Abstract
Vertebrate teeth are attached to jaws by a variety of mechanisms, including acrodont, pleurodont, and thecodont modes of attachment. Recent studies have suggested that various modes of attachment exist within each sub-category. Especially squamates feature a broad diversity of modes of attachment. Here we have investigated tooth attachment tissues in the late cretaceous mosasaur Clidastes and compared mosasaur tooth attachment with modes of attachment found in other extant reptiles. Using histologic analysis of ultrathin ground sections, four distinct mineralized tissues that anchor mosasaur teeth to the jaw were identified: (i) an acellular cementum layer at the interface between root and cellular cementum, (ii) a massive cone consisting of trabecular cellular cementum, (iii) the mineralized periodontal ligament containing mineralized Sharpey’s fibers, and (iv) the interdental ridges connecting adjacent teeth. The complex, multilayered attachment apparatus in mosasaurs was compared with attachment tissues in extant reptiles, including Iguana and Caiman. Based on our comparative analysis we postulate the presence of a quadruple-layer tissue architecture underlying reptilian tooth attachment, comprised of acellular cementum, cellular cementum, mineralized periodontal ligament, and interdental ridge (alveolar bone). We propose that the mineralization status of the periodontal ligament is a dynamic feature in vertebrate evolution subject to functional adaptation.
Introduction
The anchorage of teeth in jaws is one of the great architectural masterpieces in the design of the vertebrate body plan. Teeth commonly provide pointy tips, sharp incisal edges, or massive masticatory plateaus that allow them to exert their function related to capturing, biting, and chewing. However, for an efficient capture and partitioning of prey, tooth function is greatly enhanced through the connection with a rigid base as it is established by the load-bearing jaw bones. This connection between teeth and jaws is maintained by the periodontal attachment apparatus. The anchorage of teeth to jaws is also unique from a developmental point of view since vertebrate teeth develop essentially independent from the adjacent jaw bone through interactions between oral epithelium and migratory cranial ectomesenchyme. Only after tooth formation has begun, tooth-bound migratory tissues extend toward the jaw bone and form a connective tissue attachment that structurally and mechanically integrates the teeth with the jaw (Diekwisch 2002). The nature of this attachment greatly differs between vertebrate lineages and varies between rigid ankylosis and flexible fiber-mediated attachment.
Three basic types of tooth attachment are observed in extant reptiles (Osborn, 1984; Gaengler, 2000). The acrodont type of tooth attachment, characteristic of the Tuatara (Sphenodon punctatus) as well as agamid lizards and chameleons, is characterized by ankylosis of the tooth to the crest of the tooth-bearing element. The squamate condition is pleurodont, the tooth being ankylosed to the pleura (lingually sloping inner surface) of the tooth-bearing element (Lessman, 1952). Crocodiles feature a thecodont dentition, where the teeth are set in sockets but do not ankylose to the tooth-bearing elements. Instead, crocodile teeth show a fibrous attachment (Osborn, 1984) to the wall of the alveolus by means of the periodontal ligament. These distinctions of tooth implantation become blurred if fossil reptiles are taken into account. Acrodonty and pleurodonty can be seen as parts of the same transformation series (Estes et al., 1988), while other fossil reptiles show subthecodont or thecodont tooth implantation that involves ankylosis (Motani, 1997). The debate over the interpretation of tooth implantation and attachment has resulted in a debate over the affinities between mosasaurs and snakes, which were claimed to uniquely share a thecodont mode of tooth implantation among squamates (Lee, 1997a, b, 1998; Zaher and Rieppel, 1999). In an attempt to clarify and better characterize the nature of tooth implantation and ankylosis in mosasaurs, Caldwell et al. (2003) subjected mosasaur jaw fragments to histological analysis (see also Caldwell, 2007).
Mosasauroids are an extinct clade of marine squamates generally thought to be related to extant monitor lizards and their fossil relatives (e.g., Lee, 1997a, 1998; Rieppel et al., 2007). The clade makes its first appearance in the Fossil Record with the poorly known taxon Proaigialosaurus from the Upper Jurassic Solnhofen deposits in Southern Germany (Kuhn, 1958; referred to Aigialosauridae by Carroll, 1988), and it went extinct at the end of the Cretaceous. A first significant radiation of the clade is documented for mid-Cretaceous (Cenomanian) times, when aigialosaurs, adriosaurs, acetosaurs, coniasaurs, dolichosaurs, and pontosaurs populated coastal stretches of the neo-Tethys, predominantly in areas that today correspond to southeastern Europe and the Middle East (see Caldwell, 2006, for a recent review). During the Upper Cretaceous, with global sea level at a peak and ichthyosaurs facing extinction, the mosasaurs invaded the open sea, initiating a second rapid radiation of the clade as they adapted to numerous ecological niches offered throughout the oceans (Bell, 1997). Pelagic animals with limbs transformed to form flippers, mosasaurs include gigantic species, some attaining 14 meters (Prognathodon saturator: Dortangs et al., 2002) to 17.6 meters (Mosasaurus hoffmanni: Lingham-Soliar, 1995) in total length, thus establishing themselves at the top of the marine food chain during that time. The mosasaur feeding apparatus was indeed adapted to large prey, as is indicated by the high degree of mobility achieved by their lower jaws. The mandibular rami did not meet anteriorly in a syndesmotic symphysis, but remained independent from one another as is indicated by the smooth and rounded surface of their anterior tips. The lower jaws could thus separate at their anterior ends during feeding, and gape could be further enlarged by the presence of an intramandibular joint between the dentary and postdentary bones. Similar features are known for snakes feeding on large prey, such as boas and pythons. It is in recognition of this similarity that Cope (1869) introduced Pythonomorpha as a group that would include what he considered the two major families of mosasaurs. Although not initially part of his project, Cope (1872) later defended relationships of mosasaurs with snakes against Owen’s (1877, 1878) criticism by proposing a gradualistic scenario that would link the feeding mechanics of mosasaurs with those of macrostomatan snakes (see Rieppel et al., 2003, for further comments).
The present paper serves two functions, one to perform a detailed histological and comparative analysis of the mosasaurian attachment apparatus, and second to conduct a more generalized study of reptilian attachment tissues, comparing a mosasaur (Halisaurus sternbergi), a Green Iguana (Iguana iguana) and a Spectacled Caiman (Caiman crocodylus). The purpose of this second study is to identify similarities and differences between reptilian attachment tissues in order to identify possibly scenarios involved in the evolution of the reptilian feeding apparatus. Here we review the histology of reptilian tooth attachment in light of the findings of McIntosh et al. (2002). This study showed the crocodile (Caiman) tooth attachment to be histologically intermediate between a squamate type ankylosis (e.g. in the gecko Hemidactylus), and the mouse gomphosis, where the tooth is attached to the alveolus by the periodontal ligament. In the present paper, we have identified the mineralized periodontal ligament as an attachment tissue at the interface between mosasaur root cementum and interdental ridge, a concept that is no longer extravagant in light of the findings of McIntosh et al. (2002). This finding may have important consequences for the interpretation of the periodontium of mosasaurs, to which we now turn.
Materials and Methods
Mosasaur jaw collection and preparation
The jaw fragments sectioned for the present study come from two different specimens of mosasaurs, both held in the collections of the Field Museum of Natural History, Chicago. Specimen FMNH PR 186 represents Halisaurus sternbergi, specimen FMNH PR 484 represents an unidentified individual (Mosasauridae gen. et spec. indet.).
Ultrathin ground sections
Samples were infiltrated in Exakt Technovit 7200 VLC according to the manufacturer’s instructions. Samples were cut using an Exakt 300 parallel band saw system and an Exakt 400CS grinding and polishing device. Samples were then mounted on plastic slide holders. Surfaces were ground and polished using a graded series of Exact polishing papers with a smallest size of P2500. Thickness of ground sections was between 5 and 20μm in average.
Polarized light microscopy
For this study, ultrathin ground sections were subjected to polarized light and analyzed using an analyzing polarization filter at 120°, 240°, and 360° rotation. Micrographs were recorded digitally. An identical region in each micrograph was chosen and the distribution of birefringend tissue elements was compared between micrographs from all three angles of rotation.
Tissue preparation of two extant squamates
One Green Iguana (Iguana iguana) and an infant Spectacled Caiman (Caiman crocodylus) were obtained and sacrificed in accordance with Baylor College of Dentistry and UIC animal care regulations. Mandibles were prepared, fixed in formalin, decalcified and processed for paraffin sections. Sections measuring 5μm in thickness were then stained in Paragon-Epoxy stain (Paragon C. & C., Bronx, New York). Paragon-Epoxy is a polychromatic toluidine blue/basic fuchsin stain that has been proven particularly suitable for the demonstration of cement lines in tooth attachment tissues. Alternatively, ground sections were subjected to von Kossa’s procedure for the identification of mineralized calcium deposits.
Electron microprobe analysis
Electron microprobe analysis was conducted using a Hitachi S-3000N scanning electron microscope with an Oxford Inca EDX system and a light element X-ray detector (15mm WD). Elemental composition was determined as weight % and Ca/P ratios were calculated from five measurements per area selected.
Morphometric analysis of lacunae and fiber bundles
Five tissue sections per specimen and 10 randomly determined areas per section were selected using a Leica light microscope, a 100x lens, and a Leica digital camera system. All objects within one area were measured and their diameter was determined using our image analysis software. Mean values and standard deviation were calculated and used for comparison.
Results
Macroscopic analysis of mosasaur jaw fragments revealed distinct mineralized tissues contributing to mosasaur tooth anchorage
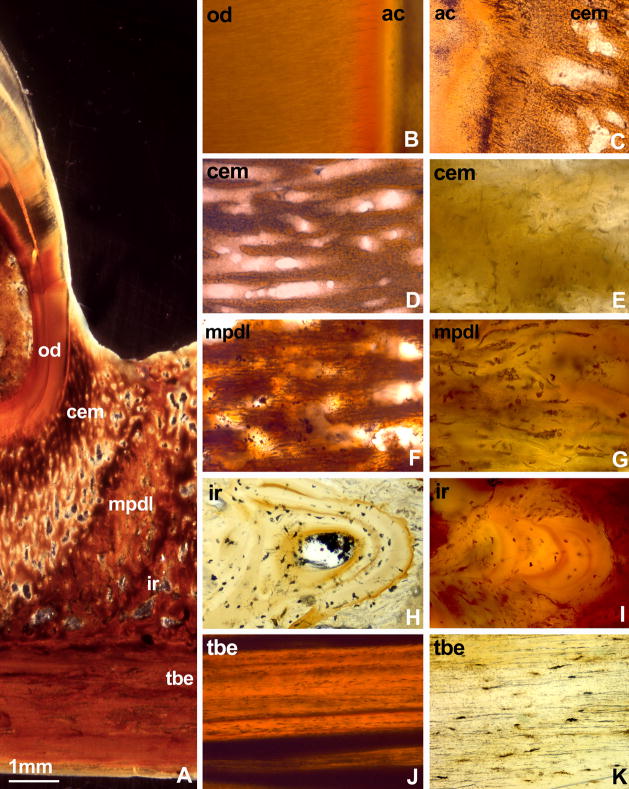
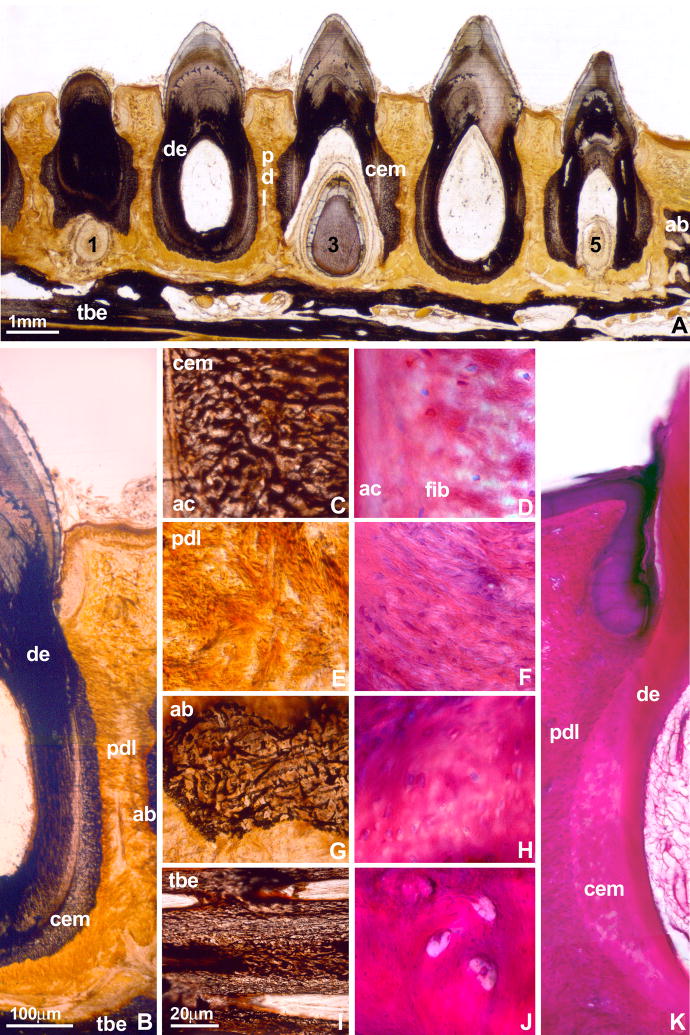
In the present study, a number of different methods of investigation were used to determine structural differences between tissue compartments of the mosasaur attachment apparatus, including transmission and incident lighting techniques of ultrathin mosasaur ground sections as well as histological examination of mosasaur attachment tissue thin sections at high magnification. Histological analysis of ultrathin ground sections by transmitted light revealed the following clearly distinguishable tissue layers contributing to tooth attachment in mosasaurs (Figs. 1–3,7): (i) a thin layer of acellular cementum between root orthodentin and the remainder of the periodontium (Figs. 3A,B), (ii) the cellular cementum cone as a trabecular tissue providing the major portion of the tooth anchorage (Figs. 3A,D,E), (iii) the mineralized periodontal ligament as a fibrous mineralized tissue between the cellular cementum and the tooth bearing element/interdental ridge (Figs. 3A,F,G), (iv) the interdental ridge containing lamellar osteons (Figs. 3A,H,I), and (v) the tooth-bearing element featuring compact bone as the basic bony element of the jaw bone (Figs. 3A,J,K). These separate tissues were also delineated on the polished section of another mosasaur tooth (Fig. 2A) and following polarized light application (Fig. 4). Cellular cementum and mineralized periodontal ligament microstructure were remarkably similar (Fig. 3D-G). However, the mineralized periodontal ligament layer contained thick bundles of Sharpey’s fibers measuring 9.06+/−3.35μm in diameter, while fibers were less pronounced in the cellular cementum layer and measured only 4.35+/−1.9μm in thickness (Figs. 3B,D).
Figure 1. Complex tooth attachment in a mosasaur (Halisaurus sternbergi).
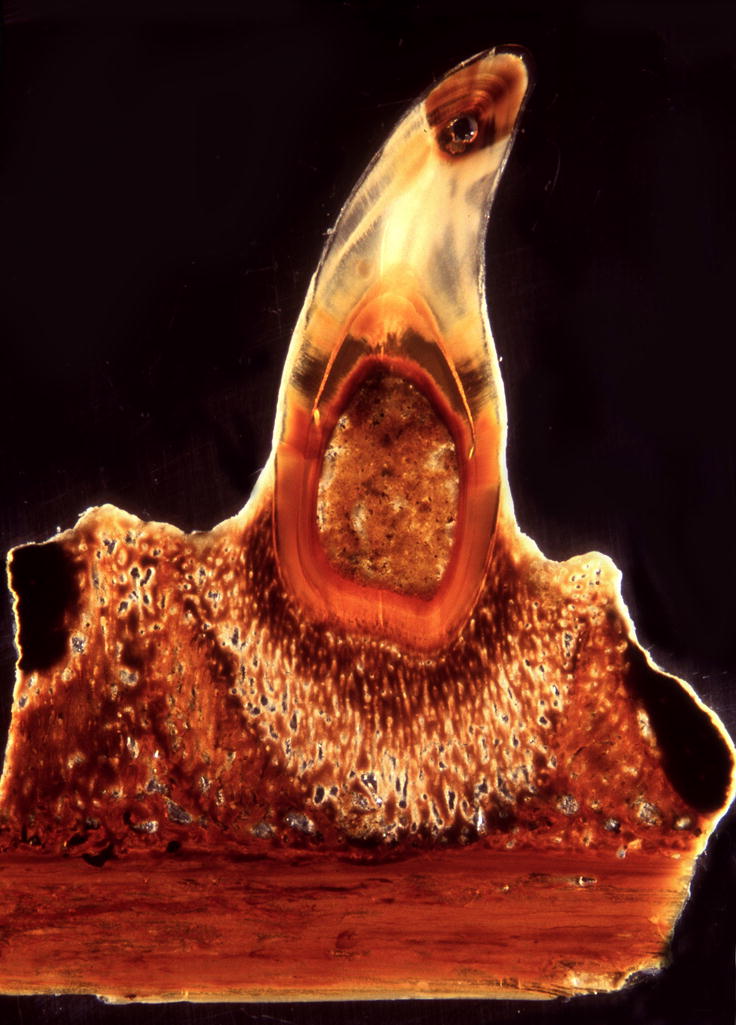
A single tooth is anchored to the jaw bone by distinct tissue layers. This complex tooth attachment pattern has been the basis for discussions about the phylogenetic placement of mosasaurids. The specimen is Halisaurus sternbergi FMNH PR 186 from the Collection of the Field Museum.
Figure 3. Details of the mosasaur (Halisaurus sternbergi) attachment apparatus as revealed using ultrathin ground sections.
Ground sections (Figs. 3A,B,D,E,F,G,I,J) and ultrathin ground sections (Figs. 3C,H,K) identified four distinct mineralized tissues between the root orthodentin (od) and the tooth bearing element (tbe), including a then layer of acellular cementum (ac), a bulky layer of cellular cementum (cem), the fiber-rich mineralized periodontal ligament (mdpl), and the compact interdental ridge (ir)(Fig. 3A). A thin layer of acellular cementum (ac) was discernible at the interface between orthodentin (od) and cellular cementum (cem)(Figs. 3B,C). The cementum layer was preserved as a spongy mineral containing thin and disoriented (4.35+/−1.9μm diameter) fibers (Figs. 3D,E). The mineralized periodontal ligament (mpdl) was characterized by the presence of thick (9.06+/−3.35μm diameter) and parallel-oriented Sharpey’s fibers embedded in a mineralized matrix (Fig. 3F,G). In contrast to the cellular cementum and the mineralized periodontal ligament, the interdental ridge (ir) contained osteons of lamellar bone and fiber bundles at its cervical border (Figs. 3A,G,H,I). In contrast, the tooth bearing element (tbe) was characterized by densely packed layers of compact bone (Figs. 3J,K).
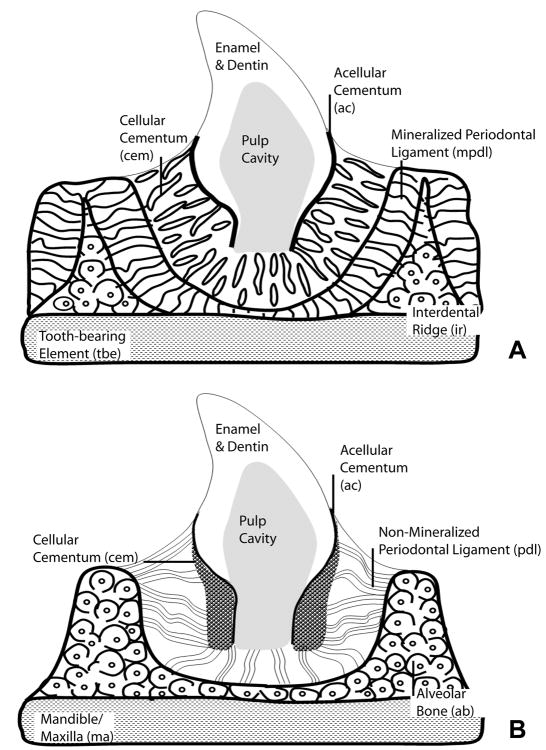
Figure 7. Schematic comparison of components of the mosasaurian (Fig. 7A) and the mammalian (Fig. 7B) attachment apparatus.
Fig. 7A. This summary sketch illustrates position and histological structure of the major tissue types involved in mosasaur tooth attachment: (i) a thin layer of acellular cementum (ac) covering the orthodentin root surface, (ii) the trabecular meshwork of the cellular cementum (cem), (iii) the fiber-rich mineralized periodontal ligament (mpdl), (iv) the interdental ridge (ir) consisting of osteons of lamellar bone, and (v) the tooth bearing element (tbe) displaying parallel layers of compact bone. We found three distinct differences between attachment tissue organization in mosasaurs (Fig. 7A) and tissue organization in crocodilian and mammalian counterparts (Fig. 7B). (i) The cellular cementum layer (cem) was reduced in thickness, especially in the coronal half of the tooth, (ii) the mineralized periodontal ligament (mpdl) was replaced by a non-mineralized (mammals) or a semi-mineralized (crocodilian) periodontal ligament (pdl), and (iii) the thin interdental ridge of mosasaurs (ir) was replaced by a solid alveolar bone theca (ab) in mammals and crocodilians. Pulp cavity and tooth crown (enamel and dentin) are labeled for orientation purposes.
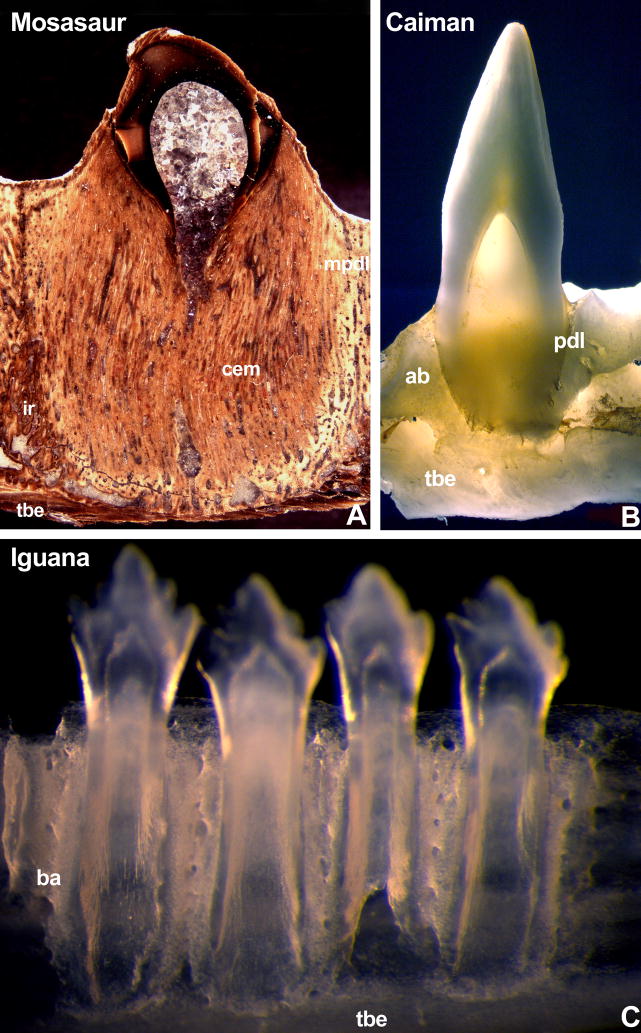
Figure 2. Diversity of tooth attachment tissues in reptilians.
In the present study we have compared (i) a massive cementum embedment (cem) connecting mosasaur tooth roots with mineralized periodontal ligament (mdpl) and interdental ridge (ir) to form a stable anchorage on the tooth bearing element (tbe)(Fig. 2A), (ii) a thin periodontal ligament (pdl) connecting crocodilian teeth with their alveolar bone sockets (ab)(Fig. 2B) and (iii) the bone of attachment (ba) that attaches teeth to the lingual surface of the tooth bearing element (tbe) characteristic of the pleurodont mode of attachment found in many squamates (Fig. 2C). The mosasaur specimen is an unidentified mosasaur (FMNH PR 484) from the collection of the Field Museum. Incident light analysis of a polished specimen clearly distinguished (i) a thick layer of fiber cementum (cem) at the interface between tooth root and jaw (light brown color), (ii) the mineralized periodontal ligament (mpdl, yellowish color), (iii) the interdental ridge (ir, brown color), and (iv) the tooth bearing element (tbe, dark-brown color).
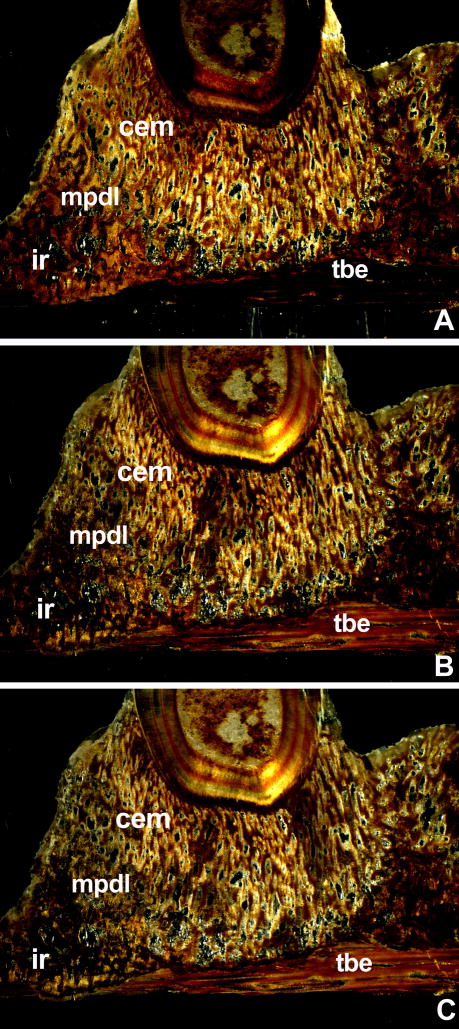
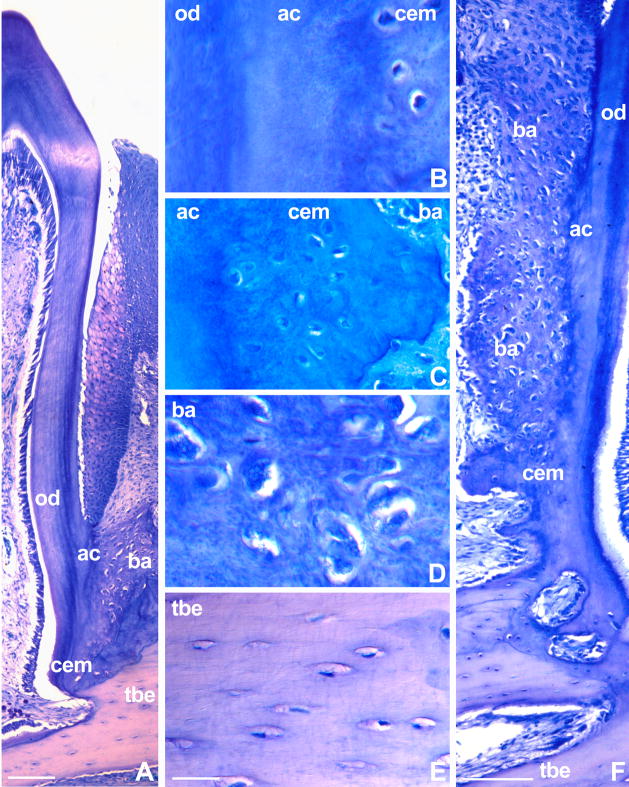
Figure 4. Polarized light microscopy of mosasaur attachment tissues.
In order to assess whether mosasaur attachment tissues were birefringent, ultrathin ground sections were analyzed between polarizers at angles of 120, 240, and 360 degree of rotation of the plane of analyzer polarization compared to the polarizer plane (Figs A,B,C). Note the distinct changes in birefringency following rotation of the polarizer. Distinct birefringence in fossilized mineralized tissues is an indicator for the presence of collagen fibers prior to fossilization. Birefringence in the fibrous cellular cementum (cem) was particularly obvious in the polarizer/analyzer setting used in Fig. 6A. Note the low level of birefringence in the interdental ridge (ir) coinciding with reduced numbers of fiber bundles. In contrast, the mineralized periodontal ligament (mpdl) displayed moderate levels of birefringence. The distribution of positively and negatively polarized tissues within the tooth bearing element (tbe) significantly changed after rotating polarizer position, indicative of the presence of parallel fiber alignment. The mosasaur used for this analysis was Halisaurus sternbergi.
Polarized light microscopy indicated birefringence of mosasaur periodontal tissues
Polarized light analysis at three angles of inclination revealed significant changes in tissue birefringence between rotated planes of polarization (120, 240, and 360 degrees)(Fig. 4). Birefringence in fossilized mineralized tissues is an indicator for the presence of collagen fibers in the tissue prior to fossilization (Shackelford et al. 1964, Wojtowicz et al. 1998). Birefringence was most obvious in the fibrous cellular cementum bulb immediate surrounding the root orthodentin, followed by the surrounding mineralized periodontal ligament (Fig. 4). There was little or no birefringence in the interdental ridge coinciding with a low density of fiber bundles. Levels of birefringence in the tooth bearing element were low and depended on the polarizer/analyzer angle of rotation (Fig. 4). The mosasaur used for this analysis was Halisaurus sternbergi.
Electron microprobe analysis revealed high calcium/phosphate ratios in all tissues of the mosasaur attachment apparatus
Calculations of calcium phosphate ratios obtained from electron microprobe analysis of mosasaur dental tissues, including interdental ridge, cellular cementum, tooth bearing element, root dentin, and enamel, resulted in values close to 2.0 (Table 1). There was no significant difference between the Ca/P ratios of those five tissues investigated and overall values were significantly higher than those of naturally occurring hydroxyapatite. Standard deviation values were higher in the cellular cementum (1.2, CEM) compared to other tissues investigated (below 0.1), indicative of varying degrees of mineralization in the cellular cementum layer. There is a possibility that Ca/P ratios were influenced by diagenetic changes caused by infiltrating fossil matrix. However, control measurements of the surrounding embedment resulted in Ca/P ratios close to infinity due to the absence of phosphorus outside of the embedded tissues, enhancing confidence in our microprobe analysis.
Table 1.
Calcium phosphate ratios in mosasaur dental and attachment tissues as revealed using SEM electron microprobe
| Area | TBE | PED | CEM | DE | ENM | CON |
|---|---|---|---|---|---|---|
| Ca/P ratio | 1.97 | 2.02 | 2.14 | 2.04 | 1.95 | 0 |
| Stand. Dev. | 0.04 | 0.08 | 1.2 | 0.04 | 0.03 | 0 |
Ca/P ratios are listed as weight %. Ca/P ratios indicated similar degrees of mineralization in fossilized tooth and attachment tissues vs. control. TBE (tooth-bearing element), MPDL (mineralized periodontal ligament), CEM (cellular cementum), DE (root dentin), ENM (enamel), and CON (control) were analyzed. TBE, PED, CEM, DE, and ENM featured high Ca/P ratios of ~ 2.0, while control ratios could not be determined due to absence of phosphorus in the surrounding embedment. Note the high standard deviation in the cellular cementum (CEM).
The bulk of the mosasaur attachment tissue immediately surrounding the orthodentin root surface consists of cellular cementum
In order to identify mosasaur tooth attachment tissues and compare them with the histology of attachment tissues in other reptilians, a comparative study between mosasaur (Halisaurus sternbergi) and caiman (Caiman crocodylus) attachment tissues was performed (Figs. 2A,B). The massive mosasaur cellular cementum layer revealed an underlying fibrous structure using transmitted light analysis of thin ground sections (Figs. 1,3A,B) while it displayed a typical mineralized tissue morphology when a polished sample was photographed in incident light (Fig. 2A). Similarly, von Kossa’s staining of caiman cementum revealed pronounced mineralized fiber bundles as part of the cementum layer (Figs. 5A,B,C) while a routine ground section dye such as Paragon emphasized the cellular structure of the cementum layer in comparison to other tissue layers (Figs. 5J,K). Nevertheless, fiber bundles were also identified in Caiman cementum stained with Paragon (Fig. 5D). Together, this analysis indicates that the bulbous cone of mineralized attachment tissues surrounding the mosasaur root orthodentin consists of a fiber-rich tissue that closely resembles cellular cementum.
Figure 5. Comparative histology of caiman periodontal attachment tissues.
Fig. 5A is a longitudinal section through a mandibular tooth row of the Spectacled Caiman (Caiman crocodylus). Successional teeth in position 1, 3, and 5 are discernable. Mineralized tissues were identified by von Kossa’s stain as calcium ions were replaced by silver deposits. While both orthodentin (de) and cellular root cementum (cem) were stained by silver precipitation, a fine line separated the intensely colored dentin (de) from the lighter color of the cellular cementum. Von Kossa’s procedure heavily reacted with the alveolar bone (ab) and the tooth bearing element (tbe). The periodontal ligament (pdl) of this juvenile specimen appears less or not mineralized. Earlier studies have demonstrated a presence of sub-microscopic mineral deposits in the caiman ligament (McIntosh et al. 2002) not likely to be detected at this level of resolution. The overview micrograph in Fig. 5B identifies root dentin (de), cellular cementum (cem), periodontal ligament (pdl), alveolar bone (ab), and the tooth bearing element (tbe). Ground sections in Figs. 5A,B,C,E,G,I) were stained using von Kossa’s recipe and sections in Figs. 5D,F,H,J, and K were subjected to Paragon’s polychrome stain. Note the trabecular structure of cellular cementum that emerged after applying von Kossa’s stain for mineralized calcium (Fig. 5C) and compare the woven organization of caiman cementum with a very similar appearance of the innermost mosasaur attachment mineralized tissue (Figs. 2A, 3A,B), which as a result was denominated as cellular cementum (cem). In contrast, light micrographs of Paragon-stained caiman cementum do not readily reveal the trabecular organization of cellular cementum, even though individual fiber bundles (fib) are distinguishable (Fig. 5D). A thin layer of acellular cementum (ac) separates cellular cementum (cem) and root orthodentin (de)(Figs. 5B–D). The remaining micrographs illustrate the microstructure of caiman periodontal ligament (pdl, Figs. 5E,F), alveolar bone (ab, Figs. 5G,H), and tooth bearing element (tbe, Figs. 5I,J). While both alveolar bone and the tooth bearing element were blackened following von Kossa’s stain (Figs. 5G,I), the periodontal ligament was not (Fig. 5I). This series of micrographs also illustrates that the trabecular microstructure of caiman cellular cementum (Figs. 5C,D) did not resemble periodontal ligament, alveolar bone, and tooth bearing element (Figs. 5G–J). Alveolar bone sockets are not pronounced in this medial section through the distal jaw portion of a juvenile caiman mandible.
While our comparative histology analysis revealed similarities between mosasaur and caiman cellular cementum, there were differences in the morphology and appearance of the other two major mosasaur attachment tissues with other reptilian attachment tissues. One of the other two distinct mosasaur attachment tissues we refer to as mineralized periodontal ligament as it consists of a mineralized support tissue that forms the interface between cellular cementum on one side and interdental ridge/tooth bearing element on the other side (Figs. 2A; 3A,D; 7). On both sides, the mineralized periodontal ligament was delineated by a layer of bundle bone (Figs. 1, 3A). In mosasaurs, the mineralized periodontal ligament displayed the trabecular histology characteristic for cellular cementum, but contained fiber bundles that were parallel oriented and more than double as thick (Fig. 3D vs. Fig. 3B). As a fiber-rich tissue that forms the interface between cementum and tooth bearing element/interdental ridge, the mineralized periodontal ligament might be equivalent to the fiber-rich periodontal ligament, especially since earlier studies showed remaining levels of calcification in the caiman periodontium (McIntosh et al. 2002).
The third major mosasaur attachment tissue is the interdental ridge (Zaher and Rieppel 1999). The mosasaur interdental ridge mostly consisted of osteons of lamellar bone (Figs. 3F,G). In comparison to the Caiman alveolar bone, the border to adjacent tissues was less delineated, and Sharpey’s fibers from the mineralized periodontal ligament infiltrated the coronal margin of the interdental ridge (Fig. 3F).
Pleurodont anchorage of iguana teeth via bone of attachment
For comparison, we performed a histological analysis of the attachment tissues of an extant squamate, the Green Iguana (Iguana iguana)(Figs. 2C, 6). Our analysis revealed several layers of mostly acellular cementum covering the surface of the root dentin and a cell-rich bone of attachment connecting adjacent teeth, while anchoring teeth to the tooth bearing element (Figs. 6A,F). The bone of attachment contained enlarged cellular lacunae, differentiating bone of attachment from other mineralized tissues of the periodontal apparatus (Fig. 2C, 6C–D). In addition, we did not observe any osteons in the iguana bone of attachment. Root cementum and bone of attachment were ankylosed with the tooth bearing element or the interdental ridge (Figs. 6A,E,F). In order to distinguish tooth attachment tissues from each other, diameters of lacunae in cross-section were compared. Lacunae within bone of attachment measured 9.75+/−1.75μm in diameter, and those in cellular cementum were slightly smaller (7.55+/−1.55μm diameter). In contrast, the tooth bearing element contained oval-shaped lacunae measuring 21.65+/−4.25μm in length and 6.1+/−0.95μm in thickness.
Figure 6. Attachment apparatus of an extant squamate, the Iguana (Iguana iguana).
Note the complex pleurodont attachment apparatus featuring several layers of acellular cementum (ac), cellular cementum (cem), and bone of attachment (ba), which connect the root dentin (od) of many squamate teeth to the tooth bearing element (tbe) and to adjacent teeth (Figs. 6A and 6F). The Iguana orthodentin root surface (od) was covered by acellular cementum (ac)(Figs. 6A,B,C,F). Cellular cementum (cem) was found only infrequently (Figs. 6A,B,C,F) and its lacunae were smaller than those found in bone of attachment (7.55+/−1.55μm diameter, Figs. 6A,B). The bone of attachment (ba) was characterized by enlarged lacunae harboring encapsulated osteocytes (9.75+/−1.75μm diameter, Figs. 6A,D,F). The tooth bearing element (tbe) featured numerous oval-shaped lacunae in parallel alignment (Fig. 6E). These oval-shaped lacunae of the tooth bearing element measured 21.65+/−4.25μm in length and 6.1+/−0.95μm in thickness. Although mineralized tissue of different origins were tightly interconnected, cement lines clearly outlined attachment tissues of different morphology. Bar =100μm (Figs. 6A,F), 20μm (Fig. 6B–E).
Discussion
In the present study we have subjected mosasaur jaw fragments to a number of mineralized tissue histology techniques to understand the mineralized tissue microarchitecture involved in mosasaur tooth anchorage. Mosasaur tooth attachment was compared with the attachment apparatus of Caiman and Iguana in order to identify the basic tissue architecture underlying reptilian tooth attachment. Both the extinct Mosasaurs and the extant Iguanas are grouped as squamates while the Caiman belongs to the Archosaur clade (Fig. 8). Using ultrathin ground sections, electron microprobe analysis, semi-thin sections, and polarized light microscopy, five unique layers of mosasaurian tooth attachment were identified: (i) a thin layer of acellular cementum between root orthodentin and the remainder of the periodontium, (ii) a trabecular cellular cementum providing the major portion of the tooth anchorage, (iii) the mineralized periodontal ligament between the cellular cementum and the interdental ridge/tooth bearing element, (iv) the interdental ridge containing lamellar osteons, and (v) the tooth-bearing element featuring compact bone as the basic bony element of the jaw bone (Table 2, Fig. 7). It appears that our cellular cementum largely corresponds to the ‘osteocementum’ of Caldwell et al. (2003), and to the “aligned cellular cementum” of Caldwell (2007), our ‘interdental ridge’ is a re-classification of Caldwell’s alveolar bone (Caldwell et al. 2003), and while we have identified an extensive mineralized fiber layer between interdental ridge and cellular cementum, the ‘mineralized periodontal ligament’, Caldwell et al. (2003) report that the non-ossified component of the periodontal ligament is unrecognizable. Instead, they find morphologies of a cribriform plate-like structure and remnants of Sharpey’s fibers, which they believe to support the presence of a periodontal membrane (Caldwell et al. 2003). However, Caldwell et al. (2003) fall short of identifying a distinct periodontal ligament tissue layer between cellular cementum and interdental ridge.
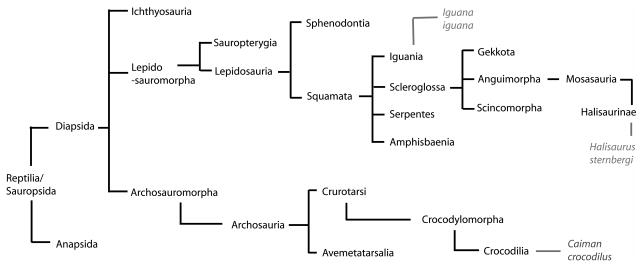
Figure 8. Cladogram of species investigated.
Both the iguana and the mosasaur (Halisaurus sternbergi) are squamates while the caiman belongs to the archosaurs. The cladogram was adapted from Benton (2004).
Table 2.
Classification of Selected Reptilian Tissues involved in Tooth Attachment
| Tissue Type | Position | Mineralization | Composition |
|---|---|---|---|
| Acellular Cementum | Immediately adjacent to root orthodentin. | Mineralized matrix | No cells |
| Cellular Cementum | Adjacent to acellular cementum | Mineralized fibers and matrix | Cells and fibers |
| Periodontal Ligament | Connecting tooth root surface with alveolar bone | Non- mineralized or partially mineralized | Cells and prominent, parallel-oriented Sharpey’s fibers |
| Mineralized Periodontal Ligament | Separate tissue layer between cellular cementum and tooth bearing element/interdental ridge | Mineralized matrix and fibers | Cells and prominent, parallel-oriented Sharpey’s fibers |
| Interdental Ridge | Between mineralized periodontal ligaments (mpdl) of adjacent teeth and between mpdl and tooth bearing element | Mineralized fibers and matrix | Osteons and lamellae, small lacunae |
| Alveolar Bone | Anchors non-mineralized periodontal ligament and connects tooth with jaw bone | Mineralized matrix | Osteons and lamellae, small lacunae |
| Bone of Attachment | Between adjacent teeth and tooth bearing element of pleurodont squamates | Mineralized matrix | Osteocytes residing in large lacunae |
| Tooth Bearing Element | Basic skull bone that supports teeth, connects to either alveolar bone or interdental ridge | Mineralized matrix | Lamellar bone, Osteocytes residing in oval- shaped lacunae |
Borders of the Theca: Interdental Ridge or Alveolar Bone?
The discussion over the condition of the mineralized tissue separating individual teeth from each other is not only of importance for the development and structure of mosasaur tooth attachment, but is also intimately linked to the debate over the presence of ‘true’ sockets and thecodonty of mosasaurian teeth. Lee (1997) classified the mosasaurian socket wall as a non-dental tissue, Zaher and Rieppel (1999) noted that tooth sockets of mosasaurs were merely apparent sockets, and Caldwell et al. (2003) thought of the mosasaurian sockets as real sockets that remain in place through multiple replacement events, similar to those found in crococilians. Our analysis revealed that the mosasaurian interdental ridge was intimately linked to the adjacent mineralized periodontal ligament by mineralized fibers and mechanical integration through ankylosis while the alveolar bone of mammals formed a stand-alone structural entity in conjunction with the jaw bone and separated from the tooth root by a non- or semi-mineralized periodontal ligament. We therefore retained Zaher and Rieppel’s nomenclature of the interdental ridge in contrast to the mammalian alveolar bone. The concept of ‘true’ thecodonty implies the presence of deep sockets which in depth exceed the length of the tooth crown, a lack of ankylosis, and the presence of genuine alveoli (Romer 1956, Motani 1997, Zaher and Rieppel 1999). In contrast, sockets of the mosasaur Halisaurus sternbergi studied here were shallow compared to the length of the crown, and teeth were ankylosed to the socket walls by means of a mineralized periodontal ligament. We noted that in many Crocodiliae, the distal regions of the jaw do not contain much alveolar bone in the midline zone, while the mesial portion of the jaw feature fairly prominent alveolar bone, suggesting that even in squamates, the alveolar bone is a variable structural element susceptible to functional evolutionary pressures. Nevertheless, histological similarities between the interdental ridge and alveolar bone such as the organization into osteons (Caldwell et al. 2003) appear to suggest that the mosasaurian interdental ridge might be an early step in the evolution of thecodont modes of tooth attachment in vertebrates.
Trabecular Fiber Cementum: the Bulk of the Mosasaurian Tooth Attachment Apparatus
Our comparitive analysis of mosasaurian and crocodilian attachment apparatus histology suggests that the large bulbous tissue mass surrounding the mosasaurian tooth root orthodentin is likely to consist of cellular cementum, in support of a classification first introduced by Caldwell et al. (2003). Depending on the type of analysis, transmitted light or incident light illumination of mosasaur ground sections versus von Kossa’s or Epoxy-Paragon stain for Caiman cementum, micrographs either highlighted a bone-like mineralized tissue layer or emphasized an underlying sub-structure of fiber bundles. The presence of mineralized fiber bundles in the cellular cementum of mosasaurs was especially prominent following transmitted light illumination, while in the Caiman, von Kossa’s procedure caused silver precipitates to outline mineralized fiber bundles, emphasizing the trabecular organization of crocodilian cellular cementum. Polarizing microscopy confirmed the presence of highly birefringent fiber bundles in the mosasaurian cellular cementum, indicative of the presence of collagen prior to fossilization, while electron microprobe analysis revealed high calcium/phosphate ratios. This cementum layer may be classified as intrinsic and extrinsic because of the strong presence of fibers within the cementum layer and the insertion of Sharpey’s fibers from the mineralized periodontal ligament. Together, these findings are consistent with the interpretation of the bulk of the mosasaurian attachment tissue surrounding the tooth root orthodentin as a cellular, trabecular fiber cementum.
The Mineralized Periodontal Ligament in Mosasaurs: a Presumptive Precursor of the Non-mineralized Mammalian Periodontal Ligament
In earlier studies (McIntosh et al. 2002) it has been proposed that the partially mineralized Caiman periodontal ligament was an evolutionary interface between the non-mineralized mammalian periodontal ligament and the rigid ankylosis that characterizes amphibians and reptilians. Here we suggest that the mosasaur ‘mineralized periodontal ligament’ represents yet another way to modularize the interface tissue between the root of the tooth and the tooth-bearing element. We have chosen the term ‘mineralized periodontal ligament’ to identify the separate mineralized tissue layer between the tooth-bearing element/interdental ridge and the cellular cementum. From a functional perspective, we expect that the mosasaurian mineralized periodontal ligament mostly functions as an attachment interface while the amphibian mineralized periodontal ligament mostly serves the purpose of elevation, but these functions are not well understood as of yet. Our electron microprobe analysis revealed that the mosasaurian mineralized periodontal ligament is highly mineralized in comparison to the periodontal ligament of crocodilians and mammals. The mineralized phase accounts for approximately 50% of its volume, the remainder consists of interstitial spaces. Moreover, the high fiber content of the mosasaurian mineralized periodontal ligament prior to fossilization as suggested by its birefringence in tandem with its trabecular structure points to highly flexible mechanical properties. In functional terms, the ‘mineralized periodontal ligament’ of mosasaurs provides a resilient mode of attachment, as does the partially mineralized periodontal ligament of Caiman, or the non-mineralized periodontal ligament of mammals.
Bone of Attachment Tissue of Pleurodont Attachment in Squamates
The mineralized tissue involved in reptile tooth ankylosis has historically been referred to as ‘bone of attachment’ (Tomes, 1874), the nature of which remained notoriously elusive. In previous studies, we have compared the spongy cell mass immediately surrounding the mosasaur root orthodentin with ‘bone of attachment’ of other squamates (e.g., Zaher and Rieppel, 1999; Rieppel and Kearney, 2005). However, our histological analysis allows us now to reclassify this tissue layer as trabecular fiber cementum (cellular cementum, osteocementum). Moreover, we found significant differences between the trabecular organization of the mosasaur fiber cementum and the structure of the ‘bone of attachment’ of Iguana, featuring numerous enlarged lacunae that contain osteocytes.
Regulators of the Mineralized State of the Periodontium
Recent studies in mammals have shed light on potential underlying mechanisms of reptilian attachment tissue diversity. Wnt1/Rosa26 molecular marker studies, fluorescent and radioactive labeling experiments have confirmed the common origin of root cementum, alveolar bone, and periodontal ligament from migratory neural crest and a tooth-bound intermediary, the dental follicle (Ten Cate et al., 1971; Chai et al., 2000; Diekwisch, 2001, 2002). The dental follicle in turn consists of at least three distinct progenitor cell populations (Luan et al. 2006a) that may contribute to attachment tissue diversity through changes in composition and gene expression. At least two specific gene products have been identified that directly affect the mineralization status of the periodontal ligament, ank1 and ameloblastin (Ho et al. 2000, Fukumoto et al. 2004). In addition, the non-mineralized status of the periodontium may be controlled by Hertwig’s Epithelial Root Sheath (HERS), an epithelial cell layer that extends to the tooth apex of crocodylians and mammals (Luan et al. 2006b). These molecular and cellular mechanisms may specifically define the mineralized tissue interface involved in tooth attachment, from the ankylosis of many squamates to the fibrous mineralized periodontal ligament of mosasaurs or to the submicroscopically mineralized state of crocodilian ligaments, and eventually to the non-mineralized periodontal ligament that provides the basis of resilient tooth anchorage in mammals. Such concept is also featured in the exoskeletal building block theory of Smith and Hall (1993) which introduces a morphogenetic system consisting of a dental cap epithelium in conjunction with dental papillary and follicular ecomesenchyme as the underlying theme of gnathostome tooth structure diversification.
Evolution of the Vertebrate Attachment Apparatus through Functional Re-organization of an Ancestral Phenotype
The history of vertebrate tooth attachment is characterized by an evolutionary transition from teleosts and amphibian ankylosis over various reptilian modes of attachment to the thecodont gomphosis of mammals, even though some teleost teeth are also attached by ligaments (reviewed in Peyer 1968, Gaengler 2000). A number of potential evolutionary benefits of thecodont attachment modes of attachment can be thought of, including a reduced chance of tooth breakage and a mechanical adaptability to antagonistic occlusal surface loads, especially in multi-cusped teeth. The studies presented here suggest that the modulation of the static or elastic state of the vertebrate attachment apparatus might be accomplished to a large extend through changes in the mineralized state of the periodontal ligament tissue layer. As shown in the previous paragraph, such changes may have been the result of changes in gene expression or caused by varying degrees of Hertwig’s Root Sheath (HERS) invasion into the mineralized attachment apparatus. Such a functional modification of a periodontal attachment tissue would be just one example for a functional reorganization of an ancestral phenotype in tandem with genetic accommodation (Luan and Diekwisch 2007). Evidence for the likelihood of such a scenario is provided by the presence of intermediates such as the semi-mineralized crocodilian attachment, in the ankylosis of the periodontium following removal of HERS, and in the change of the mineralized state of the periodontium following changes in ank1 and AMBN gene expression. In reptilians, the diversity in attachment structures indicates a significant evolutionary adaptability of reptilian taxa to various food environments. The resilient attachment apparatus in mosasaurs, maintained by a fibrous mineralized periodontal ligament and a highly trabecular cellular cementum, undoubtedly will allow the mosasaur to cope with various loads and degrees of hardness of prey encountered in Cretaceous oceans.
Acknowledgments
Funding for these studies by NIH grants DE18075 and DE19155 to XL as well as DE15425 and NSF grant MCB-0236226 to TGHD is gratefully acknowledged.
References
- Bell GL. Mosasauridae: Introduction. In: Callaway JM, Nicholls EL, editors. Ancient Marine Reptiles. Academic Press; San Diego: 1997. pp. 281–292. [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Caldwell MW. A new species of Pontosaurus (Squamata, Pythonomorpha) from the Upper Cretaceous of Lebanon and a phylogenetic analysis of Pythonomorpha. Memorie della Societa Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano. 2006;24:1–42. [Google Scholar]
- Caldwell MW. Ontogeny, anatomy and attachment of the dentition in mosasaurs (Mosasauridae: Squamata) Zool J Linnean Soc. 2007;149:701–716. [Google Scholar]
- Caldwell MW, Budney LA, Lamoureux DO. Histology of tooth attachment tissues in the Late Cretaceous mosasaurid Platecarpus. J Vertebr Paleontol. 2003;23:622–630. [Google Scholar]
- Carroll RL. Vertebrate Paleontology and Evolution. W.H. Freeman and Company; New York: 1988. [Google Scholar]
- Cope ED. On the reptilian order Pythonomorpha and Streptosauria. Proceedings of the Boston Society of Natural History. 1869;12:250–261. [Google Scholar]
- Cope ED. On the geology and paleontology of the Cretaceous strata of Kansas. In: Hayden FV, editor. Preliminary Report on the United States Geological Survey of Montana and Portions of Adjacent Territories; being a Fifth Annual Report of Progress, Part 3, PaLeontology. Washington: 1872. pp. 318–349. [Google Scholar]
- Cope ED. Professor Owen on the Pythonomorpha. Bulletin of the U.S. Geological and Geographical Survey, Territories (Hayden Survey) 1878;4:299–311. [Google Scholar]
- Diekwisch TGH. The developmental biology of cementum. Int J Dev Biol. 2001;45:695–706. [PubMed] [Google Scholar]
- Diekwisch TGH. Pathways and fate of migratory cells during late tooth organogenesis. Connect Tissue Res. 2002;43:245–56. [PubMed] [Google Scholar]
- Dortangs RW, Schulp AS, Mulder EWA, Jagt JWM, Peeters HGH, Th de Graaf D. A large new mosasaurs from the Upper Cretaceous of The Netherlands. Netherlands J Geosci/Geologie en Mijnbouw. 2002;81:1–8. [Google Scholar]
- Estes R, Queiroz K, de Gauthier J. Phylogenetic relationships within Squamata. In: Estes R, Pregill G, editors. Phylogenetic Relationships of the Lizard Families. Stanford Univ Press; Stanford, CA: 1988. pp. 119–281. [Google Scholar]
- Fukumoto S, Kiba T, Hall B, Iehara N, Nakamura Y, Longenecker G, Krebsbach PH, Nanci A, Kulkarni AB, Yamada Y. Ameloblastin is a cell adhesion molecles required for maintaining the differentiation state of ameloblasts. J Cell Biol. 2004;167:973–983. doi: 10.1083/jcb.200409077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaengler P. Evolution of tooth attachment in lower vertebrates to tetrapods. In: Teaford MF, Smith MM, Ferguson MW, editors. Development, Function and Evolution of Teeth. Cambridge University Press; Cambridge: 2000. pp. 173–185. [Google Scholar]
- Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- Kuhn O. Ein neuer Lacertilier aus dem frankischen Lithographieschiefer. Neues Jahrbuch fur Geologie und Palaontologie, Monatshefte. 1958;1958:380–382. [Google Scholar]
- Lawson R. The teeth of Hypogeophis rostratus (Amphibia, Apoda) and tooth structure in the amphibian. Proc Zool Soc Lond. 1965a;145:321–325. [Google Scholar]
- Lawson R. Tooth development and replacement of teeth in Hypogeophis rostratus. J Zool. 1965b;147:352–362. [Google Scholar]
- Lee MSY. The phylogeny of varanoid lizards and the affinities of snakes. Philos Trans R Soc Lond B Biol Sci. 1997a;352:53–91. [Google Scholar]
- Lee MSY. On snake-like dentition in mosasaurian lizards. J Nat Hist. 1997b;31:303–314. [Google Scholar]
- Lee MSY. Convergent evolution and character correlation in burrowing reptiles: towards a resolution of squamate relationships. Biol J Linn Soc. 1998;65:369–453. [Google Scholar]
- Lessmann MH. Zur labialen Pleurodontie an Lacertilier Gebissen. Anatomischer Anzeiger. 1952;99:35–67. [PubMed] [Google Scholar]
- Lingham-Soliar T. Anatomy and functional morphology of the larges marine reptile known, Mosasaurus hoffmanni (Mosasauridae, Reptilia) from the Upper Cretaceous, Upper Maastrichtian of the Netherlands. Philos Trans R Soc Lond B Biol Sci. 1995;347:155–180. [Google Scholar]
- Luan X, Diekwisch TGH. Vienna-Chicago: the cultural transformation of the model system of the un-opposed molar. BioEssays. 2007;29:819–830. doi: 10.1002/bies.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X, Ito Y, Dangaria S, Diekwisch TGH. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006a;15:595–608. doi: 10.1089/scd.2006.15.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X, Ito Y, Diekwisch TGH. Evolution and development of Hertwig’s epithelial root sheath. Dev Dyn. 2006b;235:1167–80. doi: 10.1002/dvdy.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JE, Anderton X, Flores-De-Jacobi L, Carlson DS, Shuler CF, Diekwisch TGH. Caiman periodontium as an intermediate between basal vertebrate ankylosis-type attachgment and mammalian “true” periodontium. Microsc Res Tech. 2002;59:449–459. doi: 10.1002/jemt.10222. [DOI] [PubMed] [Google Scholar]
- Motani R. Temporal and spatal distribution of tooth implantation. In: Callaway JM, Nicholls EL, editors. Ancient Marine Reptiles. Academic Press; San Diego: 1997. pp. 81–103. [Google Scholar]
- Osborn JW. From reptile to mammal: evolutionary considerations of the dentition with emphasis on tooth attachment. Symp Zool Soc Lond. 1984;52:549–574. [Google Scholar]
- Owen R. On the rank and affinities in the reptilian class of Mosasauridae Gervais. Quarterly Journal of the Geological Society of London. 1877;33:682–715. [Google Scholar]
- Owen R. On the affinities of the Mosasauridae Gervais, as exemplified in the bony structure of the fore fin. Quarterly Journal of the Geological Society of London. 1878;34:784–753. [Google Scholar]
- Peyer B. Comparative odontology. University of Chicago Press; Chicago: 1968. pp. 89–99. [Google Scholar]
- Rieppel O, Conrad JL, Maisano JA. New morphological data for Eosaniwa koehni Haubold, 1977, and a revised phylogenetic analysis. J Paleonto. 2007;81:760–769. [Google Scholar]
- Rieppel O, Kearney M. Tooth replacement in the Late Cretaceous mosasaur Clidastes. J Herpetol. 2005;39:688–692. [Google Scholar]
- Rieppel O, Zaher H. The intramandibular joint in squamates and the phylogenetic relationships of the fossil snake Pachyrhachis problematicus. Fieldiana (Geology), ns. 2000;43:1–69. [Google Scholar]
- Rieppel O, Zaher H, Tchernov E, Polcyn MJ. The anatomy and relationships of Haasiophis terrasanctus, a fossil snake with well-developed hind limbs from the mid-Cretaceous of the Middle East. J Paleonto. 2003;77:336–358. [Google Scholar]
- Romer AS. Osteology of the Reptiles. University of Chigaco Press; Chicago: 1965. p. 772. [Google Scholar]
- Shackelford JM, Wyckoff RWG. Collagen in fossil teeth and bones. J Ultrastructural Res. 1964;11:173–180. doi: 10.1016/s0022-5320(64)80101-5. [DOI] [PubMed] [Google Scholar]
- Smith MM, Hall BK. A developmental model for evolution of the vertebrate exoskeleton and teeth: the role of cranial and trunk neural crest. Evolutionary Biology. 1993;27:387–447. [Google Scholar]
- Ten Cate AR, Mills C, Solomon J. The development of the periodontium:A transplantation and autoradiographic study. Anat Rec. 1971;170:365–380. doi: 10.1002/ar.1091700312. [DOI] [PubMed] [Google Scholar]
- Tomes CS. On the structure and development of the teeth of Ophidia. Phil Trans Roy Soc Lond. 1874;165:297–302. [Google Scholar]
- Wojtowicz A, Yamauch M, Montella M, Bandiera R, Sotowski R, Ostrowski K. Persistence of birefringence and specific collagen cross-links in dentine of teeth of the ‘Nuraghi’ population living in Sardinia BC 1500–1200. Int J Osteoarchaeol. 1998;8:288–294. [Google Scholar]
- Zaher H, Rieppel O. Tooth implantation and replacement in squamates, with special reference to mosasaur lizards and snakes. Am Mus Novit. 1999;3271:1–19. [Google Scholar]