Abstract
Study Objectives:
Women undergo hormonal changes both naturally during their lives and as a result of sex hormone treatments. The objective of this study was to gain more knowledge about how these hormones affect sleep and responses to sleep loss.
Design:
Rats were ovariectomized and implanted subcutaneously with Silastic capsules containing oil vehicle, 17β-estradiol and/or progesterone. After 2 weeks, sleep/wake states were recorded during a 24-h baseline period, 6 h of total sleep deprivation induced by gentle handling during the light phase, and an 18-h recovery period.
Measurements and Results:
At baseline and particularly in the dark phase, ovariectomized rats treated with estradiol or estradiol plus progesterone spent more time awake at the expense of non-rapid eye movement sleep (NREMS) and/or REMS, whereas those given progesterone alone spent less time in REMS than ovariectomized rats receiving no hormones. Following sleep deprivation, all rats showed rebound increases in NREMS and REMS, but the relative increase in REMS was larger in females receiving hormones, especially high estradiol. In contrast, the normal increase in NREMS EEG delta power (an index of NREMS intensity) during recovery was attenuated by all hormone treatments.
Conclusions:
Estradiol promotes arousal in the active phase in sleep-satiated rats, but after sleep loss, both estradiol and progesterone selectively facilitate REMS rebound while reducing NREMS intensity. These results indicate that effects of ovarian hormones on recovery sleep differ from those on spontaneous sleep. The hormonal modulation of recovery sleep architecture may affect recovery of sleep related functions after sleep loss.
Citation:
Deurveilher S; Rusak B; Semba K. Estradiol and progesterone modulate spontaneous sleep patterns and recovery from sleep deprivation in ovariectomized rats. SLEEP 2009;32(7):865-877.
Keywords: Ovariectomy, female sex hormones, sleep homeostasis, REM sleep, EEG delta power
NATURAL CHANGES IN ESTRADIOL AND PROGESTERONE LEVELS ACROSS THE MENSTRUAL CYCLE, AND ESPECIALLY DURING PREGNANCY AND MENOPAUSE, have been associated with sleep disturbances and changes in sleep EEG in women.1,2 Hormone replacement therapy (estradiol, progesterone, or both) can improve sleep quality in postmenopausal women under baseline conditions.3–8 It is unclear, however, whether hormone replacement therapy can reduce cognitive performance deficits resulting from sleep deprivation9,10 or facilitate sleep recovery after sleep deprivation.11 Given the prevalence of chronic, partial sleep deprivation12 and the frequency with which women are given hormone treatments,13–15 it is important to determine whether and how such treatments may influence the ability to recover lost sleep.
Rodents have been used frequently as models to examine the effects of ovarian hormones on sleep. Changes in hormonal levels during the estrous cycle were associated with changes in sleep patterns,16–20 whereas in ovariectomized rats, estradiol treatment reduced sleep, especially rapid eye movement sleep (REMS), under baseline conditions.21–27 The effects of hormonal changes associated with estrous cycles on recovery sleep after sleep deprivation may not, however, parallel those on baseline sleep. Despite baseline differences in sleep, intact rats did not show estrous cycle-related differences in amounts of non-REMS (NREMS) and REMS during recovery, nor in NREMS EEG delta activity (an index of NREMS intensity and drive), after 6 h of sleep deprivation.28 In mice, neither ovariectomy29 nor lack of aromatase (an enzyme that converts testosterone into estradiol)30 affected recovery REMS or NREMS delta activity after 6 h of sleep deprivation. Although these studies suggest that endogenous sex hormones do not influence recovery sleep after 6 h of sleep deprivation in rodents, the stage of the estrous cycle at the beginning of 4 days of REMS deprivation has been reported to affect the pattern of recovery sleep.31 Nonetheless, the clinically more relevant question of whether hormone replacement after loss of ovarian hormones affects the ability to compensate for lost sleep has not been addressed directly using rodent models.
To examine whether and how replacement of estradiol and/or progesterone after ovariectomy modulates the pattern of baseline sleep and recovery sleep after 6 h of sleep deprivation, we implanted estradiol- and/or progesterone-containing capsules subcutaneously in ovariectomized adult rats. These capsules produced relatively stable physiological levels of circulating hormones. The analytic advantage of this approach is that it allows comparisons between baseline sleep and recovery sleep after sleep deprivation under identical hormonal conditions. This is not possible using intact females, because estrous cycles do not include intervals with stable hormonal levels that are long enough to accommodate lengthy baseline and recovery periods, particularly during proestrus and estrus when estradiol and progesterone levels change rapidly.32,33 Gonadally intact and hormonally untreated male rats were studied for comparison.
MATERIALS AND METHODS
Animals
Wistar rats of both sexes (Charles River Canada, St. Constant, QC, Canada), 2-2.5 months old (females: 211–264 g; males: 310–355 g at the time of surgery), were housed under a 12/12 h light/dark cycle (lights on [ = Zeitgeber Time (ZT)0] at 07:00) at 23 ± 1°C ambient temperature. Rat chow and water were available ad libitum. Animal handling protocols followed the guidelines of the Canadian Council on Animal Care and were approved by the Dalhousie University Committee on Laboratory Animals.
Surgery
Female rats were ovariectomized bilaterally under anesthesia (72 mg/kg ketamine, 3.8 mg/kg xylazine, and 0.7 mg/kg acepromazine, i.p). A midline incision was made through the skin and muscle layers in the lower abdomen. The uterine horns were pulled out of the abdominal cavity, the oviducts were clamped with forceps, and the ovaries removed. The uterine horns were replaced inside the abdomen and the muscle layers were sutured. Silastic capsules (1.6 mm inner diameter × 3.2 mm outer diameter; 35 mm in length for oil and estradiol, and 40 mm in length for progesterone (Dow Corning Corporation, Midland, MI) were inserted subcutaneously lateral to the incision in each animal. Capsules were filled with the following: sesame oil (60 μL; Catalog No. S3547, Sigma-Aldrich, St Louis, MO) (Oil group; n = 8); 10.5 μg of 17β-estradiol (Catalog No. E8875; Sigma-Aldrich) in 60 μL sesame oil (Low [diestrus] Estradiol [LE] group; n = 8); 60 μg of 17β-estradiol in 60 μL sesame oil (High [proestrus] Estradiol [HE] group; n = 8); or 40 mg of crystalline progesterone (Catalog No. P0130; Sigma-Aldrich) (Low [diestrus] Progesterone [LP] group; n = 8). All rats received one capsule except rats treated with both hormones (Low [diestrus] Estradiol + Low [diestrus] Progesterone [LEP] group; n = 8) which received 2 capsules, one containing 10.5 μg of 17β-estradiol, and the other containing 40 mg of progesterone. The 10.5- and 60-μg doses of estradiol were reported to result in blood levels of estradiol characteristic of diestrus and proestrus, respectively, 8 days after implantation.34,35 The 40-mg progesterone dose was reported to give low blood levels of progesterone equivalent to diestrus levels at one week after implantation.36 Male rats were left gonadally intact but were implanted with one oil-filled Silastic capsule as a control procedure (M group; n = 8). After capsule implantation, the skin incision was closed with surgical skin staples.
All animals were then placed in a stereotaxic apparatus and implanted with 2 miniature stainless steel screws for recording the electroencephalogram (EEG), one over the frontal cortex (1 mm rostral to bregma and 2 mm right of the midline) and the other over the occipital cortex (6 mm caudal to bregma and 2 mm left of the midline). A third screw was placed over the cerebellum to serve as a ground electrode. A pair of fluorocarbon-coated stainless steel wires with a 2–3 mm exposure were inserted into the dorsal neck muscles to record the electromyogram (EMG). All electrodes were connected to a small plastic connector (Plastics One Inc., Roanoke, VA) and the complete assembly was anchored to the skull with dental acrylic. Following surgery, animals were injected subcutaneously with an analgesic (Ketoprofen, 5 mg/kg) and an antibiotic (Duplocillin, 0.15 mL) and monitored for recovery from anesthesia before being returned to the animal colony to be housed singly.
Experimental Design and Data Acquisition
Eight to 9 days after surgery, each rat was transferred to a clear Plexiglas cage (40 × 30 × 40 cm3) placed inside an individual experimental chamber that was equipped with a fan and an incandescent light controlled by a timer to maintain the same 12/12 h light/dark cycle as in the colony room. The following day, rats were anesthetized briefly (5–10 min) with isoflurane to remove the skin staples. Rats were then connected to a flexible cable attached to a rotating commutator (Plastics One Inc.) and remained connected for 3–4 days for adaptation before polygraphic recording started.
EEG/EMG recording began at mid-light phase (ZT6) with a baseline 24-h period followed by sleep deprivation for 6 h over the second half of the light phase (ZT6-12). Sleep deprivation was conducted by introducing novel objects (plastic items such as tubes, cups, and toys of different shapes) into their cages, gently shuffling their bedding, cage tapping, and, when necessary, slowly moving their litter tray. These stimuli were applied only when the rats showed behavioral signs of sleepiness (i.e., when they were immobile, adopting a sleep posture) or when slow waves were apparent in the EEG. After sleep deprivation, EEG/EMG recording continued for 18 h to assess recovery sleep during the 12-h dark phase and the first 6 h of the next light phase.
EEG and EMG signals were amplified and band pass-filtered (EEG: 0.3–100 Hz; EMG: 10–100 Hz; Grass Telefactor, West Warwick, RI). Signals were digitized at 256 Hz and stored on a computer for off-line analysis (SleepSign, Kissei Comtec America, Irvine, CA).
Sleep-Wake Scoring and Analysis
Behavioral states were scored automatically in consecutive 10-sec epochs with each epoch identified as wakefulness (low-voltage, fast EEG activity; moderate to high EMG activity), NREMS (high-voltage EEG activity, dominated by delta waves [0.5–4 Hz]; low amplitude EMG), or REMS (EEG dominated by theta waves [4.5–8 Hz]; very low background EMG activity, with occasional muscle twitches). The automatic scoring was inspected visually off-line and corrected ( < 10% disagreement) when it did not agree with visual scoring.
EEG delta (0.5–4 Hz) power during NREMS was measured in 0.5-Hz bins using fast Fourier transform (FFT; Hanning window) for 2-sec windows. Power values were averaged over a 10-sec epoch, and the mean value at 2-h intervals was normalized to the 24-h baseline level in each animal. Epochs with artifacts (8.7% of total sleep epochs) were included in sleep-wake scoring but not in the FFT analysis.
Tissue Collection and Radioimmunoassay
At the end of the 48-h recording period, rats were given an overdose of anesthetics (see above). Blood samples were collected by cardiac puncture in heparinized tubing and then centrifuged at 3000 rpm for 10 min. Plasma was collected and kept frozen at −80°C until radioimmunoassay. Plasma concentrations of estradiol and progesterone were determined using commercial kits (Estradiol: Catalog No. KE2D1; Progesterone: TKPG1; Inter Medico, Markham, ON). The detection limit of the assays was 5 pg/mL for estradiol and 0.1 ng/mL for progesterone. The intra-assay coefficient of variation was 13.1% for estradiol and 6.5% for progesterone. All assays were conducted in a single session. Uteri were harvested immediately after blood collection and weighed as previously described.37 The uterus weight index was defined as mg of uterus/mm of uterus.
Statistical Analyses
Statistical analyses were conducted with Statview 5.0 (SAS Institute Inc., Cary, NC) and SPSS 14.0 (SPSS Inc, Chicago, IL). Sleep-wake parameters were analyzed using a 2-way repeated measures ANOVA. Serum progesterone levels and uterus weights were analyzed using one-way ANOVA. Post hoc multiple comparison analyses (LSD) were used to further analyze significant main effects and interactions. For serum estradiol levels, nonparametric Kruskal-Wallis tests were performed, and nonparametric Dunn post hoc tests were used to determine group differences. Probabilities of less than 0.05 were considered statistically significant.
RESULTS
The results reported here were derived from 48 rats, including 40 ovariectomized female rats, assigned to Oil, LE, HE, LP, and LEP groups (8 per group), and 8 gonadally intact and hormonally untreated male rats (M group).
Plasma E and P Levels, Uterus Weights, and Body Weights
The efficacy of hormone manipulations was confirmed by measuring plasma estradiol and progesterone levels and, as bioassays, uterus and body weights (Table 1). The values from the Oil and LE groups were similar to those we reported previously using the same Oil and LE treatments.37 Among the female groups, plasma estradiol levels were highest in the HE group, followed by the LE and LEP groups, and then the Oil and LP groups (H5 = 16.19, P < 0.01; HE > Oil, P < 0.05). Plasma progesterone levels were higher in the LP and LEP groups than in the Oil group (F3,21 = 3.72, P < 0.05; LP and LEP > Oil, both P < 0.05). The LE, HE, and LEP groups showed a dose-dependent increase in uterus weight indices (F4,35 = 35.26, P < 0.0001; LE, HE and LEP > Oil and LP, all P < 0.0001; HE > LE and LEP, both P < 0.01). Conversely, the LE, HE, and LEP groups gained smaller percentages of body weight than the Oil and LP groups over the 15-day period from surgery to the end of recording (all P < 0.001).
Table 1.
Plasma Estradiol and Progesterone Levels, Uterus Weight Indices, and Body Weights
| Variables | Treatment groups |
|||||
|---|---|---|---|---|---|---|
| Oil (n) | LE (n) | HE (n) | LP (n) | LEP (n) | M (n) | |
| Plasma estradiol level (pg/mL) | < 5 (7) | 13.4 (6) | 32.2a (5) | < 5 (8) | 14.0 (7) | < 5 (5) |
| Plasma progesterone level (ng/mL) | 1.7 ± 0.3 (6) | n.d. | n.d. | 3.4 ± 0.4a (8) | 4.4 ± 0.8a (6) | 2.5 ± 0.8 (5) |
| Uterus weight index (mg/mm) | 2.5 ± 0.3 (8) | 6.8 ± 0.4a (8) | 9.3 ± 0.9ab (8) | 3.2 ± 0.2bc (8) | 7.1 ± 0.4acd (8) | n.a. |
| Uterus weight/body weight ratio | 0.4 ± 0.1 | 1.2 ± 0.1a | 1.7 ± 0.2ab | 0.5 ± 0.1bc | 1.3 ± 0.1acd | n.a. |
| Body weight (g) | ||||||
| At surgery | 236 ± 6 (8) | 236 ± 4 (8) | 238 ± 4 (8) | 237 ± 4 (8) | 230 ± 4 (8) | 326 ± 6abcde (8) |
| At perfusion | 276 ± 6* (8) | 250 ± 5*a (8) | 244 ± 4*a (8) | 274 ± 8*bc (8) | 242 ± 3*ad (8) | 376 ± 8*abcde (8) |
| Gain in body weight (%) | +17 ± 1 | +6 ± 1a | +2 ± 1a | +16 ± 2bc | +5 ± 1ad | +15 ± 2bce |
All values are expressed as mean ± SEM, except for plasma estradiol levels for which the median is shown because the data sets contained values below the detection limit of the assay (5 pg/mL). The number (n) of animals contributing to each value varies because data were unavailable for some animals; the n is shown in parentheses next to each value.
Different from corresponding body weight at surgery;
different from Oil;
different from LE;
different from HE;
different from LP;
different from LEP. All P < 0.05. Fisher LSD post hoc tests used for plasma progesterone levels, uterus and body weights; nonparametric Dunn post hoc tests used for plasma E levels.
Baseline Amounts of Sleep-Wake States
Wakefulness
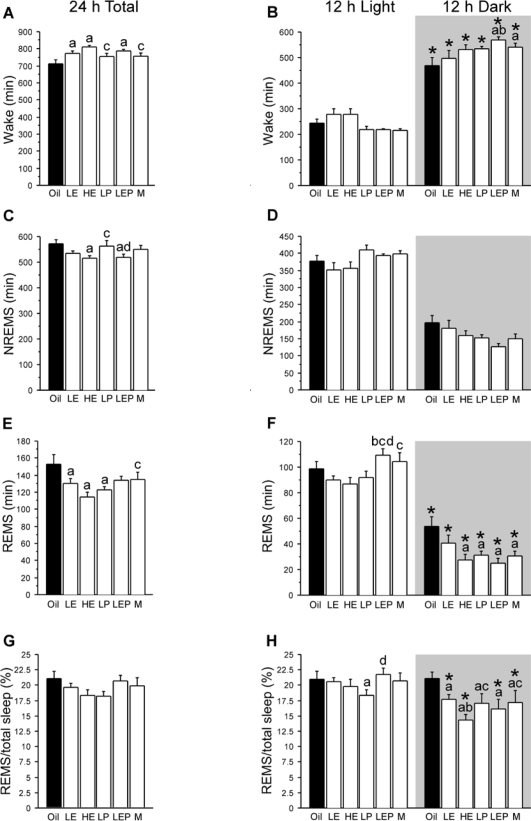
Over the 24-h baseline period (Figure 1A), the 6 groups differed in the amounts of wake (F5,42 = 4.61, P < 0.01). The LE, HE, and LEP groups had more wake (+61, +95, and +72 min, respectively; all P < 0.01) than the Oil group. This increase was prominent during the dark phase (Group × Phase interaction: F5,42 = 2.75, P < 0.05; LEP > Oil, P < 0.05; Figure 1B). Consequently, the LEP group had a significantly lower light/dark ratio (i.e., a greater light/dark difference) of wake amount than the Oil group (P < 0.05; Table 2). Additive effects of estradiol and progesterone were observed during the dark phase (LE: +28 min; LP: +65 min; and LEP: + 99 min vs. Oil; LEP > LE, +71 min, P < 0.05; Figure 1B). The M group (males) had more wake than the Oil group during the dark phase (P < 0.05).
Figure 1.
Amount (mean + SEM, min) of wake (A, B), NREMS (C, D), and REMS (E, F), and REMS as a percentage of total sleep (G, H), during baseline recording for the full 24-h period (left) and during the 12-h light and 12-h dark phases (right) in ovariectomized female rats treated with Oil (solid bars), low estradiol (LE), high estradiol (HE), low progesterone (LP), or low estradiol plus low progesterone (LEP), and in intact males (M; white bars) (n = 8 per group). The background shading indicates the dark phase. Treatments with estradiol alone (LE and HE) or combined with progesterone (LEP) increased wake and decreased NREMS and/or REMS, while treatments with progesterone alone (LP) selectively decreased REMS. These effects were more prominent during the dark phase. *Different from light phase; adifferent from Oil; bdifferent from LE; cdifferent from HE; ddifferent from LP; all P < 0.05 (Fisher LSD post hoc comparisons).
Table 2.
Light/Dark Ratio of Time Spent in Wake, NREMS, and REMS During the Baseline Recording
| Variables | Treatment groups | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Oil | LE | HE | LP | LEP | M | ||
| Wake | 0.55 ± 0.21 | 0.59 ± 0.22 | 0.53 ± 0.17 | 0.41 ± 0.08b | 0.40 ± 0.03abc | 0.40 ± 0.07b | F5,42 = 2.72, P < 0.05 |
| NREMS | 2.11 ± 0.68 | 2.35 ± 1.37 | 2.40 ± 0.88 | 2.71 ± 0.31 | 3.04 ± 0.54 | 2.89 ± 0.87 | F5,42 = 2.42, P = 0.051 |
| REMS | 2.05 ± 0.60 | 2.85 ± 1.68 | 3.62 ± 1.66a | 3.21 ± 1.36a | 4.62 ± 2.23ab | 3.80 ± 1.35a | F5,42 = 3.31, P < 0.025 |
All values are expressed as mean ± SEM, (n = 8 per group).
Different from Oil;
different from LE;
different from HE. All P < 0.05 (Fisher LSD post hoc comparisons). Note that a smaller ratio indicates a greater light/dark difference for wake amounts, whereas the opposite is the case for NREMS and REMS amounts.
NREMS
Over the 24-h baseline period (Figure 1C), the 6 groups differed in NREMS amounts (F5,42 = 2.57, P < 0.05). The HE and LEP groups had less NREMS (−57 and −54 min, P < 0.025, respectively) relative to the Oil group, mainly because of a reduction in the mean duration, but not the number, of NREMS episodes (data not shown). The Group × Phase interaction approached statistical significance (F5,42 = 2.31, P = 0.06; Figure 1D), so that the LEP, M, and then LP group in that order tended to have a higher light/dark ratio than the other groups (Table 2).
REMS
Over the 24-h baseline period (Figure 1E), the 6 groups differed in the amounts of REMS (F5,42 = 3.34, P < 0.025). The LE, HE, and LP groups had less REMS (−22, −38, and −29 min, respectively; all P < 0.05) relative to the Oil group, mainly because of a reduction in the number of REMS episodes, but not the mean duration (data not shown). The decrease in REMS time was more prominent during the dark phase (Group × Phase interaction: F5,42 = 3.68, P < 0.05; HE, LP, and LEP < Oil, all P < 0.01; Figure 1F). In addition, the LEP rats tended to show less REMS than the LE rats during the dark phase (−15 min; P = 0.051), but during the light phase, they showed significantly more REMS than the LE rats (+19 min; P < 0.025); Consequently, the light/dark ratio for REMS was higher in the HE, LP, and LEP groups than in the Oil group (all P < 0.05; Table 2), and it was higher in the LEP rats than the LE rats (P < 0.025; Table 2). The M group had less REMS than the Oil group during the dark phase (P < 0.01), resulting in a higher light/dark ratio (P < 0.05).
REMS/Total Sleep
To evaluate possible differential effects of estradiol and/or progesterone on NREMS vs. REMS, the ratio of REMS time to total sleep time was used (Figure 1G). Overall, the ratio was smaller in the dark compared to the light phase in the LE, HE, LEP, and M groups (Group × Phase interaction: F5,42 = 3.08, P < 0.025; post hoc comparisons, all P < 0.05), whereas the Oil and LP groups had similar ratios between the light and dark phases (Figure 1H). During the dark phase, the REMS/total sleep ratio was significantly smaller in all other groups relative to the Oil group (all P < 0.005), with a dose-dependent negative effect of estradiol (HE < LE, P < 0.025; Figure 1H). During the light phase, the only significant differences were found in the smaller REMS/total sleep ratio in LP vs. Oil and LEP groups (both P < 0.05). These results for REMS/total sleep ratios largely paralleled those for REMS amounts.
In summary, treatments with estradiol alone or combined with progesterone produced an increase in wake time (up to ∼1.5 h over 24 h) and a decrease in NREMS and/or REMS times, whereas treatments with progesterone alone induced a reduction particularly in REMS time. These effects were more prominent during the dark than the light phase. Males were in an intermediate range, and most closely resembled the progesterone-treated females in terms of baseline NREMS and REMS amounts.
Sleep Deprivation
Gentle interventions for 6 h during the second half of the light period kept the animals awake for > 98% of the total time in all 6 groups (Supplementary Table 1). A few brief episodes of NREMS occurred but the total NREMS time (2–5 min out of 6-h sleep deprivation) was similar among the groups. REMS was absent in all groups. The amounts of NREMS and REMS lost during sleep deprivation relative to sleep amounts during the same 6-h period of baseline recording were similar among the groups (Supplementary Table 1). More interventions were required during the second half of sleep deprivation (F1,5 = 108.62, P < 0.0001), and the time course of this increase was similar among the groups (Group × 3-h time intervals, n.s.).
Recovery Sleep after Sleep Deprivation
NREMS
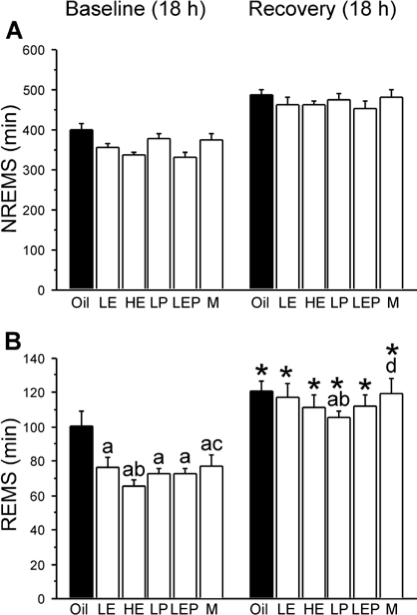
All groups spent more time in NREMS during the 18-h recovery period than during the time-matched 18-h baseline period (main effect of Condition: F1,5 = 463.22, P < 0.0001), with no significant differences among groups (Group × Condition, n.s.; Figure 2A). As shown in Figure 3, NREMS amounts increased greatly during the first 2 h after sleep deprivation in all groups, after which they declined somewhat but remained elevated above baseline levels at most time points for the rest of the dark phase in all groups. During the subsequent 6-h period in the light phase (12–18 h post-deprivation), NREMS amounts returned to baseline levels in all groups with the exception of the LEP group (Figure 3), which continued to show higher values during the second (P < 0.025) and third (P < 0.001) 2-h intervals compared to baseline. As shown in Figure 5A, the NREMS lost during the 6 h of sleep deprivation was only partially (48%–73%) recovered during the 18-h recovery period in all 6 groups, with no significant differences among groups (F5,38 = 1.56, n.s.).
Figure 2.
Amount (mean + SEM, min) of NREMS (A) and REMS (B) during the 18-h recovery period after 6 h of sleep deprivation and during the equivalent 18 h of baseline recording (left) in the Oil (solid bars), LE, HE, LP, LEP, and M (white bars) groups (n = 7 or 8 because a complete sleep recording was not available for every animal). All groups showed NREMS and REMS rebound following sleep deprivation, reaching similar absolute levels for NREMS and REMS, with the exception of the LP group, which had less absolute recovery of REMS amounts than the Oil, LE, and M groups. *Different from corresponding baseline; adifferent from Oil; bdifferent from LE; cdifferent from HE; ddifferent from LP; all P < 0.05 (Fisher LSD post hoc comparisons).
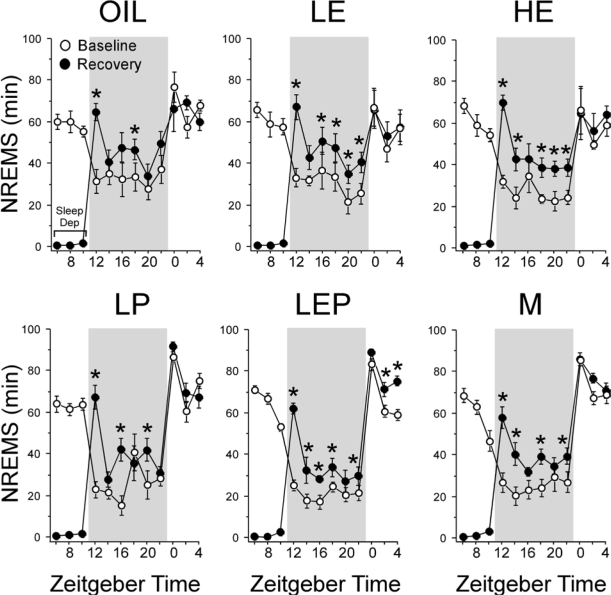
Figure 3.
Time course of NREMS amount (min) in 2-h intervals across the 24-h baseline period (white circles), during 6 h of sleep deprivation (Sleep Dep in top left panel), and during the 18 h recovery period (black circles) in the Oil, LE, HE, LP, LEP, and M groups. Data are shown as means ± SEM, with n = 7-8 per group. In all groups, NREMS amounts increased greatly during the first 2 h after sleep deprivation, then declined but remained elevated at most time points for the rest of the dark phase. During the subsequent light phase, NREMS amounts returned to baseline levels in all groups except the LEP group. *Different from corresponding baseline, P < 0.05 (Fisher LSD post hoc comparisons). Background shading indicates dark phase.
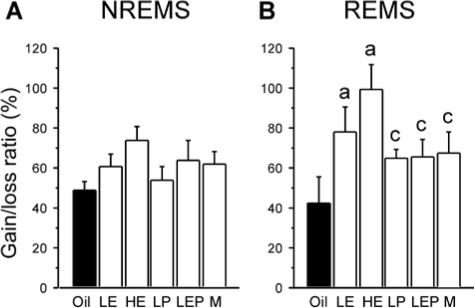
Figure 5.
Percentage of NREMS (A) and REMS (B) recovered after 6 h of sleep deprivation in the Oil (solid bars), LE, HE, LP, LEP, and M (white bars) groups. The percentage was calculated by dividing the difference between NREMS or REMS amounts during the 18-h baseline and recovery periods by the duration of NREMS or REMS lost during the 6 h deprivation period. Data are shown as means + SEM (n = 7-8 per group). The percentage gain of REMS during recovery relative to lost REMS was greater in the LE and HE groups than in the Oil group, while the percentage gain of NREMS was not significantly different among groups. aDifferent from Oil; cdifferent from HE. All P < 0.05 (Fisher LSD post hoc comparisons).
REMS
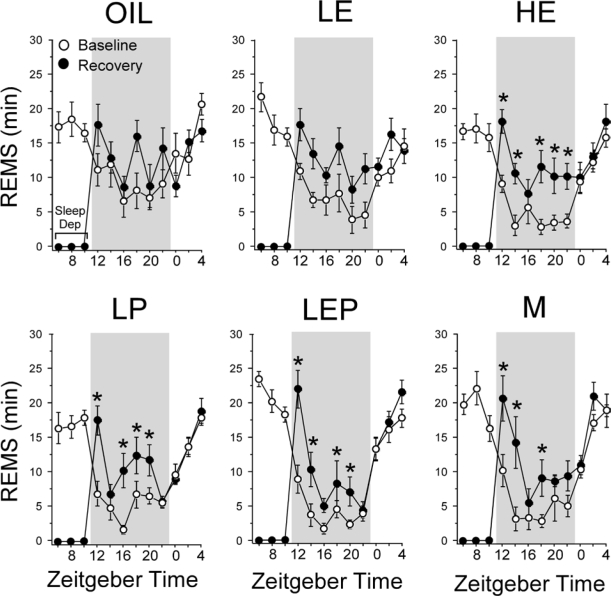
Figure 2B shows that, similar to NREMS, total REMS amounts increased over baseline levels during the 18-h recovery period (main effect of Condition: F1,5 = 299.85, P < 0.0001). However, unlike the NREMS rebound, the extent of this increase in REMS varied with hormonal condition (Group × Condition: F5,38 = 3.39, P < 0.025). During the 18-h recovery period, LE, HE, and LEP rats showed absolute amounts (in min) of REMS that were similar to that shown by the Oil rats, and the LP rats showed significantly less REMS than the Oil, LE, and M groups (all P < 0.025; Figure 2B). However, the percentage increase during the 18-h recovery period over the equivalent 18-h baseline period was smallest in the Oil rats (+23% on average), and was significantly larger in the 4 other female groups (LE, +57%; HE, +73%; LP, +45%; LEP, +57%; all P < 0.05 vs. Oil, respectively; HE > LP, P < 0.025). The M group showed an intermediate percentage increase in REMS (+49%) that was greater than that in the Oil group (P < 0.025) but smaller than that in the HE group (P < 0.05).
The time course of REMS analyzed in 2-h intervals during the 18-h recovery period (Figure 4) paralleled the time course of NREMS (Figure 3). REMS amounts increased greatly during the first 2 h following sleep deprivation; they subsequently declined but remained elevated above baseline levels for most 2-h intervals during the rest of the dark phase in all groups except the Oil group, which showed only intermittent elevations. The rebound increase in REMS did not persist into the subsequent light phase.
Figure 4.
Time course of REMS amount (min) in 2-h intervals across the 24-h baseline period (white circles), during 6 h of sleep deprivation (Sleep Dep in top left panel), and during the 18-h recovery period (black circles) in the Oil, LE, HE, LP, LEP, and M groups. Data are shown as means ± SEM, with n = 7-8 per group. Like NREMS (Fig. 3), in all groups except for the Oil group, REMS amounts increased greatly during the first 2 h following sleep deprivation, after which they declined but remained elevated at most time points during the rest of the dark phase. The Oil group showed only intermittent elevations during the same period. During the subsequent light phase, REMS amounts returned to baseline levels in all groups. *Different from corresponding baseline, P < 0.05 (Fisher LSD post hoc comparisons). Background shading indicates dark phase.
Figure 5B shows the REMS gain during the 18-h recovery period as a percentage of REMS lost during sleep deprivation. Unlike NREMS, the gain/loss ratio for REMS was markedly different among the groups (F5,38 = 3.09, P < 0.025). This ratio was highest in the HE rats, which recovered virtually all of the lost REMS (99%), followed by the LE (78%), LP, LEP (both 65%), and Oil rats (42%; HE > Oil, LP, LEP, and M, all P < 0.05; P < 0.025, LE vs. Oil). Male rats (67%) were intermediate in this range and differed significantly only from HE group (P < 0.025).
In summary, all 6 groups showed significant NREMS and REMS rebound increases following 6 h of sleep deprivation. Although the absolute levels of NREMS and REMS during recovery were fairly similar among the groups, the magnitude of increase in REMS, but not NREMS, was markedly affected by hormonal treatments. In particular, the percentage gain in REMS over baseline was greater in all hormonally treated females than in oil-treated rats, mainly because of the higher REMS baselines in the Oil group. The percentage of REMS gain during recovery relative to lost REMS was also greater in the LE and HE groups compared to the Oil group.
Recovery NREMS EEG Delta Power
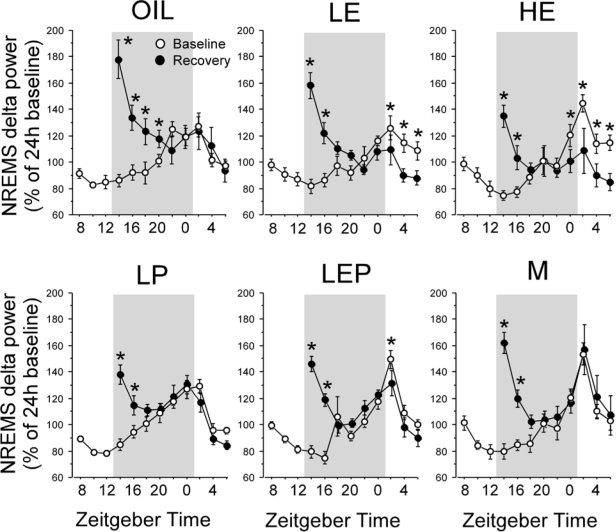
The time course of normalized NREMS delta power during the recovery period was examined in 2-h intervals (Time). As shown in Figure 6, all groups showed a maximum value of normalized NREMS delta power during the first 2 h of recovery followed by a gradual decrease in parallel with the decrease in NREMS amount. However, the time course varied among the groups (Group × Time, F40,288 = 1.89, P < 0.01). During the first 2 h after sleep deprivation, the 6 groups showed different levels of normalized NREMS delta power (F5,42 = 3.06, P < 0.025), with higher values in the Oil rats (179%) than in the HE (135%), LP (138%), and LEP (146%) rats (all P < 0.025 vs. Oil). The values in the M group (162%) were in a high range, significantly higher than in the HE group (P < 0.05). The Oil group continued to show elevated normalized NREMS delta power above baseline throughout the first 8 h of recovery, whereas the increased NREMS delta persisted only for 4 h in all other groups (Figure 6). This increase in NREMS delta power was followed by a decrease in delta power below baseline levels, i.e., a negative rebound, which started at 10 h from the start of sleep deprivation (P < 0.025) and continued throughout the 12- to 18-h post-deprivation period (P < 0.05) in the HE rats, whereas the LE rats showed a negative rebound only during the 12- to 18-h post-deprivation period (P < 0.05).
Figure 6.
Time course of normalized NREMS delta power in 2-h intervals across the 24-h baseline period (white circles) and during the 18-h recovery period (black circles) in the Oil, LE, HE, LP, LEP, and M groups after 6 h of sleep deprivation. EEG power was normalized to the 24 h baseline average at 2-h intervals in each animal. Data are shown as means ± SEM (n = 7-8 per group). During the first 2 h after sleep deprivation, the Oil group showed more normalized NREMS delta power than the other female groups, and continued to show elevated values throughout the first 8 h of recovery, while the increased NREMS delta power persisted only for 4 h in all other groups. A decrease in delta power below baseline levels was observed 10-12 h post-deprivation in the LE and HE groups. *Different from corresponding baseline, P < 0.05 (Fisher LSD post hoc comparisons). Background shading indicates dark phase.
DISCUSSION
We show that treatments with estradiol alone or combined with progesterone increased wake and decreased NREMS and/or REMS, whereas treatments with progesterone alone selectively decreased REMS during spontaneous sleep. Following 6 h of sleep deprivation, treatments with estradiol and/or progesterone enhanced REMS rebound, while decreasing NREMS EEG delta power (a measure of NREMS intensity and drive). These data indicate that estradiol and progesterone have both individual and combined effects on baseline sleep and recovery sleep following sleep deprivation, and that the hormonal effects on baseline sleep do not parallel those on recovery sleep.
The 2-week LE and HE regimens increased plasma estradiol levels to those reported for intact rats during diestrus (7-20 pg/mL on average) and proestrus (up to 50 pg/mL), respectively.32,33 Estradiol levels in some estradiol-treated rats were lower than expected, but in spite of this variability, estradiol treatments generally increased uterus weight and attenuated body weight gain, consistent with previous studies.38,39 Treatments with progesterone increased plasma progesterone levels to levels reported for diestrus (3-30 ng/mL).32,40 In contrast to previous studies,41,42 progesterone did not modulate the effects of estradiol on uterus weight or body weight even though it did affect sleep, possibly because the progesterone dose used was relatively low.
Estradiol Replacement Reduces Baseline Sleep, and Progesterone Replacement Reduces Baseline REMS in the Dark Phase
At baseline, estradiol replacement increased wake at the expense of REMS and, at high dose, also NREMS, consistent with previous studies using different estradiol regimens (see Introduction). The findings that progesterone replacement reduced REMS, and that the addition of progesterone to LE enhanced the estradiol effects, particularly in the dark phase, are novel. Overall, the hormonal effects were seen predominantly during the dark (active) phase and resulted in more robust light-dark differences in the amount of sleep, in particular REMS. The reduction in REMS time by estradiol and progesterone was due to a reduction in the number of REMS episodes, indicating that both hormones disrupted REMS initiation. This impairment could be secondary to impaired maintenance of NREMS, because the occurrence of REMS normally requires a preceding period of NREMS. This possibility is supported by findings in the HE group, which showed shorter NREMS episodes, indicating disrupted NREMS maintenance.
The wake-promoting effects of estradiol are consistent with the reduced sleep in intact animals on proestrus night, after estradiol levels peak,18,28,43 and a general arousal-promoting effect of estradiol in rodents.44 Progesterone replacement decreased REMS in the present study, whereas two previous studies using male rats reported a decrease in both REMS and wake amounts after acute injection of a high dose of progesterone (30–180 mg/kg).45,46 This discrepancy may be due to differences in dose, route of administration, durations of treatment, and/or the sex of animals.
Co-administration of estradiol and progesterone had an additive effect in increasing wake during the dark phase. No additive effect in decreasing REMS was observed during the dark phase; however, it is noteworthy that during the light phase, the LEP group had more REMS than either the LE or LP group, which may represent a rebound increase in REMS following decreased REMS during the dark phase. Similar to this observation, previous studies showed that acute injections of estradiol followed by progesterone reduced REMS during the dark phase in ovariectomized rats,22,25 but it is unclear whether there was an interaction between the 2 hormones because the effects of progesterone alone were not reported in those studies.
Little is known about the mechanisms by which ovarian hormones affect sleep/wake states. Estradiol and progesterone can have both genomic and non-genomic effects.47,48 Nuclear estrogen and progesterone receptors, which mediate genomic effects, are present in many sleep-wake regulatory nuclei, including the basal forebrain, hypothalamus/preoptic area, dorsal raphe nucleus and locus coeruleus,49–51 suggesting a possible direct action of estradiol and progesterone at some nuclei of the sleep/wake regulatory system. In fact, estradiol treatment can increase the expression of synthetic enzymes for serotonin and noradrenaline52,53 in brainstem neurons that are thought to promote wake and suppress REMS,54,55 which is consistent with the reduction of REMS after estradiol and/or progesterone treatment in the present study.
In addition, estradiol has been proposed to increase wake through the inhibition of the sleep-promoting ventrolateral preoptic nucleus by decreasing lipocalin-type prostaglandin D2 synthase18,56 and adenosine A2A receptor mRNAs57 in that nucleus. It is also possible that estradiol and progesterone promote wake by non-genomically modulating ionotropic neurotransmitter receptors58 that are involved in sleep-wake regulation. Consistent with these possibilities, estradiol modulates c-Fos expression (a marker of neuronal activation) in sleep and wake regulatory areas.18,37,59
Another potential mechanism whereby estradiol and progesterone could affect sleep is through modulation of the circadian pattern of sleep-wake or locomotor activity. Estrogen receptors are present in the suprachiasmatic nucleus (SCN), the site of the principal circadian clock in mammals,51,60,61 including SCN afferents and efferents.62,63 SCN neurons respond to estradiol with an increase in light-induced expression of transcription factors,64 shifts in the expression rhythm of clock-related genes,65 and increased cell firing in vitro.66 Estradiol replacement shortens the free-running locomotor activity rhythm in ovariectomized rats and hamsters67–69; this effect of estradiol can be antagonized by progesterone.68–70 Thus, it is possible that some of the changes in the diurnal pattern of sleep-wake states observed after estradiol/progesterone treatments might be due to altered circadian time-keeping mechanisms in the SCN.
Finally, we cannot exclude the possibility that at least some of the estradiol/progesterone effects on spontaneous sleep-wake could be secondary to the modulation by these hormones of other physiological functions, such as energy balance71 and the hypothalamic-pituitary-adrenal axis,72 which can indirectly influence sleep.
Estradiol and Progesterone Replacement Promotes REMS and Reduces NREMS EEG Delta Power During Recovery from Sleep Deprivation
In response to 6 h of sleep deprivation, all rats showed a typical increase in NREMS and REMS, reaching similar absolute levels for both, with the exception of the LP group, which showed less absolute recovery of REMS amounts than the Oil and other groups. The relative increase in REMS from baseline and the gain/loss ratio were greatly enhanced by hormonal treatments, particularly with estradiol. We interpret these data to suggest a more robust REMS homeostatic response to total sleep deprivation particularly in estradiol-treated rats. This finding supports a possible role for estradiol in promoting recovery REMS, despite its inhibitory influence on baseline REMS. The mechanisms controlling rebound REMS after sleep deprivation are not well understood and may be different from those regulating REMS expression in the absence of sleep deprivation. The median preoptic nucleus contains estrogen and progesterone receptors,73,74 and it has been proposed to play a specific role in REMS homeostasis.75 It is possible that estradiol/progesterone treatment acts via this nucleus to augment REMS amounts after sleep deprivation.
There are, however, alternative interpretations possible for these findings. One is that, compared to oil-treated rats, estradiol-treated rats at baseline had lower REMS amounts and expressed a higher proportion of their daily REMS in the light phase (i.e., they showed a higher light/dark ratio). Thus, estradiol-treated rats could actually have lost a higher proportion of their daily REMS during the sleep deprivation period that occurred in the light phase, resulting in a stronger drive for REMS during the recovery period. Another possibility is that the REMS-inhibiting effect of estradiol/progesterone observed during baseline may not be expressed in the face of increased homeostatic sleep pressure.
Although estradiol/progesterone replacements altered neither baseline NREMS delta power nor rebound NREMS amounts, they reduced recovery NREMS delta power in terms of both the initial level and the duration of the elevation. In addition, late in the recovery phase, estradiol replacement induced a negative rebound in NREMS delta power, which was completely abolished by co-administration of progesterone. These results are consistent with a recent report that postmenopausal women taking hormone therapy (estradiol + progesterone) had a smaller increase in NREMS delta power following one night of sleep deprivation than did women who were not using hormone therapy.11 It is unlikely that the reduced NREMS delta power in estradiol- and progesterone-treated rats is due to reduced sleep deprivation-induced sleep pressure in these rats for two reasons. First, all rats, regardless of hormonal treatment, required the same number of interventions to stay awake during sleep deprivation, consistent with our previous study.37 Second, the NREMS latency following sleep deprivation was actually slightly shorter in hormone-treated rats, even though the amount of sleep loss during sleep deprivation was similar among the groups. The possibility that NREMS delta activity was actively inhibited by increased REMS amounts in estradiol-treated rats would be consistent with the idea of a mutual inhibitory interaction between NREMS intensity and REMS propensity.76,77 These results suggest that the NREMS delta power rebound is attenuated by estradiol, and that this effect is modulated by progesterone.
The mechanisms underlying the reduced recovery of NREMS delta power after estradiol/progesterone treatment, like those underlying homeostatic regulation of sleep in general, are not well understood. However, the basal forebrain is one brain region implicated in sleep recovery after sleep loss,78,79 and it is possible that estradiol and progesterone act on the basal forebrain and its projections to the cortex to alter homeostatic regulation of slow wave generation.
Methodological Issues and Clinical Implications
We based our assessments of the effects of estradiol and progesterone treatments on comparisons with oil-treated ovariectomized rats, because ovariectomy dramatically reduces estradiol and progesterone levels and provides a baseline against which to compare hormonal effects. However, using ovariectomized animals as a reference group can also introduce issues of interpretation. For example, ovariectomy, by removing the negative feedback of estradiol on gonadotropin secretion, leads to high levels of plasma gonadotropin levels,32,80 which do not occur in the hormonally treated groups. These hormonal changes could influence sleep, and previous studies have reported that basal levels of REMS were increased in ovariectomized rats compared to intact females at any stage of the estrous cycle.23,43,81 Therefore, estradiol and progesterone treatments may influence sleep regulation either by restoring ovarian hormone levels and/or by restoring baseline gonadotropin levels (through feedback on gonadotropin release).
Use of intact females is another option for studying hormonal effects on sleep and sleep recovery after sleep deprivation. But because of the dynamic changes over short periods of time in ovarian hormone levels (and gonadotropins) during natural estrous cycles,32 it is difficult to identify periods of time with stable endocrine profiles lengthy enough to accommodate baseline, sleep deprivation and recovery periods. Use of intact or surgically manipulated males as controls is problematic because of the genetic and numerous epigenetic differences between the sexes. Thus, it is challenging to identify ideal controls for studying the effects of female steroid hormones on sleep in rats, and conclusions reached should be tempered by awareness of these constraints.
In the Introduction, we summarized reported differences between effects of reproductive hormones on sleep in women and in rodent models. Multiple factors in the studies cited, including differences in age, the length of hormone deprivation before replacement, the types of hormones used, the dose and duration of treatment, and the presence or absence of the ovaries, may be responsible in part for these differences. Extrapolating from rodent studies to humans will require a greater understanding of the roles of each of these factors, and the use of a variety of models. Our use of young ovariectomized rats may be appropriate as a model of chemical or surgical oophorectomy in young women being treated for cancer,82 but studies of older rodents may be more appropriate for modeling endocrine effects on sleep during human menopause, and the potential impact of hormone replacement therapy.
Conclusions
We show for the first time that estradiol/progesterone replacement can influence the ability to recover sleep after short-term sleep deprivation in ovariectomized rats, enhancing REMS recovery while reducing NREMS delta power. Importantly, these effects on recovery sleep do not parallel the hormones' effects on baseline sleep; i.e., reduced sleep during the active phase. Although some of these effects may be interrelated, it is also possible that the mechanisms regulating baseline and recovery sleep are modulated differently by ovarian hormones. This study also shows a complex interaction between the effects of estradiol and progesterone on different aspects of baseline and homeostatic sleep regulation. The mechanisms involved need to be elucidated in order to understand the impact of hormone replacement on the ability of women to respond to sleep loss with compensatory increases in sleep, and to benefit from recovery sleep in relation to performance and cognitive function.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Rusak is a research consultant for Institut de Recherches Internationales Servier. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Diane Wilkinson for conducting hormone assays, Joan Burns for assistance with blood collection, and Michelle Black for assistance with sleep deprivation and with statistics. We also thank Dr. Michael Wilkinson for his assistance and for helpful comments on an earlier version of this manuscript. This work was supported by a Canadian Institutes of Health Research grant (MOP-67085).
ABBREVIATIONS
- HE
High estradiol
- LE
Low estradiol
- LP
Low progesterone
- LEP
Low estradiol + low progesterone
- M
Males
- NREMS
Non-rapid eye movement sleep
- REMS
Rapid eye movement sleep
- SCN
Suprachiasmatic nucleus
- SEM
Standard error of the mean
- ZT
Zeitgeber time
APPENDIX
Table S1.
Effectiveness of the Sleep Deprivation Procedure
| Variables | Treatment groups |
|||||
|---|---|---|---|---|---|---|
| Oil (n) | LE (n) | HE (n) | LP (n) | LEP (n) | M (n) | |
| NREMS (min) | ||||||
| 6 h sleep deprivation (ZT7-12) | 3 ± 1 (8) | 2 ± 1 (8) | 5 ± 1 (8) | 4 ± 1 (8) | 4 ± 2 (8) | 4 ± 1 (8) |
| Baseline - sleep deprivation | −172 ± 5 | −180 ± 7 | −176 ± 7 | −185 ± 8 | −187 ± 4 | −174 ± 9 |
| REMS (min) | ||||||
| 6 h sleep deprivation (ZT7-12) | 0 ± 0 (8) | 0 ± 0 (8) | 0 ± 0 (8) | 0 ± 0 (8) | 0 ± 0 (8) | 0 ± 0 (8) |
| Baseline - sleep deprivation | −52 ± 4 | −55 ± 3 | −50 ± 3 | −51 ± 3 | −62 ± 3 | −58 ± 4 |
| Number of interventions | ||||||
| Sleep deprivation (ZT7-9) | 33 ± 9 (6) | 25 ± 5 (4) | 28 ± 5 (5) | 24 ± 4 (6) | 32 ± 5 (5) | 31 ± 4 (5) |
| Sleep deprivation (ZT10-12) | 105 ± 19 | 88 ± 12 | 112 ± 33 | 97 ± 14 | 99 ± 16 | 170 ± 26 |
| 6 h sleep deprivation (ZT7-12) | 139 ± 27 | 113 ± 14 | 140 ± 36 | 121 ± 17 | 131 ± 19 | 202 ± 27 |
For NREMS and REMS, the top line shows the mean duration in min of the sleep stage ( ± SEM) during the 6-h sleep deprivation period. The second line shows the decrease in the sleep stage relative to baseline at that time of day (baseline-sleep deprivation). The bottom panel shows the number of interventions required during sleep deprivation to prevent sleep during the first 3 h (ZT7-9), the second 3 h (ZT10-12), and the full 6-h period of deprivation (ZT7-12). ZT = zeitgeber time.
REFERENCES
- 1.Dzaja A, Arber S, Hislop J, et al. Women's sleep in health and disease. J Psychiatr Res. 2005;39:55–76. doi: 10.1016/j.jpsychires.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Moline ML, Broch L, Zak R. Sleep in women across the life cycle from adulthood through menopause. Med Clin North Am. 2004;88:705–36. doi: 10.1016/j.mcna.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Polo-Kantola P, Erkkola R, Helenius H, Irjala K, Polo O. When does estrogen replacement therapy improve sleep quality? Am J Obstet Gynecol. 1998;178:1002–9. doi: 10.1016/s0002-9378(98)70539-3. [DOI] [PubMed] [Google Scholar]
- 4.Thomson J, Oswald I. Effect of oestrogen on the sleep, mood, and anxiety of menopausal women. Br Med J. 1977;2:1317–9. doi: 10.1136/bmj.2.6098.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montplaisir J, Lorrain J, Denesle R, Petit D. Sleep in menopause: differential effects of two forms of hormone replacement therapy. Menopause. 2001;8:10–6. doi: 10.1097/00042192-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Hachul H, Bittencourt LR, Andersen ML, Haidar MA, Baracat EC, Tufik S. Effects of hormone therapy with estrogen and/or progesterone on sleep pattern in postmenopausal women. Int J Gynaecol Obstet. 2008;103:207–12. doi: 10.1016/j.ijgo.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Antonijevic IA, Stalla GK, Steiger A. Modulation of the sleep electroencephalogram by estrogen replacement in postmenopausal women. Am J Obstet Gynecol. 2000;182:277–82. doi: 10.1016/s0002-9378(00)70211-0. [DOI] [PubMed] [Google Scholar]
- 8.Schüussler P, Kluge M, Yassouridis A, et al. Progesterone reduces wakefulness in sleep EEG and has no effect on cognition in healthy postmenopausal women. Psychoneuroendocrinology. 2008;33:1124–31. doi: 10.1016/j.psyneuen.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Karakorpi M, Alhola P, Urrila AS, et al. Hormone treatment gives no benefit against cognitive changes caused by acute sleep deprivation in postmenopausal women. Neuropsychopharmacology. 2006;31:2079–88. doi: 10.1038/sj.npp.1301056. [DOI] [PubMed] [Google Scholar]
- 10.Alhola P, Kylmala M, Urrila AS, et al. Does hormone therapy affect attention and memory in sleep-deprived women? Climacteric. 2008;11:221–32. doi: 10.1080/13697130801958832. [DOI] [PubMed] [Google Scholar]
- 11.Kalleinen N, Polo O, Himanen SL, Joutsen A, Urrila AS, Polo-Kantola P. Sleep deprivation and hormone therapy in postmenopausal women. Sleep Med. 2006;7:436–47. doi: 10.1016/j.sleep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Balkin TJ, Belenky G, Drake C, Rosa R, Rosekind M. Sleep in America Poll. 2008 doi: 10.1111/j.1365-2869.2010.00890.x. National Sleep Foundation. http://www.sleepfoundation.org/atf/cf/%7Bf6bf2668-a1b4-4fe8-8d1a-a5d39340d9cb%7D/2008%20POLL%20SOF.PDF. [DOI] [PubMed]
- 13.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 14.Picardo CM, Nichols M, Edelman A, Jensen JT. Women's knowledge and sources of information on the risks and benefits of oral contraception. J Am Med Women's Assoc. 2003;58:112–6. [PubMed] [Google Scholar]
- 15.Kotz K, Alexander JL, Dennerstein L. Estrogen and androgen hormone therapy and well-being in surgically postmenopausal women. J Womens Health (Larchmt) 2006;15:898–908. doi: 10.1089/jwh.2006.15.898. [DOI] [PubMed] [Google Scholar]
- 16.Yamaoka S. Participation of limbic-hypothalamic structures in circadian rhythm of slow wave sleep and paradoxical sleep in the rat. Brain Res. 1978;151:255–68. doi: 10.1016/0006-8993(78)90883-1. [DOI] [PubMed] [Google Scholar]
- 17.Colvin GB, Whitmoyer DI, Lisk RD, Walter DO, Sawyer CH. Changes in sleep-wakefulness in female rats during circadian and estrous cycles. Brain Res. 1968;7:173–81. doi: 10.1016/0006-8993(68)90095-4. [DOI] [PubMed] [Google Scholar]
- 18.Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci. 2008;27:1780–92. doi: 10.1111/j.1460-9568.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- 19.Kleinlogel H. The female rat's sleep during oestrous cycle. Neuropsychobiology. 1983;10:228–37. doi: 10.1159/000118016. [DOI] [PubMed] [Google Scholar]
- 20.Zhang SQ, Kimura M, Inoue S. Sleep patterns in cyclic and pseudopregnant rats. Neurosci Lett. 1995;193:125–8. doi: 10.1016/0304-3940(95)11685-p. [DOI] [PubMed] [Google Scholar]
- 21.Barbe L, Faure JM, Bensch C, Dufy B, Vincent JD. The influence of ovarian steroids on the distribution of sleep elements in white female rats [in French] J Physiol (Paris) 1970;62(Suppl 2):240–1. [PubMed] [Google Scholar]
- 22.Branchey M, Branchey L, Nadler RD. Effects of estrogen and progesterone on sleep patterns of female rats. Physiol Behav. 1971;6:743–6. doi: 10.1016/0031-9384(71)90267-8. [DOI] [PubMed] [Google Scholar]
- 23.Colvin GB, Whitmoyer DI, Sawyer CH. Circadian sleep-wakefulness patterns in rats after ovariectomy and treatment with estrogen. Exp Neurol. 1969;25:616–25. doi: 10.1016/0014-4886(69)90104-6. [DOI] [PubMed] [Google Scholar]
- 24.Matsushima M, Takeichi M. Effects of intraventricular implantation of crystalline estradiol benzoate on the sleep-wakefulness circadian rhythm of ovariectomized rats. Jpn J Psychiatry Neurol. 1990;44:111–21. doi: 10.1111/j.1440-1819.1990.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamaoka S. Drug and hormonal modification of sleep rhythms in female rats: Changes in aging. In: Walker RF, Cooper RL, editors. Experimental and clinical interventions in aging. New York: Marcel Dekker; 1983. pp. 369–95. [Google Scholar]
- 26.Pawlyk AC, Alfinito PD, Johnston GH, Deecher DC. Subchronic 17alpha-ethinyl estradiol differentially affects subtypes of sleep and wakefulness in ovariectomized rats. Horm Behav. 2008;53:217–24. doi: 10.1016/j.yhbeh.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Pawlyk AC, Alfinito PD, Deecher DC. Effect of 17alpha-ethinyl estradiol on active phase rapid eye movement sleep microarchitecture. Eur J Pharmacol. 2008;591:315–8. doi: 10.1016/j.ejphar.2008.06.084. [DOI] [PubMed] [Google Scholar]
- 28.Schwierin B, Borbely AA, Tobler I. Sleep homeostasis in the female rat during the estrous cycle. Brain Res. 1998;811:96–104. doi: 10.1016/s0006-8993(98)00991-3. [DOI] [PubMed] [Google Scholar]
- 29.Paul KN, Dugovic C, Turek FW, Laposky AD. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep. 2006;29:1211–23. doi: 10.1093/sleep/29.9.1211. [DOI] [PubMed] [Google Scholar]
- 30.Vyazovskiy VV, Kopp C, Wigger E, Jones ME, Simpson ER, Tobler I. Sleep and rest regulation in young and old oestrogen-deficient female mice. J Neuroendocrinol. 2006;18:567–76. doi: 10.1111/j.1365-2826.2006.01452.x. [DOI] [PubMed] [Google Scholar]
- 31.Andersen ML, Antunes IB, Silva A, Alvarenga TA, Baracat EC, Tufik S. Effects of sleep loss on sleep architecture in Wistar rats: Gender-specific rebound sleep. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:975–83. doi: 10.1016/j.pnpbp.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–26. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 33.Henderson SR, Baker C, Fink G. Oestradiol-17beta and pituitary responsiveness to luteinizing hormone releasing factor in the rat: a study using rectangular pulses of oestradiol-17beta monitored by non-chromatographic radioimmunoassay. J Endocrinol. 1977;73:441–53. doi: 10.1677/joe.0.0730441. [DOI] [PubMed] [Google Scholar]
- 34.Dubal DB, Wise PM. Neuroprotective effects of estradiol in middle-aged female rats. Endocrinology. 2001;142:43–8. doi: 10.1210/endo.142.1.7911. [DOI] [PubMed] [Google Scholar]
- 35.Dubal DB, Kashon ML, Pettigrew LC, et al. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–8. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Mannino CA, South SM, Inturrisi CE, Quinones-Jenab V. Pharmacokinetics and effects of 17beta-estradiol and progesterone implants in ovariectomized rats. J Pain. 2005;6:809–16. doi: 10.1016/j.jpain.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Deurveilher S, Cumyn E, Peers T, Rusak B, Semba K. Estradiol replacement enhances sleep deprivation-induced c-Fos immunoreactivity in forebrain arousal regions of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1328–40. doi: 10.1152/ajpregu.90576.2008. [DOI] [PubMed] [Google Scholar]
- 38.Gogos A, Van den Buuse M. Estrogen and progesterone prevent disruption of prepulse inhibition by the serotonin-1A receptor agonist 8-hydroxy-2-dipropylaminotetralin. J Pharmacol Exp Ther. 2004;309:267–74. doi: 10.1124/jpet.103.061432. [DOI] [PubMed] [Google Scholar]
- 39.Ke HZ, Chen HK, Simmons HA, et al. Comparative effects of droloxifene, tamoxifen, and estrogen on bone, serum cholesterol, and uterine histology in the ovariectomized rat model. Bone. 1997;20:31–9. doi: 10.1016/s8756-3282(96)00313-4. [DOI] [PubMed] [Google Scholar]
- 40.Dohler KD, Wuttke W. Serum LH, FSH, prolactin and progesterone from birth to puberty in female and male rats. Endocrinology. 1974;94:1003–8. doi: 10.1210/endo-94-4-1003. [DOI] [PubMed] [Google Scholar]
- 41.Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J Comp Physiol Psychol. 1975;88:183–93. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- 42.Medlock KL, Forrester TM, Sheehan DM. Progesterone and estradiol interaction in the regulation of rat uterine weight and estrogen receptor concentration. Proc Soc Exp Biol Med. 1994;205:146–53. doi: 10.3181/00379727-205-43690. [DOI] [PubMed] [Google Scholar]
- 43.Fang J, Fishbein W. Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res. 1996;734:275–85. [PubMed] [Google Scholar]
- 44.Pfaff D, Frohlich J, Morgan M. Hormonal and genetic influences on arousal--sexual and otherwise. Trends Neurosci. 2002;25:45–50. doi: 10.1016/s0166-2236(00)02084-1. [DOI] [PubMed] [Google Scholar]
- 45.Lancel M, Faulhaber J, Holsboer F, Rupprecht R. Progesterone induces changes in sleep comparable to those of agonistic GABAA receptor modulators. Am J Physiol. 1996;271:E763–72. doi: 10.1152/ajpendo.1996.271.4.E763. [DOI] [PubMed] [Google Scholar]
- 46.Lancel M, Faulhaber J, Holsboer F, Rupprecht R. The GABA(A) receptor antagonist picrotoxin attenuates most sleep changes induced by progesterone. Psychopharmacology (Berl) 1999;141:213–9. doi: 10.1007/s002130050827. [DOI] [PubMed] [Google Scholar]
- 47.Pluchino N, Luisi M, Lenzi E, et al. Progesterone and progestins: effects on brain, allopregnanolone and beta-endorphin. J Steroid Biochem Mol Biol. 2006;102:205–13. doi: 10.1016/j.jsbmb.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 48.Zhang D, Trudeau VL. Integration of membrane and nuclear estrogen receptor signaling. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:306–15. doi: 10.1016/j.cbpa.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 49.Greco B, Allegretto EA, Tetel MJ, Blaustein JD. Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology. 2001;142:5172–81. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- 50.Curran-Rauhut MA, Petersen SL. The distribution of progestin receptor mRNA in rat brainstem. Brain Res Gene Expr Patterns. 2002;1:151–7. doi: 10.1016/s1567-133x(02)00011-x. [DOI] [PubMed] [Google Scholar]
- 51.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 52.Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006;60:288–95. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 53.Serova L, Rivkin M, Nakashima A, Sabban EL. Estradiol stimulates gene expression of norepinephrine biosynthetic enzymes in rat locus coeruleus. Neuroendocrinology. 2002;75:193–200. doi: 10.1159/000048237. [DOI] [PubMed] [Google Scholar]
- 54.Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–86. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 55.McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–30. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Mong JA, Devidze N, Goodwillie A, Pfaff DW. Reduction of lipocalin-type prostaglandin D synthase in the preoptic area of female mice mimics estradiol effects on arousal and sex behavior. Proc Natl Acad Sci U S A. 2003;100:15206–11. doi: 10.1073/pnas.2436540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ribeiro AC, Pfaff DW, Devidze N. Estradiol modulates behavioral arousal and induces changes in gene expression profiles in brain regions involved in the control of vigilance. Eur J Neurosci. 2009;29:795–801. doi: 10.1111/j.1460-9568.2009.06620.x. [DOI] [PubMed] [Google Scholar]
- 58.Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–6. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- 59.Peterfi Z, Churchill L, Hajdu I, Obal F, Jr, Krueger JM, Parducz A. Fos-immunoreactivity in the hypothalamus: dependency on the diurnal rhythm, sleep, gender, and estrogen. Neuroscience. 2004;124:695–707. doi: 10.1016/j.neuroscience.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 60.Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech Ageing Dev. 2002;123:593–601. doi: 10.1016/s0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- 61.Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kallo I. Oestrogen receptor alpha and beta immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol. 2008;20:1270–7. doi: 10.1111/j.1365-2826.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- 62.de la Iglesia HO, Blaustein JD, Bittman EL. The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. NeuroReport. 1995;6:1715–22. doi: 10.1097/00001756-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 63.de La Iglesia HO, Blaustein JD, Bittman EL. Oestrogen receptor-alpha-immunoreactive neurones project to the suprachiasmatic nucleus of the female Syrian hamster. J Neuroendocrinol. 1999;11:481–90. doi: 10.1046/j.1365-2826.1999.00341.x. [DOI] [PubMed] [Google Scholar]
- 64.Abizaid A, Mezei G, Horvath TL. Estradiol enhances light-induced expression of transcription factors in the SCN. Brain Res. 2004;1010:35–44. doi: 10.1016/j.brainres.2004.01.089. [DOI] [PubMed] [Google Scholar]
- 65.Nakamura TJ, Moriya T, Inoue S, et al. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res. 2005;82:622–30. doi: 10.1002/jnr.20677. [DOI] [PubMed] [Google Scholar]
- 66.Fatehi M, Fatehi-Hassanabad Z. Effects of 17beta-estradiol on neuronal cell excitability and neurotransmission in the suprachiasmatic nucleus of rat. Neuropsychopharmacology. 2008;33:1354–64. doi: 10.1038/sj.npp.1301523. [DOI] [PubMed] [Google Scholar]
- 67.Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol. 1981;241:R62–6. doi: 10.1152/ajpregu.1981.241.1.R62. [DOI] [PubMed] [Google Scholar]
- 68.Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196:305–7. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi JS, Menaker M. Interaction of estradiol and progesterone: effects on circadian locomotor rhythm of female golden hamsters. Am J Physiol. 1980;239:R497–504. doi: 10.1152/ajpregu.1980.239.5.R497. [DOI] [PubMed] [Google Scholar]
- 70.Rodier WI., 3rd Progesterone-estrogen interactions in the control of activity-wheel running in the female rat. J Comp Physiol Psychol. 1971;74:365–73. doi: 10.1037/h0030568. [DOI] [PubMed] [Google Scholar]
- 71.Wade GN. Sex steroids and energy balance: sites and mechanisms of action. Ann N Y Acad Sci. 1986;474:389–99. doi: 10.1111/j.1749-6632.1986.tb28029.x. [DOI] [PubMed] [Google Scholar]
- 72.Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144:311–21. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- 73.Hagihara K, Hirata S, Osada T, Hirai M, Kato J. Distribution of cells containing progesterone receptor mRNA in the female rat di- and telencephalon: an in situ hybridization study. Brain Res Mol Brain Res. 1992;14:239–49. doi: 10.1016/0169-328x(92)90179-f. [DOI] [PubMed] [Google Scholar]
- 74.Simerly RB, Carr AM, Zee MC, Lorang D. Ovarian steroid regulation of estrogen and progesterone receptor messenger ribonucleic acid in the anteroventral periventricular nucleus of the rat. J Neuroendocrinol. 1996;8:45–56. doi: 10.1111/j.1365-2826.1996.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 75.Gvilia I, Turner A, McGinty D, Szymusiak R. Preoptic area neurons and the homeostatic regulation of rapid eye movement sleep. J Neurosci. 2006;26:3037–44. doi: 10.1523/JNEUROSCI.4827-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tobler I, Borbely AA. The effect of 3-h and 6-h sleep deprivation on sleep and EEG spectra of the rat. Behav Brain Res. 1990;36:73–8. doi: 10.1016/0166-4328(90)90161-7. [DOI] [PubMed] [Google Scholar]
- 77.Endo T, Schwierin B, Borbely AA, Tobler I. Selective and total sleep deprivation: effect on the sleep EEG in the rat. Psychiatry Res. 1997;66:97–110. doi: 10.1016/s0165-1781(96)03029-6. [DOI] [PubMed] [Google Scholar]
- 78.Kaur S, Junek A, Black MA, Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J Neurosci. 2008;28:491–504. doi: 10.1523/JNEUROSCI.1585-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalinchuk AV, McCarley RW, Stenberg D, Porkka-Heiskanen T, Basheer R. The role of cholinergic basal forebrain neurons in adenosine-mediated homeostatic control of sleep: Lessons from 192 IgG-saporin lesions. Neuroscience. 2008:238–53. doi: 10.1016/j.neuroscience.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wise PM, Ratner A. Effect of ovariectomy on plasma LH, FSH, estradiol, and progesterone and medial basal hypothalamic LHRH concentrations in old and young rats. Neuroendocrinology. 1980;30:15–9. doi: 10.1159/000122968. [DOI] [PubMed] [Google Scholar]
- 81.Li H, Satinoff E. Body temperature and sleep in intact and ovariectomized female rats. Am J Physiol. 1996;271:R1753–8. doi: 10.1152/ajpregu.1996.271.6.R1753. [DOI] [PubMed] [Google Scholar]
- 82.Group EBCTC. Ovarian ablation in early breast cancer: overview of the randomised trials. Lancet. 1996;348:1189–96. [PubMed] [Google Scholar]