Abstract
Background
Although, endothelial nitric oxide (NO) synthase (eNOS) is believed to antagonize vascular remodeling induced by the angiotensin II (AngII) type-1 receptor, the exact signaling mechanism remains unclear.
Methods and Results
By expressing eNOS to vascular smooth muscle cells (VSMCs) via adenovirus, we investigated a signal transduction mechanism of the eNOS gene transfer in preventing vascular remodeling induced by AngII. We found marked inhibition of AngII-induced Rho/Rho-kinase activation and subsequent VSMC migration by eNOS gene transfer whereas Gq-dependent transactivation of the epidermal growth factor receptor by AngII remains intact. This could be explained by the specific inhibition of G12/13 activation by eNOS-mediated G12/13 phosphorylation.
Conclusion
The eNOS/NO cascade specifically targets the Rho/Rho-kinase system via inhibition of G12/13 to prevent vascular migration induced by AngII, representing a novel signal cross-talk in cardiovascular protection by NO.
Keywords: angiotensin II, vascular smooth muscle, G protein, nitric oxide synthase
Angiotensin II (AngII) has been implicated in the cardiovascular remodeling associated with hypertension, atherosclerosis, and restenosis after balloon injury. In this regard, AngII stimulates hypertrophy, proliferation, and migration of vascular smooth muscle cells (VSMCs) through a G protein–coupled receptor (GPCR), the AngII type-1 receptor (AT1).1–3 Importantly, in addition to vasoconstriction induced by AngII, other pathological effects of AngII such as induction of growth and migration are blocked by nitric oxide (NO) in vitro.4–6 Also, gene delivery of the key enzyme, endothelial NO synthase (eNOS), prevented atheroscrelosis and postangioplasty restenosis in vivo.7–10 We have recently reported that eNOS gene transfer activates the NO/cyclic GMP (cGMP)/protein kinase G (PKG) cascade and inhibits VSMC hypertrophy induced by AngII.11 However, the detailed signaling target(s) by which the eNOS cascade prevents vascular remodeling induced by AngII remain unclear.
Recent accumulating evidence suggests that induction of VSMC migration by the AT1 receptor requires multiple sets of downstream tyrosine and serine/threonine kinases.3 Among these kinases, we and others have demonstrated that extracelluar-signal regulated kinase (ERK) activated through “trans”-activation of the epidermal growth factor receptor (EGFR) and Rho-kinase (ROCK), an effecter of a small G protein, Rho, are critical for VSMC migration induced by AngII.12–15 In VSMCs, Gq and Gq-derived second messengers appear to mediate the EGFR/ERK cascade activation via the AT1 receptor,16,17 whereas G12/13 are implicated in Rho/ROCK activation by AngII.18 However, there might be cross-talk of these signals17,19 and their activation might be, at least in part, G protein–independent in other cell systems.2,20,21 Interestingly, downstream effectors of eNOS, cGMP, and PKG seem to inhibit the Rho/ROCK pathway at multiple distinct points that have not been fully characterized.22–24 However, some of the past studies demonstrated the ERK pathway activated by AngII as a target of inhibition by the NO/cGMP/PKG cascade.25
In the present study, we have tested our hypothesis that eNOS selectively targets the Rho/ROCK pathway activated by the AT1 receptor thereby preventing migration of VSMCs. We found the primary inhibitory target of eNOS was G12/13. Our data demonstrate a novel signal cross-talk of eNOS and the Rho/ROCK pathway in response to AngII stimulation, which may explain the key molecular mechanism of cardiovascular protection by the eNOS cascade.
Materials and Methods
Reagents
AngII was purchased from Sigma. L-NAME and okadaic acid was purchased from Calbiochem. Antibody to detect total eNOS was purchased from BD Transduction Laboratories. Phospho-specific antibodies to detect Ser239-phosphorylated vasodialator-stimulated phosphoprotein (VASP) and Thr853-phosphorylated myosin phosphatase target subunit-1 (MYPT1) were purchased from Upstate. Antibody for GAPDH was purchased from Chemicon International. Antibody against total MYPT1 was purchased from Covance. Phospho-specific antibody for Tyr1068-phosphorylated EGFR was purchased from Biosource International. Phospho-specific antibody for Tyr204-phosphorylated ERK, and antibodies against EGFR and ERK2 were purchased from Santa Cruz Biotechnology. Phospho-RRXS/T motif antibody (100G7) was purchased from Cell Signaling.
Cell Culture
Isolation and characterization of rat aortic VSMCs in culture were described previously.26 Bovine aortic endothelial cells (BAECs) were purchased from Cambrex. Cells were subcultured in DMEM containing 10% fetal bovine serum, penicillin and streptomycin as previously described.26 Cells at passage 3 to 12 at ≈80% confluence in culture wells were made quiescent by incubation with serum-free medium for 24 hour before the adenovirus infection.
Adenoviral Infection
Generation and characterization of replication-deficient adenovirus encoding eNOS and myc-p115RGS were described previously.27,28 The adenovirus titer (moi) was determined by Adeno-X Rapid Titer Kit (BD Biosciences). VSMCs were infected with adenovirus for 2 days as previously described.29 The infection efficiency was estimated to be 90% to 100% as defined by infection with adenovirus (20 to 100 moi) encoding green fluorescent protein.
Immunoprecipitation and Immunoblotting
Immunoprecipitation and Immunoblotting were performed as previously described.16 Cell lysates or immunoprecipitation lysates were subjected to SDS-PAGE gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane. The membranes were then exposed to primary antibodies overnight at 4°C. After incubation with the peroxidase linked secondary antibody for 1 hour at room temperature, immunoreactive proteins were visualized by a chemi-luminescence reaction kit. The results were quantified by densitometry in the linear range of film exposure using CanoScan N670U (Canon) and Un-Scan-It Gel 5.3 software (Silk Scientific).14 An example of data supporting the linearity were demonstrated in Supplemental Figure I (available online at http://atvb.ahajournals.org). Unless stated otherwise, results were expressed as % increase in which the response to AngII is defined as 100% because the basal signals are more varied depending on film exposure than the stimulated signals.
Intracellular cGMP Measurements
VSMCs infected with eNOS adenovirus for 48 hours were incubated at 37°C for 20 minutes in the presence of 0.5 mmol/L methylisobutylxanthin, and intracellular cGMP was determined by an enzyme immunoassay kit (Cayman Chemical).11
Intracellular Ca2+ Measurements
Intracellular Ca2+ was measured as described previously30 with slight modification.31 VSMCs subcultured on coverslips were loaded in HBSS with 3 µmol/L fura 2-AM at room temperature for 45 minutes in the dark, then washed three times with fura 2-free HBSS and allowed for complete de-esterification of the dye for 15 to 60 minutes. Under these conditions, compartmentalization of the dye was minimal. The coverslips were mounted in a custom-designed bath in the stage of a S300 Axiovert Nikon inverted microscope equipped with a C & L Instruments fluorimeter system. The fura 2 fluorescence was acquired at a frequency of 1 Hz and intracellular Ca2+-values were then obtained as described.30
G12/13 Activity Assay
To measure G12/13 activity, we have used an affinity precipitation assay using a tetratricopeptide repeat (TPR) domain of Ser/Thr phosphatase type 5.32 VSMCs were lysed with 500 µL of an ice-cold cell lysis buffer (20 mmol/L Hepes, pH 8.0, 2 mmol/L MgCl2, 1 mmol/L EDTA, 0.1% Triton X-100, 1 mmol/L phenylmethylsulfonyl fluoride, 10 µg/mL aprotinin, and 10 µg/mL leupeptin). Cell lysates were then incubated with GST-TPR bound to glutathione-Sepharose beads for 2 hours at 4°C. After the beads were washed with the ice-cold cell lysis buffer, the bound proteins were eluted in SDS sample buffer and analyzed by immunoblotting with anti-G12 and anti-G13 antibodies.33
Rho Activity Assay
VSMCs were lysed with an ice-cold cell lysis buffer (25 mmol/L HEPES pH7.5, 150 mmol/L NaCl, 1% Igepal CA-630, 10 mmol/L MgCl2, 1 mmol/L EDTA, 10% Glycerol). Cell lysates were incubated with GST-fused Rho-binding domain of Rhotekin which were bound to glutathione-Sepharose beads for 2 hours, and bound proteins were immunoblotted with anti-RhoA antibody.34
Migration Assay
VSMC migration was measured using a monolayer-wounding protocol in which cells migrated from a confluent area into an area that was mechanically denuded of cells. VSMCs infected with adenovirus for 2 days were scraped by a metal dental pick (DenTek) and stimulated by 100 nmol/L AngII for 24 hours with 5 mmol/L hydroxyurea to completely block proliferation.35 VSMC migration was quantified as previously reported.35
Statistical Analysis of Data
Unless stated otherwise, results are representative of at least 3 separate experiments giving similar results. Densitometry and migration data were analyzed using 1-way ANOVA. The mean±SEM was measured with a significance level of P<0.05.
Results
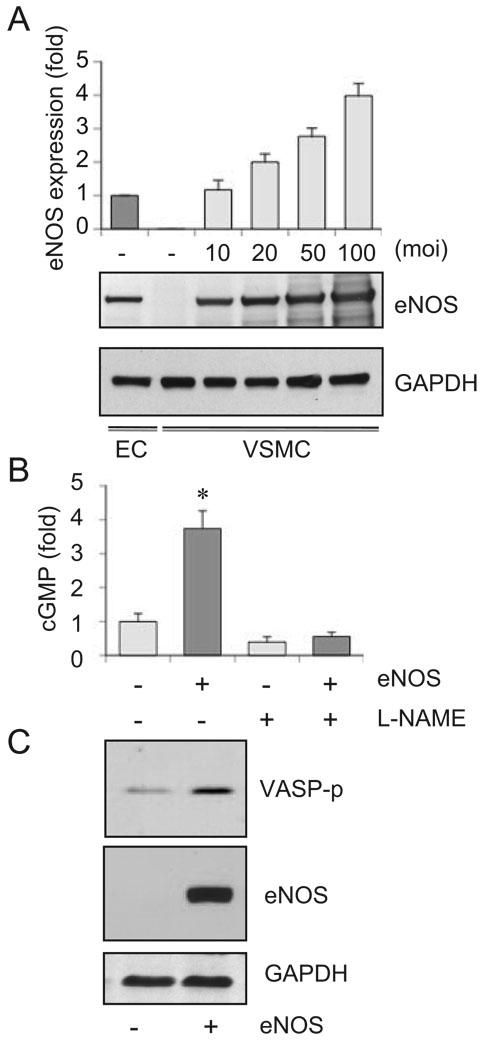
To avoid any superphysiological overexpression of eNOS, eNOS expression by the adenovirus vector was titrated with endogenous eNOS expression in bovine endothelial cells in culture (Figure 1A). 20 moi was thereafter chosen, which induced twice the amount of the endogenous eNOS expression in VSMCs. As shown in Figure 1B, the eNOS gene transfer to VSMCs resulted in increased cGMP accumulation via NO production. We also observed marked Ser239 phosphorylation of VASP, a substrate of PKG (Figure 1C). These data indicate that the exogenously-introduced eNOS was functionally coupled to activate the NO/cGMP/PKG cascade in cultured VSMCs.
Figure 1.
eNOS gene delivery to cultured VSMCs results in NO-dependent cGMP production and phosphorylation of a PKG substrate. A, VSMCs were infected with adenovirus encoding eNOS for 48 hours, and the cell lysates were subjected to immunoblot analysis as indicated (n=3). B, VSMCs were infected with adenovirus encoding eNOS or its control vector (20 moi) for 48 hours, and intracellular cGMP production was measured with or without pretreatment of L-NAME (n=4). C, VSMCs were infected with adenovirus encoding eNOS or its control vector (20 moi), and the cell lysates were subjected to immunoblot analysis as indicated (n=3).
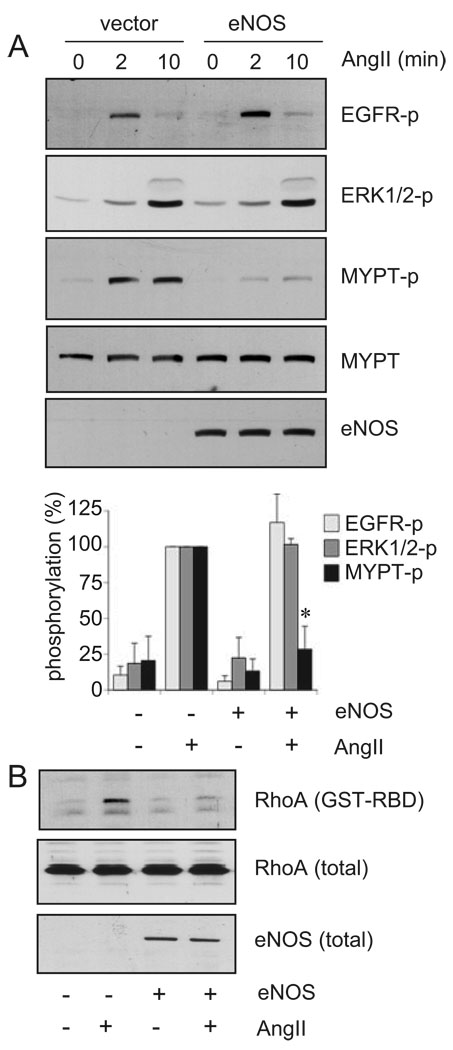
To identify the signaling target of the eNOS cascade that leads to inhibition of AngII-induced VSMC remodeling, the effects of eNOS gene transfer on the EGFR/ERK pathway and the Rho/ROCK pathway were examined. Previously, we have demonstrated that AngII-induced ERK activation and subsequent VSMC hypertrophy and migration require the EGFR transactivation mediated through the AT1 receptor.13,36 However, infection of eNOS adenovirus did not prevent EGFR transactivation or ERK activation induced by AngII in VSMCs (Figure 2A), suggesting that the EGFR/ERK pathway is not a direct inhibitory target of eNOS.
Figure 2.
eNOS gene delivery inhibits the Rho/ROCK cascade activation by AngII in VSMCs. The cells were infected with adenovirus encoding eNOS or its control vector (20 moi) for 48 hours and stimulated with 100 nmol/L AngII for 2 and 10 minutes (A) or 2 minutes (B) (n=3). A, Cell lysates were subjected to immunoblot analysis with antibodies as indicated. B, RhoA activity was evaluated by using GST-RBD.
Recently, we have demonstrated rapid activation of the Rho/ROCK pathway through the AT1 receptor in VSMCs. MYPT1 is a known substrate for ROCK and its phosphorylation status has been used to assess changes in ROCK activity.14,17 The MYPT1 phosphorylation was markedly inhibited by eNOS adenovirus infection (Figure 2A). We also found that activation of RhoA, as assessed by an affinity precipitation with GST-rhotekin fusion protein, was blocked by eNOS adenovirus (Figure 2B). Because the Rho/ROCK cascade has also been shown to mediate VSMC protein synthesis as well as migration induced by AngII,14,37 these data suggest that previous observations of inhibition of AngII-induced protein synthesis and migration by NO4,11 were mediated through the Rho/ROCK inhibition.
We have also examined the effect of eNOS gene transfer on a phosphatase inhibitor, okadaic acid–induced MYPT1 phosphorylation. The inhibitor has been used to demonstrate regulation of MYPT1 phosphorylation by a phosphatase such as PP2A.38 As shown in supplemental Figure II, okadaic acid markedly stimulated phosphorylation of MYPT1 at Thr853 whereas eNOS gene transfer did not affect MYPT-1 phosphorylation stimulated by okadaic acid in VSMCs. These results suggest a presence of a phosphatase regulation of MYPT1 phosphorylation in VSMCs and an eNOS-dependent mechanism could not compete with the phosphatase inhibitor.
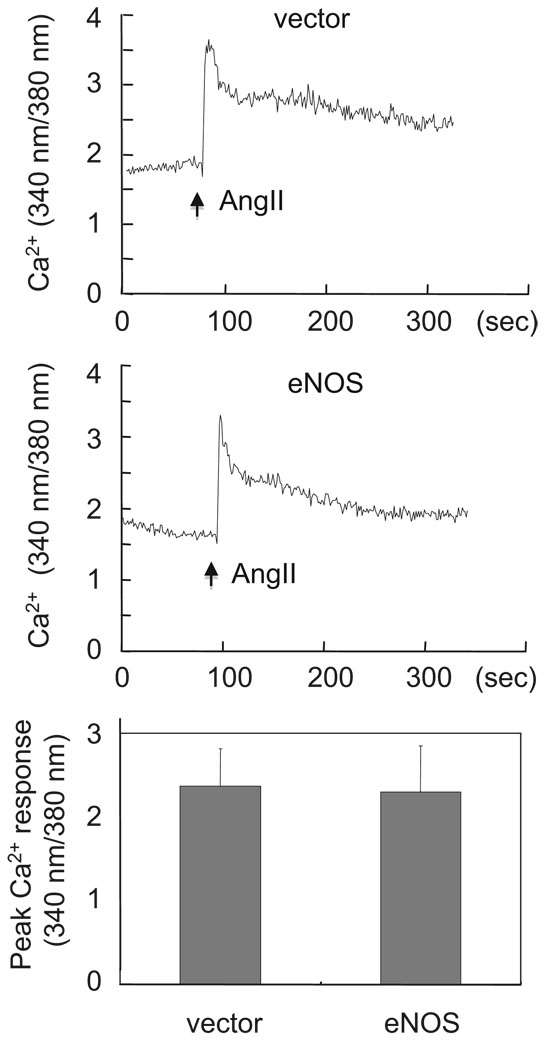
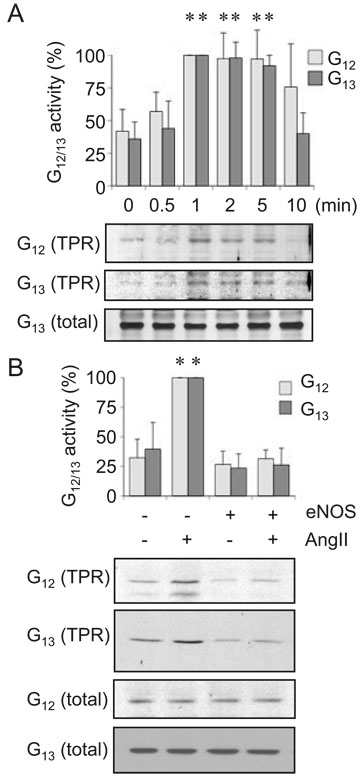
It should be noted that Gq appears to play a major role for EGFR transactivation and ERK activation by the AT1 receptor, whereas our recent analysis on MYPT1 phosphorylation by AngII suggests a requirement of G12/13 for the Rho/ROCK pathway activation by the AT1 receptor in VSMCs.17 As shown in Figure 3, eNOS adenovirus had no inhibitory effect on AngII-induced intracellular Ca2+ elevation confirming that Gq and Gq-derived second messengers are not the eNOS targets in VSMCs. Alternatively, activation of G12/13 by AngII was evaluated by an affinity precipitation assay using GST-TPR fusion protein (Figure 4). Activation of G12/13 by AngII was detected as early as 1 minute and it was consistent to 5 minutes (Figure 4A). We confirmed that the activation was mediated through the AT1 receptor by using a specific AT1 receptor antagonist (data not shown). Importantly, treatment of eNOS adenovirus markedly inhibited G12/13 activation by AngII in VSMCs (Figure 4B) suggesting that G12/13 are the primary targets of eNOS for the Rho/ROCK pathway inhibition.
Figure 3.
eNOS gene delivery did not affect intracellular Ca2+ elevation induced by AngII in VSMCs. VSMCs infected with adenovirus encoding eNOS or its control vector (20 moi) for 48 hours were loaded with fura2 and stimulated with 100 nmol/L AngII. Intracellular Ca2+ elevation was measured as indicated (n=4).
Figure 4.
eNOS gene delivery inhibits G12/13 activation by AngII in VSMCs. A, The cells were stimulated with 100 nmol/L AngII for the indicated durations. G12/13 activity was evaluated by using GST-TPR (n=3). B, VSMCs were infected with adenovirus encoding eNOS or its control vector (20 moi) for 48 hours and stimulated with 100 nmol/L AngII for 1 minute. G12/13 activity was evaluated by using GST-TPR (n=3).
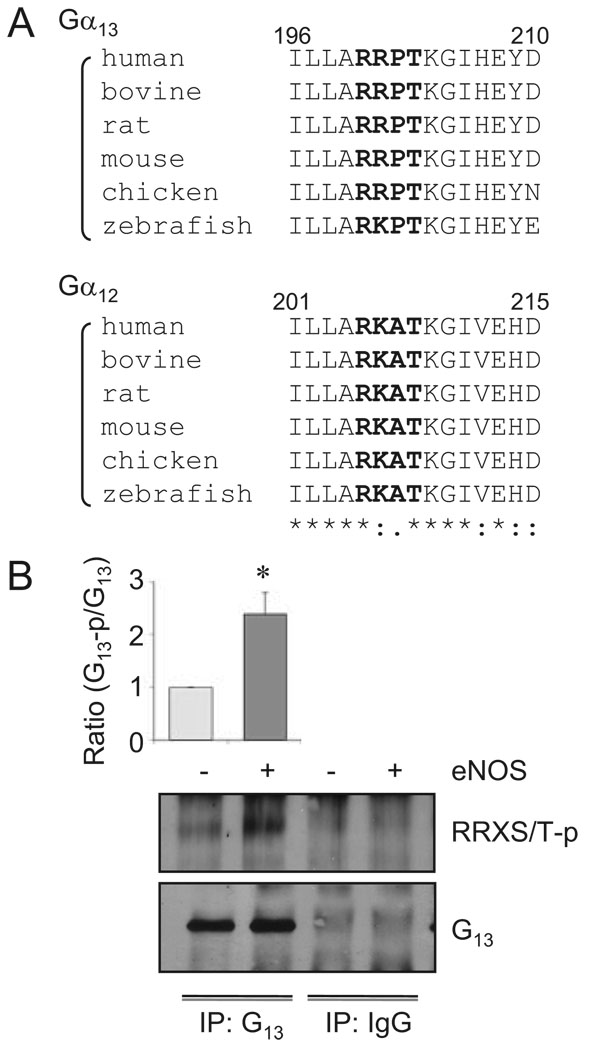
It should be noted that G13 possesses a potential phosphorylation site for PKG (R/K-R/K-X-S/T), RRPT203 (rat and human), located at the switch I region of the protein. Interestingly, the site Thr203 in G13 has been shown to be phosphorylated by PKA (consensus R/K-X1–2-S/T) leading to inhibition of G13 activity.39 Moreover, the phosphorylation motif and surrounding sequences are well conserved beyond the species in the α subunits of G12/13 (Figure 5A) but not in the α subunits of other G proteins. By using an antibody specifically to detect phosphorylation at the RRXS/T motif, we found that eNOS gene-transfer markedly enhanced G13 phosphorylation in this conserved motif in VSMCs (Figure 5B). These data suggest a potential involvement of the G12/13 phosphorylation by PKG in the mechanism by which eNOS inhibits G12/13 activity.
Figure 5.
eNOS gene transfer stimulates G13 phosphorylation at the conserved RRPT motif. A, Comparison of the amino acid sequences of Gα13 and Gα12 in various species containing a conserved PKG phosphorylation site motif, R/K-R/K-X-S/T. B, VSMCs were infected with adenovirus encoding eNOS or its control vector (20 moi) for 48 hours. The cell lysates were subjected to immunoprecipitation with G13 antibody or control IgG and subsequent immunoblot analysis with phospho-RRXS/T motif antibody and G13 antibody (n=3).
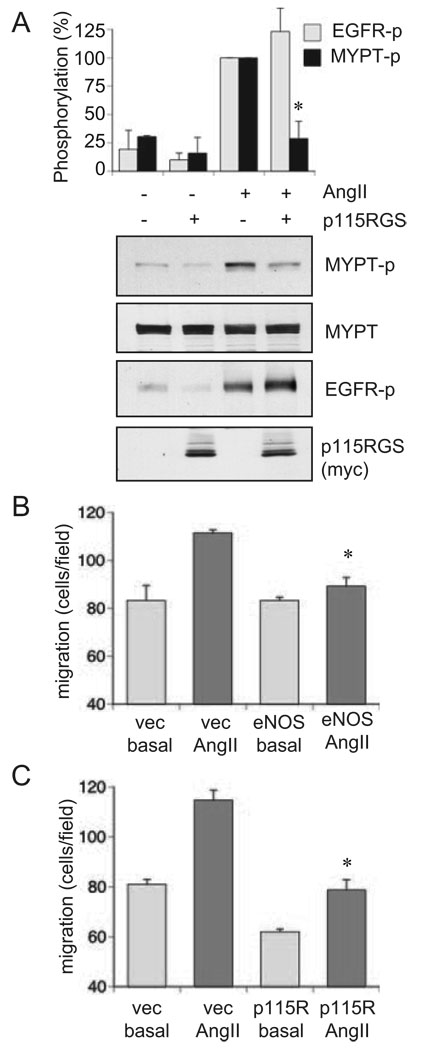
To support the specific role of G12/13 in mediating the Rho/ROCK pathway activation by AngII in VSMC, the effects of adenovirus encoding a RGS domain of p115Rho GEF, a specific G12/13 inhibitor, on MYPT1 phosphorylation and EGFR transactivation were examined. p115RGS almost completely inhibited MYPT1 phosphorylation induced by AngII without affecting EGFR transactivation by AngII (Figure 6A). Moreover, not only expression of eNOS but also p115RGS markedly inhibited VSMC migration induced by AngII (Figure 6B and 6C). Taken together, these data strongly support our hypothesis that G12/13 are the primary targets of eNOS/NO to prevent AngII-induced VSMC migration mediated through the Rho/ROCK pathway.
Figure 6.
Requirement of G12/13 activity for AngII-induced ROCK activation and VSMCs migration. A, The cells were infected with adenovirus encoding p115RGS, a selective G12/13 inhibitor, or its control vector (50 moi) for 48 hours and stimulated with 100 nmol/L AngII for 2 minutes. The cell lysates were subjected to immunoblot analysis with antibodies as indicated (n=3). B and C, Confluent VSMCs infected with adenovirus encoding (B) eNOS or the control vector (20 moi) or (C) p115RGS or the control vector (50 moi) were scraped by a metal dental pick and stimulated with AngII for 24 hours in the presence of 5 mmol/L hydroxyurea to block cell proliferation completely. The nucleus was stained with Hoechst 33342 dye, and migrated VSMCs from the wound edge were counted in 4 independent view fields (100×). Results shown are representatives from 3 independent experiments (each n=4).
Discussion
The major novel finding of the present study is that exogenously introduced eNOS in VSMCs, with its expression relatively comparable to the endogenous level, markedly inhibited AngII-induced Rho/ROCK pathway activation, and subsequent VSMC migration via its specific inhibition on G12/13 coupled to the AT1 receptor. Overexpression of eNOS such as by adenovirus has been shown to inhibit VSMC proliferation, hypertrophy, as well as migration associated with a wide variety of inhibitions of the upstream signal transductions and gene transcriptions.40–43 However, it is still difficult to conclude whether the observations were translatable to physiological consequences of eNOS-dependent vascular protection, because most of the past studies might overexpress eNOS far beyond the eNOS amount expressed in endothelial cells. For example, marked inhibition of VSMC proliferation by eNOS adenovirus were observed between 150 to 1000 moi,40,41 whereas the inhibition of thymidine incorporation, migration, and hypertrophy were even observed at 25 to 50 moi.11,42,43 Importantly, functional coupling of the gene-transferred eNOS to the NO/cGMP cascade was detectable as low as 10 to 25 moi infection.42 In the present study, we have carefully titrated the expression level of eNOS relatively comparable to the level observed in endothelial cells (20 moi), which appear to be sufficient to activate the NO/cGMP/PKG cascade as previously reported.42
Pharmacological NO production has been shown to downregulate AT1 receptor expression in VSMCs.44 Also, PKG activated through the NO/cGMP cascade has been demonstrated to inhibit intracellular Ca2+ elevation by GPCRs through several mechanisms such as inhibition of regulator of G protein 2.45–47 Alternatively, Ca2+ influx could be inhibited by NO via activation of sarco/endoplasmic reticulum calcium ATPase, which appears to be cGMP-independent.48 However, these mechanisms were not confirmed in our experiments with a moderate eNOS gene transfer. This is most likely attributable to the distinct duration and amount of the cGMP/PKG cascade activation between our study and past observations where relatively potent activation of the cascade was induced quite rapidly with pharmacological treatments.
Beside the inhibition of intracellular Ca2+ elevation, it has been recognized that the NO/cGMP/PKG cascade inhibits the Rho-kinase/ROCK-mediated Ca2+-sensitizing pathway in VSMCs.49 Although the exact molecular mechanism by which the cascade antagonizes Ca2+ sensitization induced by GPCRs remains unestablished, multiple mechanisms have been proposed including phosphorylations of RhoA and MYPT1.22,24,50,51 However, our present data rather pointed out a new mode of the Rho/ROCK pathway inhibition by eNOS through inhibition of G12/13, the key G proteins crucial for the Rho/ROCK cascade activation by GPCRs.52 This is further supported by inhibition of AngII-induced MYPT1 phosphorylation by p115RGS, a specific G12/13 inhibitor.
Recently, activation of PP2A by NO has been demonstrated.53 Although our data presenting inhibition of ROCK up-streams (G12/13 and Rho) by eNOS strongly suggest that the inhibition of MYPT1 phosphorylation is mediated through the ROCK cascade inhibition by eNOS, it may not be sufficient enough to completely rule out a partial involvement of MYPT1-targeting phosphatase activation by eNOS in VSMCs.
Regarding the molecular mechanism of G12/13 inhibition by eNOS, the AT1 phosphorylation by PKG could be excluded because the AT1 receptor does not have any consensus sequence phosphorylated by PKG. In this regard, G12/13 possess a potential phosphorylation site for PKG and PKA which is not conserved in other G proteins. Moreover, the site Thr203 in G13 has been shown to be phosphorylated by PKA leading to inhibition of G13 and subsequent Rho activation by a GPCR agonist.39 Taken together, our findings suggest a novel mechanism of the Rho/ROCK pathway inhibition by eNOS through G12/13 phosphorylation which is potentially shared by other PKA/PKG agonists in VSMCs.
Inhibition of VSMC migration by the NO/cGMP/PKG cascade has been shown to involve inhibition of matrix metalloproteases43 and CaM kinase II.54 Thus, our findings provide further mechanistic insight by which the cascade inhibits VSMC migration that is useful to consider a potential new therapy to prevent VSMC migration observed in cardiovascular diseases. Although requirement of the Rho/ROCK cascade in mediating VSMC migration has been demonstrated by us and others, 14,55,56 presentation of G12/13 activation as a critical upstream event of the migration is also one of the new elements of the present study. A potential downstream in mediating the Rho/ROCK-dependent VSMC migration may involve inhibition of myosin light chain phosphatase as expected by the MYPT phosphorylation in the present study as well as activation of LIM kinase by ROCK. Activation of LIM kinase inhibits cofilin-mediated actin depolymerization favoring increased F-actin during migration.56
The limitations of the present study include a lack of dissection of G12 and G13 in mediating VSMC migration and lack of in vivo correlations. Most of the past publications with other cell systems suggest a dominant role of G13 in mediating cell migration.57 A recent study using vascular specific G12/13-deficient mice confirmed the roles of these G proteins in mediating vascular contraction in response to AngII.18 Therefore, further research to clarify the in vivo significance of the inhibition of G12/13 function by eNOS is expected to yield important information for better treatment of cardiovascular diseases associated with the enhanced renin angiotensin system.
Supplementary Material
Acknowledgments
We thank Kyoko Hinoki for her technical assistance.
Sources of Funding
This work was supported by National Institute of Health Grants HL076770 (S.E.), HL90804 (E.B.), and HL63810 (M.V.A.). It was also supported by the American Heart Association Established Investigator Award 0740042N (S.E.), an AHA Grant-in-Aid 2455562U (M.V.A.), and by a W.W. Smith Charitable Trust Grant H0605 (S.E.). K.I. was supported by a Japan Heart Foundation Bayer Yakuhin Research Grant Abroad.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 2.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 3.Nakashima H, Suzuki H, Ohtsu H, Chao JY, Utsunomiya H, Frank GD, Eguchi S. Angiotensin II regulates vascular and endothelial dysfunction: recent topics of Angiotensin II type-1 receptor signaling in the vasculature. Curr Vasc Pharmacol. 2006;4:67–78. doi: 10.2174/157016106775203126. [DOI] [PubMed] [Google Scholar]
- 4.Dubey RK, Jackson EK, Luscher TF. Nitric oxide inhibits angiotensin II-induced migration of rat aortic smooth muscle cell. Role of cyclic-nucleotides and angiotensin1 receptors. J Clin Invest. 1995;96:141–149. doi: 10.1172/JCI118014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritchie RH, Schiebinger RJ, LaPointe MC, Marsh JD. Angiotensin II-induced hypertrophy of adult rat cardiomyocytes is blocked by nitric oxide. Am J Physiol. 1998;275:H1370–H1374. doi: 10.1152/ajpheart.1998.275.4.H1370. [DOI] [PubMed] [Google Scholar]
- 6.Yan C, Kim D, Aizawa T, Berk BC. Functional interplay between angiotensin II and nitric oxide: cyclic GMP as a key mediator. Arterioscler Thromb Vasc Biol. 2003;23:26–36. doi: 10.1161/01.atv.0000046231.17365.9d. [DOI] [PubMed] [Google Scholar]
- 7.Channon KM, Qian H, George SE. Nitric oxide synthase in atherosclerosis and vascular injury: insights from experimental gene therapy. Arterioscler Thromb Vasc Biol. 2000;20:1873–1881. doi: 10.1161/01.atv.20.8.1873. [DOI] [PubMed] [Google Scholar]
- 8.von der Leyen HE, Dzau VJ. Therapeutic potential of nitric oxide synthase gene manipulation. Circulation. 2001;103:2760–2765. doi: 10.1161/01.cir.103.22.2760. [DOI] [PubMed] [Google Scholar]
- 9.van Haperen R, de Waard M, van Deel E, Mees B, Kutryk M, van Aken T, Hamming J, Grosveld F, Duncker DJ, de Crom R. Reduction of blood pressure, plasma cholesterol, and atherosclerosis by elevated endothelial nitric oxide. J Biol Chem. 2002;277:48803–48807. doi: 10.1074/jbc.M209477200. [DOI] [PubMed] [Google Scholar]
- 10.von der Thusen JH, Fekkes ML, Passier R, van Zonneveld AJ, Mainfroid V, van Berkel TJ, Biessen EA. Adenoviral transfer of endothelial nitric oxide synthase attenuates lesion formation in a novel murine model of postangioplasty restenosis. Arterioscler Thromb Vasc Biol. 2004;24:357–362. doi: 10.1161/01.ATV.0000114235.51044.92. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Eguchi K, Ohtsu H, Higuchi S, Dhobale S, Frank GD, Motley ED, Eguchi S. Activation of endothelial nitric oxide synthase by the angiotensin II type 1 receptor. Endocrinology. 2006;147:5914–5920. doi: 10.1210/en.2006-0834. [DOI] [PubMed] [Google Scholar]
- 12.Xi XP, Graf K, Goetze S, Fleck E, Hsueh WA, Law RE. Central role of the MAPK pathway in ang II-mediated DNA synthesis and migration in rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:73–82. doi: 10.1161/01.atv.19.1.73. [DOI] [PubMed] [Google Scholar]
- 13.Saito S, Frank GD, Motley ED, Dempsey PJ, Utsunomiya H, Inagami T, Eguchi S. Metalloprotease inhibitor blocks angiotensin II-induced migration through inhibition of epidermal growth factor receptor trans-activation. Biochem Biophys Res Commun. 2002;294:1023–1029. doi: 10.1016/S0006-291X(02)00595-8. [DOI] [PubMed] [Google Scholar]
- 14.Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S, Takuwa Y, Sasaki T, Rothstein JD, Suzuki H, Nakashima H, Woolfolk EA, Motley ED, Eguchi S. Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1831–1836. doi: 10.1161/01.ATV.0000175749.41799.9b. [DOI] [PubMed] [Google Scholar]
- 15.Kyaw M, Yoshizumi M, Tsuchiya K, Kagami S, Izawa Y, Fujita Y, Ali N, Kanematsu Y, Toida K, Ishimura K, Tamaki T. Src and Cas are essentially but differentially involved in angiotensin II-stimulated migration of vascular smooth muscle cells via extracellular signal-regulated kinase 1/2 and c-Jun NH2-terminal kinase activation. Mol Pharmacol. 2004;65:832–841. doi: 10.1124/mol.65.4.832. [DOI] [PubMed] [Google Scholar]
- 16.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem. 1998;273:8890–8896. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 17.Ohtsu H, Higuchi S, Shirai H, Eguchi K, Suzuki H, Hinoki A, Brailoiu E, Eckhart AD, Frank GD, Eguchi S. Central role of Gq in the hypertrophic signal transduction of angiotensin II in vascular smooth muscle cells. Endocrinology. 2008;149:3569–3575. doi: 10.1210/en.2007-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S. G12–G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 19.Ohtsu H, Suzuki H, Nakashima H, Dhobale S, Frank GD, Motley ED, Eguchi S. Angiotensin II signal transduction through small GTP-binding proteins: mechanism and significance in vascular smooth muscle cells. Hypertension. 2006;48:534–540. doi: 10.1161/01.HYP.0000237975.90870.eb. [DOI] [PubMed] [Google Scholar]
- 20.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 21.Oro C, Qian H, Thomas WG. Type 1 angiotensin receptor pharmacology: signaling beyond G proteins. Pharmacol Ther. 2007;113:210–226. doi: 10.1016/j.pharmthera.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 23.Gudi T, Chen JC, Casteel DE, Seasholtz TM, Boss GR, Pilz RB. cGMP-dependent protein kinase inhibits serum-response element-dependent transcription by inhibiting rho activation and functions. J Biol Chem. 2002;277:37382–37393. doi: 10.1074/jbc.M204491200. [DOI] [PubMed] [Google Scholar]
- 24.Ellerbroek SM, Wennerberg K, Burridge K. Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem. 2003;278:19023–19031. doi: 10.1074/jbc.M213066200. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Yu X, Brecher P. Nitric oxide and N-acetylcysteine inhibit the activation of mitogen-activated protein kinases by angiotensin II in rat cardiac fibroblasts. J Biol Chem. 1998;273:33027–33034. doi: 10.1074/jbc.273.49.33027. [DOI] [PubMed] [Google Scholar]
- 26.Eguchi S, Matsumoto T, Motley ED, Utsunomiya H, Inagami T. Identification of an essential signaling cascade for mitogen-activated protein kinase activation by angiotensin II in cultured rat vascular smooth muscle cells. Possible requirement of Gq-mediated p21ras activation coupled to a Ca2+/calmodulin-sensitive tyrosine kinase. J Biol Chem. 1996;271:14169–14175. doi: 10.1074/jbc.271.24.14169. [DOI] [PubMed] [Google Scholar]
- 27.Scotland RS, Morales-Ruiz M, Chen Y, Yu J, Rudic RD, Fulton D, Gratton JP, Sessa WC. Functional reconstitution of endothelial nitric oxide synthase reveals the importance of serine 1179 in endothelium-dependent vasomotion. Circ Res. 2002;90:904–910. doi: 10.1161/01.res.0000016506.04193.96. [DOI] [PubMed] [Google Scholar]
- 28.Stemmle LN, Fields TA, Casey PJ. The regulator of G protein signaling domain of axin selectively interacts with Galpha12 but not Galpha13. Mol Pharmacol. 2006;70:1461–1468. doi: 10.1124/mol.106.023705. [DOI] [PubMed] [Google Scholar]
- 29.Eguchi S, Iwasaki H, Ueno H, Frank GD, Motley ED, Eguchi K, Marumo F, Hirata Y, Inagami T. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser(411) in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, Ras, extracellular signal-regulated kinase, and Akt. J Biol Chem. 1999;274:36843–36851. doi: 10.1074/jbc.274.52.36843. [DOI] [PubMed] [Google Scholar]
- 30.Filipeanu CM, Brailoiu E, Le Dun S, Dun NJ. Urotensin-II regulates intracellular calcium in dissociated rat spinal cord neurons. J Neurochem. 2002;83:879–884. doi: 10.1046/j.1471-4159.2002.01196.x. [DOI] [PubMed] [Google Scholar]
- 31.Mifune M, Ohtsu H, Suzuki H, Nakashima H, Brailoiu E, Dun NJ, Frank GD, Inagami T, Higashiyama S, Thomas WG, Eckhart AD, Dempsey PJ, Eguchi S. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J Biol Chem. 2005;280:26592–26599. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi Y, Katoh H, Mori K, Negishi M. Galpha(12) and Galpha(13) interact with Ser/Thr protein phosphatase type 5 and stimulate its phosphatase activity. Curr Biol. 2002;12:1353–1358. doi: 10.1016/s0960-9822(02)01034-5. [DOI] [PubMed] [Google Scholar]
- 33.Radhika V, Hee Ha J, Jayaraman M, Tsim ST, Dhanasekaran N. Mitogenic signaling by lysophosphatidic acid (LPA) involves Galpha12. Oncogene. 2005;24:4597–4603. doi: 10.1038/sj.onc.1208665. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto H, Takuwa N, Yokomizo T, Sugimoto N, Sakurada S, Shigematsu H, Takuwa Y. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol Cell Biol. 2000;20:9247–9261. doi: 10.1128/mcb.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarkar R, Meinberg EG, Stanley JC, Gordon D, Webb RC. Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. Circ Res. 1996;78:225–230. doi: 10.1161/01.res.78.2.225. [DOI] [PubMed] [Google Scholar]
- 36.Eguchi S, Iwasaki H, Hirata Y, Frank GD, Motley ED, Yamakawa T, Numaguchi K, Inagami T. Epidermal growth factor receptor is indispensable for c-Fos expression and protein synthesis by angiotensin II. Eur J Pharmacol. 1999;376:203–206. doi: 10.1016/s0014-2999(99)00357-x. [DOI] [PubMed] [Google Scholar]
- 37.Yamakawa T, Tanaka S, Numaguchi K, Yamakawa Y, Motley ED, Ichihara S, Inagami T. Involvement of Rho-kinase in angiotensin II-induced hypertrophy of rat vascular smooth muscle cells. Hypertension. 2000;35:313–318. doi: 10.1161/01.hyp.35.1.313. [DOI] [PubMed] [Google Scholar]
- 38.Lontay B, Kiss A, Gergely P, Hartshorne DJ, Erdodi F. Okadaic acid induces phosphorylation and translocation of myosin phosphatase target subunit 1 influencing myosin phosphorylation, stress fiber assembly and cell migration in HepG2 cells. Cell Signal. 2005;17:1265–1275. doi: 10.1016/j.cellsig.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Manganello JM, Huang JS, Kozasa T, Voyno-Yasenetskaya TA, Le Breton GC. Protein kinase A-mediated phosphorylation of the Galpha13 switch I region alters the Galphabetagamma13-G protein-coupled receptor complex and inhibits Rho activation. J Biol Chem. 2003;278:124–130. doi: 10.1074/jbc.M209219200. [DOI] [PubMed] [Google Scholar]
- 40.Tanner FC, Meier P, Greutert H, Champion C, Nabel EG, Luscher TF. Nitric oxide modulates expression of cell cycle regulatory proteins: a cytostatic strategy for inhibition of human vascular smooth muscle cell proliferation. Circulation. 2000;101:1982–1989. doi: 10.1161/01.cir.101.16.1982. [DOI] [PubMed] [Google Scholar]
- 41.D’Souza FM, Sparks RL, Chen H, Kadowitz PJ, Jeter JR., Jr Mechanism of eNOS gene transfer inhibition of vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2003;284:C191–C199. doi: 10.1152/ajpcell.00179.2002. [DOI] [PubMed] [Google Scholar]
- 42.Kullo IJ, Schwartz RS, Pompili VJ, Tsutsui M, Milstien S, Fitzpatrick LA, Katusic ZS, O’Brien T. Expression and function of recombinant endothelial NO synthase in coronary artery smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17:2405–2412. doi: 10.1161/01.atv.17.11.2405. [DOI] [PubMed] [Google Scholar]
- 43.Gurjar MV, Sharma RV, Bhalla RC. eNOS gene transfer inhibits smooth muscle cell migration and MMP-2 and MMP-9 activity. Arterioscler Thromb Vasc Biol. 1999;19:2871–2877. doi: 10.1161/01.atv.19.12.2871. [DOI] [PubMed] [Google Scholar]
- 44.Ichiki T, Usui M, Kato M, Funakoshi Y, Ito K, Egashira K, Takeshita A. Downregulation of angiotensin II type 1 receptor gene transcription by nitric oxide. Hypertension. 1998;31:342–348. doi: 10.1161/01.hyp.31.1.342. [DOI] [PubMed] [Google Scholar]
- 45.Hofmann F. The biology of cyclic GMP-dependent protein kinases. J Biol Chem. 2005;280:1–4. doi: 10.1074/jbc.R400035200. [DOI] [PubMed] [Google Scholar]
- 46.Osei-Owusu P, Sun X, Drenan RM, Steinberg TH, Blumer KJ. Regulation of RGS2 and second messenger signaling in vascular smooth muscle cells by cGMP-dependent protein kinase. J Biol Chem. 2007;282:31656–31665. doi: 10.1074/jbc.M706360200. [DOI] [PubMed] [Google Scholar]
- 47.Weber S, Bernhard D, Lukowski R, Weinmeister P, Worner R, Wegener JW, Valtcheva N, Feil S, Schlossmann J, Hofmann F, Feil R. Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circ Res. 2007;101:1096–1103. doi: 10.1161/CIRCRESAHA.107.154351. [DOI] [PubMed] [Google Scholar]
- 48.Ying J, Tong X, Pimentel DR, Weisbrod RM, Trucillo MP, Adachi T, Cohen RA. Cysteine-674 of the sarco/endoplasmic reticulum calcium ATPase is required for the inhibition of cell migration by nitric oxide. Arterioscler Thromb Vasc Biol. 2007;27:783–790. doi: 10.1161/01.ATV.0000258413.72747.23. [DOI] [PubMed] [Google Scholar]
- 49.Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91:1421–1430. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- 50.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem. 2004;279:34496–34504. doi: 10.1074/jbc.M405957200. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura K, Koga Y, Sakai H, Homma K, Ikebe M. cGMP-dependent relaxation of smooth muscle is coupled with the change in the phosphorylation of myosin phosphatase. Circ Res. 2007;101:712–722. doi: 10.1161/CIRCRESAHA.107.153981. [DOI] [PubMed] [Google Scholar]
- 52.Riobo NA, Manning DR. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends Pharmacol Sci. 2005;26:146–154. doi: 10.1016/j.tips.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Illi B, Dello Russo C, Colussi C, Rosati J, Pallaoro M, Spallotta F, Rotili D, Valente S, Ragone G, Martelli F, Biglioli P, Steinkuhler C, Gallinari P, Mai A, Capogrossi MC, Gaetano C. Nitric oxide modulates chromatin folding in human endothelial cells via protein phosphatase 2A activation and class II histone deacetylases nuclear shuttling. Circ Res. 2008;102:51–58. doi: 10.1161/CIRCRESAHA.107.157305. [DOI] [PubMed] [Google Scholar]
- 54.Zhang S, Yang Y, Kone BC, Allen JC, Kahn AM. Insulin-stimulated cyclic guanosine monophosphate inhibits vascular smooth muscle cell migration by inhibiting Ca/calmodulin-dependent protein kinase II. Circulation. 2003;107:1539–1544. doi: 10.1161/01.cir.0000056766.45109.c1. [DOI] [PubMed] [Google Scholar]
- 55.Seasholtz TM, Majumdar M, Kaplan DD, Brown JH. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circ Res. 1999;84:1186–1193. doi: 10.1161/01.res.84.10.1186. [DOI] [PubMed] [Google Scholar]
- 56.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res. 2007;100:607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- 57.Dhanasekaran DN. Transducing the signals: a G protein takes a new identity. Sci STKE. 2006;2006:pe31. doi: 10.1126/stke.3472006pe31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.