Abstract
The dense granule protein 4 (GRA4) is a granular protein from Toxoplasma gondii, and is a candidate for vaccination against this parasite. In this study, the plasmid pcDNA3.1-GRA4 (pGRA4), encoding for the GRA4 antigen, was incorporated by the dehydration-rehydration method into liposomes composed of 16 mmol/L egg phosphatidylcholine (PC), 8 mmol/L dioleoyl phosphatidylethanolamine (DOPE), and 4 mmol/L 1,2-diodeoyl-3-(trimethylammonium) propane (DOTAP). C57BL/6 mice and BALB/c mice were immunized intramuscularly three times with liposome-encapsulated pGRA4 to determine whether DNA immunization could elicit a protective immune response to T. gondii. Enzyme-linked immunosorbent assay (ELISA) of sera from immunized mice showed that liposome-encapsulated pGRA4 generated high levels of IgG antibodies to GRA4. Production of primary interferon (IFN)-γ and interleukin (IL)-2 in GRA4-stimulated splenocytes from vaccinated mice suggested a modulated Th1-type response. 72.7% of C57BL/6 mice immunized with liposome-encapsulated pGRA4 survived the challenge with 80 tissue cysts of ME49 strain, whereas C57BL/6 mice immunized with pGRA4 had only a survival rate of 54.5%. When immunized BALB/c mice were intraperitoneally challenged with 103 tachyzoites of the highly virulent RH strain, the survival time of mice immunized with liposome-encapsulated pGRA4 was markedly longer than that of other groups. Our observations show that liposome-encapsulated pGRA4 enhanced the protective effect against infection of T. gondii.
Keywords: DNA vaccine, Granule protein 4 (GRA4), Liposome, Toxoplasma gondii
INTRODUCTION
Toxoplasmosis is a parasite disease caused by the protozoan Toxoplasma gondii. It is one of the most epidemic parasitic diseases in human beings and animals. About 35%~40% of the world’s adult population is estimated to carry a Toxoplasma infection and demonstrates varying clinical manifestations. People are infected by eating infected meat or contaminated foods and by inadvertent ingestion of oocysts or sporozoites in cat feces (Tenter et al., 2000), and less frequently infected by direct recipience of tissue or blood from other contaminated humans and vertical transmission from acutely infected mothers (Feigin and Cherry, 1998). The infection of immunocompetent humans causes a mild illness or no illness. Its clinical disease is largely confined to certain risk groups (Schwartzman, 2001). Toxoplasmosis is an opportunistic disease, which is often lethal for patients with immunodeficiency such as those with acquired immune deficiency syndrome (AIDS) and bone marrow or transplant recipients. According to surveillance, approximately 4000 persons with AIDS in the USA suffering from toxoplasmic encephalitis every year, while up to 50% of all human immunodeficiency virus (HIV)-infected patients in Europe developed toxoplasmic encephalitis (Happe et al., 2002). Furthermore, toxoplasmosis may cause abortion or neonatal malformations such as birth defects, retinitis, and brain damage if acutely infected via vertical transmission (Remington et al., 2006). In order to control toxoplasmosis effectively, efforts should be directed to developing a sensitive vaccine to limit congenital infection.
It is similar in humans and animals that chronically infected individuals develop lifelong immune protection against reinfection (Brown and McLeod, 1990; Parker et al., 1991; Khan et al., 1991). Thus it should be feasible to develop a vaccine to limit congenital infection. A live vaccine based on an attenuated strain of T. gondii is currently used in animals (Buxton et al., 1991; Buxton and Innes, 1995). However, such live mutant vaccines have the capacity to regain virulence; therefore it is not suitable for use in humans. For this reason, the use of recombinant technology to develop a new vaccine arises as an interesting alternative for human immune protection.
Immunization with naked DNA can induce both humoral and cellular immune responses in animal models (Ulmer et al., 1993). It has been shown that it is predominantly a Th1-type response (Montgomery et al., 1997; Tighe et al., 1998), which is suitable for immunity against toxoplasmosis. DNA vaccination with surface antigen gene 1 (SAG1) was able to provide partial protection against T. gondii infection in mice (Aosai et al., 1999; Couper et al., 2003; Fachado et al., 2003). Immunization of C57BL/6 mice with a plasmid expressing granule protein 4 (GRA4) protected them against a lethal challenge with the 76K T. gondii strain (Desolme et al., 2000). Gene vaccination with protein GRA1, GRA7, and rhoptry protein ROP2 induced protection against infection with different virulent T. gondii strains in C3H mice but not in BALB/c and C57BL/6 mice (Vercammen et al., 2000). Although DNA vaccines showed considerable effect in murine models, naked plasmid DNA is not very immunogenic and requires adjuvants to enhance its effectiveness. Recombinant GRA4 (rGRA4) combined with cholera toxin induced partial protection for immunized C57BL/6 mice against a nonlethal challenge with the 76K T. gondii strain (Leyva et al., 2001). Administration of a plasmid encoding the granulocyte macrophage colony-stimulating factor (pGM-CSF) enhanced the protection induced by immunization with plasmid encoding the protein MIC3 (Ismael et al., 2003). Co-inoculation of plasmids expressing GRA4 (pGRA4) and SAG1 (pSAG1mut) with pGM-CSF reduced mortality of susceptible C57BL/6 mice upon oral challenge with cysts of the 76K type II strain (Mévélec et al., 2005). CTXA2/B as a genetic adjuvant enhanced the magnitude of immune responses as well as increased survival rate in mice infected with the lethal RH tachyzoites (Cong et al., 2008).
Lipid-based gene delivery systems have been the subject of much interest (Rolland, 1998). In contrast to naked DNA, liposome-encapsulated DNA is protected from the attack of nucleases in a biological milieu. Furthermore, this carrier can introduce its contents into the cytoplasm for the generation of antigen-specific cytotoxic T lymphocytes (CTLs) via membrane fusion. Thus liposome mediated DNA immunization induced an efficient antigen specific immunity (Gregoriadis et al., 1996a; 1996b; 2002). Immunization with liposome-encapsulated DNA construct encoding SAG1 and ROP1 induced humoral and cellular immune responses (Chen et al., 2003). Nasal immunization of normal mice with HIVgp160-encapsulated hemagglutinating virus of Japan (HVJ)-liposome induced high titers of gp160-specific neutralizing immunoglobulin G (IgG) in serum and IgA in nasal wash, saliva, fecal extract, and vaginal wash, along with both Th1- and Th2-type responses (Gaku et al., 2003).
In the present study, pGRA4 was incorporated by the dehydration-rehydration method into the liposome, and BALB/c and C57BL/6 mice were immunized with GRA4 encapsulated in liposome. Finally, the humoral and cellular immune responses of immunized BALB/c and C57BL/6 mice to infection of two T. gondii strains were analyzed, and the protection efficacy of GRA4 encapsulated in liposome was discussed.
MATERIALS AND METHODS
Animals
Six to eight weeks old female C57BL/6 mice and BALB/c mice were purchased from Shanghai laboratory animal center, Chinese academy of sciences, and maintained under pathogen-free conditions in the experimental animal facility at the Centre of Laboratory Animals, Zhejiang Academy of Medical Sciences.
Maintenance of parasites
RH and ME49 strains of T. gondii were used. The RH strain, a highly virulent strain for mice, was used to challenge BALB/c mice. ME49 was used to challenge C57BL/6 mice. This strain was selected because it produces many tissue cysts in the brain and is mildly virulent for mice. Each C57BL/6 mouse was administered 20 tissue cysts of ME49 by intraperitoneal injection. The brain tissue cysts were obtained before challenging.
Constructions of pGRA4
The entire GRA4 reading frame was amplified from T. gondii RH genomic DNA by polymerase chain reaction (PCR) with sense primer (GRA4-F, 5′-CGCGGGTACCATGCAGGGCACTTGGTTTTC-3′) and antisense primer (GRA4-R, 5′-CGCGGAATTCTCACTCTTTGCGCATTCTTT-3′) (Mévélec et al., 1992; 1998). The PCR product was digested with KpnI and EcoRI restriction enzymes, purified, and cloned into the corresponding sites of pcDNA3.1 (Invitrogen, Carlsbad, CA, USA), and then sequenced (Bioasia, Shanghai, China).
Expression and purification of recombinant GRA4 protein
Truncated forms of GRA4 were expressed in Escherichia coli as glutathione S-transferase fusion proteins (amino acids 24-276/296-345). The truncated frame of GRA4 was cloned into the pGEX-3X expression vector (Amersham Pharmacia Biotech, Piscataway, NJ) and then transformed E. coli DH5α. The transformed E. coli DH5α grew in lysogeny broth (LB) medium (Oxoid, Basingstok, England) at 37 °C until an optical density (OD) of 0.7 at 600 nm was reached, and then E. coli DH5α was induced to express recombinant proteins with 1 mmol/L isopropyl β-D-1-thiogalactopyranoside (IPTG). After 4 h of induction, cells were pelleted at 5000×g and then resuspended in 20 ml column buffer (10 mmol/L Tris-HCl (pH 8.0), 100 mmol/L NaCl). Cells were sonicated, and the lysates were centrifuged at 10 000×g. The soluble fractions were collected and incubated for 20 min with 5 ml of glutathione agarose beads (Sigma, St. Louis, MO). Non-specifically bound proteins were washed off with a column buffer containing 1% (w/v) triton X-100. Purified proteins were eluted with 10 mmol/L reduced glutathione (Sigma). The purity of the recombinant proteins was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% (w/v) acrylamide gels.
Purification of pGRA4 and transfection cells
The pGRA4 was purified from transformed E. coli DH5α, using Endofree plasmid giga kit (Qiagen, Chatsworth, CA), then was dissolved in sterile endotoxin-free phosphate-buffered saline (PBS, Sigma) and stored at −20 °C. The DNA concentration was determined by absorbance at 260 nm. Plasmid expression was analyzed by transfection of Cos-7 cells using a polycationic liposome reagent (Lipofectamine™, Invitrogen, California, USA) according to the manufacturer’s instructions. After 2 d, cell monolayers and supernatants were collected and stored at −20 °C. Expression of transfected cells was then analyzed by SDS-PAGE and Western blotting.
Liposome encapsulating
The dehydration-rehydration method was used to prepare liposomes (Kirby and Gregoriadis, 1984; Perrie and Gregoriadis, 2000; Perrie et al., 2003). Small unilamellar vesicles were composed of egg phosphatidylcholine (PC) (Avanti Polar Lipids, Alabaster, AL), dioleoyl phosphatidylethanolamine (DOPE) (Avanti Polar Lipids, Alabaster, AL), and 1,2-dioleoyl-3-(trimethylammonium) propane (DOTAP) (Sigma) in a molar ratio of 16:8:4. Briefly, lipids were dissolved in chloroform and dried on a rotary evaporator, and then stood for 30 min under high vacuum in a desiccator. 100 mg of pGRA4 dissolved in 1 ml of PBS were added to the dried lipid films. Formulations were stirred for 3 h at 4 °C to ensure complete hydration, and then freeze-dried overnight followed by rehydration with 50 mg of pGRA4 dissolved in 1 ml of PBS. After incubation for 2 h, formulations were centrifuged at 25 000×g for 40 min to remove non-entrapped DNA and then resuspended in PBS.
Immunization
All experiments were performed using 6~8 weeks old female C57BL/6 mice and BALB/c mice. Prior to immunization, each mouse was injected with 100 μl of 10 mmol/L cardiotoxin (Sigma-Aldrich, St. Louis, MO) at the tibialis anterior muscles of both hind legs to enhance uptake of plasmid DNA. Five days later, C57BL/6 mice were injected with 50 μg naked pGRA4 (n=19), 50 μg empty vector pcDNA3.1 (n=19), or 50 μg cationic liposome-encapsulated pGRA4 (n=19). Mice were boosted a further two times in a period of two or four weeks with the same dose. PBS plus liposomes was injected to the control mice (n=19). The BALB/c mice were immunized as described for C57BL/6 mice.
Serum was obtained by terminal exsanguination two weeks after the final injection. The preimmune serum sample was used as negative control.
Enzyme-linked immunosorbent assay for GRA4
The sera from immunized mice were evaluated by enzyme-linked immunosorbent assay (ELISA) (Chen et al., 2008; Nigro et al., 2003) in 96-well microtiter plates (Nunc, Roskilde, Denmark) coated with recombinant GRA4 (5 μg/ml) in 50 mmol/L carbonate buffer (pH 9.6), and kept overnight at 4 °C. After 2 h of blocking with 10% (v/v) fetal calf serum (FCS) at 37 °C, the plates were drained and washed three times with 0.1 mol/L PBS containing 0.05% (v/v) Tween 20 (PBST) for 1 min each time. A dilution series of each serum sample was applied to each well and incubated for 1 h at 37 °C. They were washed and incubated with alkaline phosphatase conjugated goat anti-mouse IgG (diluted 1:1000 in PBS) for 2 h at 37 °C, then washed five times as described above and bound phosphatase activity measured with p-nitrophenyl-phosphate (Sigma). The plates were incubated at room temperature for 20 min, and the OD was read at 405 nm using a plate reader. The antigen-specific antibody titer was given as the reciprocal of the highest dilution whose absorbance was 2.5-fold greater than the absorbance of the serum of mice injected with empty pcDNA3.1 at the same dilution. Results are expressed as the means of log2 titers±SD. IgG1 and IgG2a antibody determinations were performed as described for total IgG. Alkaline phosphatases conjugated anti-mouse IgG1 and IgG2a (Sigma) were used as diluted 1/500 in PBS.
Spleen cell proliferation
Two weeks after the last booster injection, spleens were aseptically collected from three mice per group. Spleen cell suspensions were prepared and adjusted to a final concentration of 5×106 cells/ml in RPMI 1640 tissue culture medium (Life Technologies, Rockville, MD) supplemented with 5% (v/v) FCS, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (10 mmol/L), L-glutamine (2 mmol/L), sodium pyruvate (1 mmol/L), β-mercaptoethanol (50 μmol/L), gentamycin (50 μg/ml), penicillin (1000 U/ml), and streptomycin (100 μg/ml). Then 3×105 cells per well were seeded into flat-bottom 96-well microtiter plates with 10 μg recombinant GRA4 protein (200 μl culture medium as a negative control, 5 μg concanavalin A/ml as a positive control). The plates were then incubated in 5% CO2 at 37 °C for 3 d. 1 μCi of [3H]thymidine per well was added for the final 18 h of culture. The cells were harvested, and radioactivity incorporation was measured with liquid scintillation counting. Results are expressed as the stimulation index (SI), which is the ratio between the mean value of counts per minute for recombinant antigen-stimulated cells and the mean value of counts per minute for nonstimulated cells.
Cytokine analysis
Two weeks after the last booster injection, spleen cells were cultured as above. The cultured supernatant was harvested after three days and stored at −70 °C. Commercial ELISA kits (mouse IL-4, mouse IL-2, mouse IFN-γ, Endogen, Pierce Biotechnology, Rockford, USA) were used to determine the levels of cytokines according to the manufacturer’s instructions.
Challenge
Fourteen days after the last booster, mice were challenged by oral infection with 80 ME49 tissue cysts. Mice were observed daily for mortality. For studies involving the determination of tissue-cyst burden in the brain, five mice of each group were given 20 ME49 strain tissue cysts via gavage. Six weeks after infection, brains were removed and homogenized in 1 ml of PBS. The mean number of cysts per brain was determined under an optical microscope. The results are expressed as mean±SD for each group. Tissue cysts were counted in three separate aliquots for each brain.
Challenge of RH-strain tachyzoites to BALB/c mice was performed by intraperitoneal injection of 103 tachyzoites.
Statistical analysis
Levels of significance between groups of mice were determined using the Wilcoxon signed-rank test for mortality and analysis of variance (ANOVA) for brain cyst loads and antibody titers. The Mann-Whitney U-test was used for survival time.
RESULTS
SDS-PAGE and Western blot
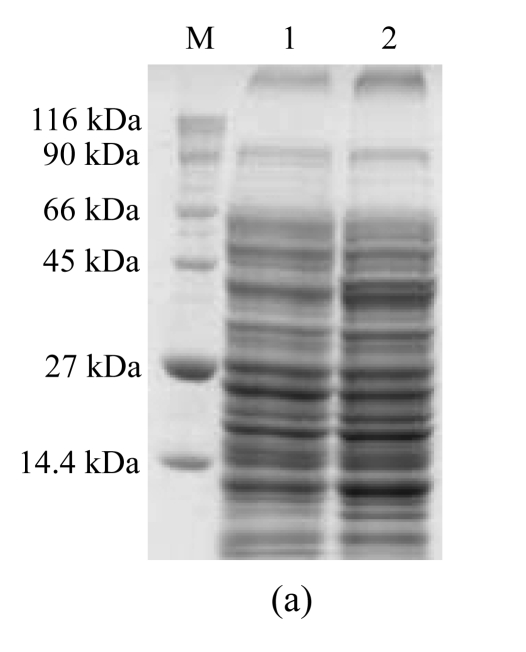
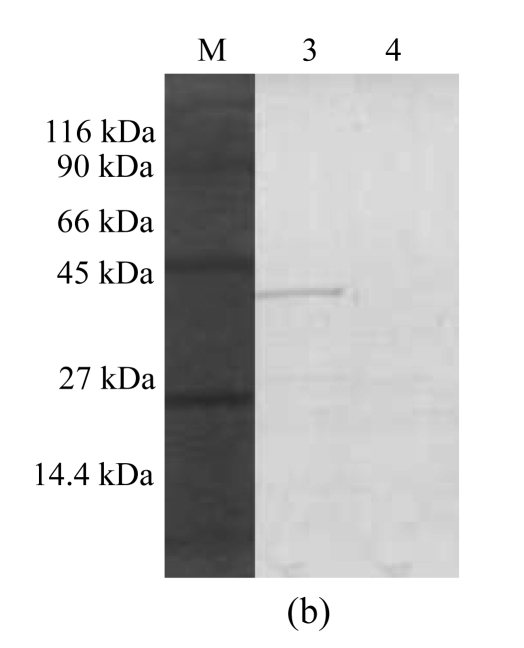
COS-7 cells were transfected with pGRA4 for 48 h. The protein extracts were then analyzed by SDS-PAGE and Western blotting, and only those cells transfected with pGRA4 showed the expected size for the complete protein (40 kDa) (Fig.1). The 40-kDa band detected in sera from infected mice provided the antigenicity of pGRA4.
Fig.1.
SDS-PAGE and Western blotting analyses of a lysate of COS-7 cells transfected with pGRA4 or with empty pcDNA3.1 as a control. A mouse anti-rGRA4 serum sample was used as the first antibody. (a) SDS-PAGE analysis (M: molecular mass; 1: lysate of COS-7 cells transfected with empty pcDNA3.1; 2: lysate of COS-7 cells transfected with pGRA4); (b) Western blotting analysis (M: molecular mass; 3: lysate of COS-7 cells transfected with pGRA4; 4: lysate of COS-7 cells transfected with empty pcDNA3.1
Humoral response
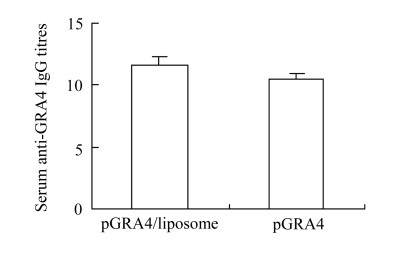
Sera from immunized mice were collected prior to challenge and analyzed by ELISA for specific antibody response. Intramuscular immunization of mice resulted in the development of high titers of GRA4-specific antibody in the mice immunized with pGRA4 or liposome-encapsulated pGRA4 (Fig.2) by ELISA with the recombinant GRA4 protein. Although the IgG antibody titer was higher in the sera of mice inoculated with cationic liposome-encapsulated pGRA4 than in the sera of mice immunized with naked pGRA4, there were no statistically significant differences between the two groups (P>0.05). In order to characterize whether a Th1 or Th2 response was elicited in immunized mice, the distribution of IgG subclass was analyzed against T. gondii (Table 1). Immunization with pGRA4 or liposome-encapsulated pGRA4 elicited mainly IgG2a anti-T. gondii in BALB/c mice. No IgG1 was detected in the sera of immunized BALB/c mice.
Fig.2.
Serum antigen-specific total IgG titres following intramuscular administration of pGRA4 or pGRA4/liposome complexes. Values are represented as the mean of log2(reciprocal endpoint titre). Error bars represent standard deviation (SD)
Table 1.
Determination of the specific anti-T. gondii IgG subclass profile in the sera of BALB/c mice immunized with pcDNA3.1, naked pGRA4 and pGRA4/liposome complexes
| Immunization regimen | OD405 |
||
| IgG | IgG1 | IgG2a | |
| Control pcDNA3.1 | 0.209±0.018 | 0.178±0.013 | 0.195±0.018 |
| pGRA4 | 0.791±0.064 | 0.176±0.012 | 0.563±0.039 |
| pGRA4/liposome | 0.853±0.080 | 0.186±0.019 | 0.621±0.045 |
Each group comprised eleven mice. The results are expressed as mean±SD and represent one of three similar experiments
Cytokine production
The cultured supernatants of splenocytes from mice immunized with pGRA4 or cationic liposome-encapsulated pGRA4 were harvested at different time and assessed for IL-2, IL-4 and IFN-γ activities. Large amounts of IFN-γ were detected in the restimulation splenocyte cultures of pGRA4 or cationic liposome-encapsulated pGRA4, whereas there was no detectable production of IL-4 (Table 2).
Table 2.
Cellular immune responses of spleen cells from immunized mice
| Immunization regimen | Proliferation SIa | Cytokine production (pg/ml)b |
||
| IL-2 | IL-4 | IFN-γ | ||
| Untreated | <20 | <20 | <20 | |
| Control pcDNA3.1 | 1.2±0.2 | 40±11 | <20 | 156±35 |
| pGRA4 | 4.0±0.3 | 125±23 | <20 | 1204±60 |
| pGRA4/liposome | 9.8±0.8 | 168±10 | <20 | 1851±84 |
SI: stimulation index, expressed as the ratio between the mean counts per minute for triplicate stimulated cultures and the mean counts per minute for triplicate unstimulated cultures
The culture supernatants were examined for cytokine production
Values for IL-2, IL-4 are taken from 24 h of culturing, values for IFN-γ from 96 h of culturing
Protection of mice against challenge with T. gondii following DNA vaccination
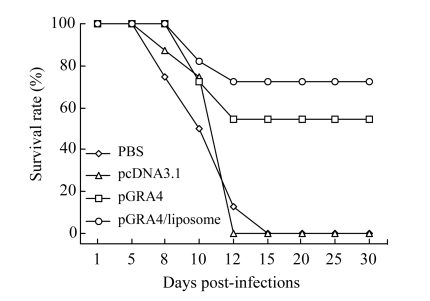
C57BL/6 mice immunized with pGRA4 dramatically increased their survival rate (Fig.3) (54.5%, n=11, P<0.01). A significant increase of the survival rate (72.7%, n=11, P<0.01) was observed in the mice injected with cationic liposome-encapsulated pGRA4. None of mice was untreated or injected with empty pcDNA3.1 survived infection.
Fig.3.
Survival curves of immunized C57BL/6 mice after challenge with 80 tissue cysts of T. gondii ME49 strain
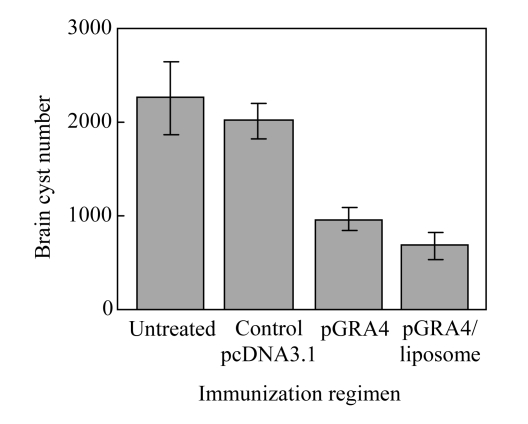
In addition to determining whether it is possible to produce protection against mortality from acute toxoplasmosis, we were interested in determining whether the vaccination would provide any protection against the formation of T. gondii tissue cysts in the brain. In these experiments, all mice in both controls and vaccinated groups survived for six weeks, the animals were killed and their brains were removed for enumeration of T. gondii tissue cysts (Fig.4). The number of tissue cysts in the liposome-encapsulated pGRA4 group was significantly decreased compared with control groups (P<0.01).
Fig.4.
Enumeration of Toxoplasma gondii tissue cysts in brains of immunized and control C57BL/6 mice. Error bars represent standard deviation (SD)
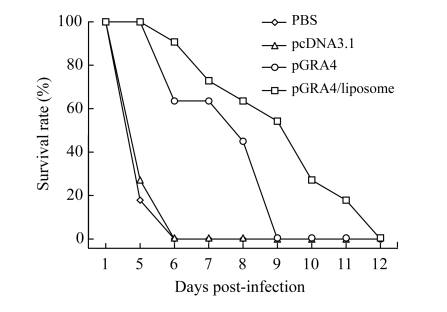
BALB/c mice were immunized in the same way as C57BL/6 mice and challenged intraperitoneally with 103 tachyzoite of RH strain in four weeks after the last immunization. Mortality was checked daily for one month (Fig.5). All mice died within 12 d. The survival time of the mice immunized with cationic liposome-encapsulated pGRA4 was markedly longer than that of control groups (P<0.01).
Fig.5.
Survival curves of immunized BALB/c mice after challenge with 103 tachyzoite forms of RH T. gondii strain
DISCUSSION
This study showed that the GRA4 DNA vaccine encapsulated in liposomes could elicit a broad range of immune responses that were capable of decreasing mortality of animals infected with T. gondii. It also demonstrated that the vaccine was capable of reducing the levels of tissue cysts in the brains of infected animals.
These results are consistent with the study by Mévélec et al.(2005) in which co-inoculation of pGRA4 and pSAG1mut with pGM-CSF reduced mortality of susceptible C57BL/6 mice upon oral challenge with cysts of the 76K type II strain. In addition, Martin et al.(2004) observed that the challenge of recombinant GRA4 protein or recombinant GRA4 and ROP2 proteins vaccinated C57BL/6 and C3H mice with ME49 cysts resulted in fewer brain cysts than the controls, whereas vaccination with recombinant ROP2 protein alone only conferred protection to C3H mice. Immunization with pGRA4 showed a protective level similar to that of recombinant GRA4 protein combined with alum. These data suggest that GRA4 is a good candidate for the development of a vaccine against toxoplasmosis. In our study, pGRA4 was incorporated by the dehydration-rehydration method into liposomes to enhance the protective effect. The challenge of C57BL/6 mice with a lethal dose of tissue cysts of the moderately virulent ME49 strain gave a conclusive indication that DNA vaccination afforded partial protection against acute toxoplasmosis: 72.7% C57BL/6 mice immunized with liposome-encapsulated pGRA4 and 54.5% C57BL/6 mice immunized with pGRA4 survived. When the immunized BALB/c mice were intraperitoneally challenged with 103 tachyzoites of the highly virulent RH strain, the survival time of mice immunized with liposome-encapsulated pGRA4 was markedly longer than that of other groups. A further validation of the efficacy of DNA vaccination is found in the comparison of the number of brain tissue cysts in vaccinated animals (Fig.3). Although the liposome-encapsulated pGRA4 did not completely eliminate tissue cysts in the vaccinated animals, it did lead to a substantial reduction in the cyst burden. These results demonstrated that liposome-encapsulated pGRA4 did enhance the protective effect against infection of T. gondii.
For T. gondii, it has demonstrated that a Th1-type response is the result of a naturally occurring infection (Denkers and Gazzinelli, 1998). Thus, it appears that a vaccination protocol that directs a Th1 rather than a Th2 response is desirable. Mice exhibit a preferential production of IgG2a when the response is Th1-type. As shown in Figs.3 and 4, DNA vaccination with pGRA4 and cationic liposome-encapsulated pGRA4 leads to eliciting mainly IgG2a, and no IgG1 has been detected in the sera of immunized BALB/c and C57BL/6 mice. This indicates that the response is a Th1-type response. The results of the cytokine production by in vitro stimulation of splenocytes support the above conclusion (Table 2). Both IL-2 and IFN-γ were produced by in vitro stimulation of splenocytes from immunized mice, whereas there was no detectable production of IL-4, which was consistent with a Th1-type response.
Gene delivery systems have been the subject of much interest, with cationic lipids offering potential for gene delivery by selected routes of immunization (Caplen et al., 1995; Wheeler et al., 1996; Liu et al., 1996). Gene delivery system is quite a new research field in T. gondii. Stanley et al.(2004) demonstrated that a crude extract of Toxoplasma tachyzoite antigen encapsulated into a poly(D,L-lactide-co-glycolide) (PLG) microparticle delivery system induced both cell-mediated immunity and strong antigen-specific mucosal IgA using the intranasal route of administration of sheep. GRA1 protein vaccine loaded chitosan particles and boosting with GRA1 pDNA vaccine resulted in high anti-GRA1 antibodies (Bivas-Benita et al., 2003). Intraperitoneal challenge with Toxoplasma cysts resulted in significant reduction of brain cysts in immunized CBA/J mice using GRA2 and GRA6 formulated in monophosphoryl lipid A (MPL) adjuvant (Golkar et al., 2007). Compared to above carriers, liposome can better protect DNA encapsulated in it from the attack of nucleases in a biological milieu. Furthermore, this carrier introduced its contents into the cytoplasm for the generation of antigen-specific cytotoxic T lymphocytes (CTLs) via membrane fusion (Gregoriadis et al., 1996a; 1996b; Perrie et al., 2001). Thus, liposomal delivery systems offer significant versatility for the incorporation of components with potentially desirable effects within the endosome. Chen et al.(2003) observed BALB/c mouse immunization with liposome-encapsulated plasmid encoding SAG1 and ROP1 induced humoral and cellular immune response. All these works demonstrate the importance of gene-delivery system in T. gondii, but the protective effect is not clear while the challenge study has not been researched in these works. In our study, we chose RH and ME49 strains of T. gondii to challenge BALB/c mice and C57BL/6 mice, respectively. When challenged with ME49 stain of T. gondii, liposome-encapsulated pGRA4 was more capable of decreasing mortality of mice infected with T. gondii and reducing the levels of tissue cysts in the brains of infected mice compared to the naked pGRA4. When challenged with virulent RH strain, mice immunized with liposome-encapsulated pGRA4 exhibited prolonged survival time compared with naked pGRA4-immunized groups. Our data indicated that liposome-encapsulated pGRA4 induced more effective humoral and cellular immune responses to infection of T. gondii than the naked pGRA4 in vaccinated mice.
The initial goal of this study was to improve the ability of GRA4 to elicit a protective immune response by DNA vaccination. Our results reinforce the value of liposome to be used in immunization against T. gondii. We consider that combinations with other effective antigens and adjuvant like pGM-CSF should also be taken into account in the future. Therefore, our results demonstrate that a gene-delivery system offers a promising approach as a vaccine against toxoplasmosis.
Footnotes
Project supported by the Science Foundation of the Health Bureau of Zhejiang Province, China (Nos. 2003QN003 and 2005A001) and the Science Foundation of the Science and Technology Department of Zhejiang Province, China (No. 2006C13022)
References
- 1.Aosai F, Mun HS, Norose K, Chen M, Hata H, Kobayashi M, Kiuchi M, Stauss HJ, Yano A. Protective immunity induced by vaccination with SAG1 gene-transfected cells against Toxoplasma gondii infection in mice. Microbiology and Immunology. 1999;43(1):87–91. doi: 10.1111/j.1348-0421.1999.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 2.Bivas-Benita M, Laloup M, Versteyhe S, Dewitc J, de Braekeleerc J, Jongertc E, Borchard G. Generation of Toxoplasma gondii GRA1 protein and DNA vaccine loaded chitosan particles: preparation, characterization, and preliminary in vivo studies. International Journal of Pharmaceutics. 2003;266(1-2):17–27. doi: 10.1016/S0378-5173(03)00377-6. [DOI] [PubMed] [Google Scholar]
- 3.Brown CR, McLeod R. Class I MHC genes and CD8+ T cells determine cyst numbers in Toxoplasma gondii infection. The Journal of Immunology. 1990;145(10):3438–3441. [PubMed] [Google Scholar]
- 4.Buxton D, Innes EA. A commercial vaccine for ovine toxoplasmosis. Parasitology. 1995;110(s1):S11–S16. doi: 10.1017/S003118200000144X. [DOI] [PubMed] [Google Scholar]
- 5.Buxton D, Thomson K, Maley S, Wright S, Bos HJ. Vaccination of sheep with a live incomplete strain (S48) of Toxoplasma gondii and their immunity to challenge when pregnant. Vet Rec. 1991;129(5):89–93. doi: 10.1136/vr.129.5.89. [DOI] [PubMed] [Google Scholar]
- 6.Caplen NJ, Alton EW, Middleton PG, Dorin JR, Stevenson BJ, Gao X, Durham SR, Jeffery PK, Hodson ME, Coutelle C. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nature Medicine. 1995;1(1):39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- 7.Chen HF, Chen GJ, Zheng H, Guo H. Induction of immune responses in mice by vaccination with liposome-entrapped DNA complexes encoding Toxoplasma gondii SAG1 and ROP1 genes. Chinese Medicine Journal. 2003;116(10):1561–1566. [PubMed] [Google Scholar]
- 8.Chen R, Lu SH, Lou D, Lin AF, Zeng XJ, Ding ZY, Wen LY, Ohta N, Wang JF, Fu C. Evaluation of a rapid ELISA technique for detection of circulating antigens of Toxoplasma gondii . Microbiology and Immunology. 2008;52(3):180–187. doi: 10.1111/j.1348-0421.2008.00020.x. [DOI] [PubMed] [Google Scholar]
- 9.Cong H, Gu QM, Hong EY, Wang JW, Zhao QL, Zhou HY, Li Y, Zhang JQ. Multi-epitope DNA vaccine linked to the A(2)/B subunit of cholera toxin protect mice against Toxoplasma gondii . Vaccine. 2008;26(31):3913–3921. doi: 10.1016/j.vaccine.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 10.Couper KN, Nielsen HV, Petersen E, Roberts F, Roberts CW, Alexander J. DNA vaccination with the immunodominant tachyzoite surface antigen (SAG-1) protects against adult acquired Toxoplasma gondii infection but does not prevent maternofetal transmission. Vaccine. 2003;21(21-22):2813–2820. doi: 10.1016/S0264-410X(03)00163-4. [DOI] [PubMed] [Google Scholar]
- 11.Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clinical Microbiology Reviews. 1998;11(4):569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desolme B, Mévélec MN, Buzoni-Gatel D, Bout D. Induction of protective immunity against toxoplasmosis in mice by DNA immunization with a plasmid encoding Toxoplasma gondii GRA4 gene. Vaccine. 2000;18(23):2512–2521. doi: 10.1016/S0264-410X(00)00035-9. [DOI] [PubMed] [Google Scholar]
- 13.Fachado A, Rodriguez A, Angel SO, Pinto D, Vila I, Acosta A, Amendoeira RM, Lannes-Vieira J. Protective effect of a naked DNA vaccine cocktail against lethal toxoplasmosis in mice. Vaccine. 2003;21(13-14):1327–1335. doi: 10.1016/S0264-410X(02)00692-8. [DOI] [PubMed] [Google Scholar]
- 14.Feigin RD, Cherry JD. Toxoplasmosis. In: Bergelson J, editor. Textbook of Pediatric Infectious Diseases. 4th Ed. Philadelphia: WB Saunders; 1998. p. 2921. [Google Scholar]
- 15.Gaku S, Takachika H, Yoko N, Kenji S, Kohich I, Yoshiki S, Hidemi T, Mitsuo H, Jun K, Hiroshi K. HIV mucosal vaccine: nasal immunization with gp160-encapsulated hemagglutinating virus of Japan-liposome induces antigen-specific CTLs and neutralizing antibody responses. The Journal of Immunology. 2003;170:495–502. doi: 10.4049/jimmunol.170.1.495. [DOI] [PubMed] [Google Scholar]
- 16.Golkar M, Shokrgozar MA, Rafati S, Musset K, Assmar M, Sadaie R, Cesbron-Delauw MF, Mercier C. Evaluation of protective effect of recombinant dense granule antigens GRA2 and GRA6 formulated in monophosphoryl lipid A (MPL) adjuvant against Toxoplasma chronic infection in mice. Vaccine. 2007;25(21):4301–4311. doi: 10.1016/j.vaccine.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 17.Gregoriadis G, Saffie R, Hart SL. High yield incorporation of plasmid DNA within liposomes: effect on DNA integrity and transfection. Journal of Drug Targeting. 1996;3(6):469–475. doi: 10.3109/10611869609015966. [DOI] [PubMed] [Google Scholar]
- 18.Gregoriadis G, Gursel I, Gursel M, McCormack B. Liposomes as immunological adjuvants and vaccine carriers. Journal of Controlled Release. 1996;41(1-2):49–56. doi: 10.1016/0168-3659(96)01355-7. [DOI] [Google Scholar]
- 19.Gregoriadis G, Bacon A, Caparros-Wanderley W, McCormack B. A role for liposomes in genetic vaccination. Vaccine. 2002;20(S5):B1–B9. doi: 10.1016/S0264-410X(02)00514-5. [DOI] [PubMed] [Google Scholar]
- 20.Happe S, Fischer A, Heese CH, Reichelt D, Gruneberg U, Freund M. HIV-associated cerebral toxoplasmosis: review and retrospective analysis of 36 patients. Der Nervenarzt. 2002;73(12):1174–1178. doi: 10.1007/s00115-002-1416-y. [DOI] [PubMed] [Google Scholar]
- 21.Ismael AB, Sekkai D, Collin C, Bout D, Mévélec MN. The MIC3 gene of Toxoplasma gondii is a novel potent vaccine candidate against toxoplasmosis. Infection and Immunity. 2003;71(11):6222–6228. doi: 10.1128/IAI.71.11.6222-6228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan IA, Ely KH, Kasper LH. A purified parasite antigen (P30) mediates CD8+ T-cell immunity against fatal Toxoplasma gondii infection in mice. The Journal of Immunology. 1991;147:3501–3506. [PubMed] [Google Scholar]
- 23.Kirby C, Gregoriadis G. Dehydration-rehydration vesicles: a simple method for high yield drug entrapment in liposomes. Biotechnology. 1984;2(11):979–984. doi: 10.1038/nbt1184-979. [DOI] [Google Scholar]
- 24.Leyva R, Herion P, Saavedra R. Genetic immunization with plasmid DNA coding for the ROP2 protein of Toxoplasma gondii . Parasitology Research. 2001;87(1):70–79. doi: 10.1007/s004360000296. [DOI] [PubMed] [Google Scholar]
- 25.Liu F, Yang J, Huang L, Liu D. New cationic lipid formulations for gene transfer. Pharmaceutical Research. 1996;13(12):1856–1860. doi: 10.1023/A:1016041326636. [DOI] [PubMed] [Google Scholar]
- 26.Martin V, Supanitsky A, Echeverria PC, Litwin S, Tanos T, de Roodt AR, Guarnera EA, Angel SO. Recombinant GRA4 or ROP2 protein combined with alum or the gra4 gene provides partial protection in chronic murine models of toxoplasmosis. Clinical and Diagnostic Laboratory Immunology. 2004;11(4):704–710. doi: 10.1128/CDLI.11.4.704-710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mévélec MN, Chardés T, Mercereau-Puijalon O, Bourguin I, Achbarou A, Dubremetz JF, Bout D. Molecular cloning of GRA4, a Toxoplasma gondii dense granule protein, recognized by mucosal IgA anti-bodies. Molecular and Biochemical Parasitology. 1992;56(2):227–238. doi: 10.1016/0166-6851(92)90172-G. [DOI] [PubMed] [Google Scholar]
- 28.Mévélec MN, Merecerau-Puijalon O, Buzoni-Gatel D, Bourguin I, Chardés T, Dubremetz JF, Bout D. Mapping of B epitopes in Gra4, a dense granule antigen of Toxoplasma gondii and protection studies using recombinant proteins administered by the oral route. Parasite Immunology. 1998;20:183–195. [PubMed] [Google Scholar]
- 29.Mévélec MN, Bout D, Desolme B, Marchand H, Magné R, Bruneel O, Buzoni-Gatel D. Evaluation of protective effect of DNA vaccination with genes encoding antigens GRA4 and SAG1 associated with GM-CSF plasmid, against acute, chronical and congenital toxoplasmosis in mice. Vaccine. 2005;23(36):4489–4499. doi: 10.1016/j.vaccine.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery DL, Ulmer JB, Donnelly JJ, Liu MA. DNA vaccines. Pharmacology & Therapeutics. 1997;74(2):195–207. doi: 10.1016/S0163-7258(97)82003-7. [DOI] [PubMed] [Google Scholar]
- 31.Nigro M, Gutierrez A, Hoffer AM, Clemente MH, Kaufer F, Carral L, Martin V, Guarnera EA, Angel SO. Evaluation of Toxoplasma gondii recombinant proteins for the diagnosis of recently acquired toxoplasmosis by an immunoglobulin G analysis. Diagnostic Microbiology and Infectious Disease. 2003;47(4):609–613. doi: 10.1016/S0732-8893(03)00156-1. [DOI] [PubMed] [Google Scholar]
- 32.Parker SJ, Roberts CW, Alexander J. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clinical and Experimental Immunology. 1991;84(2):207–212. doi: 10.1111/j.1365-2249.1991.tb08150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrie Y, Gregoriadis G. Liposome-entrapped plasmid DNA: characterisation studies. Biochimica et Biophysica Acta (BBA) 2000;1475(2):125–132. doi: 10.1016/S0304-4165(00)00055-6. [DOI] [PubMed] [Google Scholar]
- 34.Perrie Y, Frederik PM, Gregoriadis G. Liposome mediated DNA vaccine: the effect of vesicle composition. Vaccine. 2001;19(23-24):3301–3310. doi: 10.1016/S0264-410X(00)00432-1. [DOI] [PubMed] [Google Scholar]
- 35.Perrie Y, McNeil S, Vangala A. Liposome-mediated DNA immunisation via the subcutaneous route. Journal of Drug Targeting. 2003;11(8-10):555–563. doi: 10.1080/10611860410001670071. [DOI] [PubMed] [Google Scholar]
- 36.Remington JS, Mcleod R, Thulliez P, et al. Infection Diseases of the Fetus and Newborn Infant. 6th Ed. Philadelphia: Elsevier Saunders; 2006. pp. 947–1091. [DOI] [Google Scholar]
- 37.Rolland AP. From genes to gene medicines: recent advances in nonviral gene delivery. Critical Reviews in Therapeutic Drug Carrier Systems. 1998;15(2):143–198. doi: 10.1615/critrevtherdrugcarriersyst.v15.i2.20. [DOI] [PubMed] [Google Scholar]
- 38.Schwartzman JD. Toxoplasmosis. Current Infectious Disease Reports. 2001;3(1):85–89. doi: 10.1007/s11908-001-0063-y. [DOI] [PubMed] [Google Scholar]
- 39.Stanley AC, Buxton D, Innes EA, Huntley JF. Intranasal immunisation with Toxoplasma gondii tachyzoite antigen encapsulated into PLG microspheres induces humoral and cell-mediated immunity in sheep. Vaccine. 2004;22(29-30):3929–3941. doi: 10.1016/j.vaccine.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii from animals to humans. International Journal for Parasitology. 2000;30(12-13):1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tighe HM, Roman CM, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunology Today. 1998;19(2):89–97. doi: 10.1016/S0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 42.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Darki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259(5102):1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 43.Vercammen M, Scorza T, Huygen K, de Braekeleer J, Diet R, Jacobs D. DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infection and Immunity. 2000;68(1):38–45. doi: 10.1128/IAI.68.1.38-45.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wheeler CJ, Felgner PL, Tsai YJ, Marshall J, Sukhu L, Doh SG, Hartikka J, Nietupski J, Manthorpe M, Nichols M, et al. A novel cationic lipid greatly enhances plasmid DNA delivery and expression in mouse lung. Proceedings of the National Academy of Sciences USA. 1996;93(21):11454–11459. doi: 10.1073/pnas.93.21.11454. [DOI] [PMC free article] [PubMed] [Google Scholar]