signal (including RA receptors and binding proteins) or decline in RA receptor expression with age (Etchamendy et al 2001). In such cases RA or other retinoids will be protective and there may be conditions where retinoids have a therapeutic role in depression. This may be true for other neuropsychiatric disorders, for instance vitamin A deficiency results in a decline in learning and memory (Etchamendy et al 2003{Cocco, 2002 #6082)} which could be attenuated by retinoid treatment. Retinoids have also been suggested as a possible treatment of schizophrenia in particular for the negative symptoms of this disorder (Sharma 2005) which overlap with the symptoms of depression.
A number of important future approaches arise from these results. RA signaling within the orbitofrontal cortex has yet to be examined in animal models. An important question will be whether primary alterations in RA signaling in the orbitofrontal cortex mediate symptoms of depression, or whether a primary alteration in the hippocampus leads to downstream changes in orbitofrontal function. Studies are also required to assess the effects of clinical administration of RA on the human brain and behavior. Given the likely effects of isotretinoin on prefrontal cortical function as well as the hippocampus, behavioral symptoms in addition to depression should be investigated, including impulsivity and aggression, as well as cognitive assessments of learning and memory. These studies can be performed in a properly controlled and cost-effective way and are essential to understand the potential involvement of RA in the pathophysiology of depression, as well as to develop strategies to provide a safe and effective route of RA administration in the treatment of skin disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- International Union of Nutritional Sciences, Committee 1/I, Nomenclature: Generic descriptors and trivial names for vitamins and related compounds. Recommendations 1976. Nutritional Abstracts Review Series A. 1978;48:831–835. [Google Scholar]
- Australian Adverse Drug Reactions Committee: Depression with isotretinoin. Australian Adverse Drug Reactions Bulletin. 1998a:17. [Google Scholar]
- Irish Medicines Board: Drug Safety Newsletter 1998b [Google Scholar]
- Isotretinoin and depression. Canadian Adverse Drug Reaction Newsletter. 1999:9. [Google Scholar]
- Food and Drug Administration Center for Drug Evaluation and Research. Meeting of the Dermatologic and Opthalmic Drugs Advisory Committee. September 19 2000; Dermatologic and Opthalmic Drugs Advisory Committee; Washington, D.C.. 2000a. [Google Scholar]
- 2000b;2005 http://www.fda.gov/ohrms/dockets/ac/00/backgrd/3639b1c_05.pdf.
- Danish Medicines Agencies: Psychic adverse reactions to the use of the acne medication isotretinoin. DKMA 2002 [Google Scholar]
- Australian Adverse Drug Reactions Committee. Acne, isotretinoin, and suicidality. Australian Adverse Drug Reactions Bulletin. 2005:24. [Google Scholar]
- Alemayehu W. Pseudotumor cerebri (Toxic effect of the “magic bullet”) Ethiopian Medical Journal. 1995;33:265–270. [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- APA. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C.: American Psychiatric Press; 2000. [Google Scholar]
- Arican O, Sasmaz S, Ozbulut O. Increased suicidal tendency in a case of psoriasis vulgaris under acitretin treatment. Journal of the European Academy of Dermatology & Venereology. 2006;20:464. doi: 10.1111/j.1468-3083.2006.01447.x. [DOI] [PubMed] [Google Scholar]
- Asson-Batres MA, Zeng MS, Savchenko V, Aderoju A, McKanna J. Vitamin A deficiency leads to increased cell proliferation in olfactory epithelium of mature rats. J Neurobiol. 2003;54:539–554. doi: 10.1002/neu.10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MP, Dougall N, Ross M, Murray C, O’Carroll RE, Moffoot A, et al. Single photon emission tomography with 99mTc-exametazime in major depression and the pattern of brain activity underlying the psychotic/neurotic continuum. J Affect Disord. 1992;26:31–43. doi: 10.1016/0165-0327(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Azoulay L, Blais L, Berard A. Isotretinoin and the risk of depression in patients with acne: A case-crossover study. Pharmacoepidemiology and Drug Safety. 2006;15:S261. doi: 10.4088/jcp.v69n0403. [DOI] [PubMed] [Google Scholar]
- Barak Y, Wohl Y, Greenberg Y, Bar Dayan Y, Friedman T, Shoval G, et al. Affective psychosis following Accutane (isotretinoin) treatment. Int Clin Psychopharmacol. 2005;20:39–41. doi: 10.1097/00004850-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Bass MH. Journal of the Mount Sinai Hospital. Vol. 26. New York: 1959. The relation of Vitamin A intake to cerebrospinal fluid pressure: A review; pp. 421–423. [PubMed] [Google Scholar]
- Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Phelps ME, Mazziotta JC, Schwartz JM, Gerner RH, Selin CE, et al. Cerebral metabolic rates for glucose in mood disorders. Arch Gen Psychiatry. 1985;42:441–447. doi: 10.1001/archpsyc.1985.01790280019002. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Dolan RJ, Friston KJ, Frackowiak RSJ. Positron emission tomography in the study of brain metabolism in psychiatric and neuropsychiatric disorders. Br J Psychiatry. 1990;157:82–95. [PubMed] [Google Scholar]
- Bench CJ, Frackowiak RSJ, Dolan RJ. Changes in regional cerebral blood flow on recovery from depression. Psychol Med. 1995;25:247–251. doi: 10.1017/s0033291700036151. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RSJ, Dolan RJ. The anatomy of melancholia: focal abnormalities of cerebral blood flow in major depression. Psychol Med. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- Bendich A, Langseth L. Safety of Vitamin A. Am J Clin Nutr. 1989;49:358–371. doi: 10.1093/ajcn/49.2.358. [DOI] [PubMed] [Google Scholar]
- Benkelfat C, Bradwejn J, Meyer E, Ellenbogen M, Milot S, Gjedde A, et al. Functional neuroanatomy of CCK sub 4-induced anxiety in normal healthy volunteers. Am J Psychiatry. 1995;152:1180–1184. doi: 10.1176/ajp.152.8.1180. [DOI] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Bigby M, Stern RS. Adverse reactions to isotretinoin. J Am Acad Dermatol. 1988;18:543–552. doi: 10.1016/s0190-9622(88)70078-x. [DOI] [PubMed] [Google Scholar]
- Biver F, Goldman S, Delvenne V, Luxen A, De Maertelaer V, Hubain P, et al. Frontal and parietal metabolic disturbances in unipolar depression. Biol Psychiatry. 1994;36:381–388. doi: 10.1016/0006-3223(94)91213-0. [DOI] [PubMed] [Google Scholar]
- Blaner WS. Cellular metabolism and actions of 13-cis-retinoic acid. J Am Acad Dermatol. 2001;45:S129–135. doi: 10.1067/mjd.2001.113714. [DOI] [PubMed] [Google Scholar]
- Blomhoff R. Introduction: Overview of Vitamin A Metabolism and Function. In: Blomhoff R, editor. Vitamin A in Health and Disease. New York: Marcel Dekker; 1994. pp. 1–35. [Google Scholar]
- Blumenthal JA, Bayak MA, Moore KA. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- Bowden C, Theodorou AE, Cheetham SC, Lowther S, Katona CL, Crompton MR, et al. Dopamine D1 and D2 receptor binding sites in brain samples from depressed suicides and controls. Brain Res. 1997;752:227–233. doi: 10.1016/s0006-8993(96)01460-6. [DOI] [PubMed] [Google Scholar]
- Bravard P, Krug M, Rzeznick JC. Isotretinoine et depression: soyons vigilants. Nouvelle Dermatology. 1993;12:215. [Google Scholar]
- Bremner JD. Structural changes in the brain in depression and relationship to symptom recurrence. CNS Spectrums. 2002;7:129–139. doi: 10.1017/s1092852900017442. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Fani N, Ashraf A, Votaw JR, Brummer ME, Cummins T, et al. Functional brain imaging alterations in acne patients treated with isotretinoin. Am J Psychiatry. 2005;162:983–991. doi: 10.1176/appi.ajp.162.5.983. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Salomon RM, Staib L, Ng CK, Miller HL, et al. PET measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Arch Gen Psychiatry. 1997;54:364–374. doi: 10.1001/archpsyc.1997.01830160092012. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller H, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–117. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Ng CK, Vermetten E, Nazeer A, Oren D, et al. Regional brain metabolic correlates of positron emission tomographic measurement of alpha-methylparatyrosine-induced depressive symptoms: Implications for the neural circuitry of depression. JAMA. 2003;289:3125–3134. doi: 10.1001/jama.289.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Vaccarino LV, Charney DS. Deficits in hippocampal and anterior cingulate function during verbal declarative memory encoding in mid-life depression. Am J Psychiatry. 2004;161:637–645. doi: 10.1176/appi.ajp.161.4.637. [DOI] [PubMed] [Google Scholar]
- Bruno NP, Beacham BE, Burnett JW. Adverse effects of isotretinoin therapy. Cutis. 1984;33:484–489. [PubMed] [Google Scholar]
- Buchsbaum MS, DeLisi LE, Holcomb H, Cappelletti J, King AC, Johnson J, et al. Anteroposterior gradients in cerebral glucose use in schizophrenia and affective disorders. Arch Gen Psychiatry. 1984;41:1159–1166. doi: 10.1001/archpsyc.1984.01790230045007. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, DeLisi LE, Holcomb H, Kessler RC, Johnson J, King AC, et al. Frontal cortex and basal ganglia metabolic rates assessed by positron emission tomography with [F-18] 2-deoxyglucose in affective illness. J Affect Disord. 1986;10:137–152. doi: 10.1016/0165-0327(86)90036-4. [DOI] [PubMed] [Google Scholar]
- Burket JM, Storrs FJ. Nodulocystic infantile infantile acne occurring in a kindred of steatocystoma. Arch Dermatol. 1987;123:432–33. [PubMed] [Google Scholar]
- Byrne A, Costello M, Greene E, Zibin T. Isotretinoin therapy and depression-evidence for an association. Irish J Psychosom Med. 1998;15:58–60. [Google Scholar]
- Byrne A, Hnatko G. Depression associated with isotretinoin therapy [letter] Can J Psychiat. 1995a;40:567. doi: 10.1177/070674379504000915. [DOI] [PubMed] [Google Scholar]
- Byrne A, Hnatko G. Depression associated with isotretinoin therapy. Can J Psychiat. 1995b;40:567. doi: 10.1177/070674379504000915. [DOI] [PubMed] [Google Scholar]
- Cassileth BR, Lusk EJ, Tenaglia AN. A psychological comparison of patients with malignant melanoma and other dermatologic disorders. J Am Acad Dermatol. 1982;7:742–746. doi: 10.1016/s0190-9622(82)70155-0. [DOI] [PubMed] [Google Scholar]
- CDC. Suicide Rates. 2006;2007 [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb Journal. 1996;10:940–954. [PubMed] [Google Scholar]
- Charest A, Wainer BH, Albert PR. Cloning and differentiationinduced expression of a murine serotonin1A receptor in a septal cell line. J Neurosci. 1993;13:5164–5171. doi: 10.1523/JNEUROSCI.13-12-05164.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- Chia CY, Lane W, Chibnall J, Allen A, Siegfried E. Isotretinoin therapy and mood changes in adolescents with moderate to severe acne. Arch Dermatol. 2005;141:557–560. doi: 10.1001/archderm.141.5.557. [DOI] [PubMed] [Google Scholar]
- Chiang MY, Misner D, Kempermann G, Schikorski T, Giguere V, Sucov HM, et al. An essential role for retinoid receptors RAR beta and RXR gamma in long- term potentiation and depression. Neuron. 1998;21:1353–1361. doi: 10.1016/s0896-6273(00)80654-6. [DOI] [PubMed] [Google Scholar]
- Coberly S, Lammer E, Alashari M. Retinoic acid embryopathy: case report and review of literature. Pediatrics, Pathology and Laboratory Medicine. 1996;16:823–836. [PubMed] [Google Scholar]
- Cocco S, Diaz G, Stancampiano R, Diana A, Carta M, Curreli R, et al. Vitamin A deficiency produces spatial learning and memory impairment in rats. Neuroscience. 2002;115:475–582. doi: 10.1016/s0306-4522(02)00423-2. [DOI] [PubMed] [Google Scholar]
- Colbert MC, Linney E, LaMantia AS. Local sources of retinoic acid coincide with retinoid-mediated transgene activity during embryonic development. Proc Natl Acad Sci USA. 1993;90:6572–6576. doi: 10.1073/pnas.90.14.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran J, Maden M. Nerve growth factor acts via retinoic acid synthesis to stimulate neurite outgrowth. Nat Neurosci. 1999;2:307–308. doi: 10.1038/7214. [DOI] [PubMed] [Google Scholar]
- Cott AD, Wisner KL. Isotretinoin treatment of a woman with bipolar disorder. J Clin Psychiatry. 1999;60:407–408. doi: 10.4088/jcp.v60n0611a. [DOI] [PubMed] [Google Scholar]
- Cotterill JA, Cunliff WJ. Suicide in dermatological patients. Brit J Dermatol. 1997;137:246–250. doi: 10.1046/j.1365-2133.1997.18131897.x. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38:157–160. doi: 10.1016/s0896-6273(03)00195-8. [DOI] [PubMed] [Google Scholar]
- Crandall J, Sakai Y, Zhang J, Koul O, Mineur Y, Crusio WE, et al. 13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc Natl Acad Sci USA. 2004;101:5111–5116. doi: 10.1073/pnas.0306336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliff WJ, van der Kerkhof PCM, Caputo R, Caicchini S, Cooper A, Fyrand OL, et al. Roaccutane treatment guidelines: Results of an international survey. Pharm Treatment. 1997;194:351–357. doi: 10.1159/000246134. [DOI] [PubMed] [Google Scholar]
- D’Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disorder. 2002;4:183–194. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: Clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry. 2000;61:S7–11. [PubMed] [Google Scholar]
- Diehl DJ, Gershon S. The role of dopamine in mood disorders. Compr Psychiat. 1992;33:115–120. doi: 10.1016/0010-440x(92)90007-d. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Friston KJ. Positron emission tomography in psychiatric and neuropsychiatric disorders. Seminars in Neurology. 1989;9:330–337. doi: 10.1055/s-2008-1041342. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JRJ, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Neuroanatomical circuits in depression: Implications for treatment mechanisms. Psychopharmacol Bull. 1992;28:261–274. [PubMed] [Google Scholar]
- Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. European Journal of Biochemistry. 2000;267:4315–4324. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- Duke EE, Guenther L. Psychiatric reactions to the retinoids. Can J Dermatol. 1993;5:467. [Google Scholar]
- Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Kramer M, Anthony JC, Dryman A, Shapiro S, Locke BZ. The incidence of specific DIS/DSM-III mental disorders: data from the NIMH Epidemiologic Catchment Area Program. Acta Psychiatr Scand. 1989;79:163–178. doi: 10.1111/j.1600-0447.1989.tb08584.x. [DOI] [PubMed] [Google Scholar]
- Eckhoff C, Nau H. Identification and quantitation of all-trans- and 13-cis-retinoic acid and 13-cis-4-oxoretinoic acid in human plasma. Journal of Lipid Research. 1990;31:1445–1454. [PubMed] [Google Scholar]
- Etchamendy N, Enderlin V, Marighetto A, Pallet V, Higueret P, Jaffard R. Vitamin A deficiency and relational memory deficit in adult mice: relationships with changes in brain retinoid signalling. Behavioral Brain Research. 2003;145:37–49. doi: 10.1016/s0166-4328(03)00099-8. [DOI] [PubMed] [Google Scholar]
- Etchamendy N, Enderlin V, Marighetto A, Vouimba RM, Pallet V, Jaffard R, et al. Alleviation of a selective age-related relational memory deficit in mice by pharmacologically induced normalization of brain retinoid signaling. J Neurosci. 2001;21:6423–6429. doi: 10.1523/JNEUROSCI.21-16-06423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferahbas A, Turan MT, Esel E, Utas S, Kutlugun C, Kilic CG. A pilot study evaluating anxiety and depressive scores in acne patients treated with isotretinoin. Journal of Dermatological Treatment. 2004;15:153–157. doi: 10.1080/09546630410027472. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Cisneros FJ, Goug BJ, Ali SF. Four weeks of oral isotretinoin treatment causes few signs of general toxicity in male and female Sprague-Dawley rats. Food and Chemical Toxicology. 2005a;43:1289–1296. doi: 10.1016/j.fct.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Cisneros FJ, Gough B, Hanig JP, Berry KJ. Chronic oral treatment with 13-cis-retinoic acid (isotretinoin) or all - trans - retinoic acid does not alter depression-like behaviors in rats. Toxicological Sciences. 2005b;87:451–459. doi: 10.1093/toxsci/kfi262. [DOI] [PubMed] [Google Scholar]
- Fishbane S, Frei GL, Finger M, Dressler R, Silbiger S. Hypervitaminosis A in two hemodialysis patients. Am J Kidney Dis. 1995;25L:346–349. doi: 10.1016/0272-6386(95)90020-9. [DOI] [PubMed] [Google Scholar]
- Fiskerstrand CE, Lovejoy EA, Quinn JP. An intronic polymorphic domain often associated with susceptibility to affective disorders has allele dependent differential enhancer activity in embryonic stem cells. Federation of European Biochemical Societies Letters. 1999;458:171–174. doi: 10.1016/s0014-5793(99)01150-3. [DOI] [PubMed] [Google Scholar]
- Friedman T, Wohl Y, Knobler HY, Lubin G, Brenner S, Levi Y, et al. Increased mental health services related to isotretinoin treatment: A 5-year analysis. Eur Neuropsychopharm. 2005 doi: 10.1016/j.euroneuro.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Garcia R. Stress, metaplasticity, and antidepressants. Current Molecular Medicine. 2002;2:629–638. doi: 10.2174/1566524023362023. [DOI] [PubMed] [Google Scholar]
- Garrie SA, Garrie EV. Anxiety and skin diseases. Cutis. 1978;22:205–208. [PubMed] [Google Scholar]
- Gatti S, Serri F. Acute depression from isotretinoin. J Am Acad Dermatol. 1991;25:132. doi: 10.1016/s0190-9622(08)80509-9. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry. 1995;152:341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. Depression. 1994;2:59–72. [Google Scholar]
- Gerber A, Raab AP, Sobel AE. Vitamin A poisoning in adults: With description of a case. Am J Med. 1954;16:729–745. doi: 10.1016/0002-9343(54)90281-8. [DOI] [PubMed] [Google Scholar]
- Gomber S, Chellani H. Acute toxicity of vitamin A administered with measles vaccine. Indian Pediatrics. 1996;33:1053–1055. [PubMed] [Google Scholar]
- Goulden V, Layton AM, Cunliffe WJ. Current indications for isotretinoin as a treatment for acne vulgaris. Pharm Treatment. 1995;190:284–287. doi: 10.1159/000246717. [DOI] [PubMed] [Google Scholar]
- Gupta MA, Gupta AK, Schork NJ, Ellis CN, Voorhees JJ. Psychiatric aspects of the treatment of mild to moderate facial acne: Some preliminary observations. International Journal of Dermatology. 1990;29:719–721. doi: 10.1111/j.1365-4362.1990.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Haupt R. Akute symptomatische psychose bei Vitamin A-Intoxikation. Der Nervaenarzt. 1977;48:91–95. [PubMed] [Google Scholar]
- Hazen PG, Carney JF, Walker AE, Stewart JJ. Depression-a side effect of 13-cis-retinoic acid therapy. J Am Acad Dermatol. 1983;9:278–279. doi: 10.1016/s0190-9622(83)80154-6. [DOI] [PubMed] [Google Scholar]
- Henderson CA, Highet AS. Depression induced by etrinate [letter] Brit Med J. 1989;298:96. doi: 10.1136/bmj.298.6678.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn FA, Vollmayr B. Neurogenesis and depression: etiology or epiphenomenon? Biol Psychiatry. 2004;56:146–150. doi: 10.1016/j.biopsych.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Hepburn NC. Deliberate self-poisoning with isotretinoin. Brit J Dermatol. 1990;122:840–841. doi: 10.1111/j.1365-2133.1990.tb06279.x. [DOI] [PubMed] [Google Scholar]
- Hersom K, Neary MP, Levaux HP, Klaskala W, Strauss JS. Isotretinoin and antidepressant pharmacotherapy: A prescription sequence symmetry analysis. J Am Acad Dermatol. 2003;49:424–432. doi: 10.1067/s0190-9622(03)02087-5. [DOI] [PubMed] [Google Scholar]
- Hull PR, D’Arcy C. Isotretinoin use and subsequent depression and suicide: Presenting the evidence. American Journal of Clinical Dermatology. 2003;4:493–505. doi: 10.2165/00128071-200304070-00005. [DOI] [PubMed] [Google Scholar]
- Hull PR, D’Arcy C. Isotretinoin use and subsequent depression and suicide: Presenting the evidence. American Journal of Clinical Dermatology. 2004;4:493–505. doi: 10.2165/00128071-200304070-00005. [DOI] [PubMed] [Google Scholar]
- Hull PR, D’Arcy C. Acne, depression, and suicide. Dermatologic Clinics. 2005;23:665–674. doi: 10.1016/j.det.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Hull PR, Demkiw-Bartel C. Isotretinoin use in acne: prospective evaluation of adverse events. J Cut Med Surg. 2000;4:66–70. doi: 10.1177/120347540000400205. [DOI] [PubMed] [Google Scholar]
- Hunter K, Maden M, Summerbell D, Eriksson U, Holder N. Retinoic acid stimulates neurite outgrowth in the amphibian spinal cord. Proc Natl Acad Sci USA. 1991;88:3666–3670. doi: 10.1073/pnas.88.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz TA, Clark C, Murphy E, Klonoff H, Martin WRW, Pate BD. Regional cerebral glucose metabolism in major depressive disorder. Can J Psychiat. 1990;35:684–688. doi: 10.1177/070674379003500807. [DOI] [PubMed] [Google Scholar]
- Husson M, Enderlin V, Alfos S, Boucheron C, Pallet V, Higueret P. Expression of neurogranin and neuromodulin is affected in the striatum of vitamin A-deprived rats. Brain Research & Molecular Brain Research. 2004;123:7–17. doi: 10.1016/j.molbrainres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Idres N, Marill J, Flexor MA, Chabot GG. Activation of retinoic acid receptor-dependent transcription by all-trans-retinoic acid metabolites and isomers. J Biol Chem. 2002;277:31491–31498. doi: 10.1074/jbc.M205016200. [DOI] [PubMed] [Google Scholar]
- Jacobs BL. Adult brain neurogenesis and depression. Brain and Behavioral Immunity. 2002;16:602–609. doi: 10.1016/s0889-1591(02)00015-6. [DOI] [PubMed] [Google Scholar]
- Jacobs DG, Deutsch N, Brewer M. Suicide, depression, and isotretinoin: Is there a causal link? J Am Acad Dermatol. 2001;45:S168. doi: 10.1067/mjd.2001.118233. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Lie DC, Decicco KL, Shi Y, Deluca LM, Gage FH, et al. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:3902–3907. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, R C, Hotte M, Naudon L, Gurden H, Spedding M. Plasticity at hippocampal to prefrontal cortex synapses is impaired by loss of dopamine and stress: importance for psychiatric diseases. Neurotoxicology Research. 2004;6:233–244. doi: 10.1007/BF03033225. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH, Taylor JR. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Prog Brain Res. 2000;126:433–453. doi: 10.1016/S0079-6123(00)26028-7. [DOI] [PubMed] [Google Scholar]
- Jick SS, Kremers HM, Vasilakis-Scaramozza C. Isotretinoin use and risk of depression: Psychotic symptoms, suicide, and attempted suicide. Arch Dermatol. 2000;136:1231–1236. doi: 10.1001/archderm.136.10.1231. [DOI] [PubMed] [Google Scholar]
- Kane MA, Chen N, Sparks S, Napoli JL. Quantification of endogenous retinoic acid in limited biological samples by LC/MS/MS. Biochem J. 2005;388:363–369. doi: 10.1042/BJ20041867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P, Chambon P, Leid M. Role of Nuclear Retinoic Acid Receptors in the Regulation of Gene Expression. In: Blomhoff R, editor. Vitamin A in Health and Disease. New York: Marcel Dekker; 1994. [Google Scholar]
- Kellett SC, Gawkrodger DJ. The psychological and emotional impact of acne and the effect of treatment with isotretinoin. Brit J Dermatol. 1999:273–282. doi: 10.1046/j.1365-2133.1999.02662.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Regulation of adult hippocampal neurogenesis - implications for novel theories of major depression. Bipolar Disorder. 2002;4:17–33. doi: 10.1034/j.1399-5618.2002.40101.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Krezel W, Ghyselinck N, Samad TA, Dupe V, Kastner P, Borrelli E, et al. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science. 1998;279:863–867. doi: 10.1126/science.279.5352.863. [DOI] [PubMed] [Google Scholar]
- Krezel W, Kastner P, Chambon P. Differential expression of retinoid receptors in the adult mouse central nervous system. Neuroscience. 1999;89:1291–1300. doi: 10.1016/s0306-4522(98)00342-x. [DOI] [PubMed] [Google Scholar]
- La Placa M. Acute depression from isotretinoin Another case. European Academy of Dermatology and Venereology. 2005;19:380–399. doi: 10.1111/j.1468-3083.2004.01120.x. [DOI] [PubMed] [Google Scholar]
- Lacerda ALT, Keshavan MS, Hardan AY, Yorbik O, Brambilla P, Sassi RB, et al. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol Psychiatry. 2004;55:353–358. doi: 10.1016/j.biopsych.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Lai TJ, Payne ME, Byrum CE, Steffens D, Krishnan KR. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48:971–975. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- Lammer EJ, Armstrong DL. Malformations of hindbrain structures among humans exposed to isotretinoin (13-cis-retinoic acid) during early embryogenesis. In: Morris-Kay GM, editor. Retinoids in normal development and teratogenesis. Oxford: Oxford University Press; 1992. [Google Scholar]
- Landy D. Pibloktoq (hysteria) and Inuit nutrition: Possible implication of hypervitaminosis A. Social Science and Medicine. 1985;21:173–185. doi: 10.1016/0277-9536(85)90087-5. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern CE, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Lee SH, Payne ME, Steffens DC, McQuoid DR, Lai TJ, Provenzale JM, et al. Subcortical lesion severity and orbitofrontal cortex volume in geriatric depression. Biol Psychiatry. 2003;54:529–533. doi: 10.1016/s0006-3223(03)00063-5. [DOI] [PubMed] [Google Scholar]
- Lengqvist J, Mata de Urquiza A, Bergman AC, Wilson TM, Sjovall J, Perlmann T, et al. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Molecular and Cellular Proteomics. 2004;3:692–703. doi: 10.1074/mcp.M400003-MCP200. [DOI] [PubMed] [Google Scholar]
- Lindberg G, Bingefors K, Ranstam J, Rastam L, Melander A. Use of calcium channel blockers and risk of suicide: Ecological findings confirmed in population based cohort study. Brit Med J. 1998;316:741–745. doi: 10.1136/bmj.316.7133.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemayr H. Isotretinoin intoxication in attempted suicide. Acta Dermato-Venereologica. 1986;66:452–453. [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, et al. Function of the retinoic acid receptors (RAR) during development (1) Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- Lombaert A, Carton H. Benign intracranial hypertension due to A-hypervitaminosis in adults and adolescents. European Neurology. 1976;14:340–350. doi: 10.1159/000114758. [DOI] [PubMed] [Google Scholar]
- Luo T, Wagner E, Crandall JE, Drager UC. A retinoic-acid critical period in the early postnatal mouse brain. Biol Psychiatry. 2004a;56:971–980. doi: 10.1016/j.biopsych.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Luo T, Wagner E, Grun F, Drager UC. Retinoic acid signaling in the brain marks formation of optic projections, maturation of the dorsal telencephalon, and function of limbic sites. J Comp Neurol. 2004b;470:297–316. doi: 10.1002/cne.20013. [DOI] [PubMed] [Google Scholar]
- Maden M. Role and distribution of retinoic acid during CNS development. International Review of Cytology. 2001;209:1–77. doi: 10.1016/s0074-7696(01)09010-6. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie J, See G. Acute hypervitaminosis A of the infant Its clinical manifestation with benign acute hydrocephaly and pronounced bulge of the fontanel A clinical and biologic study. American Journal of Diseases of Children. 1954;73:731–736. doi: 10.1001/archpedi.1954.02050090719008. [DOI] [PubMed] [Google Scholar]
- Marqueling AL, Zane LT. Depression and suicidal behavior in acne patients treated with isotretinoin: A systematic review. Seminars in Cutaneous Medicine and Surgery. 2005;24:92–102. doi: 10.1016/j.sder.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Martinot JL, Hardy P, Feline A, Huret J-D, Mazoyer B, Attar-Levy D, et al. Left prefrontal glucose hypometabolism in the depressed state: A confirmation. Am J Psychiatry. 1990;147:1313–1317. doi: 10.1176/ajp.147.10.1313. [DOI] [PubMed] [Google Scholar]
- Mata De Urquiza A, Solomin L, Perlmann T. Feedback-inducible nuclear-receptor-driven reporter gene expression in transgenic mice. Proc Natl Acad Sci USA. 1999;96:13270–13275. doi: 10.1073/pnas.96.23.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka I, Kumagai M, Kurihara K. Differential and coordinated regulation of expression of norepinephrine transporter in catecholaminergic cells in culture. Brain Res. 1997;776:881–888. doi: 10.1016/s0006-8993(97)01016-0. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Frontal lobe dysfunction in secondary depression. J Neuropsychiatry Clin Neurosci. 1994;6:428–442. doi: 10.1176/jnp.6.4.428. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lewis PJ, Regenold W, Wagner HN. Paralimbic hypoperfusion in unipolar depression. J Nucl Med. 1994;35:929–934. [PubMed] [Google Scholar]
- Mayberg HS, Starkstein SE, Peyser CE, Brandt J, Dannals RF, Folstein SE. Paralimbic frontal lobe hypometabolism in depression associated with Huntington’s disease. Neurology. 1992;42:1791. doi: 10.1212/wnl.42.9.1791. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Starkstein SE, Sadzot B, Preziosi T, Andrezejewski PL, Dannals RF, et al. Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson’s Disease. Ann Neurol. 1990;28:57–64. doi: 10.1002/ana.410280111. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Drager UC. High levels of a retinoic acid-generating dehydrogenase in the meso-telencephalic dopamine system. Proc Natl Acad Sci USA. 1994;91:7772–7776. doi: 10.1073/pnas.91.16.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery P, Zhang J, Crandall JE. Retinoic acid signaling and function in the adult hippocampus. J Neurobiol. 2006;66:780–791. doi: 10.1002/neu.20237. [DOI] [PubMed] [Google Scholar]
- McCaffery PJ, Adams J, Maden M, Rosa-Molinar E. Too much of a good thing: retinoic acid as an endogenous regulator of neural differentiation and exogenous teratogen. European Journal of Neuroscience. 2003;18:457–472. doi: 10.1046/j.1460-9568.2003.02765.x. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Price LH. Depression associated with Vitamin A intoxication. Psychosomatics. 1992;33:117. doi: 10.1016/S0033-3182(92)72033-7. [DOI] [PubMed] [Google Scholar]
- Medansky RS, Handler RM, Medansky DL. Self-evaluation of acne and emotion: a pilot study. Psychosomatics. 1981;22:379–383. doi: 10.1016/S0033-3182(81)73507-2. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, et al. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Mervaala E, Fohr J, Konogen M, Valkonen-Korhonen M, Vainio P, Partanen J, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30:117–125. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- Mey J, McCaffery P. Retinoic acid signaling in the nervous system of adult vertebrates. Neuroscientist. 2004;10:409–421. doi: 10.1177/1073858404263520. [DOI] [PubMed] [Google Scholar]
- Meyskens FLJ. Short clinical reports. J Am Acad Dermatol. 1982;6:732–733. [PubMed] [Google Scholar]
- Middelkoop T. Roaccutane (Isotretinoin) and the risk of suicide: Case report and a review of the literature and pharmacovigilance reports. J Pharm Practice. 1999;12:374–378. [Google Scholar]
- Misner DL, Jacobs S, Shimizu Y, de Urquiza AM, Solomin L, Perlmann T, et al. Vitamin A deprivation results in reversible loss of hippocampal long-term synaptic plasticity. Proc Natl Acad Sci USA. 2001;98:11714–11719. doi: 10.1073/pnas.191369798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Romanski LM, LeDoux JE. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Neary MP, Klaskala W, McLane J. Epidemiological study of adverse events in Accutane users and matched non-users: retrospective analysis of major US health plan claims database. Pharmacoepidemiology and Drug Safety. 2001;10:S141. [Google Scholar]
- Ng CH, Schweitzer I. The association between depression and isotretinoin use in acne. Aust N Z J Psych. 2003;37:78–81. doi: 10.1046/j.1440-1614.2003.01111.x. [DOI] [PubMed] [Google Scholar]
- Ng CH, Tam MM, Celi E, Tate B, Scheitzer I. Prospective study of depressive symptoms and quality of life in acne vulgaris patients treated with isotretinoin compared to antibiotic and topical therapy. Australasian Journal of Dermatology. 2002;45:262–268. doi: 10.1046/j.1440-0960.2002.00612.x. [DOI] [PubMed] [Google Scholar]
- Ng CH, Tam MM, Hook SJ. Acne, isotretinoin treatment and acute depression. World Journal of Biological Psychiatry. 2001;2:159–161. doi: 10.3109/15622970109026803. [DOI] [PubMed] [Google Scholar]
- O’Connell MJ, Chua R, Hoyos B, Buck J, Chen YQ, Derguini F, et al. Retro-retinoids in regulated cell growth and death. Journal of Experimental Medicine. 1996;184:549–555. doi: 10.1084/jem.184.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly KC, Shumaker J, Gonzalez-Lima F, Lane MA, Bailey SJ. Chronic administration of 13-cis-retinoic acid increases depression-related behavior in mice. Neuropsychopharmacology. 2006;31:1919–1927. doi: 10.1038/sj.npp.1300998. [DOI] [PubMed] [Google Scholar]
- Oliver TK, Havener WH. Eye manifestations of chronic Vitamin A intoxication. AMA Arch Opthalmol. 1958;60:19–22. doi: 10.1001/archopht.1958.00940080033004. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfanos CE, Zouboulis CC, Almond-Roesler B, Geilen CC. Current use and future potential role of retinoids in dermatology. Drugs. 1997;53:358–384. doi: 10.2165/00003495-199753030-00003. [DOI] [PubMed] [Google Scholar]
- Pasquariello PS, Schut L, Borns P. Benign increased intracranial hypertension due to chronic vitamin A overdosage in a 26-month-old child. Clinical Pediatrics. 1977;16:379–382. doi: 10.1177/000992287701600415. [DOI] [PubMed] [Google Scholar]
- Patten SB, Love EJ. Drug-induced depression Incidence, avoidance and management. Drug Safety. 1994;10:203–219. doi: 10.2165/00002018-199410030-00003. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Pezze MA, Ferger B, Zhang WN, Murphy CA, Feldon J, et al. Activation of dopaminergic neurotransmission in the medial prefrontal cortex by N-methyl-d-aspartate stimulation of the ventral hippocampus in rats. Neuroscience. 2005;132:219–232. doi: 10.1016/j.neuroscience.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Rajkowska NA, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Melzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, et al. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- Restak RM. Pseudotumor cerebri, psychosis, and hypervitaminosis A. J Nerv Ment Dis. 1972;155:155–172. doi: 10.1097/00005053-197207000-00008. [DOI] [PubMed] [Google Scholar]
- Ring RA, Bench CJ, Trimble MR, Brooks DJ, Frackowiak RSJ, Dolan RJ. Depression in Parkinson’s Disease: A positron emission study. Br J Psychiatry. 1994;165:333–339. doi: 10.1192/bjp.165.3.333. [DOI] [PubMed] [Google Scholar]
- Robusto O. Depressao por antiacneico. Acta Medica Portuguesa. 2002;15:325–326. [PubMed] [Google Scholar]
- Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex. 2004;14:224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- Rodahl K, Moore T. Vitamin A content and toxicity of bear and seal liver. Biochem J. 1943;37:166–168. doi: 10.1042/bj0370166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, De Luca LM. A new metabolite of retinol: all-trans-4-oxo-retinol as a receptor activator and differentiation agent. Nutrition Reviews. 1996;54:355–356. doi: 10.1111/j.1753-4887.1996.tb03802.x. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes and Development. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Rowe A. Retinoid X receptors. International Journal of Biochemistry and Cell Biology. 1997;29:275–278. doi: 10.1016/s1357-2725(96)00101-x. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Peck GL, Squillace KM, Gantt GG. Reduced anxiety and depression in cystic acnes patients after successful treatment with oral isotretinoin. J Acad Dermatol. 1987;17:25–32. doi: 10.1016/s0190-9622(87)70166-2. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prohovnik I, Moeller JR, Brown RP, Apter S, Prudic J, et al. Regional cerebral blood flow in mood disorders, I: comparison of major depressives and normal controls at rest. Arch Gen Psychiatry. 1990;47:60–70. doi: 10.1001/archpsyc.1990.01810130062009. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Crandall JE, Brodsky J, McCaffery P. 13-cis Retinoic acid (accutane) suppresses hippocampal cell survival in mice. Ann NY Acad Sci. 2004;1021:436–440. doi: 10.1196/annals.1308.059. [DOI] [PubMed] [Google Scholar]
- Samad TA, Krezel W, Chambon P, Borrelli E. Regulation of dopaminergic pathways by retinoids: activation of the D2 receptor promoter by members of the retinoic acid receptor-retinoid X receptor family. Proc Natl Acad Sci USA. 1997;94:14349–14354. doi: 10.1073/pnas.94.26.14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sani BP, Venepally PR, Levin AA. Didehydroretinoic acid: retinoid receptor-mediated transcriptional activation and binding properties. Biochemistry and Pharmacology. 1997;53:1049–1053. doi: 10.1016/s0006-2952(97)00076-2. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochemical Research. 2003;28:1735–1742. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Scheinman PL, Peck GL, Rubinow DR, DiGiovanna JJ, Abangan DL, Ravin PD. Acute depression from isotretinoin. J Am Acad Dermatol. 1990;22:1112–1114. doi: 10.1016/s0190-9622(08)81018-3. [DOI] [PubMed] [Google Scholar]
- Scott AIF, Dougall N, Ross M, O’Carroll RE, Riddle W, Ebmeier KP, et al. Short-term effects of electroconvulsive treatment on the uptake of [Tc-99m] exametazine into brain in major depression shown with single photon emission tomography. J Affect Disord. 1994;30:27–34. doi: 10.1016/0165-0327(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Seigel NJ, Spackman TJ. Chronic hypervitaminosis A with intracranial hypertension and low cerebrospinal fluid concentration of protein. Clinical Pediatrics. 1972;11:580–584. doi: 10.1177/000992287201101011. [DOI] [PubMed] [Google Scholar]
- Selhorst JB, Waybright EA, Jennings S, Corbett JJ. Liver lover’s headache: Pseudotumor cerebri and vitamin A intoxication. JAMA. 1984;252:3365. [PubMed] [Google Scholar]
- Sharma RP. Schizophrenia, epigenetics and ligand-activated nuclear receptors: a framework for chromatin therapeutics. Schizophrenia Research. 2005;72:79–90. doi: 10.1016/j.schres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Shaw EW, Niccoli JZ. Hypervitaminosis A: Report of a case in an adult male. Ann Intern Med. 1953;39:131–134. doi: 10.7326/0003-4819-39-1-131. [DOI] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanhavi M, Mintun MA, Gado MU. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang P, Gado M, Csernansky J, Vannier M. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TW, Shealy YF, Strother DL, Hill DL. Nonenzymatic isomerization of all-trans- and 13-cis-retinoids catalyzed by sulfhydryl groups. Drug Metabolism and Disposition. 1986;14:698–702. [PubMed] [Google Scholar]
- Shuster S, Fisher GH, Harris E, Binnel D. The effect of skin disease on self-image. Brit J Dermatol. 1978;99:18–19. [PubMed] [Google Scholar]
- Silva AJ. Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol. 2003;54:224–237. doi: 10.1002/neu.10169. [DOI] [PubMed] [Google Scholar]
- Spector RH, Carlisle J. Pseudotumor cerebri caused by a synthetic vitamin A preparation. Neurology. 1984;34:1509–1511. doi: 10.1212/wnl.34.11.1509. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Roberts AB, Goodman DS. The Retinoids: Biology, Chemistry, and Medicine. New York: Raven Press; 1994. [Google Scholar]
- Steffens DC, Byrum CE, McQuoid DR, Greenburg DL, Payne ME, Blichington TT, et al. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Krishnan KRR. Structural neuroimaging and mood disorders: Recent findings, implications for classification, and future directions. Biol Psychiatry. 1998;43:705–712. doi: 10.1016/s0006-3223(98)00084-5. [DOI] [PubMed] [Google Scholar]
- Stimson WH. Vitamin A intoxication in adults: Report of a case with a summary of the literature. N Engl J Med. 1961;265:369–373. [Google Scholar]
- Stoll AL, Severus E, Freeman MP, Rueter S, Zboyan HA, Diamond E, et al. Omega 3 fatty acids in bipolar disorder: A preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- Strom BL. Pharmacoepidemiology. 4. New York: Wiley; 2005. [Google Scholar]
- Tang G, Russell RM. Formation of all-trans-retinoic acid and 13-cis-retinoic acid from all-trans retinyl palmitate in humans. Journal of Nutrition and Biochemistry. 1991:2. [Google Scholar]
- Thompson Haskell G, Maynard TM, Shatzmiller RA, Lamantia AS. Retinoic acid signaling at sites of plasticity in the mature central nervous system. J Comp Neurol. 2002;452:228–241. doi: 10.1002/cne.10369. [DOI] [PubMed] [Google Scholar]
- Toresson H, Mata de Urquiza A, Fagerstrom C, Perlmann T, Campbell KA. Retinoids are produced by glia in the lateral ganglionic eminence and regulate striatal neuron differentiation. Development. 1999a;126:1317–1326. doi: 10.1242/dev.126.6.1317. [DOI] [PubMed] [Google Scholar]
- Toresson H, Mata de Urquiza A, Fagerstrom C, Perlmann T, Campbell KA. Retinoids are produced by glia in the lateral ganglionic eminence and regulate striatal neuron differentiation. Development. 1999b;126:1317–1326. doi: 10.1242/dev.126.6.1317. [DOI] [PubMed] [Google Scholar]
- Tsukada M, Schroder M, Roos TC, Chandraratna RA, Reichert U, Merk HF, et al. 13-cis retinoic acid exerts its specific activity on human sebocytes through selective intracellular isomerization to all-trans retinoic acid and binding to retinoid acid receptors. Journal of Investigative Dermatology. 2000;115:321–327. doi: 10.1046/j.1523-1747.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, et al. Hippocampal volume in primary unipolar major depression: A magnetic resonance imaging study. Biol Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- Valdenaire O, Maus-Moatti M, Vincent JD, Mallet J, Vernier P. Retinoic acid regulates the developmental expression of dopamine D2 receptor in rat striatal primary cultures. J Neurochem. 1998;71:929–936. doi: 10.1046/j.1471-4159.1998.71030929.x. [DOI] [PubMed] [Google Scholar]
- Van der Meeren HLM, van der Schaar WW, van den Hurk CMAM. The psychological impact of severe acne. Cutis. 1985;7:84–86. [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Villalobos D, Ellis M, Snodgrass WR. Isotretinoin (Accutane)-associated psychosis. Vet Human Toxicol. 1989;31:362. [Google Scholar]
- Vincent SL, Khan Y, Benes FM. Cellular colocalization of dopamine D1 and D2 receptors in rat medial prefrontal cortex. Synapse. 1995;19:112–120. doi: 10.1002/syn.890190207. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Wang B, Campbell KA. The homeobox gene Gsh2 is required for retinoid production in the embryonic mouse telencephalon. Development. 2004;131:4013–4020. doi: 10.1242/dev.01272. [DOI] [PubMed] [Google Scholar]
- Wagner E, Luo T, Drager UC. Retinoic acid synthesis in the postnatal mouse brain marks distinct developmental stages and functional systems. Cereb Cortex. 2002;12:1244–1253. doi: 10.1093/cercor/12.12.1244. [DOI] [PubMed] [Google Scholar]
- Wagner E, Luo T, Sakai Y, Parada LF, Drager UC, Radttonpipcc EJNJ. Retinoic acid delineates the topography of neuronal plasticity in postnatal cerebral cortex. European Journal of Neuroscience. 2006;24:329–340. doi: 10.1111/j.1460-9568.2006.04934.x. [DOI] [PubMed] [Google Scholar]
- Wang HF, Liu FC. Regulation of multiple dopamine signal transduction molecules by retinoids in the developing striatum. Neuroscience. 2005;134:97–105. doi: 10.1016/j.neuroscience.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Wang TW, Zhang H, Parent JM. Retinoic acid regulates postnatal neurogenesis in the murine subventricular zone-olfactory bulb pathway. Development. 2005;132:2721–2732. doi: 10.1242/dev.01867. [DOI] [PubMed] [Google Scholar]
- Weigan U-W, Chou RC. Pharmacokinetics of oral isotretinoin. J Am Acad Dermatol. 1998;39:S8–12. doi: 10.1016/s0190-9622(98)70438-4. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Best AR, Sullivan RM. Plasticitiy in the olfactory system: lessons for the neurobiology of memory. Neuroscientist. 2004;10:513–524. doi: 10.1177/1073858404267048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G. Vitamin A functions in the regulation of the dopaminergic system in the brain and pituitary gland. Nutrition Reviews. 1998;56:354–355. doi: 10.1111/j.1753-4887.1998.tb01678.x. [DOI] [PubMed] [Google Scholar]
- Wolf G. The visual cycle of the cone photoreceptors of the retina. Nutrition Reviews. 2004;62:283–286. doi: 10.1111/j.1753-4887.2004.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Wooltorton E. Accutane (isotretinoin) and psychiatric adverse effects. Canadian Medical Association Journal. 2003;168:66. [PMC free article] [PubMed] [Google Scholar]
- Wu G, Fang Y, Lu ZH, Ledeen RW. Induction of axon-like and dendrite-like processes in neuroblastoma cells. Journal of Neurocytology. 1998;27:1–14. doi: 10.1023/a:1006910001869. [DOI] [PubMed] [Google Scholar]
- Wysowski DK, Pitts M, Beitz J. An analysis of reports of depression and suicide in patients treated with isotretinoin. J Am Acad Dermatol. 2001a;45:515–519. doi: 10.1067/mjd.2001.117730. [DOI] [PubMed] [Google Scholar]
- Wysowski DK, Pitts M, Beitz J. Depression and suicide in patients treated with isotretinoin. N Engl J Med. 2001b;344:460. doi: 10.1056/NEJM200102083440616. [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Lindqvist E, Mata de Urquiza A, Tomac A, Eriksson U, Perlmann T, et al. Role of retinoids in the CNS: differential expression of retinoid binding proteins and receptors and evidence for presence of retinoic acid. European Journal of Neuroscience. 1999;11:407–416. doi: 10.1046/j.1460-9568.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Simon A, Giacobini MMJ, Ericksson U, Olson L. Localization of cellular retinoid-binding proteins suggests specific roles for retinoids in the adult central nervous system. Neuroscience. 1994;62:899–918. doi: 10.1016/0306-4522(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Zetterström RH, Tomac A, Eriksdotter-Nilsson M, Nennesmo I, Eriksson U, Olson L. Localization of retinoid-binding proteins and retinoid receptors suggests a function for retinoic acid in developing and adult dopamine-innervated brain areas. Society for Neuroscience Abstracts. 1994;20:1688. [Google Scholar]
- Zhang J, Smith D, Yamamoto M, Ma L, McCaffery P. The meninges is a source of retinoic acid for the late-developing hindbrain. J Neurosci. 2003;23:7610–7620. doi: 10.1523/JNEUROSCI.23-20-07610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


