Abstract
This paper describes an α-amination process of aryl ketones using CuCl as catalyst and di-tert-butyldiaziridinone as the nitrogen source. A variety of imidazolinone derivatives are prepared in moderate yields under mild conditions. A possible catalytic cycle is proposed for this reaction.
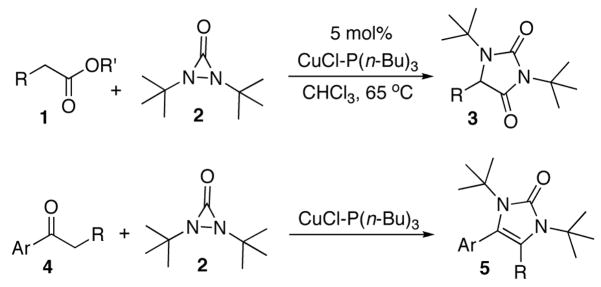
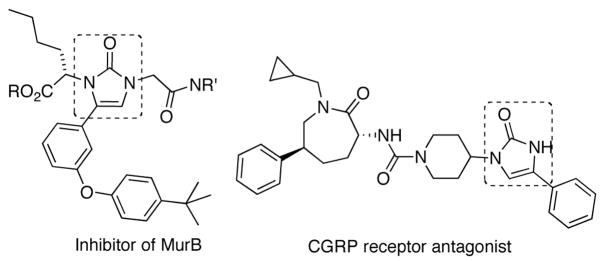
Amines and their derivatives are very important functional moieties contained in many natural products, pharmaceutical agents, and chemical materials. A variety of efficient methods have been developed to introduce nitrogens.1 The formation of C-N bonds by direct C-H amination is highly attractive, and great progress has been achieved in this area.2,3 In our studies on amination with diaziridinones, recently we have found that esters (1) can be directly aminated at the α position to form hydantoins (3) with di-tert-butyldiaziridinone (2)4–7 and CuCl (Scheme 1).8 In further investigation on C-H amination with di-tert-butyldiaziridinone (2), we have found that aryl ketone 4 can also be aminated at the α position to form imidazolinone 5 directly using CuCl as catalyst (Scheme 1). Imidazolinones are important moieties that are present in various biologically active compounds,9 such as MurB inhibitor9b and CGRP receptor antagonist9d (Figure 1). In general, imidazolinones can be prepared by cyclization of α-amino derivatives of ketones, aldehydes, and related compounds,9,10 or by derivatization of imidazolinones.11 The current direct α-amination of ketones provides a valuable alternative method for the synthesis of imidazolinones. Herein we wish to report our studies on this subject.
Scheme 1.
α-Amination of ester and ketone.
Figure 1.
Biologically active compounds containing imidazolinones.
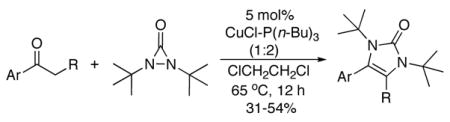
When acetophenone 4a was initially treated with di-tertbutyldiaziridinone (2) and 5 mol% of CuCl-P(n-Bu)3 (1:1) in CDCl3 at 65 °C in an NMR tube for 12 h, imidazolinone 5a was formed in 39% yield. The yield can be improved to 46% using 5 mol% of CuCl-P(n-Bu)3 (1:2) in dry 1,2- dichloroethane and by slow addition of di-tertbutyldiaziridinone over 8 h (Table 1, entry 1). As shown in Table 1, various substituted acetophenones were successfully α-aminated to give the corresponding imidazolinone derivatives in moderate yields (Table 1, entries 2-7) (the X-ray structure of 5b is shown in Supporting Information). The substituent on the aryl ring appears to have little influence on the reaction. Methyl 2-naphthyl ketone and methyl 2-thienyl ketone were also effective substrates that yielded the corresponding α-amination products with moderate yields (Table 1, entries 8 and 9). Butyrophenone was also α-aminated to afford the imidazolinone albeit in low yield (Table 1, entry 10). In all these cases, the relatively low yields obtained are largely due to relatively low conversions of ketone substrates. When the reaction was carried out at a larger scale (4.0 mmol), slightly lower yields were obtained (Table 1, entries 1 and 8). The aryl group of the ketone substrate appears to be important, and α,®-unsaturated ketones such as (E)-4-phenylbut-3-en-2-one and dialkyl ketones such as octan-2-one are not effective substrates under the current reaction conditions. The deprotection of the resulting imidazolinone product was also investigated with compound 5a. Treating 5a with CF3CO2H at 65 °C for 5 h led to a selective removal of one tert-butyl group to give compound 6 in 97% yield (Scheme 2).4a,d,8 The structure of 6 was confirmed by the X-ray analysis (see Supporting Information). However, removal of two tert-butyl groups under more forcing conditions led to a mixture of unidentified products. More effective deprotection procedures need to be further developed.
Table 1.
Cu(I)-Catalyzed α-Amination of Ketonesa
| Entry | Substrate (4) | Product (5) | Yield (%)b |
|---|---|---|---|
| 1 |
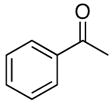 4a |
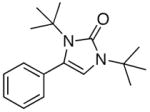 5a |
46 (42)c |
| 2 |
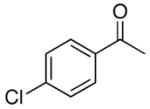 4b |
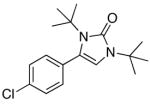 5b |
50 |
| 3 |
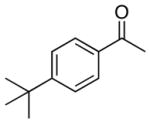 4c |
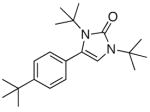 5c |
52 |
| 4 |
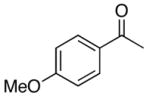 4d |
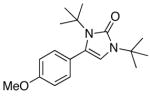 5d |
50 |
| 5 |
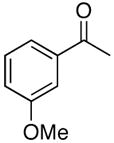 4e |
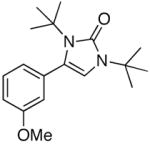 5e |
51 |
| 6 |
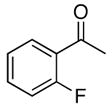 4f |
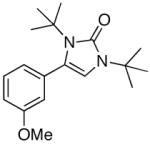 5f |
41 |
| 7 |
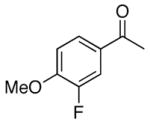 4g |
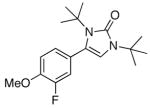 5g |
44 |
| 8 |
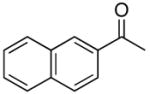 4h |
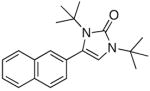 5h |
54 (49)c |
| 9 |
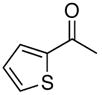 4i |
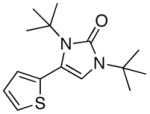 5i |
49 |
| 10 |
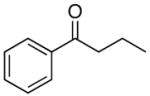 4j |
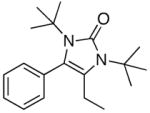 5j |
31 |
All reactions were carried out with ketone (0.4 mmol), 1,2-di-tertbutyldiaziridinone (2) (0.80 mmol) (added slowly over 8 h with syringe pump), CuCl-P(n-Bu)3 (1:2) (0.02 mmol) in DCE (0.1 mL) at 65 °C under argon for 12 h unless otherwise stated. For entries 6 and 9, 60 °C was used.
Isolated yield based on ketone.
The reaction was carried out with 4 mmol of the ketone.
Scheme 2.
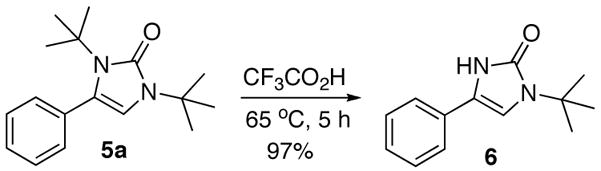
Selective deprotection of imidazolinone 5a
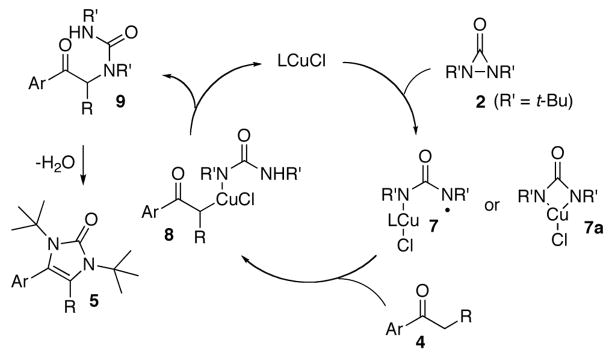
While an exact reaction mechanism awaits further study, a plausible catalytic cycle similar to the formation of hydantoin is proposed in Scheme 3.8 The N-N bond of di-tertbutyldiaziridinone (2) is probably reduced initially by CuCl to form species 74c,12–15 or 7a, which then abstracts a hydrogen or proton from ketone 4 to form 8. Species 8 undergoes a reductive elimination to give compound 9 and regenerate CuCl catalyst.16,17 Imidazolinone 5 is formed by cyclization of compound 9 and loss of water under the reaction conditions.
Scheme 3.
A proposed catalytic cycle for the α-amination.
In summary, a variety of aryl ketones can be successfully α-aminated to directly form imidazolinones in moderate yields using CuCl as catalyst and di-tert-butyldiaziridinone (2) as nitrogen source under mild reaction conditions. The current α-amination process can be potentially useful for the synthesis of biologically active imidazolinone derivatives.
Experimental Section
Representative α-amination procedure on 0.4 mmol scale (Table 1, entry 1)
To a 1.5 mL vial equipped with a stir bar was added CuCl (0.002 g, 0.02 mmol). The sealed vial was evacuated and filled with Ar three times, followed by addition of 1,2- dichloroethane (0.10 mL) and tri-n-butylphosphine (0.01 mL, 0.04 mmol). After the mixture was stirred at room temperature for 10 min, acetophenone (4a) (0.048 g, 0.40 mmol) was added. The reaction mixture was warmed to 65 °C using an oil bath with stirring, and di-tert-butyldiaziridinone (2) (0.136 g, 0.80 mmol) was added by syringe pump over 8 h. The reaction mixture was stirred at this temperature for an additional 4 h and purified by flash chromatography (silica gel, petroleum ether:ethyl ether = 5:1) to give imidazolinone 5a as a white solid (0.050 g, 46%). mp. 128–129 °C; IR (film) 1669 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.31 (s, 5H), 6.05 (s, 1H), 1.54 (s, 9H), 1.42 (s, 9H); 13C NMR (75 MHz, CDCl3) δ 153.7, 134.9, 130.5, 127.88, 127.85, 122.8, 108.2, 58.3, 54.7, 30.4, 28.3; HRMS Calcd for C17H24N2O (M+): 272.1889. Found: 272.1890.
Representative α-amination procedure on 4.0 mmol scale (Table 1, entry 8)
To a 10 mL round-bottomed flask equipped with a stir bar was added CuCl (0.020 g, 0.20 mmol) and methyl 2-naphthyl ketone (4h) (0.68 g, 4.0 mmol). The sealed flask was evacuated and filled with Ar three times, followed by addition of 1,2-dichloroethane (1.0 mL) and tri-n-butylphosphine (0.10 mL, 0.40 mmol). After stirring at room temperature for 10 min, the reaction mixture was warmed to 65 °C using an oil bath, and then di-tert-butyldiaziridinone (2) (1.36 g, 8.0 mmol) was added by syringe pump over 8 h. The reaction mixture was stirred at this temperature for an additional 4 h and purified by flash chromatography (silica gel, petroleum ether:ethyl ether = 5:1) to give unreacted methyl 2-naphthyl ketone (4h) (0.22 g) and imidazolinone 5h (0.455 g, 49%) as a white solid. mp. 117–118 °C; IR (film) 1677 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.86- 7.76 (m, 4H), 7.52-7.42 (m, 3H), 6.13 (s, 1H), 1.57 (s, 9H), 1.46 (s, 9H); 13C NMR (75 MHz, CDCl3) δ 153.9, 133.1, 132.8, 132.4, 129.0, 128.3, 128.0, 127.8, 127.1, 126.6, 126.4, 122.9, 108.8, 58.5, 54.8, 30.4, 28.3; HRMS Calcd for C21H26N2O (M+): 322.2045. Found: 322.2044.
Deprotection of 5a (Scheme 2)
A mixture of 5a (0.30 g, 1.1 mmol) and CF3CO2H (3.0 mL) was stirred at 65 °C under argon atmosphere for 5 h, concentrated, and purified by flash chromatography (sílica gel, diethyl ether) to give compound 6 as a white solid (0.23 g, 97%). mp. 170–171 °C; IR (film) 3151, 1676 cm−1; 1H NMR (300 MHz, CDCl3) δ 11.90 (s, 1H), 7.54 (d, J = 7.8 Hz, 2H), 7.35 (dd, J = 7.8, 7.2 Hz, 2H), 7.21 (t, J = 7.2 Hz, 1H), 6.65 (d, J = 2.7 Hz, 1H), 1.64 (s, 9H); 13C NMR (75 MHz, CDCl3) δ 155.0, 129.9, 128.9, 126.6, 123.2, 121.7, 105.0, 55.0, 28.5; HRMS Calcd for C13H16N2O (M+): 216.1263. Found: 216.1265.
Supplementary Material
Acknowledgments
We are grateful to the generous financial support from the General Medical Sciences of the National Institutes of Health (GM083944-02).
Footnotes
Supporting Information Available: The spectroscopic and analytic data of compounds 5b-g, i, j, the X-ray data of compounds 5b and 6 along with the 1H and 13C NMR spectra of imidazolinone products 5 and 6 (46 pages). This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.For recent leading reviews, see: Osborn HMI, Sweeney J. Tetrahedron: Asymmetry. 1997;8:1693.Lucet D, Gall TL, Mioskowski C. Angew Chem Int Ed. 1998;37:2580. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L.Wolfe JP, Wagaw S, Marcoux JF, Buchwald SL. Acc Chem Res. 1998;31:805.Hartwig JF. Acc Chem Res. 1998;31:852.Ley SV, Thomas AW. Angew Chem Int Ed. 2003;42:5400. doi: 10.1002/anie.200300594.Hultzsch KC. Adv Synth Catal. 2005;347:367.Muzart J. Tetrahedron. 2005;61:4179.Kotti SRSS, Timmons C, Li G. Chem Biol Drug Des. 2006;67:101. doi: 10.1111/j.1747-0285.2006.00347.x.Wolfe JP. Eur J Org Chem. 2007:571.Singh GS, D’hooghe M, De Kimpe N. Chem Rev. 2007;107:2080. doi: 10.1021/cr0680033.Beccalli EM, Broggini G, Martinelli M, Sottocornola S. Chem Rev. 2007;107:5318. doi: 10.1021/cr068006f.
- 2.For leading reviews, see: Davies HML, Long MS. Angew Chem Int Ed. 2005;44:3518. doi: 10.1002/anie.200500554.Espino CG, Du Bois J. In: Modern Rhodium- Catalyzed Organic Reactions. Evans PA, editor. WILEY-VCH; Weinheim, Germany: 2005. Chapter 17.Dick AR, Sanford MST. etrahedron. 2006;62:2439.
- 3.For recent leading references on sp3 C-H amination, see: Espino CG, Wehn PM, Chow J, Du Bois J. J Am Chem Soc. 2001;123:6935.Leung SKY, Huang JS, Liang JL, Che CM, Zhou ZY. Angew Chem Int Ed. 2003;42:340. doi: 10.1002/anie.200390111.Díaz-Requejo MM, Belderraín TR, Nicasio MC, Trofimenko S, Pérez PJ. J Am Chem Soc. 2003;125:12078. doi: 10.1021/ja037072l.Lebel H, Huard K, Lectard S. J Am Chem Soc. 2005;127:14198. doi: 10.1021/ja0552850.Liang C, Robert-Peillard F, Fruit C, Müller P, Dodd RH, Dauban P. Angew Chem Int Ed. 2006;45:4641. doi: 10.1002/anie.200601248.Fiori KW, Du Bois J. J Am Chem Soc. 2007;129:562. doi: 10.1021/ja0650450.Lebel H, Huard K. Org Lett. 2007;9:639. doi: 10.1021/ol062953t.Li Z, Capretto DA, Rahaman R, He C. Angew Chem Int Ed. 2007;46:5184. doi: 10.1002/anie.200700760.Liang C, Collet F, Robert-Peillard F, Müller P, Dodd RH, Dauban P. J Am Chem Soc. 2008;130:343. doi: 10.1021/ja076519d.Hasegawa Y, Watanabe M, Gridnev ID, Ikariya T. J Am Chem Soc. 2008;130:2158. doi: 10.1021/ja710273s.Reed SA, White MC. J Am Chem Soc. 2008;130:3316. doi: 10.1021/ja710206u.
- 4.For leading references on Pd(0) and Cu(I)-catalyzed diamination of olefins with di-tert-butyldiaziridinone, see: Du H, Zhao B, Shi Y. J Am Chem Soc. 2007;129:762. doi: 10.1021/ja0680562.Du H, Yuan W, Zhao B, Shi Y. J Am Chem Soc. 2007;129:7496. doi: 10.1021/ja072080d.Yuan W, Du H, Zhao B, Shi Y. Org Lett. 2007;9:2589. doi: 10.1021/ol071105a.Du H, Yuan W, Zhao B, Shi Y. J Am Chem Soc. 2007;129:11688. doi: 10.1021/ja074698t.Du H, Zhao B, Shi Y. J Am Chem Soc. 2008;130:8590. doi: 10.1021/ja8027394.Du H, Zhao B, Yuan W, Shi Y. Org Lett. 2008;10:4231. doi: 10.1021/ol801605w.
- 5.For a leading review on diaziridinones, see: Heine HW. In: The Chemistry of Heterocyclic Compounds. Hassner A, editor. John Wiley & Sons, Inc; USA: 1983. p. 547.
- 6.Greene FD, Stowell JC, Bergmark WR. J Org Chem. 1969;34:2254. [Google Scholar]
- 7.For leading references on the reaction of diaziridines with ketenes, see: Shevtsov AV, Petukhova VYu, Strelenko YuA, Lyssenko KA, Makhova NN, Tartakovsky VA. Russ Chem Bull, Int Ed. 2005;54:1021.Shevtsov AV, Ananikov VP, Makhova NN. Russ J Org Chem. 2007;43:1101.
- 8.Zhao B, Du H, Shi Y. J Am Chem Soc. 2008;130:7220. doi: 10.1021/ja802242h. [DOI] [PubMed] [Google Scholar]
- 9.For leading references, see: Carling RW, Moore KW, Moyes CR, Jones EA, Bonner K, Emms F, Marwood R, Patel S, Patel S, Fletcher AE, Beer M, Sohal B, Pike A, Leeson PD. J Med Chem. 1999;42:2706. doi: 10.1021/jm991029k.Bronson JJ, DenBleyker KL, Falk PL, Mate RA, Ho HT, Pucci MJ, Snyder LB. Bioorg Med Chem Lett. 2003;13:873. doi: 10.1016/s0960-894x(02)01076-4.Burgey CS, Stump CA, Nguyen DN, Deng JZ, Quigley AG, Norton BR, Bell IM, Mosser SD, Salvatore CA, Rutledge RZ, Kane SA, Koblan KS, Vacca JP, Graham SL, Williams TM. Biorg Med Chem Lett. 2006;16:5052. doi: 10.1016/j.bmcl.2006.07.044.Shaw AW, Paone DV, Nguyen DN, Stump CA, Burgey CS, Mosser SD, Salvatore CA, Rutledge RZ, Kane SA, Koblan KS, Graham SL, Vacca JP, Williams TM. Bioorg Med Chem Lett. 2007;17:4795. doi: 10.1016/j.bmcl.2007.06.062.Congiu C, Cocco MT, Onnis V. Bioorg Med Chem Lett. 2008;18:989. doi: 10.1016/j.bmcl.2007.12.023.Watanabe K, Morinaka Y, Hayashi Y, Shinoda M, Nishi H, Fukushima N, Watanabe T, Ishibashi A, Yuki S, Tanaka M. Bioorg Med Chem Lett. 2008;18:1478. doi: 10.1016/j.bmcl.2007.12.064.Xue N, Yang X, Wu R, Chen J, He Q, Yang B, Lu X, Hu Y. Bioorg Med Chem. 2008;16:2550. doi: 10.1016/j.bmc.2007.11.048.
- 10.For leading references, see: Chawla HM, Pathak M. Tetrahedron. 1990;46:1331.Markwalder JA, Pottorf RS, Seitz SP. Synlett. 1997:521.Akester J, Cui J, Fraenkel G. J Org Chem. 1997;62:431. doi: 10.1021/jo961972l.Wendeborn S, Winkler T, Foisy I. Tetrahedron Lett. 2000;41:6387.Fevig JM, Pinto DJ, Han Q, Quan ML, Pruitt JR, Jacobson IC, Galemmo RA, Jr, Wang S, Orwat MJ, Bostrom LL, Knabb RM, Wong PC, Lam PYS, Wexler RR. Bioorg Med Chem Lett. 2001;11:641. doi: 10.1016/s0960-894x(01)00029-4.Sosa ACB, Yakushijin K, Horne DA. J Org Chem. 2002;67:4498. doi: 10.1021/jo020063v.Lee SH, Yoshida K, Matsushita H, Clapham B, Koch G, Zimmermann J, Janda KD. J Org Chem. 2004;69:8829. doi: 10.1021/jo048353u.Parcher BW, Erion DM, Dang Q. Tetrahedron Lett. 2004;45:2677.Siamaki AR, Black DA, Arndtsen BA. J Org Chem. 2008;73:1135. doi: 10.1021/jo701875b.
- 11.For leading references, see: Wamhoff H, Kleimann W, Kunz G, Theis CH. Angew Chem. 1981;93:601.Lu J, Tan X, Chen C. J Am Chem Soc. 2007;129:7768. doi: 10.1021/ja072844p.Sheppeck JE, II, Gilmore JL, Tebben A, Xue C-B, Liu R-Q, Decicco CP, Duan JJ-W. Bioorg Med Chem Lett. 2007;17:2769. doi: 10.1016/j.bmcl.2007.02.076.
- 12.For a leading review on metal-promoted radical reactions, see: Iqbal J, Bhatia B, Nayyar NK. Chem Rev. 1994;94:519.
- 13.For leading reviews on CuX-catalyzed atom transfer reactions, see: Patten TE, Matyjaszewski K. Acc Chem Res. 1999;32:895.Clark AJ. Chem Soc Rev. 2002;31:1. doi: 10.1039/b107811a.
- 14.For leading references on nitrogen-centered radicals, see: Stella, L. In Radicals in Organic Synthesis; Renaud, P.; Sibi, M. P. Ed.; Wiley-VCH: Weinheim, 2001, Vol. 2, p 407. Guin J, Mück-Lichtenfeld C, Grimme S, Studer A. J Am Chem Soc. 2007;129:4498. doi: 10.1021/ja0692581.
- 15.For leading references on Cu(I)-catalyzed homolytic cleavage of N-O bonds of oxaziridines, see: Aubé J, Peng X, Wang Y, Takusagawa F. J Am Chem Soc. 1992;114:5466.Aubé J Chem Soc Rev. 1997;26:269.Black DStC, Edwards GL, Laaman SM. Tetrahedron Lett. 1998;39:5853.Black DStC, Edwards GL, Laaman SM. Synthesis. 2006:1981.
- 16.For leading references on Cu(II)-mediated oxidative coupling reactions, see: Schmittel M, Haeuseler A. J Organomet Chem. 2002;661:169.Baran PS, Richter JM. J Am Chem Soc. 2004;126:7450. doi: 10.1021/ja047874w.Richter JM, Whitefield BW, Maimone TJ, Lin DW, Castroviejo MP, Baran PS. J Am Chem Soc. 2007;129:12857. doi: 10.1021/ja074392m.
- 17.For a leading review on organocopper reagents, see: Lipshutz BH, Sengupta S. Org React. 1992;41:135.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.