Abstract
The mammalian natural killer gene complex (NKC) contains several families of type II transmembrane C-type lectin-like receptors (CLRs) that are best known for their involvement in the detection of virally infected or transformed cells, through the recognition of endogenous (or self) proteinacious ligands. However, certain CLR families within the NKC, particularly those expressed by myeloid cells, recognize structurally diverse ligands and perform a variety of other immune and homoeostatic functions. One such family is the ‘Dectin-1 cluster’ of CLRs, which includes MICL, CLEC-2, CLEC12B, CLEC9A, CLEC-1, Dectin-1 and LOX-1. Here, we review each of these CLRs, exploring our current understanding of their ligands and functions and highlighting where they have provided new insights into the underlying mechanisms of immunity and homeostasis.
Keywords: C-type lectin, myeloid cells, ITAM, ITIM, signaling, homeostasis
Introduction
C-type lectins are a superfamily of proteins that contain one or more C-type lectin-like domains (CTLDs), and have been divided into 17 groups based on their phylogeny and domain organization (Zelensky & Gready, 2005). The CTLD consists of a distinctive protein fold that is generated through disulfide linkages between conserved cysteine residues, but despite the similarity in structure, these domains recognize diverse and structurally unrelated ligands. These receptors can be loosely classified as ‘classical’ and ‘nonclassical’ C-type lectins based on their ability to recognize carbohydrate and noncarbohydrate ligands, respectively. In addition, these receptors can be either membrane bound or soluble, being secreted from cells into the extracellular milieu.
Of particular interest are the group V transmembrane C-type lectins encoded within the natural killer gene complex (NKC), on human chromosome 12 and mouse chromosome 6, which are best known for their recognition of endogenous proteinacious ligands and involvement in the detection of virally infected or transformed cells (Yokoyama & Plougastel, 2003). However, some CLR subfamilies, especially those expressed by myeloid cells, recognize structurally diverse ligands and perform a variety of other immune and homoeostatic functions, and are often encoded within a single gene cluster. One such subfamily is the ‘Dectin-1 cluster’ which is primarily, but not exclusively, expressed by myeloid cells [macrophages, dendritic cells (DC) and neutrophils] (Fig. 1). These type II transmembrane receptors, which include MICL, CLEC-2, CLEC12B, CLEC9A, CLEC-1, Dectin-1 and LOX-1, comprise a single extracellular CTLD, a stalk region of variable length and a cytoplasmic tail containing various signaling motifs (Fig. 2). Here, we will briefly review each of these CLRs in the order they are found in the genome (Fig. 1), highlighting their roles in immunity and homeostasis. A summary of the functions, ligands and expression of each receptor is shown in Table 1.
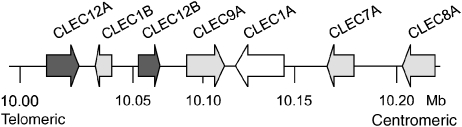
Fig. 1.
Cartoon representation of the genomic organization of the ‘Dectin-1 cluster’ in the NKC on human chromosome 12. Activation receptors are shown in light grey, inhibitory receptors in dark grey, and those whose function is unclear are shown in white.
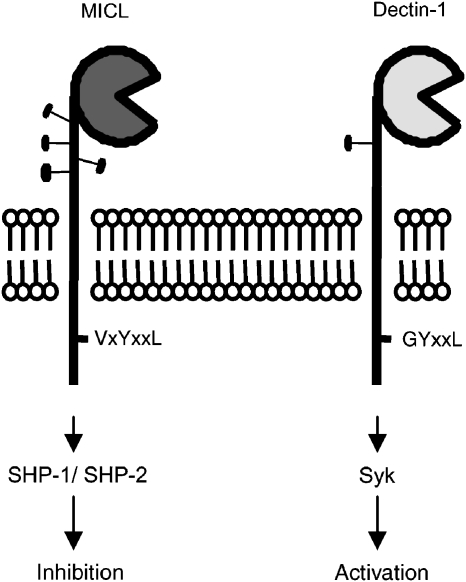
Fig. 2.
Cartoon representation of the typical structure of group V CLRs. Dectin-1 and MICL are shown as representatives of activation and inhibitory receptors that are found in this cluster. Proximal signaling components are also indicated. Lollipop structures indicate sites of N-linked glycosylation.
Table 1.
Selected ligands and expression profiles of the Dectin-1 cluster of C-type lectin receptors
| Official name | Alternate names | Cellular expression (human) | Exogenous ligands | Endogenous ligands | Cellular function |
|---|---|---|---|---|---|
| CLEC12A | MICL, DCAL2, CLL1, KLRL1 | Myeloid cells | ? | Yes but identity unknown | Inhibition |
| CLEC1B | CLEC-2 | Platelets Myeloid cells? | Rhodocytin, HIV | Podoplanin | Activation |
| CLEC12B | Macrophage antigen H | Macrophages | ? | ? | Inhibition |
| CLEC9A | DNGR1 | BDCA3+ DC, monocyte subsets, B-cells | ? | ? | Activation |
| CLEC1A | CLEC-1 | DC | ? | ? | ? |
| CLEC7A | Dectin-1 | Myeloid cells, T-cell subsets, B cells, mast cells, eosinophils | β-Glucan, Mycobacterial ligand | T-cells Apoptotic cells | Activation |
| CLEC8A | LOX-1 | Endothelium, smooth muscle, platelets, fibroblasts, macrophages | Gram-positive Gram-negative bacteria | ox-LDL modified lipoprotiens Aged/apoptotic cells Advanced glycation end-products HSP70 | Activation |
‘?’ refers to unknown data.
Myeloid inhibitory C-type lectin-like receptor (MICL) (CLEC12A)
MICL was originally identified by homology to Dectin-1 and its position within the Dectin-1 cluster (Marshall et al., 2004). MICL was subsequently identified by three other groups and named C-type lectin-like molecule-1 (CLL-1), dendritic-cell-associated C-type lectin 2 (DCAL-2) and killer cell lectin-like receptor 1 (KLRL1) (Bakker et al., 2004; Han et al., 2004; Chen et al., 2006). Human MICL is variably spliced, generating at least three isoforms, and highly N-glycosylated, and although it contains cysteine residues in its stalk region, the receptor appears to be expressed as a monomer. Murine MICL, however, is expressed as a dimer and is not heavily glycosylated (Pyz et al., 2008). Both murine and human MICL are predominantly expressed by myeloid cells (granulocytes, monocytes, macrophages and DCs) and expression of this receptor is downregulated following inflammatory conditions, including those triggered by microbial components (Marshall et al., 2004, 2006; Pyz et al., 2008). In humans, MICL has been identified as a marker for acute myeloid leukemia (Bakker et al., 2004).
MICL contains a single ITIM in its cytoplasmic tail that can associate with the signaling phosphatases SHP-1 and SHP-2 (Han et al., 2004; Marshall et al., 2004; Pyz et al., 2008), and is able to inhibit cellular activation (Marshall et al., 2004). MICL has also been reported to inhibit NK cell cytotoxicity (Han et al., 2004), although the receptor is not expressed on NK cells (Marshall et al., 2006), and antibody-mediated cross-linking of MICL on human monocyte-derived DCs has been shown to affect DC function, by altering cytokine production, cellular maturation and chemokine receptor expression, suggesting a role for this receptor in the control of immune responses (Chen et al., 2006). However, the mechanisms and significance of these effects are unclear. Recently murine MICL was shown to recognize an endogenous ligand that was present in many tissues, suggesting that the receptor may also play a role in homeostasis, but the identity of this molecule has yet to be determined (Pyz et al., 2008).
CLEC-2 (CLEC1B)
CLEC-2 was identified from a computational screen for CLRs at the same time as CLEC-1 (discussed below) (Colonna et al., 2000). The expression of CLEC-2 has been described on platelets, megakaryocytes and liver sinusoidal endothelial cells, although reverse transcriptase (RT)-PCR analysis indicates that the receptor may also be expressed on monocytes, DC and granulocytes (Colonna et al., 2000; Chaipan et al., 2006; Suzuki-Inoue et al., 2006). CLEC-2 is differentially glycosylated and, in mouse, is expressed in at least three isoforms, which are generated by alternative splicing and which possess different expression profiles and subcellular localizations (Suzuki-Inoue et al., 2006; Xie et al., 2008). Furthermore, full-length mCLEC-2 is proteolytically cleaved from the membrane, generating a soluble homodimeric form (Xie et al., 2008).
Activation of CLEC-2 triggers platelet activation and aggregation, an activity that is mediated by the cytoplasmic immunoreceptor tyrosine-based activation (ITAM)-like motif of CLEC-2 (Suzuki-Inoue et al., 2006). This single tyrosine-based motif is highly similar to that found in Dectin-1, and can recruit and signal via spleen tyrosine kinase (Syk) (Suzuki-Inoue et al., 2006). In addition to Syk, Tec family kinases, Src, PLCγ and Rac1 have been implicated in the signaling pathway activated by CLEC-2 (Fuller et al., 2007; Pleines et al., 2008).
A number of endogenous and exogenous ligands for CLEC-2 have been identified, including the snake venom toxin, rhodocytin (Suzuki-Inoue et al., 2006). Rhodocytin assembles as a tetramer and it is thought that recognition by CLEC-2 induces clustering of the receptor, triggering downstream signaling and platelet activation (Watson et al., 2007, 2008; Hooley et al., 2008). CLEC-2 can also bind HIV-1, and may capture and transfer infectious HIV-1 in cooperation with DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin) (Chaipan et al., 2006).
One endogenous ligand, podoplanin, has also been identified for CLEC-2 (Suzuki-Inoue et al., 2007). Podoplanin is a mucin-type sialoglycoprotein that is expressed on a variety of cell types, but not on blood vessel endothelium, and has been implicated in tumor cell-induced platelet aggregation, tumor metastasis and lymphatic vessel formation. It has been suggested that the interaction of CLEC-2 with podoplanin, which involves O-glycans, may be involved in promoting tumor growth and/or metastasis, and could therefore be a target for therapeutic intervention (Suzuki-Inoue et al., 2007; Christou et al., 2008; Kato et al., 2008). Despite the identification of all these ligands, however, the physiological role of CLEC-2 is still unknown.
CLEC12B
Little is known about CLEC12B, which was identified in the Dectin-1 cluster through computational analysis (Sobanov et al., 2001). RT-PCR analysis has indicated that human CLEC12B is widely expressed at low levels in various human tissues, except the brain, and that the receptor is alternatively spliced, generating at least two isoforms, one of which lacks part of the carbohydrate recognition domain (CRD) and would be predicted to be nonfunctional (Hoffmann et al., 2007). This nonfunctional isoform appears to be preferentially expressed in certain tissues, including the lung, mammary gland and ovary. Although the receptor was not detected on any peripheral blood leukocyte, its expression was induced upon the differentiation of monocytes into macrophages (Hoffmann et al., 2007). CLEC12B possesses an ITIM sequence (VxYxxL) within its cytoplasmic tail, which can recruit the inhibitory phosphatases, SHP-1 and SHP-2, and, using a model system, was shown to be able to inhibit certain cellular functions. More analysis of CLEC12B is required to understand its physiological role.
CLEC9A
CLEC9A (which has also been termed DNGR1) is the most recently characterized receptor from the Dectin-1 cluster and is expressed in many tissues, as determined by RT-PCR analysis (Caminschi et al., 2008; Huysamen et al., 2008; Sancho et al., 2008). In peripheral blood, human CLEC9A is found primarily on the surface of BDCA3+ DCs, although this receptor was also detected on small subsets of monocytes and B-cells. There are at least five isoforms of CLEC9A in mice, generated through alternative splicing, and the receptor is expressed on CD8+ DCs, which are thought to be the equivalent of human BDCA3+ cells, and also at low levels on plasmacytoid DC. Human CLEC9A and selected murine isoforms are expressed at the cell surface as glycosylated dimers.
The cytoplasmic tail of CLEC9A contains a single tyrosine residue within a sequence showing similarity to the ITAM-like sequence of Dectin-1, suggesting that CLEC9A may function as an activation receptor. Indeed, using receptor chimeras in transfected myeloid cells, CLEC9A was shown to be capable of inducing inflammatory cytokine production and signal via Syk kinase (Huysamen et al., 2008). Although this receptor did not mediate particle uptake via phagocytosis, CLEC9A was capable of internalizing bound antigens via endocytosis and directing them to the endosomal/lysosomal pathway (Huysamen et al., 2008; Sancho et al., 2008). Interestingly, antigens targeted to CLEC9A could induce humoral, CD4+ and CD8+ T-cell responses, even in the absence of adjuvant, suggesting that this receptor could be exploited as a novel target on CD8+ or BDCA3+ DCs to drive these responses (Caminschi et al., 2008; Sancho et al., 2008). In fact, in a murine model, the cross-presentation of tumor antigens targeted to CLEC9A was found to elicit potent antitumor responses (Sancho et al., 2008). However, the ligand(s) and physiological function of this receptor are still unknown.
CLEC-1 (CLEC1A)
Virtually nothing is known about CLEC-1, a receptor originally identified through a computational search for NK-related CLRs (Colonna et al., 2000). From analysis of tissue mRNA, the human receptor was shown to be expressed in the placenta, lung, bone marrow, thymus and heart. At a cellular level, the receptor was detected by RT-PCR in unstimulated as well as tumor necrosis factor (TNF) or CD40L-stimulated DCs, and in endothelial cells. However, CLEC-1 was not detected in peripheral blood monocytes (PBMCs), granulocytes, B, T, NK cells or monocytes (Colonna et al., 2000; Sobanov et al., 2001). CLEC-1 may be expressed in at least two isoforms, and the receptor contains a cytoplasmic tyrosine through which it may induce intracellular signaling, although this residue is not within a recognizable signaling motif. Unlike other members of the Dectin-1 cluster, however, CLEC-1 is not expressed at the cell surface, at least in transfected cells.
Dectin-1 (CLEC7A)
The β-glucan receptor, Dectin-1, is one of the best characterized receptors in the ‘Dectin-1 cluster’ and was originally identified as a DC-specific molecule, from which its name ‘dendritic-cell-associated C-type lectin-1’ was derived (Ariizumi et al., 2000; Brown, 2006). Dectin-1 is alternatively spliced generating two major and a number of minor isoforms, which have different functionalities, and the receptor is N-glycosylated, a posttranslation modification that contributes to its surface expression and function. Dectin-1 is predominantly expressed on monocytes, macrophages, neutrophils and microglia, but also weakly on a subset of T cells, and in humans, B cells, mast cells and eosinophils (Brown, 2006).
Dectin-1 specifically recognizes (1,3)-linked β-glucans in a calcium-independent manner and is the primary receptor for these carbohydrates on leukocytes (Brown, 2006; Palma et al., 2006; Adams et al., 2008). The crystal structure of the CTLD of Dectin-1 has been determined and mutational analyses have indicated that two residues (Trp221 and His223) are essentially required for β-glucan binding, and these two residues flank a groove on the CTLD that could be the binding site (Adachi et al., 2004; Brown et al., 2007). However, the exact mechanism of β-glucan recognition by Dectin-1 is still unknown.
Recognition of β-glucans by Dectin-1 leads to the induction of numerous cellular responses, including the respiratory burst, ligand uptake by endocytosis and phagocytosis, the production of arachidonic acid metabolites, DC maturation and the induction of numerous cytokines and chemokines, including TNF, CXCL2, IL-23, IL-6, IL-10 and IL-2 (Brown, 2006). Signaling from Dectin-1 is sufficient for many of these responses, but others, such as the respiratory burst and proinflammatory cytokine production, require, or are enhanced by, cooperative signaling from Myd88-coupled TLRs (Brown, 2006; Dennehy et al., 2008). However, the ability of Dectin-1 to induce DC maturation and the production of cytokines, such as IL-23, can directly couple innate and adaptive immunity, independently of the TLRs (Leibundgut-Landmann et al., 2007). Recent data also suggest that collaborative signaling between Dectin-1 and MyD88-coupled TLRs is far more extensive than first appreciated, and results in the enhanced production of IL-23, while downregulating the production of IL-12 (Gerosa et al., 2008).
The ability of β-glucans to induce these cellular responses is also cell-type dependent. In macrophages (Dennehy et al., 2008), for example, particulate β-glucans are unable to stimulate TNF production without costimulation of the TLRs, yet in DCs, β-glucan alone is sufficient to trigger the production of this cytokine (Leibundgut-Landmann et al., 2007). Although the underlying reasons for these differences are not yet fully understood, they have recently been shown to be related, in part, to phagocytosis and the actions of cytokines, such as granulocyte monocyte colony stimulating factor (GM-CSF) (Rosas et al., 2008).
Signaling from Dectin-1 is mediated by the cytoplasmic tail, which contains an ITAM-like motif. Upon ligand binding, this motif becomes tyrosine phosphorylated by Src family kinases, leading to the recruitment of Syk, which initiates subsequent downstream signaling pathways. The interaction between Syk and Dectin-1 is unusual, in that it involves only one tyrosine, and is not fully understood, but may involve the bridging of two Dectin-1 monomers (Rogers et al., 2005). This type of interaction is now known to occur in at least two other Dectin-1 cluster receptors, CLEC-2 and CLEC9A. Downstream signaling involves the novel adaptor CARD9, as well as activation of MAP kinases, NFAT and nuclear factor-kappa B (NFκB) (Rogers et al., 2005; Gross et al., 2006; Goodridge et al., 2007; Slack et al., 2007) There are also Syk-independent pathways of Dectin-1 signaling, which may be cell-type specific, but the signaling mechanisms involved in these responses are largely uncharacterized (Brown, 2006).
Dectin-1 has been shown to recognize several fungal species, including Saccharomyces, Candida, Pneumocystis, Coccidiodes, Penicillium and Aspergillus, but not Cryptococcus (Brown, 2006; Nakamura et al., 2007, 2008). Recognition of these organisms by Dectin-1 triggers many protective responses, such as fungal uptake by phagocytosis and killing via the respiratory burst. These interactions also induce the production of cytokines and chemokines, and while many of these are known to be protective in fungal infections (such as TNF, CXCL2, IL-1β, IL-1α, CCL3, GM-CSF, G-CSF and IL-6), others (such as IL-10 and IL-23) are paradoxically nonprotective (Brown, 2006). Although the reason for production of these nonprotective cytokines is not yet clear, there may be immunological benefits. IL-10, for example, inhibits antifungal immunity yet may be required to limit inflammatory pathology and to promote fungal persistence and long-term immunity.
The role of Dectin-1 has been examined in vivo, and although the data are not entirely consistent, they support a role for this receptor in antifungal immunity. Dectin-1-deficient mice, on a 129Sv background, displayed increased susceptibility to systemic infection with Candida albicans, which resulted from inflammatory defects and reduced fungal killing (Taylor et al., 2007). However, Dectin-1 knockout mice on a C57BL6 background were not susceptible to Candida, but were found to show differences in fungal burdens during infection with P. carinii, which resulted from defects in the respiratory burst (Saijo et al., 2007). However, C57BL6 mice deficient in CARD9, a downstream signaling component of Dectin-1, were susceptible to candidiasis, although this may also involve signaling pathways induced from other receptors (Gross et al., 2006). Furthermore, in wild-type C57BL6 mice, inhibition of Dectin-1 during pulmonary challenge with Aspergillus fumigatus resulted in reduced lung inflammation and increased fungal burden (Steele et al., 2005). In line with these findings, differential expression of Dectin-1 isoforms in the various mouse strains has also been linked to susceptibility to Coccidoides (Del Pilar Jimenez et al., 2008).
Further support for a role in Dectin-1 in antifungal immunity comes from the growing body of evidence which indicates that fungal pathogens may actively avoid immune recognition by masking their β-glucans (Gantner et al., 2005). Hyphae of Aspergillus and Candida, for example, do not expose β-glucans on their surface and enhancing the exposure of these carbohydrates, through treatment with caspofungin, has been shown to improve antifungal responses (Wheeler & Fink, 2006). Other examples include Histoplasma, which masks its β-glucan under a layer of α-glucan, and Paracoccidioides, which switches from β-glucan to α-glucan upon infection of the host.
Dectin-1 may also play other roles in addition to antifungal immunity. Three recent publications have suggested that Dectin-1 can recognize an unidentified ligand on mycobacteria, promoting bacterial uptake and the induction of a number of cytokines and chemokines, including TNF, IL-6, RANTES and G-CSF (Yadav & Schorey, 2006; Rothfuchs et al., 2007; Shin et al., 2008). These interactions also induce the production of IL-12 (Rothfuchs et al., 2007), which is of particular interest, as this T-helper type 1-promoting cytokine is essential for the control of mycobacterial infection.
Dectin-1 can also recognize an endogenous ligand on T-cells, stimulating cellular activation and proliferation, and may therefore act as a costimulatory molecule (Brown, 2006). This function of Dectin-1 is supported by its expression on antigen presenting cells in the T-cell areas of lymphoid tissues. The recognition of the endogenous ligand has also recently been shown to mediate the recognition and uptake of apoptotic cells, and the cross-presentation of cellular antigens (Weck et al., 2008). Recognition of the endogenous ligand occurs at a distinct binding site on Dectin-1, as its binding is not inhibitable by β-glucans. However, the nature of the endogenous ligand, which may be a protein, is still unknown.
Lectin-like oxidized LDL receptor (LOX-1) (CLEC8A)
LOX-1 was the first member of the Dectin-1 cluster to be identified and is well characterized, being originally isolated from a bovine aortic endothelial cDNA expression library screened for receptors for oxidized LDL (OxLDL) (Sawamura et al., 1997). LOX-1 is glycosylated, a posttranslational modification that contributes to cell-surface expression and ligand recognition, and the receptor forms homodimers that may multimerize through noncovalent interactions involving the neck region, aiding in ligand binding (Mehta et al., 2006; Dunn et al., 2008). LOX-1 can also be prototypically cleaved at the membrane proximal sites in the neck domain, producing a soluble form whose function is unknown. LOX-1 is expressed on vascular endothelial cells, smooth muscle cells, platelets, fibroblasts and macrophages and its expression can be upregulated by a variety of proinflammatory, oxidative and mechanical stimuli and during several pathological conditions in vivo, such as diabetes, hyperlipidemia, atherosclerosis and hypertension (reviewed in Chen & Du, 2007; Dunn et al., 2008). Importantly, expression of LOX-1 can also be upregulated following binding of OxLDL, which may exacerbate the development of LOX-1-mediated diseases, such as atherosclerosis.
Although a part of the Dectin-1 C-type lectin cluster, LOX-1 is considered to be a member (class E) of the scavenger receptor family. In addition to OxLDL, LOX-1 recognizes numerous other structurally diverse ligands including modified lipoproteins, selected anionic polymers and phosphopolipids, aged and apoptotic cells, activated platelets, advanced glycation endproducts, heat shock protein (HSP)70, and gram-positive and gram-negative bacteria (Chen & Du, 2007). Ligand recognition is thought to involve electrostatic interactions with positively charged residues that are exposed on the face of the CRD of LOX-1 with negatively charged regions in the ligands (Mehta et al., 2006; Dunn et al., 2008).
Despite lacking classical signaling motifs in its cytoplasmic tail, LOX-1 can mediate or modulate a variety of cellular functions, including endocytosis, phagocytosis, cytokine production, CD40 and CD40 ligand levels, apoptosis, the activation of NFκB and production of reactive oxygen species (Mehta et al., 2006; Chen et al., 2007; Dunn et al., 2008). LOX-1 can also act as a cell-adhesion molecule involved in leukocyte recruitment during inflammation and, through its ability to recognize HSP70, has been implicated in DC-mediated antigen cross-presentation (Delneste et al., 2002; Mehta et al., 2006; Dunn et al., 2008). Although the signaling pathways leading to these responses are not fully understood, various downstream components have been implicated, including phosphoinositide 3-kinase, p38 mitogen-activated protein kinase and protein kinase Cα. Recently, LOX-1-mediated internalization of Ox-LDL was shown to occur through a clathrin-independent mechanism involving a novel cytoplasmic tripeptide motif of the receptor (Murphy et al., 2008).
Much interest in LOX-1 has focused on its involvement in vascular disease, particularly the role of this receptor in the development of atherosclerosis. Many of the responses that are mediated by LOX-1 have been linked to pathological, proatherogenic, changes in the vascular endothelium, and the upregulation of LOX-1 in atherosclerotic lesions may result in a positive feedback loop that promotes disease development (Mehta et al., 2006; Dunn et al., 2008). In fact, increased release of soluble LOX-1 has been proposed to be a marker of acute coronary syndrome. Mouse models in which LOX-1 has been deleted, or overexpressed, also support a role for this receptor in the development of atherosclerosis (Inoue et al., 2005; Mehta et al., 2007; Hu et al., 2008). Furthermore, although controversial, genetic linkage studies have implicated human polymorphisms of LOX-1 with susceptibility to cardiovascular disease (Mehta et al., 2006; Dunn et al., 2008; Knowles et al., 2008). Finally there is also evidence that LOX-1 may be involved in thrombosis, myocardial ischemia reperfusion injury and hypertension and the receptor may be involved in generating inflammatory responses during microbial infection (Mehta et al., 2006; Hu et al., 2008).
Concluding remarks
Despite considerable similarity in structure, the Dectin-1 cluster of receptors recognizes a diverse range of structurally unrelated ligands and mediates a wide variety of cellular functions. While our understanding is still in its infancy, the study of these receptors has revealed a number of new insights into the underlying mechanisms of immunity and homeostasis. One of the many remaining challenges is to fully elucidate the functions and ligands of the ‘orphan’ receptors within this cluster, a task that should be greatly aided by the generation and characterization of receptor deficient mice.
Acknowledgments
We thank the Wellcome Trust, National Research Foundation (South Africa), University of Cape Town and CANSA (South Africa) for funding. G.D.B. is Wellcome Senior Fellow in South Africa. C.H. was supported by scholarships from Harry Crossley, KW Johnston Research and Marion Beatrice.
Statement
Reuse of this article is permitted in accordance with the Creative Commons Deed, Attribution 2.5, which does not permit commercial exploitation.
References
- Adachi Y, Ishii T, Ikeda Y, Hoshino A, Tamura H, Aketagawa J, Tanaka S, Ohno N. Characterization of beta-glucan recognition site on C-type lectin, dectin 1. Infect Immun. 2004;72:4159–4171. doi: 10.1128/IAI.72.7.4159-4171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams EL, Rice PJ, Graves B, et al. Differential high affinity interaction of Dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side chain branching. J Pharmacol Exp Ther. 2008;325:115–123. doi: 10.1124/jpet.107.133124. [DOI] [PubMed] [Google Scholar]
- Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R, III, Kumamoto T, Edelbaum D, Morita A, Bergstresser PR, Takashima A. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem. 2000;275:20157–20167. doi: 10.1074/jbc.M909512199. [DOI] [PubMed] [Google Scholar]
- Bakker AB, van den Oudenrijn S, Bakker AQ, et al. C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res. 2004;64:8443–8450. doi: 10.1158/0008-5472.CAN-04-1659. [DOI] [PubMed] [Google Scholar]
- Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- Brown J, O'Callaghan CA, Marshall AS, Gilbert RJ, Siebold C, Gordon S, Brown GD, Jones EY. Structure of the fungal beta-glucan-binding immune receptor dectin-1: implications for function. Protein Sci. 2007;16:1042–1052. doi: 10.1110/ps.072791207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminschi I, Proietto AI, Ahmet F, et al. The dendritic cell subtype restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112:3264–3273. doi: 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaipan C, Soilleux EJ, Simpson P, et al. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80:8951–8960. doi: 10.1128/JVI.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Floyd H, Olson NE, Magaletti D, Li C, Draves K, Clark EA. Dendritic-cell-associated C-type lectin 2 (DCAL-2) alters dendritic-cell maturation and cytokine production. Blood. 2006;107:1459–1467. doi: 10.1182/blood-2005-08-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XP, Du GH. Lectin-like oxidized low-density lipoprotein receptor-1: protein, ligands, expression and pathophysiological significance. Chin Med J (England) 2007;120:421–426. [PubMed] [Google Scholar]
- Chen XP, Xun KL, Wu Q, Zhang TT, Shi JS, Du GH. Oxidized low density lipoprotein receptor-1 mediates oxidized low density lipoprotein-induced apoptosis in human umbilical vein endothelial cells: role of reactive oxygen species. Vasc Pharmacol. 2007;47:1–9. doi: 10.1016/j.vph.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Christou CM, Pearce AC, Watson AA, Mistry AR, Pollitt AY, Fenton-May AE, Johnson LA, Jackson DG, Watson SP, O'Callaghan CA. Renal cells activate the platelet receptor CLEC-2 through podoplanin. Biochem J. 2008;411:133–140. doi: 10.1042/BJ20071216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Samaridis J, Angman L. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur J Immunol. 2000;30:697–704. doi: 10.1002/1521-4141(200002)30:2<697::AID-IMMU697>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Delneste Y, Magistrelli G, Gauchat J, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- Del Pilar Jimenez AM, Viriyakosol S, Walls L, Datta SK, Kirkland T, Heinsbroek SE, Brown G, Fierer J. Susceptibility to Coccidioides species in C57BL/6 mice is associated with expression of a truncated splice variant of Dectin-1 (Clec7a) Genes Immun. 2008;9:338–348. doi: 10.1038/gene.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennehy KM, Ferwerda G, Faro-Trindade I, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S, Vohra RS, Murphy JE, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. The lectin-like oxidized low-density-lipoprotein receptor: a pro-inflammatory factor in vascular disease. Biochem J. 2008;409:349–355. doi: 10.1042/BJ20071196. [DOI] [PubMed] [Google Scholar]
- Fuller GL, Williams JA, Tomlinson MG, Eble JA, Hanna SL, Pohlmann S, Suzuki-Inoue K, Ozaki Y, Watson SP, Pearce AC. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282:12397–12409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005;24:1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerosa F, Baldani-Guerra B, Lyakh LA, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–3115. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, Forster I, Ruland J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–656. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]
- Han Y, Zhang M, Li N, Chen T, Zhang Y, Wan T, Cao X. KLRL1, a novel killer cell lectinlike receptor, inhibits natural killer cell cytotoxicity. Blood. 2004;104:2858–2866. doi: 10.1182/blood-2004-03-0878. [DOI] [PubMed] [Google Scholar]
- Hoffmann SC, Schellack C, Textor S, Konold S, Schmitz D, Cerwenka A, Pflanz S, Watzl C. Identification of CLEC12B, an inhibitory receptor on myeloid cells. J Biol Chem. 2007;282:22370–22375. doi: 10.1074/jbc.M704250200. [DOI] [PubMed] [Google Scholar]
- Hooley E, Papagrigoriou E, Navdaev A, Pandey AV, Clemetson JM, Clemetson KJ, Emsley J. The crystal structure of the platelet activator aggretin reveals a novel (alphabeta)2 dimeric structure. Biochemistry. 2008;47:7831–7837. doi: 10.1021/bi800528t. [DOI] [PubMed] [Google Scholar]
- Hu C, Dandapat A, Sun L, Chen J, Marwali MR, Romeo F, Sawamura T, Mehta JL. LOX-1 deletion decreases collagen accumulation in atherosclerotic plaque in low-density lipoprotein receptor knockout mice fed a high-cholesterol diet. Cardiovasc Res. 2008;79:287–293. doi: 10.1093/cvr/cvn110. [DOI] [PubMed] [Google Scholar]
- Huysamen C, Willment JA, Dennehy KM, Brown GD. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem. 2008;283:16693–16701. doi: 10.1074/jbc.M709923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Arai Y, Kurihara H, Kita T, Sawamura T. Overexpression of lectin-like oxidized low-density lipoprotein receptor-1 induces intramyocardial vasculopathy in apolipoprotein E-null mice. Circ Res. 2005;97:176–184. doi: 10.1161/01.RES.0000174286.73200.d4. [DOI] [PubMed] [Google Scholar]
- Kato Y, Kaneko MK, Kunita A, et al. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci. 2008;99:54–61. doi: 10.1111/j.1349-7006.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles JW, Assimes TL, Boerwinkle E, et al. Failure to replicate an association of SNPs in the oxidized LDL receptor gene (OLR1) with CAD. BMC Med Genet. 2008;9:23. doi: 10.1186/1471-2350-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibundgut-Landmann S, Gross O, Robinson MJ, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- Marshall AS, Willment JA, Lin HH, Williams DL, Gordon S, Brown GD. Identification and characterization of a novel human myeloid inhibitory C-type Lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J Biol Chem. 2004;279:14792–14802. doi: 10.1074/jbc.M313127200. [DOI] [PubMed] [Google Scholar]
- Marshall AS, Willment JA, Pyz E, Dennehy KM, Reid DM, Dri P, Gordon S, Wong SY, Brown GD. Human MICL (CLEC12A) is differentially glycosylated and is down-regulated following cellular activation. Eur J Immunol. 2006;36:2159–2169. doi: 10.1002/eji.200535628. [DOI] [PubMed] [Google Scholar]
- Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69:36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Mehta JL, Sanada N, Hu CP, et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100:1634–1642. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- Murphy JE, Vohra RS, Dunn S, Holloway ZG, Monaco AP, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. Oxidised LDL internalisation by the LOX-1 scavenger receptor is dependent on a novel cytoplasmic motif and is regulated by dynamin-2. J Cell Sci. 2008;121:2136–2147. doi: 10.1242/jcs.020917. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kinjo T, Saijo S, Miyazato A, Adachi Y, Ohno N, Fujita J, Kaku M, Iwakura Y, Kawakami K. Dectin-1 is not required for the host defense to Cryptococcus neoformans. Microbiol Immunol. 2007;51:1115–1119. doi: 10.1111/j.1348-0421.2007.tb04007.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Miyazato A, Koguchi Y, et al. Toll-like receptor 2 (TLR2) and dectin-1 contribute to the production of IL-12p40 by bone marrow-derived dendritic cells infected with Penicillium marneffei. Microbes Infect. 2008;10:1223–1227. doi: 10.1016/j.micinf.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Palma AS, Feizi T, Zhang Y, et al. Ligands for the beta-glucan receptor, Dectin-1, assigned using “designer” microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides. J Biol Chem. 2006;281:5771–5779. doi: 10.1074/jbc.M511461200. [DOI] [PubMed] [Google Scholar]
- Pleines I, Elvers M, Strehl A, Pozgajova M, Varga-Szabo D, May F, Chrostek-Grashoff A, Brakebusch C, Nieswandt B. Rac1 is essential for phospholipase C-gamma2 activation in platelets. Pflug Arch. 2008 doi: 10.1007/s00424-008-0573-7. DOI 10.1007/s00424-008-0573-7. [DOI] [PubMed] [Google Scholar]
- Pyz E, Huysamen C, Marshall AS, Gordon S, Taylor PR, Brown GD. Characterisation of murine MICL (CLEC12A) and evidence for an endogenous ligand. Eur J Immunol. 2008;38:1157–1163. doi: 10.1002/eji.200738057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NC, Slack EC, Edwards AD, et al. Syk-dependent cytokine induction by dectin-1 reveals a novel pattern recognition pathway for C-type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Rosas M, Liddiard K, Kimberg M, Faro-Trindade I, McDonald JU, Williams DL, Brown GD, Taylor PR. The induction of inflammation by dectin-1 in vivo is dependent on myeloid cell programming and the progression of phagocytosis. J Immunol. 2008;181:3549–3557. doi: 10.4049/jimmunol.181.5.3549. [DOI] [PubMed] [Google Scholar]
- Rothfuchs AG, Bafica A, Feng CG, Egen JG, Williams DL, Brown GD, Sher A. Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J Immunol. 2007;179:3463–3471. doi: 10.4049/jimmunol.179.6.3463. [DOI] [PubMed] [Google Scholar]
- Saijo S, Fujikado N, Furuta T, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR, Reis e Sousa C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- Shin DM, Yang CS, Yuk JM, Lee JY, Kim KH, Shin SJ, Takahara K, Lee SJ, Jo EK. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. 2008;10:1608–1621. doi: 10.1111/j.1462-5822.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- Slack EC, Robinson MJ, Hernanz-Falcon P, Brown GD, Williams DL, Schweighoffer E, Tybulewicz VL, Reis e Sousa C. Syk-dependent ERK activation regulates IL-2 and IL-10 production by DC stimulated with zymosan. Eur J Immunol. 2007;37:1600–1612. doi: 10.1002/eji.200636830. [DOI] [PubMed] [Google Scholar]
- Sobanov Y, Bernreiter A, Derdak S, Mechtcheriakova D, Schweighofer B, Duchler M, Kalthoff F, Hofer E. A novel cluster of lectin-like receptor genes expressed in monocytic, dendritic and endothelial cells maps close to the NK receptor genes in the human NK gene complex. Eur J Immunol. 2001;31:3493–3503. doi: 10.1002/1521-4141(200112)31:12<3493::aid-immu3493>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. The Beta-Glucan receptor Dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005;1:e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki-Inoue K, Fuller GL, Garcia A, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, Yamazaki Y, Narimatsu H, Ozaki Y. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Tsoni SV, Willment JA, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AA, Brown J, Harlos K, Eble JA, Walter TS, O'Callaghan CA. The crystal structure and mutational binding analysis of the extracellular domain of the platelet-activating receptor CLEC-2. J Biol Chem. 2007;282:3165–3172. doi: 10.1074/jbc.M610383200. [DOI] [PubMed] [Google Scholar]
- Watson AA, Eble JA, O'Callaghan CA. Crystal structure of rhodocytin, a ligand for the platelet-activating receptor CLEC-2. Protein Sci. 2008;17:1611–1616. doi: 10.1110/ps.035568.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck MM, Appel S, Werth D, Sinzger C, Bringmann A, Grunebach F, Brossart P. hDectin-1 is involved in uptake and cross-presentation of cellular antigens. Blood. 2008;111:4264–4272. doi: 10.1182/blood-2006-10-051375. [DOI] [PubMed] [Google Scholar]
- Wheeler RT, Fink GR. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2006;2:e35. doi: 10.1371/journal.ppat.0020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Wu T, Guo L, et al. Molecular characterization of two novel isoforms and a soluble form of mouse CLEC-2. Biochem Biophys Res Commun. 2008;371:180–184. doi: 10.1016/j.bbrc.2008.03.070. [DOI] [PubMed] [Google Scholar]
- Yadav M, Schorey JS. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304–316. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]