Abstract
Inflammation and oxidative stress are pathogenic mediators of many diseases, but therapeutic targets remain elusive. In the vasculature, abdominal aortic aneurysm (AAA) formation critically involves inflammaton and matrix degradation. Cyclophilin A (CyPA, encoded by Ppia) is highly expressed in vascular smooth muscle cells (VSMC), is secreted in response to reactive oxygen species (ROS), and promotes inflammation. Using the angiotensin II (AngII)-induced AAA model in Apoe−/− mice, we show that Apoe−/−Ppia−/− mice were completely protected from AngII–induced AAA formation, in contrast to Apoe−/−Ppia+/+ mice. Apoe−/−Ppia−/− mice showed decreased inflammatory cytokine expression, elastic lamina degradation, and aortic expansion. These features were not altered by reconstitution of bone marrow cells from Ppia+/+ mice. Mechanistic studies demonstrated that VSMC-derived intracellular and extracellular CyPA were required for ROS generation and matrix metalloproteinase-2 activation. These data define a novel role for CyPA in AAA formation and suggest CyPA is a new target for cardiovascular therapies.
Introduction
Inflammation and oxidative stress are pathogenic mediators of many diseases, but therapeutic targets remain elusive. In the vasculature, abdominal aortic aneurysm (AAA) formation critically involves inflammaton and matrix degradation. Key mechanisms include vascular smooth muscle cells (VSMC) senescence1, oxidative stress2,3, increased local production of proinflammatory cytokines4 and increased activities of matrix metalloproteinases (MMPs)5,6. In animal models of AAA, genetic and pharmacological inhibition of ROS production7,8 and MMPs9,10 suppressed aneurysm formation. There is a strong mechanistic link between increased ROS and MMP activity 11–13, suggesting that therapies to limit ROS generation may be useful.
Angiotensin II (AngII) induces ROS through NADPH oxidases14 and activates MMPs15. AngII infusion into apolipoprotein E–deficient (Apoe−/−) mice for 4 weeks promotes AAA formation16,17.
Cyclophilin A (CyPA, encoded by Ppia) is a chaperone protein that binds cyclosporine18 and is abundantly expressed in VSMC19. Our lab showed that ROS stimulate secretion of CyPA from VSMC. Extracellular CyPA stimulates VSMC migration and proliferation19,20; endothelial cell adhesion molecule expression, and inflammatory cell chemotaxis19,21,22. Based on these CyPA functions we determined its role in AngII-induced AAA23. We found that AAA formation in the AngII-induced Apoe−/− mice model was completely prevented in the Ppia−/− background. Mechanistically CyPA deficiency significantly decreased inflammatory cell recruitment, ROS production and MMP activation.
Results
CyPA deficiency blocks AngII-induced AAA formation in vivo
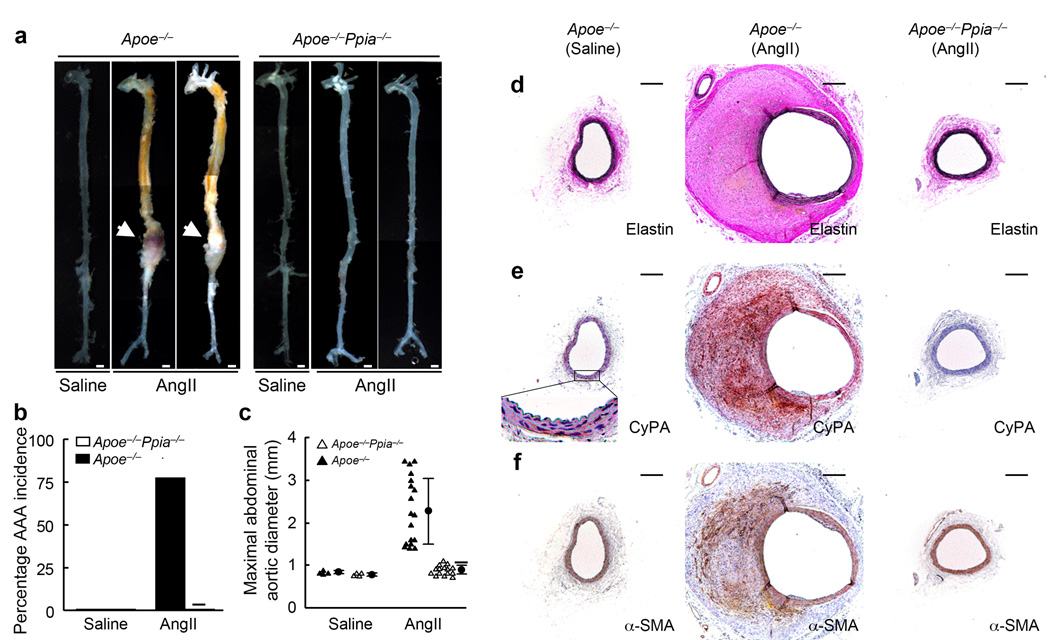
As previously reported4,16,24,25 we found that treatment with AngII for 4 weeks promoted AAA formation in Apoe−/− mice (Fig. 1a–c). To define the role of CyPA in AAA formation, we established Apoe−/−Ppia−/− mice (double-knockout) mice and treated these animals with AngII for 4 weeks. AngII increased systolic blood pressure and total cholesterol, but there were no differences between Apoe−/− mice and Apoe−/−Ppia−/− mice (Supplement Table 1a). There were no gross differences in the aortas of control Apoe−/− and Apoe−/−Ppia−/− mice (saline-infused mice, Fig. 1a). Strikingly, after AngII infusion, Apoe−/−Ppia−/− mice had no AAA incidence, in contrast to 78% AAA incidence in Apoe−/− mice (Fig. 1a,b). There was also a significant decrease in maximal aortic diameter (Fig. 1c) and aortic weight (Supplement Table 1a) in Apoe−/−Ppia−/− mice after treatment with AngII. These results suggest that CyPA is required for AAA formation induced by AngII.
Figure 1.
CyPA deficiency prevents AngII-induced AAA formation. Apoe−/− and Apoe−/−Ppia−/− mice were infused with AngII or saline for 4 weeks. (a) Representative photographs showing macroscopic features of aneurysms induced by AngII. The arrows indicate typical AAA in Apoe−/− mice. Scale bars, 1 mm. (b) The incidence of AngII-induced AAA was significantly reduced in Apoe−/−Ppia−/− mice (n = 15) compared with Apoe−/− mice (n = 18). There was no AAA formation in the control group (saline infusion) in both Apoe−/− and Apoe−/−Ppia−/− mice (n=4, respectively). (c) Maximal abdominal aortic diameter was significantly reduced in Apoe−/−Ppia−/− mice after AngII infusion for 4 weeks. Triangles represent individual mice; circles represent the mean; error bars denote SD. ★P < 0.01 compared with AngII-infused Apoe−/− mice. (d) Elastin van Gieson staining of aortic cross-sections of Apoe−/− and Apoe−/−Ppia−/− mice after AngII infusion for 4 weeks. (e, f) The predominant cellular component in the AAA expressing CyPA was VSMC as revealed by immunostaining for CyPA (e) and α-smooth muscle actin (α-SMA) (f), in serial sections. All aortic sections were from the suprarenal aorta. Scale bars, 300 µm.
Morphologically, the aortas of Apoe−/−Ppia−/− mice infused with saline (Fig. 1d–f) did not differ from aortas of control Apoe−/− mice (not shown). In Apoe−/− mice infused with AngII (Fig. 1d–f) there was a dramatic increase in aortic size of both the lumen and wall. The aortic wall developed a tissue mass composed of organized thrombus, small blood vessels, extracellular matrix and spindle-shaped cells as described by Daugherty’s group24. Most of the cells that were positive for CyPA (Fig. 1e) concomitantly exhibited immunoreactivity for α-smooth muscle actin (α-SMA, Fig. 1f), suggesting that these were VSMC19. In contrast, the aortas of Apoe−/−Ppia−/− mice infused with AngII showed no significant tissue mass or enlargement. These results suggest that CyPA deficiency confers protection from the early stages of AAA formation.
Over the 4 weeks of the experiment, 35% of the Apoe−/− mice infused with AngII died while none of the Apoe−/−Ppia−/− mice died (Supplement Fig. 1a). Gross and histological examination of the dead animals revealed aortic rupture (Supplement Fig. 1b–d). As expected, the elastic lamina was frequently disrupted and degraded in Apoe−/− mice (Supplement Fig. 1e). In contrast, CyPA deficiency completely prevented elastic lamina degradation (Supplement Fig. 1f). Based on a semi-quantitative analysis of elastin degradation (Supplement Fig. 1g), CyPA deficiency completely blocked elastin degradation after AngII treatment for 4 weeks (Supplement Fig. 1h). These data suggest that protection from elastin degradation is an important mechanism for inhibition of AAA in Apoe−/−Ppia−/− mice.
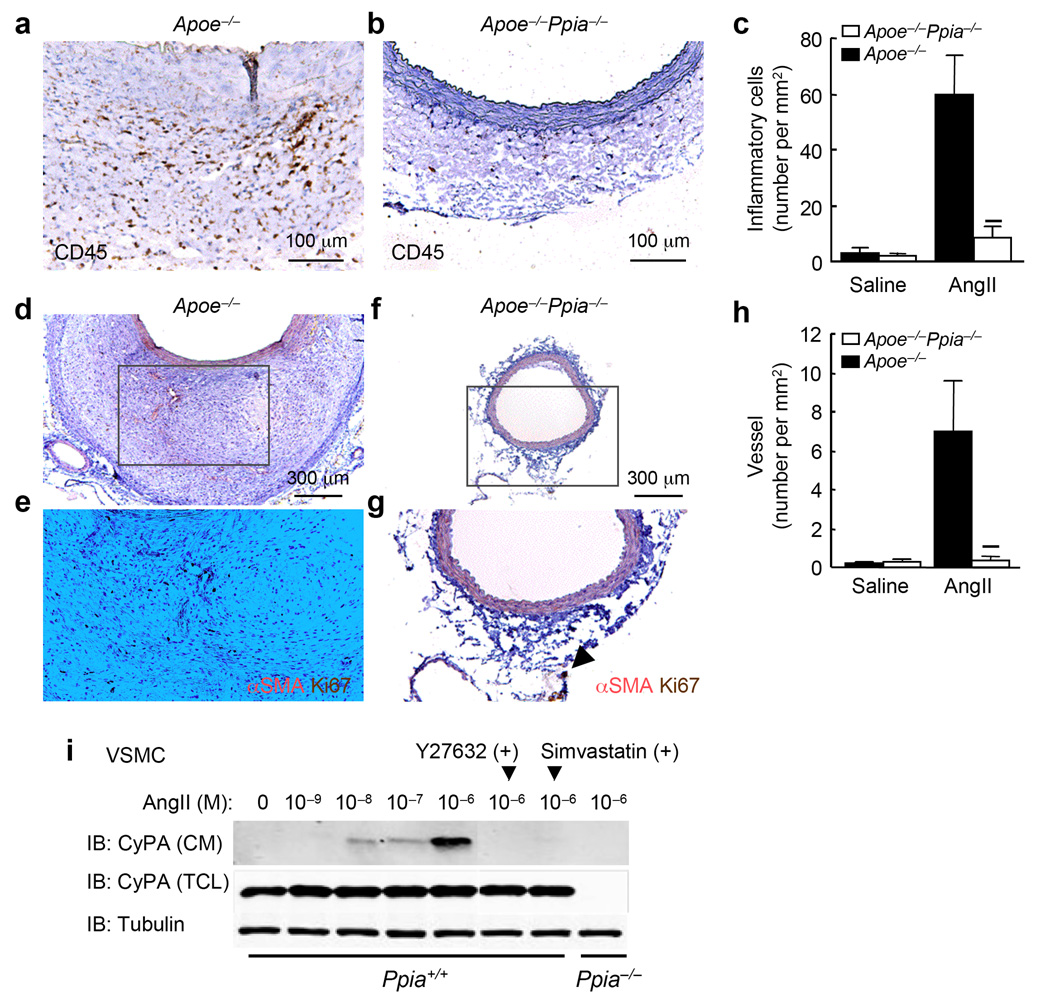
To ascertain whether AngII induced vascular inflammation was CyPA-dependent, we examined inflammatory cell migration and microvessel formation. Inflammatory cell migration, assessed by CD45+ cell number, was significantly reduced in Apoe−/−Ppia−/− mice compared with Apoe−/− mice (Fig. 2a–c). The number of microvessels in the aortic wall was also dramatically reduced in Apoe−/−Ppia−/− mice (Fig. 2d–h), consistent with the reduced inflammatory responses.
Figure 2.
CyPA deficiency reduces AngII-induced inflammatory cell accumulation and microvessel formation. (a,b) Representative CD45 staining of suprarenal aorta from Apoe−/− and Apoe−/−Ppia−/− mice infused with AngII for 4 weeks. (c) Number of migrating CD45+ cells in the aortic wall in Apoe−/−(n = 9) and Apoe−/−Ppia−/− (n = 7) mice. (d–g) Representative immunostaining of α-smooth muscle actin (α-SMA) and Ki67 in suprarenal aorta. (h) Number of proliferating microvessels in the aortic wall. Results are mean ± SD. ★P < 0.01 compared with Apoe−/− mice. (i) CyPA is secreted from mouse VSMC in response to AngII. Pretreatment with Rho kinase inhibitor Y27632 (30 µM) and simvastatin (30 µM) for 30 min reduced CyPA secretion. CM, conditioned media; TCL, total cell lysate.
To characterize the mechanisms by which CyPA participates in the inflammatory response, we first analyzed the secretion of proinflammatory molecules by cytokine/chemokine array in vitro. AngII treatment strikingly induced the secretion of proinflammatory cytokines such as MCP-1 and IL-6, as well as chemokines such as RANTES and SDF-1; whereas CyPA deficiency effectively blocked the induction of these molecules (Supplement Fig. 2a). We next showed that CyPA secretion was stimulated by AngII in mouse aortic VSMC (Fig. 2i). CyPA secretion was maximal at 1 µM AngII (Fig. 2i). Pretreatment with Y27632 (Rho kinase inhibitor) or simvastatin significantly reduced CyPA secretion (Fig. 2i), consistent with our previous report21. We studied MCP-1 expression in the aortic wall because of its known role in macrophage migration and AAA formation24,26. In saline-infused aortas, MCP-1 appeared to be more highly expressed in Apoe−/− than in Apoe−/−Ppia−/− media (Supplement Fig. 2b,c). In response to AngII, MCP-1 was highly expressed in Apoe−/− aortas (Supplement Fig. 2d), especially in the adventitia. In contrast, MCP-1 was markedly decreased in the adventitia of Apoe−/−Ppia−/− aortas (Supplement Fig. 2e). The adventitial location of MCP-1 in response to AngII is consistent with its function as a chemokine for monocytes. Additionally, in cultured aortic VSMC, AngII stimulated MCP-1 secretion was markedly decreased in Ppia−/− cells (Supplement Fig. 2f), while other AngII signal events such as ERK1/2 activation did not differ.
Vascular CyPA, not bone marrow-derived CyPA, is essential for AAA formation
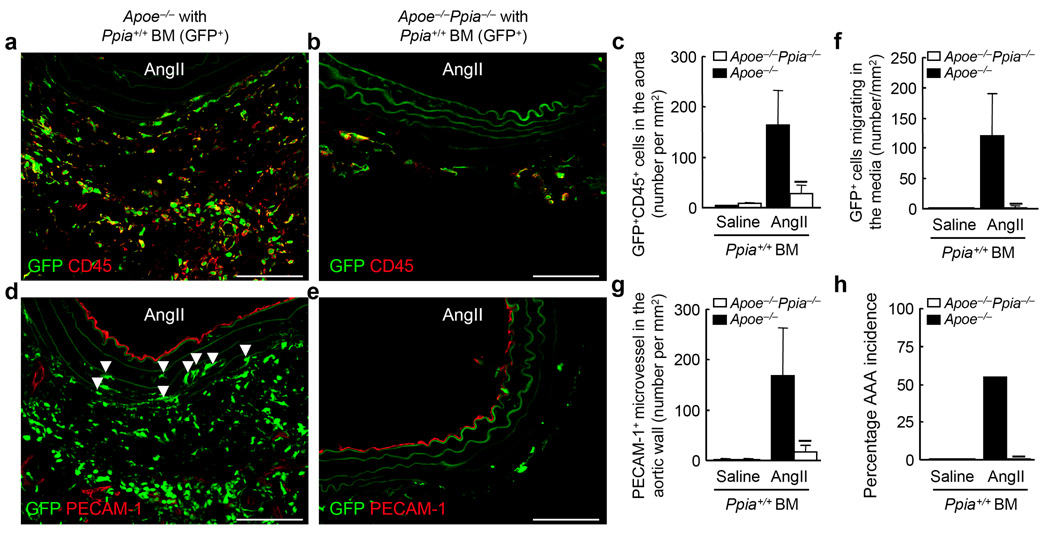
CyPA has been reported to play a crucial role in regulating the survival, proliferation, and differentiation of antigen-presenting cells by augmenting antigen uptake and presentation27. CyPA has also been reported to stimulate migration of bone marrow-derived cells in vitro22. Hematopoietic cells, especially macrophages, are involved in AAA formation4,24. We hypothesized that CyPA deficiency may impair macrophage differentiation and activation and thus prevent AAA formation by AngII. To test this possibility, Ppia+/+ GFP+ bone marrow cells were transplanted into irradiated Apoe−/− mice or Apoe−/−Ppia−/− mice. After 42 d of engraftment the mice were treated with AngII. There was no significant difference in the reconstitution ratio (%GFP+ cells in the peripheral blood) in GFP+ marrow-transplanted Apoe−/−Ppia−/− mice compared with GFP+ marrow-transplanted Apoe−/− mice (99.5 ± 0.3% vs. 99.6 ± 0.2%, respectively). There was no significant difference in the blood pressure of chimeric mice (Supplement Table 1b). However, the number of bone marrow-derived inflammatory cells (GFP+CD45+ double-positive cells) present in the aortic wall was significantly less in Apoe−/−Ppia−/− mice compared with Apoe−/− mice (Fig. 3a–c). Parenthetically, we observed both GFP+CD45+ cells and GFP+CD45− cells in the AAA lesions after AngII infusion. Recent papers have shown that both non-hematopoietic cells (CD45−) and hematopoietic cells (CD45+), are mobilized from the bone marrow, and contribute to remodeling of the vascular wall. The presence of GFP+CD45− cells in AngII-induced AAA lesions suggested that CyPA plays a crucial role in recruiting non-hematopoietic cells from the bone marrow. The number of bone marrow-derived macrophages (GFP+Mac-1+) was also significantly less in the Apoe−/−Ppia−/− recipient mice (Supplement Fig. 3a–c).
Figure 3.
Bone marrow (BM) reconstitution shows key role for vascular-derived CyPA in AAA formation. Ppia+/+ BM cells (GFP+) were transplanted into irradiated Apoe−/− or Apoe−/−Ppia−/− mice as described. (a,b) Representative CD45 staining (Alexa Fluor 546, red) of suprarenal aorta from Apoe−/− and Apoe−/−Ppia−/− mice transplanted with Ppia+/+ BM, and infused with AngII for 4 weeks. (c) Number of migrating GFP+CD45+ double-positive cells in the aortic wall in Apoe−/− and Apoe−/−Ppia−/− mice. (d,e) Representative PECAM-1 staining (Alexa Fluor 546, red) of suprarenal aorta from Apoe−/− and Apoe−/−Ppia−/− mice transplanted with Ppia+/+ BM and infused with AngII for 4 weeks. Elastic lamina in the aortic wall demonstrate green auto-fluorescence. Arrows indicate migrating GFP+ cells in the media (d). Number of migrating GFP+ cells in the media (f) or PECAM-1+ microvessels (g) were dramatically higher in the aortic wall of Apoe−/− compared to Apoe−/−Ppia−/− mice. (h) The incidence of AAA in Apoe−/− (n = 9) was much higher than Apoe−/−Ppia−/− (n = 8) mice reconstituted with Ppia+/+ bone marrow after AngII infusion for 4 weeks. Results are mean ± SD. ★P < 0.01 compared with Apoe−/− mice. Scale bars, 100 µm.
Migration of bone marrow-derived cells into the media was frequently observed in Apoe−/− recipient mice (Fig. 3d, arrows). In contrast, there were few GFP+ cells in the media of Apoe−/−Ppia−/− recipient mice (Fig. 3e,f), suggesting the importance of VSMC-derived CyPA for inflammatory cell migration. Furthermore, microvessel formation assessed by PECAM-1 staining was significantly less in Apoe−/−Ppia−/− recipient mice (Fig. 3d,e,g), supporting the concept that the reduced inflammatory responses in Apoe−/−Ppia−/− mice are due to CyPA deficiency. Consistent with this idea, the incidence of AAA was 56% in Ppia+/+ marrow-transplanted Apoe−/− mice (Fig. 3h), versus 0% in Apoe−/−Ppia−/− mice after transplantation of Ppia+/+ bone marrow cells. Finally, we prepared chimeric mice with Ppia−/− bone marrow (Supplement Fig. 3d). The incidence of AAA was 60% in Ppia−/− marrow-transplanted Apoe−/− mice, while the incidence of AAA in Apoe−/−Ppia−/− mice was still 0%. These data suggest that CyPA expression by vascular cells, rather than bone marrow-derived cells, is critical for development of AAA.
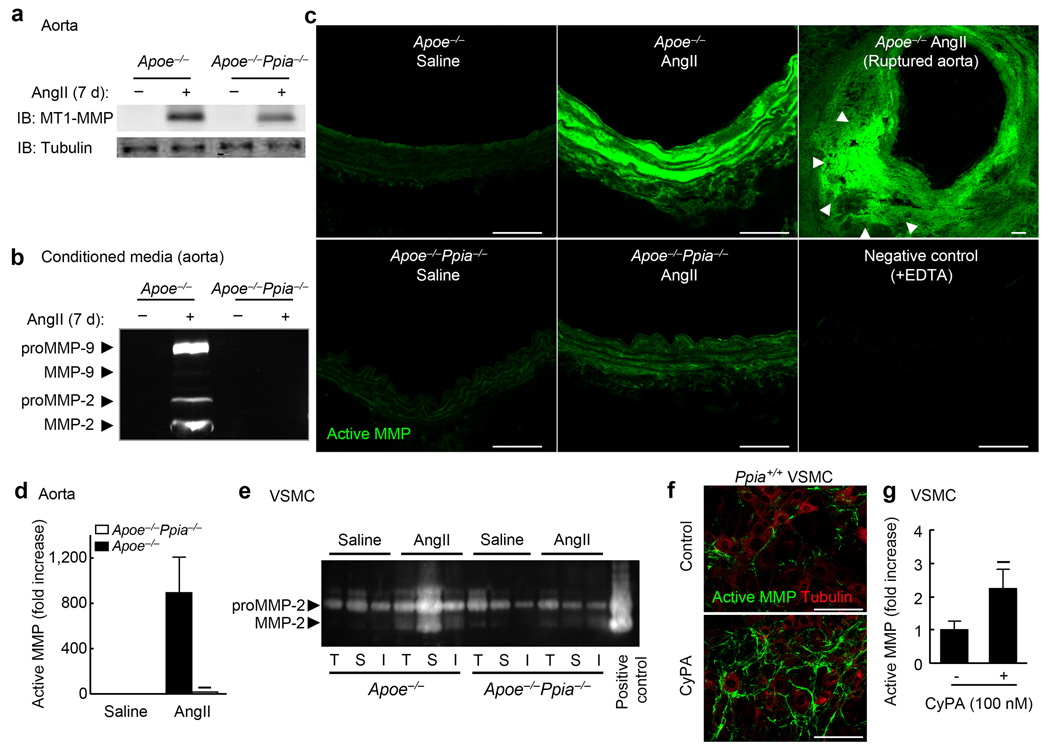
CyPA deficiency prevents AngII-induced MMP activation in vivo and in vitro
AAA development and aortic rupture depend on macrophage-derived MMP-9 and VSMC-derived MMP-224,28,29, which are enzymatically cleaved and activated by MT1-MMP30. Secreted CyPA may activate MMPs through the extracellular MMP protein inducer (EMMPRIN)31. Therefore, we anticipated decreased MMP activity in the absence of CyPA. We performed western blotting for MMP-2 using a MMP-2 mouse monoclonal antibody that recognizes the ~72 kDa latent and the 66 kDa active forms of MMP-2. Western blotting revealed significantly reduced MMP-2 activity in Ppia−/− VSMC after AngII treatment (Supplement Fig. 4a). MT1-MMP expression in the VSMC membrane fraction revealed a significant increase in WT VSMC compared with Ppia−/− VSMC in response to AngII treatment (Supplement Fig. 4b), suggesting a key role for CyPA in MT1-MMP translocation to the cell membrane. Consistent with these findings, AngII-induced activation of MT1-MMP was significantly elevated in WT VSMC compared with Ppia−/− VSMC (Supplement Fig. 4c). We next studied MMP function in the aortas of Apoe−/− and Apoe−/−Ppia−/− mice. Basal expression of MT1-MMP was low in the aortas of Apoe−/− and Apoe−/−Ppia−/− mice (Fig. 4a). While MT1-MMP expression was significantly increased in the aortas of both Apoe−/− and Apoe−/−Ppia−/− mice after AngII infusion (Fig. 4a), the increase was significantly less in aortas from Apoe−/−Ppia−/− mice. Organ culture of Apoe−/− mice aortas after AngII infusion showed high activities of proMMP-9, proMMP-2 and activated MMP-2 by zymography in conditioned media (Fig. 4b). In contrast, there was no MMP activity in conditioned media from Apoe−/−Ppia−/− mice after AngII-treatment (Fig. 4b). In situ zymography supported these observations (Fig. 4c). MMP activity was negligible in saline-treated aortas (green fluorescence). Following AngII-treatment, the media and adventitia of Apoe−/− mice showed much higher MMP activity compared to Apoe−/−Ppia−/− mice (Fig. 4c). Interestingly, the ruptured aorta of Apoe−/− mice revealed tremendous MMP activity, especially in the false lumen.
Figure 4.
CyPA is crucial for secretion and activation of MMPs. (a) Representative western blot of MT1-MMP expression in mouse aorta after 7 d infusion of AngII. (b) Gelatin zymography for conditioned media from whole aorta organ culture. Aortas from Apoe−/− and Apoe−/−Ppia−/− mice infused with saline or AngII were incubated in media for 20 h. (c) In situ zymography for gelatinase activity. Aortas from Apoe−/− and Apoe−/−Ppia−/− mice infused with saline or AngII for 7 days were analysed. Scale bars, 100 µm. (d) Densitometric analysis of MMP activity (DQ gelatin) changes relative to the density of MMP activity in control Apoe−/− mice (saline-infused). Results are mean ± SD. ★P < 0.01 compared with Apoe−/− mice. (e) Gelatin zymography for VSMC harvested separately from the thoracic aorta (T), suprarenal aorta (S), and infrarenal aorta (I) of Apoe−/− and Apoe−/−Ppia−/− mice. VSMC from Apoe−/− and Apoe−/−Ppia−/− mice harvested from different portions of aorta were stimulated with AngII (1 µM) for 24 h. (f) Representative in situ zymography (DQ gelatin) of Ppia+/+ VSMC and immunostaining with α-tubulin after stimulation with CyPA (100 nM) for 4 h. (g) Densitometric analysis of MMP activity changes relative to the density of MMP activity in control VSMC. ★P < 0.01 vs. control VSMC. Results are mean ± SD of 6 independent experiments.
To elucidate the biological properties of VSMC in AAA-prone versus AAA-resistant areas, we harvested and cultured VSMC from thoracic, suprarenal, and infrarenal aorta, and compared MMP activities in response to AngII (Fig. 4e). There was no difference in the activities of MMP-2 in cells from aortas treated with saline, assessed by gelatin zymography (Fig. 4e). AngII treatment significantly increased activities of MMP-2 in Apoe−/− VSMC, especially in VSMC from the suprarenal aorta (Fig. 4e, AngII-S). In contrast, MMP-2 activity induced by AngII was significantly less in Apoe−/−Ppia−/− VSMC regardless of the aortic location (Fig. 4e). Treatment of VSMC with CyPA augmented MMP activity by ~2-fold, assessed by in situ zymography (Fig. 4f,g), demonstrating the importance of extracellular CyPA for MMP activation in VSMC. Consistent with these data, in situ zymography showed that active MMP was much greater in the media of suprarenal aorta than infrarenal and thoracic aorta (Supplement Fig. 5a–c). These in vivo and in vitro data demonstrate that CyPA in VSMC is crucial for activation of MMPs.
CyPA deficiency prevents AngII-induced ROS production in vivo and in vitro
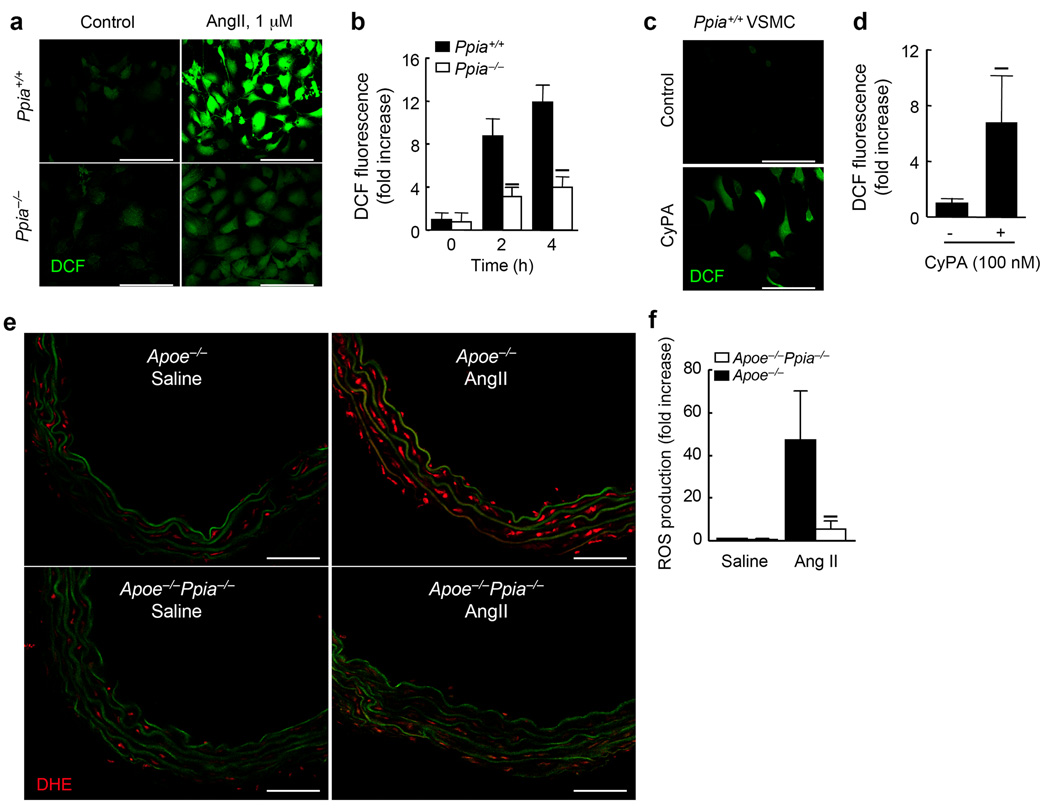
We next investigated the mechanism by which CyPA deficiency decreases MMP expression, secretion and activation. ROS play a crucial role in activating VSMC MMPs in 32 in a p47phox-dependent manner33. Therefore, we studied the effect of CyPA deficiency on VSMC ROS production induced by AngII. First, we compared activation of ERK1/2 by AngII and found no significant difference between Ppia+/+ and Ppia−/− VSMC (data not shown). In response to AngII for 4 h, Ppia+/+ mouse VSMC increased ROS production by 12-fold as assessed by dichlorofluorescein (DCF) (Fig. 5a,b). Ppia−/− VSMC showed significantly less ROS induction (Fig. 5a,b). Additionally, treatment of Ppia+/+ VSMC with CyPA significantly augmented ROS production after 4 h (Fig. 5c,d) suggesting that AngII-mediated CyPA secretion contributes to ROS production.
Figure 5.
AngII-induced ROS formation in VSMC requires CyPA. (a) Representative DCF staining of mouse aortic VSMC. AngII-induced ROS generation was decreased in CyPA-deficient VSMC. (b) Densitometric analysis of DCF fluorescence in response to AngII shows ~60% reduction in Ppia−/− VSMC at 4 h. Results are mean ± SD of 5 independent experiments. ★P < 0.01 compared with Ppia+/+ VSMC. (c) Representative DCF staining of Ppia+/+ VSMC in response to 100 nM CyPA. (d) Densitometric analysis of DCF fluorescence in Ppia+/+ VSMC in response to 100 nM CyPA. Results are mean ± SD of 5 independent experiments. ★P < 0.01 compared with control VSMC. (e) In situ dihydroethidium (DHE) staining of mouse aorta showed decreased DHE staining in Apoe−/−Ppia−/− aortas. Aortas from Apoe−/− and Apoe−/−Ppia−/− mice infused with saline or AngII for 7 d were analysed. Media green fluorescence is from elastin fiber autofluorescence, which appeared both in control and AngII-treated aorta. All sections are shown with the lumen above. Scale bars, 100 µm. (f) Densitometric analysis of DHE fluorescence relative to control Apoe−/− mice (saline-infused). Results are mean ± SD. ★P < 0.01 compared with Apoe−/− mice.
To evaluate the effect of CyPA deficiency on ROS generation in vivo, aortic sections were incubated with dihydroethidium (DHE), which in the presence of superoxide forms oxy-ethidium. In saline-infused aorta, ROS production (red fluorescence) was very low in both Apoe−/− and Apoe−/−Ppia−/− mice (Fig. 5e). After 7 d of AngII treatment, oxyethidium fluorescence was markedly increased in Apoe−/− mice aorta (Fig. 5e,f). In contrast, in Apoe−/−Ppia−/− mice ROS production was not induced by AngII (Fig. 5e,f). These in vivo and in vitro data suggest that AngII-induced ROS production in VSMC is enhanced by both intracellular and extracellular CyPA.
VSMC-derived CyPA promotes AAA formation in vivo
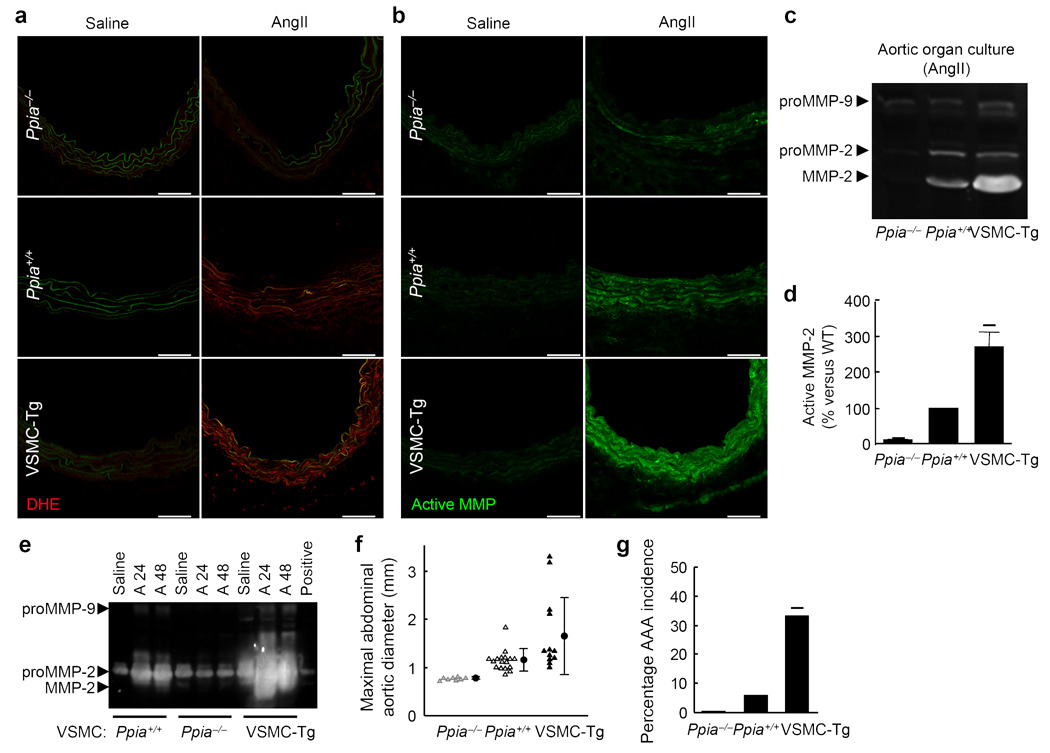
To provide further evidence that VSMC-derived CyPA regulates ROS production and MMP activity, we created VSMC-specific CyPA overexpressing mice (VSMC-Tg). We previously showed that CyPA expression is ~3-fold greater in arteries of VSMC-Tg mice versus WT (Ppia+/+) mice34. In saline-infused mice, there was no basal difference in oxy-ethidium fluorescence (red fluorescence) between WT, Ppia−/− and VSMC-Tg aorta (Fig. 6a). However, after AngII-infusion for 7 d, oxy-ethidium fluorescence was significantly higher in VSMC-Tg aorta (Fig. 6a) than in WT (intermediate) and Ppia−/− (lowest) aortas.
Figure 6.
VSMC-derived CyPA plays a crucial role for aortic ROS production, MMP-2 activation, and AAA formation. (a,b) DHE staining and in situ zymography of supra-renal aorta after treatment with saline or AngII for 7 d. There was increased DHE fluorescence in response to AngII with relative levels: VSMC-Tg > Ppia+/+ > Ppia−/−. All sections are shown with the lumen above. Scale bars, 100 µm. (c) Representative gelatin zymography of conditioned media from mouse aorta after AngII-infusion for 7 d. (d) Active MMP-2 in conditioned media from AngII-treated aortic organ culture shows relative activity: VSMC-Tg > Ppia+/+ > Ppia−/−. ★P < 0.01 vs. Ppia+/+ aorta. Results are mean ± SD of 3 independent experiments. (e) Representative gelatin zymography of aortic VSMC from Ppia+/+, Ppia−/−, VSMC-Tg mice after treatment with saline, AngII for 24 h (A24), or AngII for 48 h (A48). Positive; MMP-2 positive control. (f) Maximal abdominal aortic diameter significantly increased in VSMC-Tg mice 4 weeks after AngII infusion. Triangles represent individual mice; circles represent the mean; error bars denote SD. (g) The incidence of AngII-induced AAA was significantly increased in VSMC-Tg mice (n = 12) compared with Ppia+/+ mice (n = 17). There was no AAA induction in Ppia−/− mice (n = 8). ★P < 0.01 compared with AngII-infused Ppia+/+ mice.
There was no basal difference in MMP activity (green fluorescence) between WT, Ppia−/− and VSMC-Tg aorta in saline-infused mice (Fig. 6b). However, after AngII-infusion MMP activity was significantly less in Ppia−/− compared with WT aorta, and significantly greater in VSMC-Tg aorta (Fig. 6b). We next assayed AngII-mediated activation of MMP-2 and MMP-9 by gel zymography (Fig. 6c). Active MMP-2 in the conditioned media after organ culture of aorta was significantly augmented in VSMC-Tg compared with WT aorta, and significantly decreased in Ppia−/− aorta (Fig. 6c,d). These results were supported by a similar experiment using cultured VSMC harvested from mouse aorta (Fig. 6e). MMP-2 activity was significantly augmented in VSMC from VSMC-Tg mice compared with those from WT or Ppia−/− mice, (Fig. 6e). These data support the concept that VSMC-derived CyPA is an important mediator of AngII-induced MMP-2 activation.
To provide additional support for the pathogenic role of CyPA in AAA formation we investigated the effects of AngII infusion in VSMC-Tg mice. We tried to cross VSMC-Tg onto the Apoe−/− background, but did not obtain any viable pups. There was no significant difference in the aortic weight and diameter between Ppia−/−, WT, and VSMC-Tg mice in control, saline-infused, mice (not shown). In response to AngII infusion on Apoe+/+ background, the maximum aortic diameter increased significantly in VSMC-Tg by ~2-fold compared to Ppia−/− and WT (Fig. 6f), with a highly significant increase in AAA incidence (Fig. 6g). These results support the concept that VSMC-derived CyPA is critical for MMP-2 activation and AAA formation induced by AngII infusion.
Finally, we evaluated the role of CyPA in human AAA lesions (Supplement Fig. 6). CyPA was highly expressed throughout the aortic wall of AAA lesions, especially in areas that express active MMP (Supplement Fig. 6a,b). We performed organ culture to determine the effect of AngII treatment on CyPA secretion. AngII significantly increased secretion of CyPA from human AAA lesions (Supplement Fig. 6c). We next harvested VSMC from human AAA tissues and characterized them as highly expressing CyPA (Fig. 6d–e). In response to AngII, MMP activity was also strongly increased (Supplement Fig. 6f–g). This activity was shown to be MMP-2 by gel zymography (Fig. 6h–i). A key role for CyPA PPIase activity was shown by the marked decrease in MMP-2 activation by treatment with CsA (Supplement Fig. 6h–i). These results suggest a crucial role for CyPA in MMP activation in human AAA lesions.
Discussion
The major finding of the present study is that CyPA is a novel mediator of abdominal aortic aneurysm (AAA) formation. We characterized four pathologic mechanisms by which vascular CyPA promotes AAA formation (Supplement Fig. 7). First, AngII-induced ROS promoted secretion of CyPA and proMMP-2. Second, secreted extracellular CyPA contributed to ROS production synergistically with AngII in VSMC. Third, CyPA promoted activation of MMP-2, by inducing MT1-MMP and augmenting ROS generation. Fourth, CyPA stimulated recruitment of CD45+ inflammatory cells. The source of CyPA responsible for AAA formation appeared to be cells resident in the vessel wall, especially VSMC since no AAA were observed in Apoe−/−Ppia−/− mice after transplantation with Ppia+/+ bone marrow cells. Also, overexpression of CyPA in VSMC enhanced vascular ROS production, MMP activation, and AAA formation. Therefore, we propose a key role for vascular CyPA in AAA formation and other cardiovascular diseases associated with inflammation.
Daugherty’s group characterized the temporal events in AngII-induced AAA formation24. First, they described medial accumulation of macrophages in regions of elastin degradation. Second, medial dissection occurred with dilation of the lumen and thrombus formation. Third, an inflammatory response comprised of macrophages and T and B lymphocytes was observed. Fourth, a repair response including elastin fiber regeneration and reendothelialization occurred; and finally there was neovascularization of the thrombus and vascular wall.
VSMC appear to be essential for AngII-induced AAA formation. Expression of the AT1a receptor, responsible for CyPA secretion, ROS generation and MMP activity, is highest in VSMC. In situ measurements of ROS generation and MMP activity were greatest in medial cells that stained for αSMA. Cultured VSMC from transgenic mice and human AAA lesions recapitulated the findings of increased ROS and MMP activation. Finally, bone marrow transplantation showed a minor role for hematopoietic cells. Specifically, our data suggest that VSMC-derived CyPA initiated AAA formation by promoting accumulation of macrophages. Apoe−/−Ppia−/− mice had significantly attenuated vascular ROS production, MMP activation, and MCP-1 secretion resulting in decreased macrophage accumulation. Overexpression of CyPA in VSMC enhanced ROS production and MMP activation, and caused AAA formation even in Apoe+/+ mice (Fig. 6f). Finally, transplantation of bone marrow cells from Ppia+/+ mice into Apoe−/−Ppia−/− mice did not induce AAA formation, indicating that cells resident in the vessel wall were essential for AAA formation.
Our novel data show that extracellular CyPA induces ROS production in VSMC, which is consistent with our previous report that extracellular CyPA stimulates at least 3 signaling pathways (ERK1/2, Akt and JAK) in VSMC19. These signaling pathways have been shown to be important for ROS production2,3. Furthermore, ROS stimulate secretion of CyPA from VSMC19,21. These reports and the present data suggest that CyPA plays a crucial role in VSMC through ROS generation. AngII is thought to induce the generation of ROS and thereby activates MMPs26, thus leading to the onset of vascular inflammatory cell migration and AAA formation7,16,24.
In the present study, CyPA deficiency reduced secretion of proMMP-2 and MMP-2 as well as MT1-MMP expression, all of which can be explained by reduced ROS production. Additionally, AngII has been shown to generate ROS and activate MMP-2 in a p47phox-dependent manner in the same model7,15,35. VSMC-derived MMP-2 promotes degradation of collagen and elastin, contributing to the AAA formation29,30. Expression of MT1-MMP is important for activation of MMP-2 in AngII-induced AAA formation36. Besides enzymatic cleavage and activation of MMP-2 by cell surface expressed MT1-MMP30, ROS have been shown to directly activate MMP-232. AAA formation results from the synergistic activation of ROS production, MT1-MMP, and MMP-2. Therefore, CyPA appears to be a central mediator of AngII-mediated AAA formation.
The identification of CyPA as a mediator of tissue damage associated with inflammation and oxidative stress provides insight into the mechanisms of several therapies. For example, the Rho-kinase inhibitor Y27632, and simvastatin significantly reduced CyPA secretion from VSMC. Rho-kinase is an important therapeutic target in cardiovascular disease37 and Rho-kinase inhibition has been reported to reduce AngII-induced AAA formation38. AT1a receptor blockers and ACE inhibitors have been shown to prevent AAA formation in mice39–41. Based on the present study, reduced CyPA secretion may partially contribute to the therapeutic effect of these drugs on AAA formation. Because inflammation and oxidative stress contribute to tissue damage in several situations such as ischemia-reperfusion injury in the brain, heart and kidney, future studies of CyPA-mediated function in appropriate models may reveal a significant role in other diseases.
EMMPRIN, a putative CyPA receptor, was identified as a tumor cell membrane protein that is expressed in VSMC, activated by ROS and stimulates MMP production42. A recent paper demonstrated ROS-dependent increases in EMMPRIN43, which may be activated by binding of extracellular CyPA31. Moreover, it has been demonstrated that EMMPRIN is strongly expressed in human AAA lesions44. Therefore, it is logical to propose that agents which prevent CyPA binding to its receptors may have therapeutic potential. In summary, these reports and the present study suggest that extracellular CyPA and its receptor(s) represent novel therapeutic targets, particularly for AAA progression.
Methods
Analysis and quantification of AAAs
All animal experiments were conducted in accordance with experimental protocols that were approved by the Institutional Animal Care and Use Committee at the University of Rochester. AngII-infused AAA models were employed to assess the effect of CyPA deficiency on AAA development in Apoe−/− mice16. Six- to 8 week old male Apoe−/−Ppia+/+ littermate control mice and Apoe−/−Ppia−/− mice on a normal chow diet were infused with 1,000 ng min−1 kg AngII (MP Biomedicals) or saline for 4 weeks. AngII was dissolved in sterile saline and infused using Alzet osmotic pumps (Model 2004, DURECT Corp.). Mice were anesthetized with an intraperitoneal injection of ketamine (80 mg kg−1) and xylazine (5 mg kg−1). Pumps were placed into the subcutaneous space of ketamine and xylazine anesthetized mice through a small incision in the back of the neck that was closed with suture. All incision sites healed rapidly without any infection. To determine the effect of CyPA deficiency on AngII–induced aneurysm formation, we quantified AAA incidence and size16,17. The maximum width of the abdominal aorta was measured with Image Pro Plus software (Media Cybernetics Inc.). Aneurysm incidence was quantified based on a definition of an external width of the suprarenal aorta that was increased by 50% or greater compared with aortas from saline-infused mice.
ROS analysis
After treatment with AngII (1 µM), VSMC were washed with PBS and loaded with 2,7-dichlorofluorescein diacetate (H2DCF-DA) (5 µM; Molecular Probes) for 30 min. Aortas were perfused with PBS (pH 7.4) at 100 mmHg for 5 minutes at 4°C. Aortic tissue was harvested, and the abdominal aorta (supra renal) were embedded in OCT (Tissue-Tek; Miles Inc., Elkhart, Illinois, USA) and snap-frozen. Dihydroethidine hydrochloride (5 µM, Molecular Probes) was topically applied to the freshly cut frozen aortic sections (10 µm) for 30 min at 37°C to reveal the presence of ROS as red fluorescence (585 nm) by confocal microscopy (Olympus, FLUOVIEW)33.
MMP activity
The evaluation of MMP activities in response to AngII was performed as described5,10,11. To verify the role of CyPA in AngII-induced MMPs activation, VSMC were treated with AngII (1 µM) in culture medium. Likewise, aortas of mice infused with AngII for 7 d were incubated for 20 h in culture medium. Thereafter medium was collected and concentrated to yield conditioned medium (CM). CM was electrophoresed in SDS-PAGE gels containing 0.8 mg ml−1 gelatin (Sigma-Aldrich). Gels were incubated for 12 h (37°C) in zymography buffer (50 mmol l−1 Tris (pH 8.0), 10 mmol l−1 CaCl2, 0.05% Brij 35), and stained with Coomassie brilliant blue. For in situ zymography, freshly cut frozen aortic sections (supra renal aorta, 10 µm) or VSMC cultured on glass bottom dish were incubated with a fluorogenic gelatin substrate (DQ gelatin, Molecular Probes) according to the manufacturer’s protocol. Proteolytic activity was detected as green fluorescence by confocal microscopy (Olympus, FLUOVIEW). After fixation, VSMC were immunostained with α-tubulin.
Statistical analyses
Quantitative results are expressed as mean ± SD. Comparisons of parameters among 2 groups were made by the unpaired Student’s t-test. Comparisons of parameters among the 3 groups were made by one-way analysis of variance (ANOVA), and comparisons of different parameters between the 2 genotypes were made by two-way analysis of variance (ANOVA), followed by a post hoc analysis using the Bonferroni test. Statistical significance was evaluated with StatView (StatView 5.0, SAS Institute Inc.). A value of P<0.05 was considered to be statistically significant.
Additional methods
Detailed methodology is described in the Supplementary Methods online.
Supplementary Material
Acknowledgements
This work was supported by NIH grant HL49192 (to B.C. Berk) and Japan Heart Foundation / Bayer Yakuhin Research Grant Abroad (to K. Satoh). We are grateful to the Aab Cardiovascular Research Institute members for useful suggestions and R. Winterkorn, M.A. Georger, and A.T. Paxhia for technical assistance.
References
- 1.Kunieda T, et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 2.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 3.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 4.Bruemmer D, et al. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun J, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura K, et al. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat Med. 2005;11:1330–1338. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]
- 7.Thomas M, et al. Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation. 2006;114:404–413. doi: 10.1161/CIRCULATIONAHA.105.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavazzi G, et al. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension. 2007;50:189–196. doi: 10.1161/HYPERTENSIONAHA.107.089706. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RW, Baxter BT. MMP inhibition in abdominal aortic aneurysms. Ann. NY Acad. Sci. 1999;878:159–178. doi: 10.1111/j.1749-6632.1999.tb07682.x. [DOI] [PubMed] [Google Scholar]
- 10.Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23:483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- 11.Valentin F, Bueb JL, Kieffer P, Tschirhart E, Atkinson J. Oxidative stress activates MMP-2 in cultured human coronary smooth muscle cells. Fundam Clin Pharmacol. 2005;19:661–667. doi: 10.1111/j.1472-8206.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- 12.Clempus RE, Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res. 2006;71:216–225. doi: 10.1016/j.cardiores.2006.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCormick ML, Gavrila D, Weintraub NL. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:461–469. doi: 10.1161/01.ATV.0000257552.94483.14. [DOI] [PubMed] [Google Scholar]
- 14.Rajagopalan S, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browatzki M, et al. Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-kappaB and activator protein 1 in a redox-sensitive manner. J Vasc Res. 2005;42:415–423. doi: 10.1159/000087451. [DOI] [PubMed] [Google Scholar]
- 16.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 18.Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 19.Jin ZG, et al. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 20.Liao DF, et al. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem. 2000;275:189–196. doi: 10.1074/jbc.275.1.189. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki J, Jin ZG, Meoli DF, Matoba T, Berk BC. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res. 2006;98:811–817. doi: 10.1161/01.RES.0000216405.85080.a6. [DOI] [PubMed] [Google Scholar]
- 22.Khromykh LM, et al. Cyclophilin A produced by thymocytes regulates the migration of murine bone marrow cells. Cell Immunol. 2007;249:46–53. doi: 10.1016/j.cellimm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605–2613. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- 24.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 25.Gavrila D, et al. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:1671–1677. doi: 10.1161/01.ATV.0000172631.50972.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM. Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res. 1998;83:952–959. doi: 10.1161/01.res.83.9.952. [DOI] [PubMed] [Google Scholar]
- 27.Bharadwaj U, et al. Effects of Cyclophilin A on Myeloblastic Cell Line KG-1 Derived Dendritic Like Cells (DLC) Through p38 MAP Kinase Activation(1,2) J Surg Res. 2005;127:29–38. doi: 10.1016/j.jss.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Pyo R, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longo GM, et al. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 31.Yurchenko V, et al. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002;277:22959–22965. doi: 10.1074/jbc.M201593200. [DOI] [PubMed] [Google Scholar]
- 32.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res. 2005;97:533–540. doi: 10.1161/01.RES.0000181759.63239.21. [DOI] [PubMed] [Google Scholar]
- 34.Satoh K, et al. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117:3088–3098. doi: 10.1161/CIRCULATIONAHA.107.756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luchtefeld M, et al. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun. 2005;328:183–188. doi: 10.1016/j.bbrc.2004.12.152. [DOI] [PubMed] [Google Scholar]
- 36.Eagleton MJ, et al. Early increased MT1-MMP expression and late MMP-2 and MMP-9 activity during Angiotensin II induced aneurysm formation. J Surg Res. 2006;135:345–351. doi: 10.1016/j.jss.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 37.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25:1767–1775. doi: 10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- 38.Wang YX, et al. Fasudil, a Rho-kinase inhibitor, attenuates angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice by inhibiting apoptosis and proteolysis. Circulation. 2005;111:2219–2226. doi: 10.1161/01.CIR.0000163544.17221.BE. [DOI] [PubMed] [Google Scholar]
- 39.Habashi JP, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone Marrow Transplantation Reveals That Recipient AT1a Receptors Are Required to Initiate Angiotensin II-Induced Atherosclerosis and Aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–384. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 41.Ejiri J, et al. Oxidative stress in the pathogenesis of thoracic aortic aneurysm: protective role of statin and angiotensin II type 1 receptor blocker. Cardiovasc Res. 2003;59:988–996. doi: 10.1016/s0008-6363(03)00523-6. [DOI] [PubMed] [Google Scholar]
- 42.Guo H, et al. Characterization of the gene for human EMMPRIN, a tumor cell surface inducer of matrix metalloproteinases. Gene. 1998;220:99–108. doi: 10.1016/s0378-1119(98)00400-4. [DOI] [PubMed] [Google Scholar]
- 43.Haug C, Lenz C, Diaz F, Bachem MG. Oxidized low-density lipoproteins stimulate extracellular matrix metalloproteinase Inducer (EMMPRIN) release by coronary smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:1823–1829. doi: 10.1161/01.ATV.0000142806.59283.11. [DOI] [PubMed] [Google Scholar]
- 44.Chen XF, et al. Extracellular matrix metalloproteinase inducer (EMMPRIN) is present in smooth muscle cells of human aneurysmal aorta and is induced by Angiotensin II in vitro. Clin Sci (Lond) 2008 doi: 10.1042/CS20080235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.