Abstract
The aim of this multicentre study was to compare T1 with T2 weighted MRI scans of the labyrinth after meningitis and to investigate whether waiting with scanning improved the reliability of diagnosing an ongoing process such as cochlear osteogenesis. Forty-five patients were included who suffered from meningitis induced hearing loss (radiological imaging <1 year after meningitis). Twenty-one gadolinium enhanced T1 and 45 T2 weighted MRI scans were scored by two radiologists regarding the condition of the labyrinth. These radiological observations were compared with the condition of the cochlea as described during cochlear implantation. A higher percentage of agreement with surgery was found for T2 (both radiologists 73%) than for T1 weighted MRI scans (radiologist 1: 62%, radiologist 2: 67%), but this difference is not significant. There was no significant difference between early (0–3 months) and late (>3 months) scanning, showing that radiological imaging soon after meningitis allows early diagnosis without suffering from a lower agreement with surgical findings.
Keywords: Cochlear implantation, MRI, Cochlear osteogenesis, Cochlear ossification, Meningitis
Introduction
Bacterial meningitis is an infamous cause of acquired hearing loss leading to profound bilateral hearing loss in up to 4% of those affected [1, 2]. The incidence of meningitis induced hearing loss is different for varying causative agents such as S. pneumoniae (31–36%), N. meningitidis (8–11%) and H. influenza (6–11%) [1–3]. Meningitis induced hearing loss is probably the result of the spread of infection to the inner ear via the cochlear aqueduct or modiolus and develops at an early stage of meningitis. The ensuing labyrinthitis is thought to be responsible for sensorineural hearing loss [4, 5]. When meningitis has led to a profound sensorineural hearing loss, cochlear implantation may provide auditory rehabilitation. Cochlear implantation is more successful and less current is needed when insertion of all electrodes in a patent cochlear lumen is achieved [6, 7]. This is more demanding when the route of insertion is obstructed by osteoneogenesis, especially at the end stage of this process when a lumen may only be created by drilling out hard bone in the cochlea. Such a situation occurs when labyrinthitis progresses to labyrinthitis ossificans. Some degree of ossification of the cochlea has been described in 56–80% of people with postmeningitic deafness [7, 8]. In animal models it has been shown that the sequence of events, which starts with an inflammation that progresses to fibrosis and ultimately to ossification of the cochlea, commences the first week after the onset of meningitis and bone deposition can continue for a year [9, 10].
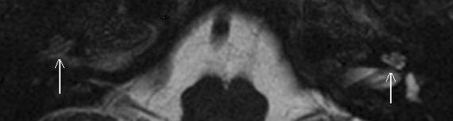
Information on the condition of the cochlea acquired via radiological imaging before the operation can assist the surgeon in choosing the cochlea in which the likelihood of optimal insertion of all electrodes is highest [11]. The timing when to implant a patient with osteoneogenesis is an important instrument in limiting the degree of hindrance during implantation because of the progressive character of cochlear osteoneogenesis. When the process of osteoneogenesis of the cochleae (including its early stage of fibrosis) is identified one might consider implanting bilaterally to prevent losing the cochlear lumen of the second ear for implantation in the future [12]. Another important reason to limit the period between meningitis and cochlear implantation as much as possible is pointed out by Durisin et al. [13], who indicate that audiological performance is better when the duration of deafness is minimized in children deafened by meningitis. However, the duration between meningitis and cochlear implantation is mainly determined by when an accurate diagnosis can be made. To establish preoperatively whether a patient deafened by meningitis suffers from osteoneogenesis of the cochlea and to what extent, one is dependent on radiological imaging. In several studies high resolution computed tomography (HRCT) of the temporal bone is found to be equivalent to T2 weighted MRI when evaluating gross bony alterations of the cochlea [14, 15]. When there is rapid ossification of the cochlea, a CT-scan might be useful even at an early stage as pointed out by Aschendorff et al. [16], but one never knows whether such bony alterations will already be apparent if the osteoneogenesis is still ongoing. The advantage of T2 weighted MRI is that it provides an impression of fluid displacement due to anatomical changes in the cochlea and is therefore capable of showing the preceding stage of fibrosis as well as ossification [17–19]. Figure 1 shows an example of a normal as well as an affected cochlea, as seen on a T2 weighted MRI scan. Gadolinium enhanced T1 weighted MRI is especially geared towards establishing the presence of active labyrinthitis. This is due to its capability to show increased perfusion of the striae vascularis indicative of local inflammation [19–21]. A normal cochlea as well as a cochlea displaying pathologically increased perfusion, as seen on a T1 weighted MRI scan are shown in Fig. 2. Extensive bone deposition in the cochlea impedes complete electrode insertion more severely than the preceding stages of minimal bone deposition and fibrosis of the cochlea. Magnetic resonance imaging is well suited to detect depositions that are not yet calcified. This makes it particularly useful if one aims to use radiological imaging in diagnosing the process of cochlear osteoneogenesis at a stage when the cochlear lumen is not yet severely compromised.
Fig. 1.
T2 weighted MRI scan of a patient with one normal and one abnormal cochlea. The cochlea that can been on the right side of the T2 weighted MRI scan shown above (which is actually the left cochlea of the patient) displays the bright intensity of a normal cochlea. On the left, the cochlea of this patient can still be recognised but the intensity of the signal is decreased which is indicative of cochlear pathology
Fig. 2.
T1 weighted MRI scan of a patient with one normal and one abnormal cochlea. The cochlea on the right side of the T1 weighted MRI scan with gadolinium (which is actually the left cochlea of the patient) cannot be seen. This is the normal healthy situation. On the left, the cochlea of this patient can be clearly seen due to increased perfusion indicative of cochlear pathology
In the present multicentre study the preoperative radiological findings of postmeningitic cochlear implant candidates are compared with the condition of the cochlea as encountered during surgery. The main objectives in the study are (1) to compare T1 with T2 weighted MRI scans of the labyrinth, (2) to investigate whether waiting with scanning improves the reliability of diagnosing an ongoing process such as meningitic osteoneogenesis, (3) to investigate whether the outcome is different when only the cochlea instead of the whole labyrinth is observed.
Materials and methods
The study was set up as a multicentre study to increase the number of patients (radiological data) that could be included. Four European cochlear implant centres cooperated for this study: the Medical University of Hannover, the University Hospital of Freiburg, the Sint-Augustinus University Hospital and the Radboud University Medical Centre Nijmegen.
The data were included when a subject met the following inclusion criteria: profound hearing loss due to meningitis, availability of an MRI scan made within 1 year after the meningitic episode, availability of the surgical report of the cochlear implantation. A total of 45 patients were included (Table 1). This resulted in 45 T2 weighted MRI scans and 21 T1 weighted MRI scans.
Table 1.
Patient characteristics
| Total number of patients | 45 |
| Male:female | 22:23 |
| Causative agent of meningitis |
S. pneumoniae 31 N. meningitidis 3 E.coli 2 H.influenza 1 M. tuberculosis 1 K. pneumoniae 1 Unknown 6 |
| Percentage of cochlea with osteoneogenesis encountered during surgery | 76% |
| Age at time of meningitis (years) |
Mean 9 Range 0–65 |
| Period between meningitis and T1 MRI scan (months) |
Mean 3 Range 0–11 |
| Period between meningitis and T2 MRI scan (months) |
Mean 4 Range 0–12 |
All gadolinium enhanced T1 weighted MRI scans were made using a T1 Turbo Spin Echo (TSE) sequence. High resolution 3D constructive interference in steady state (CISS) sequence was used in all T2 weighted MRI scans. The MRI scans were made using 1.5 Tesla MRI scanners. The slice thickness varied between 0.8 and 3 millimetre for the gadolinium enhanced T1 weighted MRI and between 0.7 and 1 millimetre for the T2 weighted images.
Two senior radiologists, both specialized in radiological imaging of the temporal bone, observed and scored the scans independently of one another. Only transverse scans were observed. The correlation between both radiologists was determined using the kappa score. The scores of each radiologist are presented separately (Table 2). The labyrinth was looked at in detail and the scores of the different regions were eventually summarized in a score for the condition of the cochlea and for the condition of the whole labyrinth (cochlea plus vestibulum and semicircular canals). The labyrinth was scored as abnormal by the radiologist when any anatomical disruption or change in perfusion (T1) was observed. Without such an alteration the anatomy was scored as normal.
Table 2.
Scoring of the labyrinth (cochlea plus vestibulum and semicircular canals) and of the cochlea
| Type of radiological imaging | Anatomical region | Radiologist | Number of participants/scans | Agreement with surgical findings (%) | 95% Confidence interval | Positive predictive value | Negative predictive value | Correlation (kappa) between radiologists (range 0–1) |
|---|---|---|---|---|---|---|---|---|
| T1 weighted MRI | Labyrinth | 1 | 21 | 62 | 38–82 | 0.75 | 0.33 | 0.88 |
| 2 | 21 | 67 | 43–85 | 0.75 | 0.40 | |||
| Cochlea | 1 | 21 | 57 | 34–78 | 0.71 | 0.25 | 0.89 | |
| 2 | 21 | 62 | 38–82 | 0.73 | 0.33 | |||
| T2 weighted MRI | Labyrinth | 1 | 45 | 73 | 58–85 | 0.78 | 0.50 | 0.72 |
| 2 | 45 | 73 | 58–85 | 0.80 | 0.50 | |||
| Cochlea | 1 | 45 | 71 | 56–84 | 0.88 | 0.47 | 0.80 | |
| 2 | 45 | 68 | 52–81 | 0.82 | 0.44 | |||
| T2 and T1 weighted MRI combined | Labyrinth | 1 | 21 | 67 | 43–85 | 0.70 | 0.33 | 0.64 |
| 2 | 21 | 71 | 41–91 | 0.71 | 0.50 |
The condition of the cochlea as experienced by the surgeon was scored to be normal (clear lumen) or abnormal (fibrosis, ossification) after reviewing the surgical notes of the various surgeons involved. The condition of the cochlea as described by the surgeon is mainly based on what was encountered in the scala tympani, especially in the basal turn. This is due to the fact the scala tympani was always opened and described during surgery even when implantation eventually took place in the scala vestibuli.
The observations of the radiologists were compared with the observation by the surgeon of the cochlea during implantation; the latter is used as gold standard.
Although the radiologists scored both ears for each patient, only the observation of the cochlea that was implanted was included. In the case of bilaterally implanted patients only the cochlea that was implanted first was included to avoid bias.
In the present study the degree of agreement with surgical findings and the positive and negative predictive values were determined for the radiological observations of two overlapping anatomical regions: the cochlea and the complete membranous labyrinth (cochlea and vestibular system). The radiologists observed these anatomical regions on a gadolinium enhanced T1 and on a T2 weighted MRI scan.
The observations on T1 and T2 weighted MRI scans were compared to each other with regard to the agreement with surgery. The scores of both MRI modalities were also compared with the score of T1 and T2 combined to investigate additional value. In the latter case the scores of T1 and T2 weighted MRI were converted into a single ‘combined’ score for MRI which was said to be abnormal when pathology was observed on T1 or on T2 weighted MRI.
For most comparisons in this study the judgement of the whole labyrinth was used because the vestibular system constitutes one continuous system with the cochlea and it has been shown that if one area is affected then this is likely to be more widespread [22]. To check whether judging the whole labyrinth or just the cochlea would indeed influence the outcome we compared the differences in observations of the whole labyrinth versus just the cochlea regarding the agreement with surgery.
The impact of the duration between the meningitic episode and the moment of radiological imaging on the degree of agreement with surgical findings was analysed by dividing the T1 and T2 weighted MRI scans in early (0–3 months) and late (>3 months) groups and comparing these two groups.
The McNemar test was used to compare the levels of agreement with surgical findings of T1 and T2 weighted MRI and with the combined MRI score. The importance of the timing of the MRI was determined by comparing the early (first 3 months) with the late (>3 months) group using Fisher’s exact test. The level of significance used was 0.05.
Results
Radiological abnormalities in the labyrinth were found by radiologist 1 on 16 of the 21 T1 weighted MRI scans (76%) and on 37 of the 45 T2 weighted MRI scans (82%). Radiologist 2 detected abnormalities on 16 of the 21 T1 weighted scans (76%) and on 35 of the 45 T2 weighted scans (78%). Meningitis was caused by S. pneumoniae (Table 1) in 79% of the patients in which the causative agent was identified. In 76% of the patients in this study an abnormal cochlear lumen was encountered during cochlear implantation. Surgery was performed within 3 months after the MRI scans were made in 22 cases (17 pathological cochleae) and in the remaining 23 cases cochlear implantation took place more than 3 months after the MRI scans were made (17 pathological cochleae).
For radiologists 1 and 2 there was no significant difference between their observations on T1 and T2 weighted MRI of the labyrinth in terms of the degree of agreement of both types of MRI with surgical findings.
Radiologist 1: 62% agreement T1 MRI versus 73% agreement T2 MRI (P = 1.00).
Radiologist 2: 67% agreement T1 MRI versus 73% agreement T2 MRI (P = 1.00).
The combined score on T1 and T2 weighted scans for radiologist 1 agreed with surgical findings in 67%. This was not significantly better than T1 weighted scans of the labyrinth (62%) and not significantly worse than the agreement of T2 weighted MRI (73%) (P = 1.00 for both comparisons). The scores on T1 and T2 weighted MRI overlapped in 76% of the cases. When the scores of T1 and T2 weighted MRI disagreed it turned out that the T1 weighted MRI was correct (agreeing with surgery) in three cases and the T2 weighted MRI in two cases.
When the combined score of T1 and T2 weighted MRI of the labyrinth was taken as a point of reference for radiologist 2, it was found that observations on T1 (67%) or T2 (73%) weighted scans did not differ significantly (P = 1.00 for both comparisons) from this combined MRI (71%) score with regard to the agreement with surgery. The T1 and T2 weighted MRI had the same score in 80% of the cases. Of the four cases that did not overlap T1 MRI was correct in two cases and T2 in the other two cases.
When radiologist 1 only observed the cochlea instead of the whole labyrinth the degree of agreement with surgery was lower, but not significantly so for both types of MRI.
Radiologist 1: 57% agreement T1 cochlea versus 62% agreement T1 labyrinth (P = 1.00). 71% agreement T2 cochlea versus 73% agreement T2 labyrinth (P = 1.00).
Similar outcomes were found for radiologist 2. Radiologist 2: 62% agreement T1 cochlea versus 67% agreement T1 labyrinth (P = 1.00). Radiologist 2: 68% agreement T2 cochlea versus 73% agreement T2 labyrinth (P = 1.00).
For the positive predictive value and negative predictive values see Table 2. The positive predictive value and negative predictive value did not change significantly either when the observation was limited to the cochlea on T1 or T2 weighted MRI (for all comparisons P > 0.05).
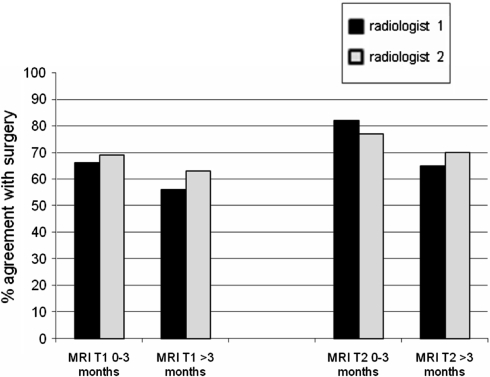
Whether T1 weighted MRI scans of the labyrinth were made within or after the first 3 months following meningitis did not have a significant influence on the agreement of the observations by radiologists 1 and 2 with surgical findings.
Radiologist 1: 66% agreement 0–3 months versus 56% agreement >3 months (P = 0.66).
Radiologist 2: 69% agreement 0–3 months versus 63% agreement >3 months (P = 1.00).
Thirteen T1 weighted MRI scans were made within 0–3 months after the meningitic episode, the other eight were made later than 3 months.
A similar pattern was found for T2 weighted MRI scans in which the duration between scanning and the meningitic episode did not significantly influence the agreement between the scans and surgical findings (Table 3).
Radiologist 1: 82% agreement 0–3 months versus 65% agreement >3 months (P = 0.31).
Radiologist 2: 77% agreement 0–3 months versus 70% agreement >3 months (P = 0.74).
Table 3.
Impact of the duration of the period between meningitis and radiological imaging
| Type of radiological imaging | Radiologist | Period between meningitis and radiological imaging (months) | Number of participants/scans | Agreement with surgical findings (%) |
|---|---|---|---|---|
| T1 weighted MRI of total labyrinth | 1 | 0–3 | 13 | 66 |
| >3 | 8 | 56 | ||
| 2 | 0–3 | 13 | 69 | |
| >3 | 8 | 63 | ||
| T2 weighted MRI of total labyrinth | 1 | 0–3 | 22 | 82 |
| >3 | 23 | 65 | ||
| 2 | 0–3 | 22 | 77 | |
| >3 | 23 | 70 |
Of the total 45 T2 weighted MRI scans 22 were made 0–3 months postmeningitic, 23 were made 3–12 months after the meningitic episode.
Discussion
This study was conducted to provide more knowledge on how and when to use magnetic resonance imaging in patients with meningitis induced severe hearing loss. The clinical relevance is reflected in the objective to accurately and timely diagnose the process of osteoneogenesis and thus limit its potential interference with cochlear implantation.
The MRI findings in this study are compatible with findings on the use of MRI scans of the labyrinth in other studies with regard to the agreement between imaging and surgical findings [14, 15]. Not many radiological studies focus exclusively on the condition of the cochlea after meningitis which makes a direct comparison to other MRI studies of patients with a mixed aetiology of deafness only of limited value. A study that does target the same group was carried out by Chan et al. [22], who suggested looking at the lateral semicircular canal as well as at the cochlear basal turn when predicting the presence of cochlear osteoneogenesis on MRI. This recommendation, not to limit the observation to the cochlea alone, is supported by the present study in which the agreement between radiological observations and surgical findings was consistently higher when the whole labyrinth instead of only the cochlea was judged (Table 2; Fig. 3). It is worth noting that the higher scores for the radiological judgment of the whole labyrinth were found for all parameters apart for the positive predictive value on T2 weighted MRI. This higher positive predictive value might have reflected the presence of cases in which the osteoneogenesis was limited to extra-cochlear parts of the labyrinth and therefore not encountered during surgery.
Fig. 3.
Observations of the radiologists
The high positive predictive value of both MRI modalities reflects their value when one aims to make sure that those who are operated on are indeed affected. It is much more difficult on the other hand to rule out osteoneogenesis when it is not seen on MRI as pointed out by the lower negative predictive values. This is why repeated scanning after a first negative MRI would be justified.
The data in this study indicate that not withstanding the fact that for both radiologists T2 weighted MRI scans consistently show a higher agreement with what is found during surgery no significant difference could be established between gadolinium enhanced T1 MRI and T2 weighted MRI in predicting pathology in the cochlea (Table 2; Fig. 3). The theoretical argument for making a T1 weighted MRI is that it is well suited to detect the active labyrinthitis that precedes ossification and thereby provide early information on abnormalities in the cochlea. In this study the T1 weighted MRI scans made in the early stage after meningitis did indeed show a higher agreement with surgery than those made at a later stage (Table 3; Fig. 4), but this difference is not significant. It is interesting to note that even in the ‘early’ first 3 months T1 weighted MRI does not show a higher percentage of agreement with surgery than T2 weighted MR scans made at the same time (Fig. 4). From the data in this study no additional value of combining the findings on T1 and T2 weighted MRI could be established (Table 2). One could therefore wonder if it is useful to make a T1 weighted MRI in addition to a T2 weighted MRI. The present study does not show a significant inferiority of T1 weighted MRI, but neither does it find a specific indication for using T1 weighted MRI when analysing a labyrinth after meningitis has occurred. Especially when trying to optimize the surgeons’ preoperative knowledge regarding the surgical options left in a pathological cochlea it can be valuable to distinguish the scala tympani from the scala vestibuli so the latter can also be considered for implantation when on MRI the scala vestibuli is still clear and the scala tympani is not. Such scalar differentiation is only possible on T2 weighted MRI as was indeed noticed in this study as well (data not used for analysis and thus not shown). Furthermore, one might want to consider that the utilisation of gadolinium is not entirely without risk and extra caution is warranted in very young children (who form a big group amongst cochlear implant candidates) due to their renal immaturity [23].
Fig. 4.
Moment of radiological imaging and its impact on the agreement between radiological observations and surgical findings
The reason this study included a comparison between early and late radiological imaging (Table 3; Fig. 4) is that radiological imaging in patients deafened by meningitis provides an image of the labyrinth at one moment on the timeline of osteoneogenesis. When this moment takes place is clinically relevant if one takes into account that for easy insertion of all electrodes of the cochlear implant a minimization of the period of potential damage to the cochlea seems preferable. For valuable radiological imaging the reverse appears plausible because the shorter the period of potential damage to the cochlea the more subtle an anatomical disruption might show up on T2 weighted MRI. T1weighted MRI on the other hand could be expected to be more suited for early use to detect early active labyrinthitis. The data in this study indicate that there is no need for concessions. There was no significant difference regarding the degree of agreement with surgical findings when the MRI scans were made the first 3 months instead of longer after the meningitic episode. The lack of superiority of radiological imaging at a later stage (>3 months) found in this study means that early radiological imaging can save valuable time without compromising the reliability of preoperative diagnosis. Such early diagnosis shortens the selection procedure for cochlear implantation which is likely to benefit the performance of the patient with a cochlear implant [13].
The results in this study show clear trends regarding which MRI modality would be preferred and when to use it. The size of the group and paired analysis limit the statistical power needed to provide hard significant results. This warrants caution when interpreting the data, but should not obscure the consistent trends that are shown. Conclusively, one can state that no significant superiority of T1, T2 weighted MRI or a combination of both was found although the observations on T2 weighted MRI consistently had the highest percentage of agreement with surgical findings. This study shows that when early cochlear implantation is preferred the resulting short period of time between the meningitic episode and the moment of radiological imaging does not jeopardize the diagnostic value of preoperative radiological imaging.
Acknowledgment
The authors would like to thank Mr. M. Durisin of the Medical University of Hannover for his effort in collecting the data and Mr T. De Boo of the epidemiological department of the University Medical Centre Nijmegen for his advice and support with the statistical analysis.
Conflict of interest statement
None of the authors had any financial interest in the current research. There was no conflict of interest for any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s00405-009-0977-9
References
- 1.Fortnum HM. Hearing impairment after bacterial meningitis: a review. Arch Dis Child. 1992;67(9):1128–1133. doi: 10.1136/adc.67.9.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dodge PR, Davis H, Feigin RD, Holmes SJ, Kaplan SL, Jubelirer DP, Stechenberg BW, Hirsh SK. Prospective evaluation of hearing impairment as a sequela of acute bacterial meningitis. N Engl J Med. 1984;311(14):869–874. doi: 10.1056/NEJM198410043111401. [DOI] [PubMed] [Google Scholar]
- 3.Kutz JW, Simon LM, Chennupati SK, Giannoni CM, Manolidis S. Clinical predictors for hearing loss in children with bacterial meningitis. Arch Otolaryngol Head Neck Surg. 2006;132(9):941–945. doi: 10.1001/archotol.132.9.941. [DOI] [PubMed] [Google Scholar]
- 4.Merchant SN, Gopen Q. A human temporal bone study of acute bacterial meningogenic labyrinthitis. Am J Otol. 1996;17(3):375–385. [PubMed] [Google Scholar]
- 5.Kesser BW, Hashisaki GT, Spindel JH, Ruth RA, Scheld WM. Time course of hearing loss in an animal model of pneumococcal meningitis. Otolaryngol Head Neck Surg. 1999;120(5):628–637. doi: 10.1053/hn.1999.v120.a92772. [DOI] [PubMed] [Google Scholar]
- 6.Rotteveel LJ, Snik AF, Vermeulen AM, Mylanus EA. Three-year follow-up of children with postmeningitic deafness and partial cochlear implant insertion. Clin Otolaryngol. 2005;30(3):242–248. doi: 10.1111/j.1365-2273.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberg LS, Luxford WM, Becker TS, House WF. Electrical stimulation of the auditory system in children deafened by meningitis. Otolaryngol Head Neck Surg. 1984;92(6):700–705. doi: 10.1177/019459988409200619. [DOI] [PubMed] [Google Scholar]
- 8.Axon PR, Temple RH, Saeed SR, Ramsden RT. Cochlear ossification after meningitis. Am J Otol. 1998;19(6):724–729. [PubMed] [Google Scholar]
- 9.Nabili V, Brodie HA, Neverov NI, Tinling SP. Chronology of labyrinthitis ossificans induced by Streptococcus pneumoniae meningitis. Laryngoscope. 1999;109(6):931–935. doi: 10.1097/00005537-199906000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Brodie HA, Thompson TC, Vassilian L, Lee BN. Induction of labyrinthitis ossificans after pneumococcal meningitis: an animal model. Otolaryngol Head Neck Surg. 1998;118(1):15–21. doi: 10.1016/S0194-5998(98)70369-9. [DOI] [PubMed] [Google Scholar]
- 11.Laszig R, Chang SO, Kubo T, Ramos MA, Frijns JH, Briggs R, Haynes DS. APSCI panel discussion I: imaging and surgical issues. Ear Hear. 2007;28(2):119S–123S. doi: 10.1097/AUD.0b013e318031548b. [DOI] [PubMed] [Google Scholar]
- 12.Offeciers E, Morera C, Muller J, Huarte A, Shallop J, Cavalle L. International consensus on bilateral cochlear implants and bimodal stimulation. Acta Otolaryngol. 2005;125(9):918–919. doi: 10.1080/00016480510044412. [DOI] [PubMed] [Google Scholar]
- 13.Durisin M, Arnoldner C, Stover T, et al. Audiological performance in cochlear implanted patients deafened by meningitis depending on duration of deafness. Eur.Arch.Otorhinolaryngol. 2008;265:381–388. doi: 10.1007/s00405-008-0584-1. [DOI] [PubMed] [Google Scholar]
- 14.Gleeson TG, Lacy PD, Bresnihan M, Gaffney R, Brennan P, Viani L. High resolution computed tomography and magnetic resonance imaging in the pre-operative assessment of cochlear implant patients. J Laryngol Otol. 2003;117(9):692–695. doi: 10.1258/002221503322334495. [DOI] [PubMed] [Google Scholar]
- 15.Bettman R, Beek E, Van Olphen A, Zonneveld F, Huizing E. MRI versus CT in assessment of cochlear patency in cochlear implant candidates. Acta Otolaryngol. 2004;124(5):577–581. doi: 10.1080/00016480310016848. [DOI] [PubMed] [Google Scholar]
- 16.Aschendorff A, Klenzner T, Laszig R. Deafness after bacterial meningitis: an emergency for early imaging and cochlear implant surgery. Otolaryngol Head Neck Surg. 2005;133(6):995–996. doi: 10.1016/j.otohns.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 17.Parry DA, Booth T, Roland PS. Advantages of magnetic resonance imaging over computed tomography in preoperative evaluation of pediatric cochlear implant candidates. Otol Neurotol. 2005;26(5):976–982. doi: 10.1097/01.mao.0000185049.61770.da. [DOI] [PubMed] [Google Scholar]
- 18.Dahm MC, Mack MG, Tykocinski M, Vogl TJ. Submillimeter imaging and reconstruction of the inner ear. Am J Otol. 1997;18(6 Suppl):S54–S56. [PubMed] [Google Scholar]
- 19.Seitz J, Held P, Waldeck A, Strotzer M, Volk M, Strutz J, Feuerbach S. Value of high-resolution MR in patients scheduled for cochlear implantation. Acta Radiol. 2001;42(6):568–573. doi: 10.1080/028418501127347395. [DOI] [PubMed] [Google Scholar]
- 20.Valvassori GE. Imaging of otosclerosis. Otolaryngol Clin North Am. 1993;26(3):359–371. [PubMed] [Google Scholar]
- 21.Ziyeh S, Berlis A, Ross UH, Reinhardt MJ, Schumacher M. MRI of active otosclerosis. Neuroradiology. 1997;39(6):453–457. doi: 10.1007/s002340050445. [DOI] [PubMed] [Google Scholar]
- 22.Chan CC, Saunders DE, Chong WK, Hartley BE, Raglan E, Rajput K. Advancement in post-meningitic lateral semicircular canal labyrinthitis ossificans. J Laryngol Otol. 2007;121(2):105–109. doi: 10.1017/S002221510600377X. [DOI] [PubMed] [Google Scholar]
- 23.Lin SP, Brown JJ. MR contrast agents: physical and pharmacologic basics. J Magn Reson Imaging. 2007;25(5):884–899. doi: 10.1002/jmri.20955. [DOI] [PubMed] [Google Scholar]