Abstract
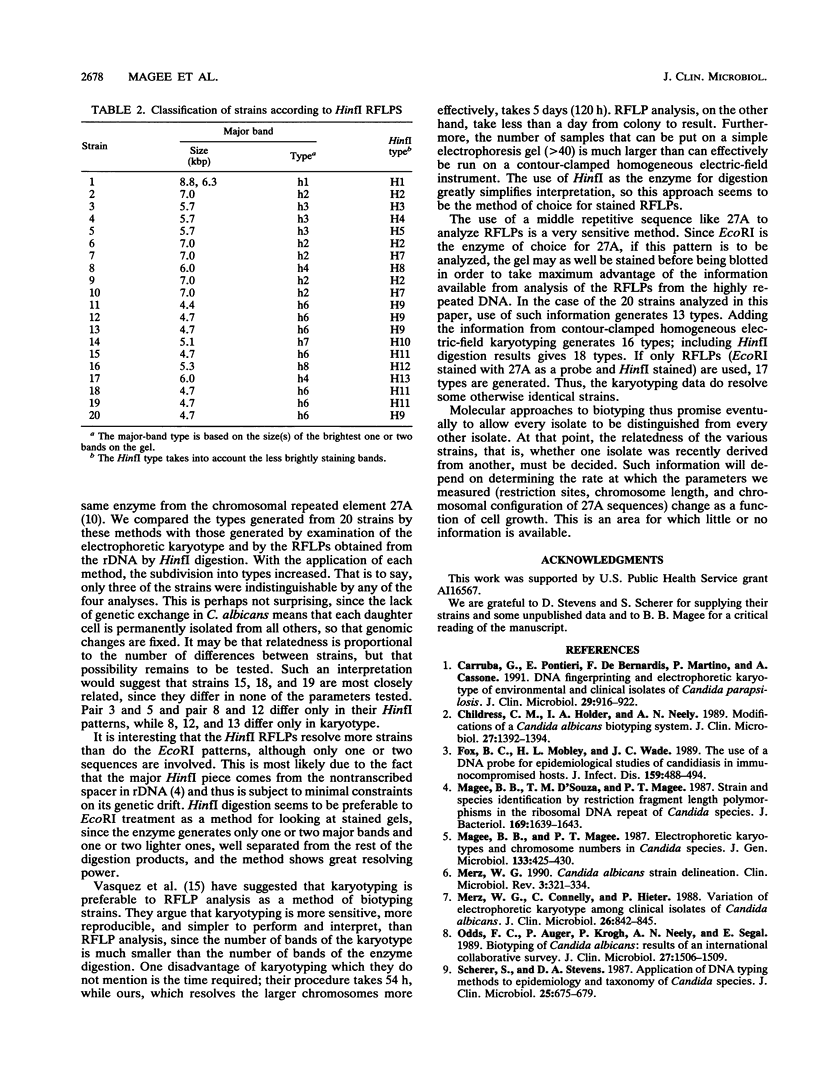
Four molecular approaches to determining the types of Candida albicans strains were compared. The strains used were those whose repeated DNA (ribosomal and mitochondrial) EcoRI restriction fragment length polymorphisms (RFLP) were determined by Stevens et al. (D. A. Stevens, F. C. Odds, and S. Scherer, Rev. Infect. Dis. 12:258-266, 1990). Scherer and Stevens (S. Scherer and D. A. Stevens, Proc. Natl. Acad. Sci. USA 85:1452-1456, 1988) used the same strains to examine the Southern blots of genomic EcoRI digests probed with the repeated sequence 27A. The results of these investigators were compared with determinations of RFLPs generated from repeated DNA by the enzyme HinfI and examination of the karyotypes of strains under two sets of conditions, one for the smaller chromosomes and one for the larger ones. Analysis of RFLPs of repeated DNA is most convenient but shows the lowest degree of resolution. Use of the repeated sequence and use of karyotype have very high resolution, but the former method is more convenient than the latter. HinfI digestion is more sensitive than EcoRI digestion but equally convenient. By using all four methods, separate types were identified for 18 of the 20 strains examined.
Full text
PDF

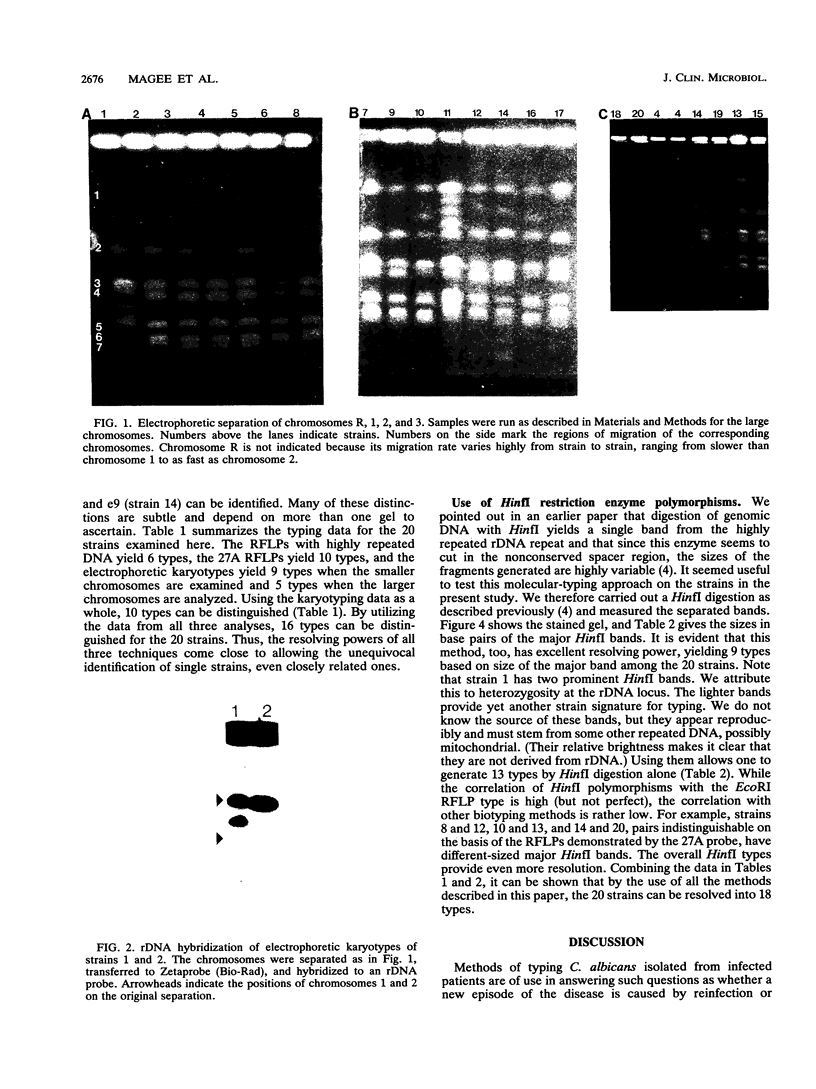
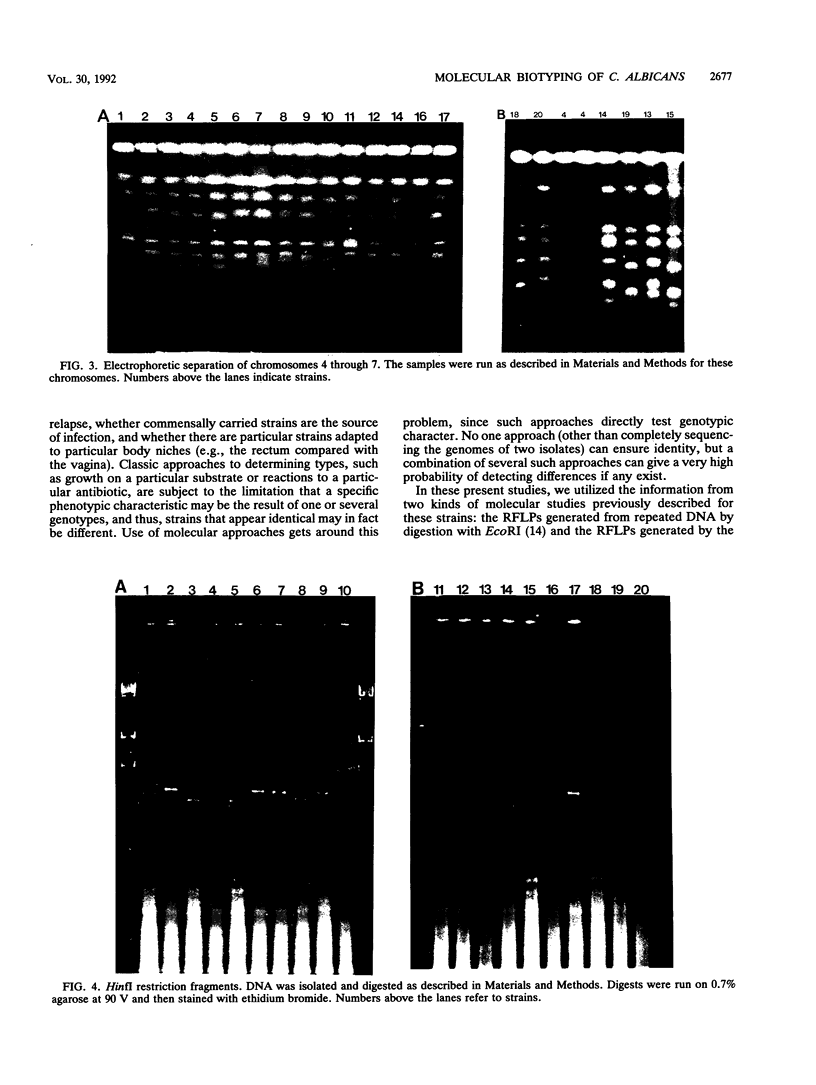

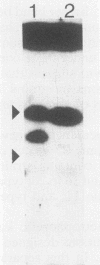
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carruba G., Pontieri E., De Bernardis F., Martino P., Cassone A. DNA fingerprinting and electrophoretic karyotype of environmental and clinical isolates of Candida parapsilosis. J Clin Microbiol. 1991 May;29(5):916–922. doi: 10.1128/jcm.29.5.916-922.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress C. M., Holder I. A., Neely A. N. Modifications of a Candida albicans biotyping system. J Clin Microbiol. 1989 Jun;27(6):1392–1394. doi: 10.1128/jcm.27.6.1392-1394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B. C., Mobley H. L., Wade J. C. The use of a DNA probe for epidemiological studies of candidiasis in immunocompromised hosts. J Infect Dis. 1989 Mar;159(3):488–494. doi: 10.1093/infdis/159.3.488. [DOI] [PubMed] [Google Scholar]
- Magee B. B., D'Souza T. M., Magee P. T. Strain and species identification by restriction fragment length polymorphisms in the ribosomal DNA repeat of Candida species. J Bacteriol. 1987 Apr;169(4):1639–1643. doi: 10.1128/jb.169.4.1639-1643.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee B. B., Magee P. T. Electrophoretic karyotypes and chromosome numbers in Candida species. J Gen Microbiol. 1987 Feb;133(2):425–430. doi: 10.1099/00221287-133-2-425. [DOI] [PubMed] [Google Scholar]
- Merz W. G. Candida albicans strain delineation. Clin Microbiol Rev. 1990 Oct;3(4):321–334. doi: 10.1128/cmr.3.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz W. G., Connelly C., Hieter P. Variation of electrophoretic karyotypes among clinical isolates of Candida albicans. J Clin Microbiol. 1988 May;26(5):842–845. doi: 10.1128/jcm.26.5.842-845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C., Auger P., Krogh P., Neely A. N., Segal E. Biotyping of Candida albicans: results of an international collaborative survey. J Clin Microbiol. 1989 Jul;27(7):1506–1509. doi: 10.1128/jcm.27.7.1506-1509.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. A Candida albicans dispersed, repeated gene family and its epidemiologic applications. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1452–1456. doi: 10.1073/pnas.85.5.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J Clin Microbiol. 1987 Apr;25(4):675–679. doi: 10.1128/jcm.25.4.675-679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell R. G., Hermans I. F., Wilkins R. J., Corner B. E. Chromosomal variations in Candida albicans. Nucleic Acids Res. 1987 Apr 24;15(8):3625–3625. doi: 10.1093/nar/15.8.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Galask R., Isley S., Rao T. V., Stone D., Hicks J., Schmid J., Mac K., Hanna C. Switching of Candida albicans during successive episodes of recurrent vaginitis. J Clin Microbiol. 1989 Apr;27(4):681–690. doi: 10.1128/jcm.27.4.681-690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Staebell M., Langtimm C., Pfaller M., Hicks J., Rao T. V. Multiple Candida strains in the course of a single systemic infection. J Clin Microbiol. 1988 Aug;26(8):1448–1459. doi: 10.1128/jcm.26.8.1448-1459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. A., Odds F. C., Scherer S. Application of DNA typing methods to Candida albicans epidemiology and correlations with phenotype. Rev Infect Dis. 1990 Mar-Apr;12(2):258–266. doi: 10.1093/clinids/12.2.258. [DOI] [PubMed] [Google Scholar]
- Vazquez J. A., Beckley A., Sobel J. D., Zervos M. J. Comparison of restriction enzyme analysis and pulsed-field gradient gel electrophoresis as typing systems for Candida albicans. J Clin Microbiol. 1991 May;29(5):962–967. doi: 10.1128/jcm.29.5.962-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickes B., Staudinger J., Magee B. B., Kwon-Chung K. J., Magee P. T., Scherer S. Physical and genetic mapping of Candida albicans: several genes previously assigned to chromosome 1 map to chromosome R, the rDNA-containing linkage group. Infect Immun. 1991 Jul;59(7):2480–2484. doi: 10.1128/iai.59.7.2480-2484.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]