Abstract
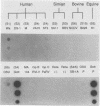
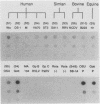
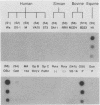
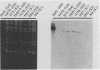
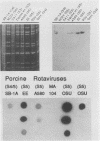
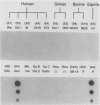
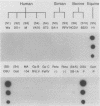
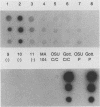
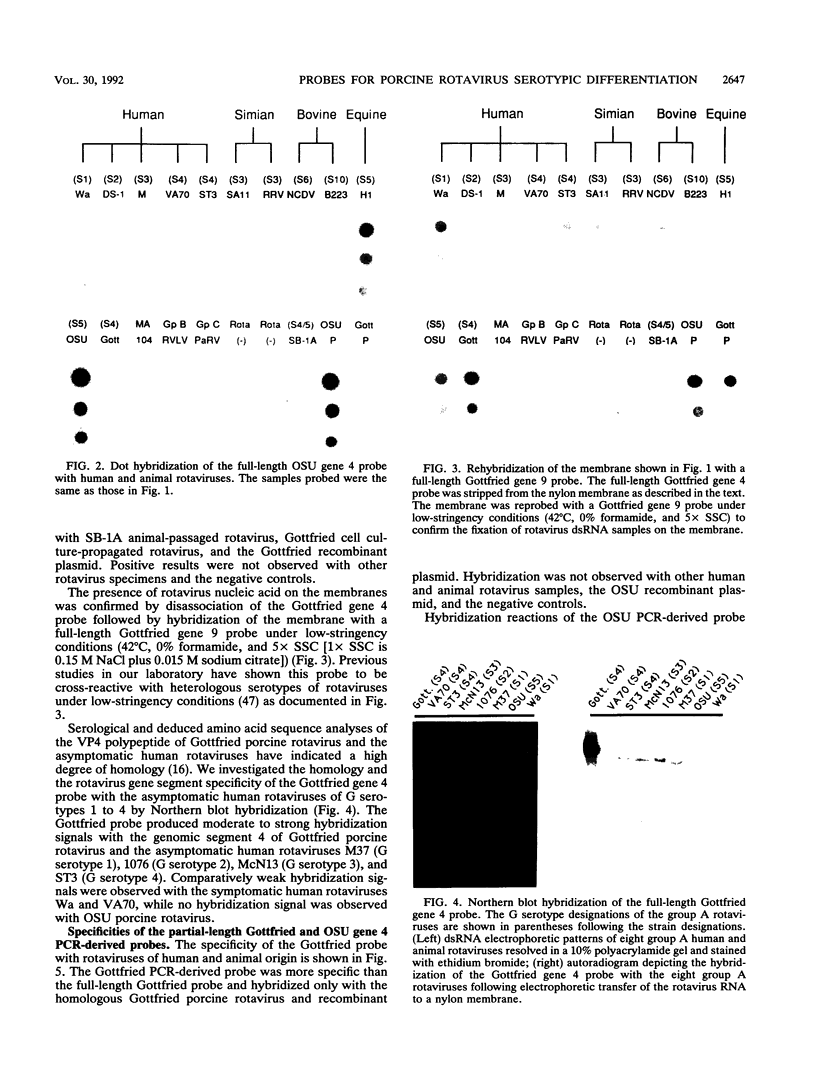
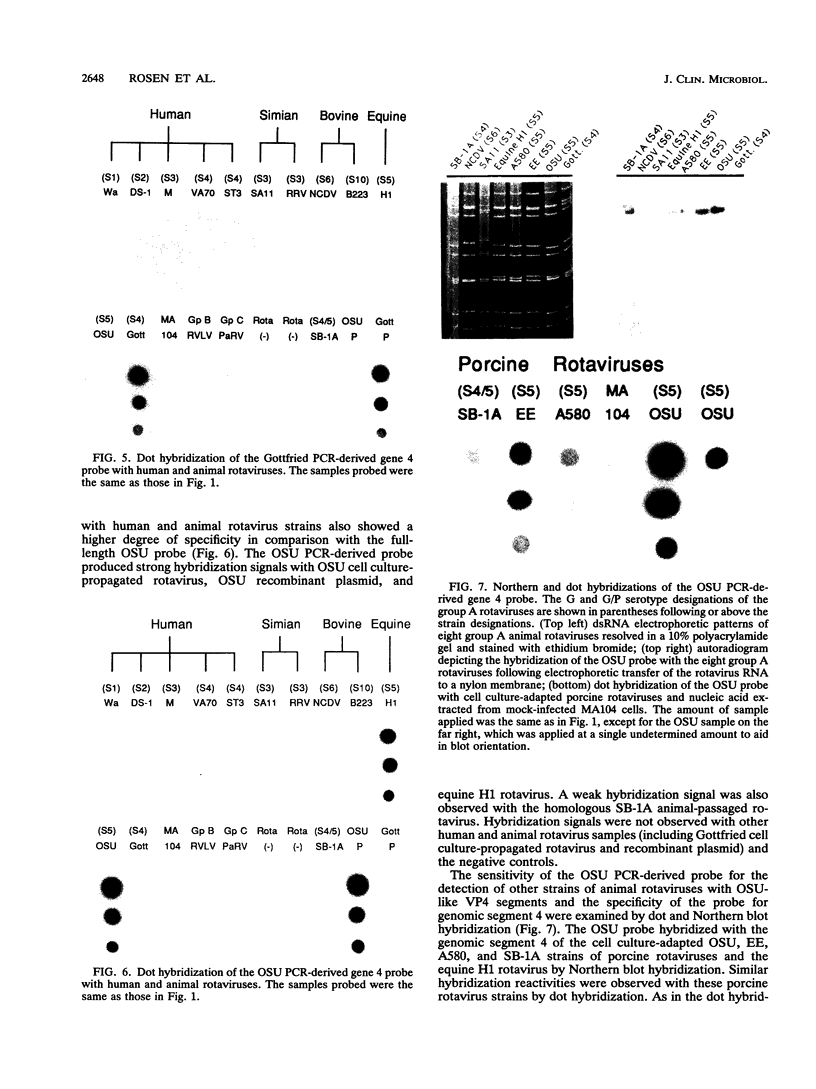
To determine the VP4 (P type) specificity of porcine rotaviruses, full- and partial-length gene 4 probes were produced from cloned Gottfried and OSU porcine rotavirus genomic segment 4 cDNAs. The gene 4 segments from the prototype Gottfried (VP7 serotype 4) and OSU (VP7 serotype 5) porcine rotavirus strains were selected for study because of their distinct P types and the occurrence of rotaviruses with similar serotypes among swine. Partial-length gene 4 cDNAs were produced and amplified by the polymerase chain reaction (PCR) and encompassed portions of the variable region (nucleotides 211 to 612) of VP8 encoded by genomic segment 4. The hybridization stringency conditions necessary for optimal probe specificity and sensitivity were determined by dot or Northern (RNA) blot hybridizations against a diverse group of human and animal rotaviruses of heterologous group A serotypes and against representative group B and C porcine rotaviruses. The PCR-derived gene 4 probes were more specific than the full-length gene 4 probes but demonstrated equivalent sensitivity. The Gottfried PCR-derived probe hybridized with Gottfried, SB2, SB3, and SB5 G serotype 4 porcine rotaviruses. The OSU PCR-derived probe hybridized with OSU, EE, A580, and SB-1A porcine rotaviruses and equine H1 rotavirus. Results of the hybridization reactions of the PCR-derived gene 4 probes with selected porcine rotavirus strains agreed with previous serological or genetic analyses, indicating their suitability as diagnostic reagents.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellinzoni R. B., Mattion N. M., Matson D. O., Blackhall J., La Torre J. L., Scodeller E. A., Urasawa S., Taniguchi K., Estes M. K. Porcine rotaviruses antigenically related to human rotavirus serotypes 1 and 2. J Clin Microbiol. 1990 Mar;28(3):633–636. doi: 10.1128/jcm.28.3.633-636.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohl E. H., Theil K. W., Saif L. J. Isolation and serotyping of porcine rotaviruses and antigenic comparison with other rotaviruses. J Clin Microbiol. 1984 Feb;19(2):105–111. doi: 10.1128/jcm.19.2.105-111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning G. F., Chalmers R. M., Fitzgerald T. A., Corley K. T., Campbell I., Snodgrass D. R. Rotavirus serotype G3 predominates in horses. J Clin Microbiol. 1992 Jan;30(1):59–62. doi: 10.1128/jcm.30.1.59-62.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning G. F., Chalmers R. M., Fitzgerald T. A., Snodgrass D. R. Serological and genomic characterization of L338, a novel equine group A rotavirus G serotype. J Gen Virol. 1991 May;72(Pt 5):1059–1064. doi: 10.1099/0022-1317-72-5-1059. [DOI] [PubMed] [Google Scholar]
- Browning G. F., Fitzgerald T. A., Chalmers R. M., Snodgrass D. R. A novel group A rotavirus G serotype: serological and genomic characterization of equine isolate FI23. J Clin Microbiol. 1991 Sep;29(9):2043–2046. doi: 10.1128/jcm.29.9.2043-2046.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S. Variation in neutralization epitopes of human rotaviruses in relation to genomic RNA polymorphism. Virology. 1987 Aug;159(2):209–216. doi: 10.1016/0042-6822(87)90457-0. [DOI] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Lazdins I., Tregear G. W., Holmes I. H. Location of the major antigenic sites involved in rotavirus serotype-specific neutralization. Proc Natl Acad Sci U S A. 1986 May;83(10):3465–3468. doi: 10.1073/pnas.83.10.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., López S., Arias C. Structural polypeptides of simian rotavirus SA11 and the effect of trypsin. J Virol. 1981 Jan;37(1):156–160. doi: 10.1128/jvi.37.1.156-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Flores J., Hoshino Y., Boeggeman E., Purcell R., Chanock R. M., Kapikian A. Z. Genetic relatedness among animal rotaviruses. Arch Virol. 1986;87(3-4):273–285. doi: 10.1007/BF01315305. [DOI] [PubMed] [Google Scholar]
- Gaul S. K., Simpson T. F., Woode G. N., Fulton R. W. Antigenic relationships among some animal rotaviruses: virus neutralization in vitro and cross-protection in piglets. J Clin Microbiol. 1982 Sep;16(3):495–503. doi: 10.1128/jcm.16.3.495-503.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Green K., Nishikawa K., Taniguchi K., Jones R., Kapikian A. Z., Chanock R. M. Sequence of the fourth gene of human rotaviruses recovered from asymptomatic or symptomatic infections. J Virol. 1988 Aug;62(8):2978–2984. doi: 10.1128/jvi.62.8.2978-2984.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Larralde G., Kapikian A. Z., Chanock R. M. Antigenic relationships among human rotaviruses as determined by outer capsid protein VP4. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7155–7159. doi: 10.1073/pnas.87.18.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Nishikawa K., Hoshino Y., Taniguchi K. Similarity of the outer capsid protein VP4 of the Gottfried strain of porcine rotavirus to that of asymptomatic human rotavirus strains. J Virol. 1990 Jan;64(1):414–418. doi: 10.1128/jvi.64.1.414-418.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. Y., Sears J. F., Taniguchi K., Midthun K., Hoshino Y., Gorziglia M., Nishikawa K., Urasawa S., Kapikian A. Z., Chanock R. M. Prediction of human rotavirus serotype by nucleotide sequence analysis of the VP7 protein gene. J Virol. 1988 May;62(5):1819–1823. doi: 10.1128/jvi.62.5.1819-1823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Flores J., Kalica A. R., Wyatt R. G., Jones R. Gene coding assignments for growth restriction, neutralization and subgroup specificities of the W and DS-1 strains of human rotavirus. J Gen Virol. 1983 Feb;64(Pt 2):313–320. doi: 10.1099/0022-1317-64-2-313. [DOI] [PubMed] [Google Scholar]
- Hardy M. E., Woode G. N., Xu Z. C., Williams J. D., Conner M. E., Dwyer R. M., Powell D. G. Analysis of serotypes and electropherotypes of equine rotaviruses isolated in the United States. J Clin Microbiol. 1991 May;29(5):889–893. doi: 10.1128/jcm.29.5.889-893.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Saif L. J., Sereno M. M., Chanock R. M., Kapikian A. Z. Infection immunity of piglets to either VP3 or VP7 outer capsid protein confers resistance to challenge with a virulent rotavirus bearing the corresponding antigen. J Virol. 1988 Mar;62(3):744–748. doi: 10.1128/jvi.62.3.744-748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Chanock R. M., Kapikian A. Z. Analysis by plaque reduction neutralization assay of intertypic rotaviruses suggests that gene reassortment occurs in vivo. J Clin Microbiol. 1987 Feb;25(2):290–294. doi: 10.1128/jcm.25.2.290-294.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Isolation and characterization of an equine rotavirus. J Clin Microbiol. 1983 Sep;18(3):585–591. doi: 10.1128/jcm.18.3.585-591.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. E., Paul P. S., Gorziglia M., Rosenbusch R. Development of specific nucleic acid probes for the differentiation of porcine rotavirus serotypes. Vet Microbiol. 1990 Sep;24(3-4):307–326. doi: 10.1016/0378-1135(90)90180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. Y., Saif L. J., Miller K. L. Reactivity of VP4-specific monoclonal antibodies to a serotype 4 porcine rotavirus with distinct serotypes of human (symptomatic and asymptomatic) and animal rotaviruses. J Clin Microbiol. 1989 Dec;27(12):2744–2750. doi: 10.1128/jcm.27.12.2744-2750.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larralde G., Li B. G., Kapikian A. Z., Gorziglia M. Serotype-specific epitope(s) present on the VP8 subunit of rotavirus VP4 protein. J Virol. 1991 Jun;65(6):3213–3218. doi: 10.1128/jvi.65.6.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdins I., Sonza S., Dyall-Smith M. L., Coulson B. S., Holmes I. H. Demonstration of an immunodominant neutralization site by analysis of antigenic variants of SA11 rotavirus. J Virol. 1985 Oct;56(1):317–319. doi: 10.1128/jvi.56.1.317-319.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liprandi F., Rodriguez I., Piña C., Larralde G., Gorziglia M. VP4 monotype specificities among porcine rotavirus strains of the same VP4 serotype. J Virol. 1991 Mar;65(3):1658–1661. doi: 10.1128/jvi.65.3.1658-1661.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López S., López I., Romero P., Méndez E., Soberón X., Arias C. F. Rotavirus YM gene 4: analysis of its deduced amino acid sequence and prediction of the secondary structure of the VP4 protein. J Virol. 1991 Jul;65(7):3738–3745. doi: 10.1128/jvi.65.7.3738-3745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Valdesuso J., Hoshino Y., Flores J., Kapikian A. Z., Chanock R. M. Analysis by RNA-RNA hybridization assay of intertypic rotaviruses suggests that gene reassortment occurs in vivo. J Clin Microbiol. 1987 Feb;25(2):295–300. doi: 10.1128/jcm.25.2.295-300.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesha H. S., Holmes I. H. Direct serotyping of porcine rotaviruses using VP7-specific monoclonal antibodies by an enzyme immunoassay. J Med Virol. 1991 Nov;35(3):206–211. doi: 10.1002/jmv.1890350311. [DOI] [PubMed] [Google Scholar]
- Nagesha H. S., Holmes I. H. New porcine rotavirus serotype antigenically related to human rotavirus serotype 3. J Clin Microbiol. 1988 Feb;26(2):171–174. doi: 10.1128/jcm.26.2.171-174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesha H. S., Holmes I. H. VP4 relationships between porcine and other rotavirus serotypes. Arch Virol. 1991;116(1-4):107–118. doi: 10.1007/BF01319235. [DOI] [PubMed] [Google Scholar]
- Nagesha H. S., Huang J., Hum C. P., Holmes I. H. A porcine rotavirus strain with dual VP7 serotype specificity. Virology. 1990 Mar;175(1):319–322. doi: 10.1016/0042-6822(90)90215-d. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Fukuhara N., Liprandi F., Green K., Kapikian A. Z., Chanock R. M., Gorziglia M. VP4 protein of porcine rotavirus strain OSU expressed by a baculovirus recombinant induces neutralizing antibodies. Virology. 1989 Dec;173(2):631–637. doi: 10.1016/0042-6822(89)90575-8. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Gorziglia M. The nucleotide sequence of the VP3 gene of porcine rotavirus OSU. Nucleic Acids Res. 1988 Dec 23;16(24):11847–11847. doi: 10.1093/nar/16.24.11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G., Greenberg H. B., Clark H. F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986 Jan;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Blavat G., Greenberg H. B. Reassortant rotaviruses containing structural proteins vp3 and vp7 from different parents induce antibodies protective against each parental serotype. J Virol. 1986 Nov;60(2):491–496. doi: 10.1128/jvi.60.2.491-496.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P. S., Lyoo Y. S., Andrews J. J., Hill H. T. Isolation of two new serotypes of porcine rotavirus from pigs with diarrhea. Arch Virol. 1988;100(1-2):139–143. doi: 10.1007/BF01310917. [DOI] [PubMed] [Google Scholar]
- Rosen B. I., Saif L. J., Jackwood D. J., Gorziglia M. Hybridization probes for the detection and differentiation of two serotypes of porcine rotavirus. Vet Microbiol. 1990 Sep;24(3-4):327–339. doi: 10.1016/0378-1135(90)90181-t. [DOI] [PubMed] [Google Scholar]
- Rosen B. I., Saif L. J., Jackwood D. J., Gorziglia M. Serotypic differentiation of group A rotaviruses with porcine rotavirus gene 9 probes. J Clin Microbiol. 1990 Nov;28(11):2526–2533. doi: 10.1128/jcm.28.11.2526-2533.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L. J., Theil K. W., Bohl E. H. Morphogenesis of porcine rotavirus in porcine kidney cell cultures and intestinal epithelial cells. J Gen Virol. 1978 May;39(2):205–217. doi: 10.1099/0022-1317-39-2-205. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Vo P. T., Offit P. A., Coulson B. S., Greenberg H. B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986 Dec;155(2):434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- Tanaka T. N., Conner M. E., Graham D. Y., Estes M. K. Molecular characterization of three rabbit rotavirus strains. Arch Virol. 1988;98(3-4):253–265. doi: 10.1007/BF01322173. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Kobayashi N., Gorziglia M., Urasawa S. Nucleotide sequence of VP4 and VP7 genes of human rotaviruses with subgroup I specificity and long RNA pattern: implication for new G serotype specificity. J Virol. 1990 Nov;64(11):5640–5644. doi: 10.1128/jvi.64.11.5640-5644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]