Abstract
Identification of mechanisms underlying endometriosis pathogenesis will facilitate understanding and treatment of infertility and pain associated with this disorder. Herein, we investigated the expression of steroidogenic pathway enzymes and key decidualization biomarkers in endometrial tissue and in eutopic endometrial stromal fibroblasts (hESFs) from women with vs. those without endometriosis, and subsequently treated in vitro with 8-bromo-cAMP (8-Br-cAMP) or progesterone (P4). Real-time quantitative PCR, immunohistochemistry, ELISA, and radiometric aromatase activity assay were used. The results demonstrate significantly increased (14.5-fold; P = 0.037) expression of aromatase in eutopic endometrium of women with disease. In 8-Br-cAMP-treated hESF from eutopic endometrium of women with endometriosis, the balance in estradiol (E2) and P4 biosynthetic and metabolizing enzymes is disturbed (decreased HSD3B1 and HSD17B2, and increased HSD17B1 and aromatase), with the equilibrium being shifted towards an E2-enriched milieu. However, hESF from the same group of women treated with P4 did not demonstrate such responsiveness. Lower expression of IGFBP1 and prolactin mRNA and protein was observed in hESF from women with vs. those without endometriosis in response to 8-Br-cAMP, but not P4, suggesting a blunted response of these decidual biomarkers to activation of the PKA pathway in eutopic endometrium in women with disease. The dichotomy of 8-Br-cAMP regulation of select steroidogenic enzymes leading to an enriched E2 milieu within the endometrium and a blunted response of decidual biomarkers to this decidualizing agent of hESF from women with endometriosis suggests resistance to full decidualization of the stromal fibroblasts and mechanisms underlying implantation failure and the pathophysiology of this disorder.
Keywords: endometrial fibroblasts, endometriosis, eutopic endometrium, steroidogenesis
Altered expression of some members of steroidogenic pathway and markers of decidualization in endometrial stromal fibroblasts from women with vs. without endometriosis suggests establishment of altered hormone environment within eutopic endometrium.
INTRODUCTION
Endometriosis is a common, benign, estrogen-dependent gynecologic disorder affecting primarily women of reproductive age. It is a major public health problem due to the morbidities associated with accompanying infertility and/or pelvic pain in affected women and an estimated cost for associated healthcare for diagnosis and treatment in 2002 totaling about $22 billion in the United States alone [1]. Infertility in women with endometriosis is believed to be related to the proinflammatory milieu in the pelvis affecting oocyte quality, fertilization, embryo development, and impaired implantation due, in part, to compromised decidualization of endometrial stromal fibroblasts (hESFs) [2, 3].
Decidualization is a process of morphological and molecular differentiation/maturation of endometrial stromal cells, especially hESFs, which is essential for embryonic implantation. Human endometrium undergoes decidualization in the absence of an implanting embryo and occurs under the influence of progesterone (P4) and involves the protein kinase A (PKA) pathway [4–6]. Decidualization of cultured hESF in vitro can be achieved by activation of the PKA pathway (relaxin, hCG, prostaglandins, cAMP analogues) and treatment with P4, IL-1β, and activin A [5, 7–10]. Classical biochemical markers of decidualized hESF are prolactin (PRL) and insulin-like growth factor binding protein 1 (IGFBP1) [8, 11–13], and recent microarray studies have revealed numerous genes and gene families that are regulated in hESF treated in vitro with decidualizing stimuli [5, 14, 15].
Endometrial transcriptomic analysis has revealed molecular dysregulation of the proliferative-to-secretory transition in women with endometriosis, suggesting resistance to P4 action [16]. Also, impaired decidualization in response to cAMP and activation of PKA pathway in eutopic and ectopic endometrium, by unknown mechanisms, have been described in the setting of endometriosis [17]. Furthermore, several enzymes in the biosynthesis and metabolism of estradiol (E2) and P4 are dysregulated in ectopic and eutopic endometrium of women with disease in response to steroid hormones [18–24]. These include increased expression of aromatase (CYP19A1, which catalyzes conversion of androstenedione (A4) to estrone [E1]), 17β-hydroxysteroid dehydrogenase type 1 (HSD17B1), which catalyzes conversion of E1 to E2 [18, 19], and steroidogenic acute regulatory protein (STAR) [20]. In addition, 17-HSDβ type 2 (HSD17B2), which converts E2 to E1, is normally highly up-regulated in early secretory endometrium, the result of P4 action [22, 25], but its mRNA is markedly down-regulated in endometriotic lesions and in eutopic endometrium in the early secretory phase compared with normal endometrium [21]. Endometriosis is an estrogen-dependent disorder, and excessive expression of CYP19A1 and local E2 production [23, 24, 26–28] and decreased metabolism to E1 suggest that the endogenous endometrium is inherently abnormal, with a persistent presence of E2 locally and that ectopic lesions autonomously have the capacity to produce E2 for continued autocrine growth and survival. Herein, we investigate expression of enzymes in the steroidogenic pathway [29] in hESF from women with vs. those without endometriosis after decidualizing stimuli. The data support dysregulation of some members of the steroidogenic pathway that may contribute to the pathogenesis of the disorder and associated morbidities of pain and infertility.
MATERIALS AND METHODS
Collection of Tissue and Isolation and Culture of Endometrial Stromal Cells
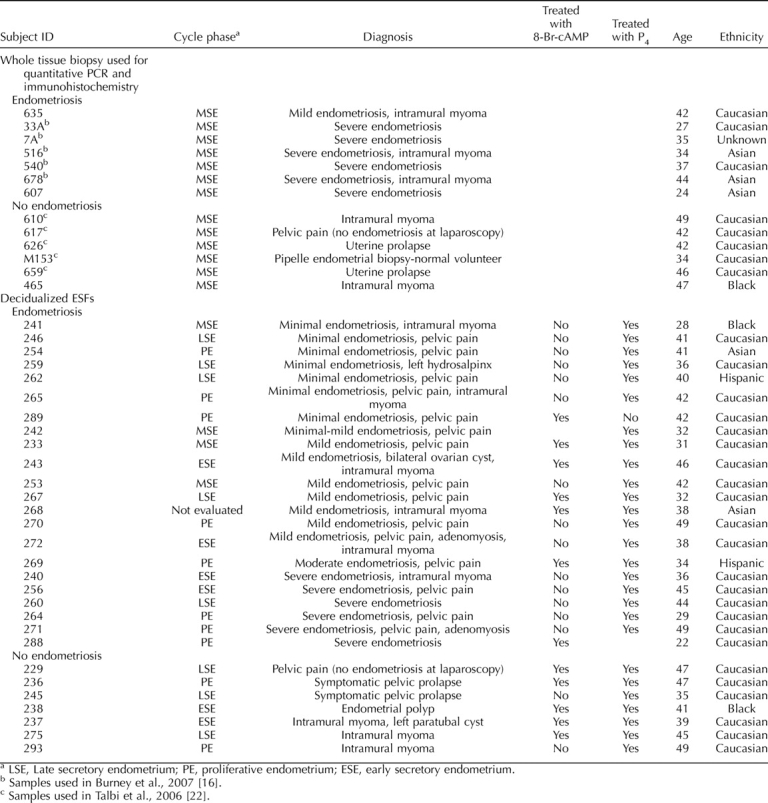
Endometrial biopsies were obtained from a total of 29 women with endometriosis (severity of disease: minimal, n = 7; minimal-mild, n = 1; mild, n = 8; moderate, n = 1; and severe, n = 12). The diagnosis of endometriosis was based on visualization of lesions found during laparoscopy, and was also confirmed by histology. Staging of endometriosis was defined according to the revised American Fertility Society classification system [30]. The participating subjects were 22–49 yr old, not pregnant, and did not use any hormonal medication within 3 mo before surgery. Controls were endometrial tissue samples obtained from 13 cycling, premenopausal subjects (31–49 yr old) undergoing endometrial biopsy or hysterectomy for benign reasons, such as fibroids, pelvic organ prolapse, or pelvic pain (Table 1). Control subjects had regular menstrual cycles (25–35 days), were documented not to be pregnant, had no history of endometriosis, and had not been on hormonal treatment for at least 3 mo before tissue sampling. Samples were collected at room temperature in PBS, transported to the laboratory, and processed as described below. The study was approved by the University of California, San Francisco (UCSF) Committee on Human Research. Written informed consent was obtained from subjects. Samples were also obtained through the UCSF/National Institutes of Health Human Endometrial Tissue and DNA Bank, with appropriate institutional review, approvals, and written informed consent from all participating subjects.
TABLE 1.
Characteristics of subjects and endometrial biopsy samples in the study.
Of 13 biopsies from subjects without endometriosis, 6 were used for experiments requiring whole tissue (gene expression and immunohistochemistry in midsecretory endometrial [MSE] tissue). Of 29 endometrial samples from women with endometriosis, 7 were used for whole tissue analyses (gene expression and immunohistochemistry) (Table 1). All the other samples were used to isolate and culture hESF. Endometrial tissue was digested with collagenase as previously described [31]. Human ESF were separated from epithelium based on size and plated with Dulbecco modified Eagle medium (DMEM)/molecular cell developmental biology medium (MCDB)-105 containing 10% charcoal-stripped fetal bovine serum (FBS), insulin (5 μg/ml), gentamicin, penicillin, and streptomycin. At passage 2, cells were plated in 60-mm plates according to study design (see below) and cultured to near confluence in the same medium. Thereafter, medium was changed to low-serum medium (DMEM/MCDB-105 medium containing ascorbic acid, transferrin, and gentamicin with 2% charcoal-stripped FBS) and cultured for 24 h prior to the onset of treatment. No exogenous growth factors were added to the medium. The optimal concentration of FBS in the culture medium was determined as 2% (data not shown). All experiments were conducted using second-passage cells.
The purity of the hESF at passage 2 was evaluated by immunohistochemistry using antibodies against cytokeratin (epithelial cell marker), vimentin (stromal cell marker), and CD45 (leukocyte marker) (data not shown). All cultures were 99% pure stromal fibroblasts, which is consistent with earlier reports [32, 33].
Decidualization Protocols
Dose response and time course of treatment with 8-bromo-cAMP and P4.
Cells were cultured in low-serum medium, and dose-response (0.1 μM, 1 μM, 10 μM, 0.1 mM, 0.5 mM, 1 mM) and time-course (24, 48, 96 h) studies were conducted in response to 8-bromo-cAMP (8-Br-cAMP), with endpoints being the decidualization markers, PRL and IGFBP1. As a result, 96 h of stimulation with 0.5 mM 8-Br-cAMP treatment was found to be optimal for those endpoints (data not shown). To determine the concentration of P4 optimal for decidualization of hESF in vitro, hESF were cultured for 14 days with E2 (10 nM) and increasing P4 concentrations (1 nM, 10 nM, 100 nM, 1 μM, 10 μM) using cells from the same patients as in the 8-Br-cAMP dose-response and time-course experiments. The optimal concentration of P4 was determined to be 1 μM (data not shown). Preliminary studies were conducted to determine whether, by passage 2, cells had a “memory” of the cycle stage in which they were procured. Cycle-specific genes (DKK1, NUDT1, IGFBP1, PRL, and LEFTY2) were barely detectable and did not differ in their expression among cells prepared from different cycle stages (data not shown), demonstrating that the hESF are naïve in their response in the experimental treatment protocols. However, cells retained memory of their having been from endometrium from women with endometriosis, despite two (or more) passages (see Results).
Treatment of hESFs with decidualzing stimuli.
Confluent hESF were treated with 0.5 mM 8-Br-cAMP (hereafter referred to as cAMP) for 96 h. Time “zero” (t = 0) control samples were collected before initiation of treatment. Cells cultured for the corresponding time periods without treatment served as additional controls. Cultures at each time point and each treatment were performed in duplicate. Cells were lysed in RLT lysis buffer (Qiagen, Valencia, CA) containing β-mercaptoethanol and conditioned media (CM) were collected after 96 h of incubation. Human ESFs from the same subject were also treated with E2 (10 nM; Sigma, St. Louis, MO) alone, E2 (10 nM) plus P4 (1 μM, Sigma), or vehicle control. Cell lysates and CM were collected at 14 days of stimulation. Cells and the CM were also harvested at t = 0 control. The culture media were changed every other day.
Total RNA Isolation
Cells were lysed in RLT lysis buffer + 0.1% β-mercaptoethanol. Total RNA was purified using Qiagen RNeasy Plus Mini kit (Qiagen) according to the manufacturer's instructions. Samples were stored in RNase-free H2O and quantified by spectroscopy, and the purity was analyzed by the 260/280 absorbance ratio. For quantitative RT-PCR analysis, 1 μg of RNA was converted to cDNA using the iScript cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA). Duplicate mRNAs were pooled from each set of treatments. There was no significant difference between the duplicates (data not shown). The real-time RT-PCR reaction was carried out for 40 cycles with primers listed in Table 2.
TABLE 2.
Primer sequences used in real-time PCR experiments.
Enzyme-Linked Immunosorbent Assay
CM from cultured hESF were subjected to ELISA to determine IGFBP1 and PRL concentrations, according to the manufacturer's instructions (Diagnostic Systems Labs, Webster, TX). All samples were assayed in duplicate. A standard curve was run in each experiment. Levels of IGFBP1 and PRL for each sample were normalized to total RNA.
Statistical Evaluation
Statistical analysis for the ELISA data was performed using a two-tailed Student t-test, and, for the quantitative RT-PCR, we used the non-parametric Mann-Whitney test. Significance was determined at P ≤ 0.05.
Immunohistochemistry and Immunocytochemistry
Immunostaining was performed for HSD3B using frozen MSE tissue samples (n = 4 from women with endometriosis and n = 4 from women without endometriosis) and for HSD17B1 and HSD17B2 using formalin-fixed, paraffin-embedded endometrial MSE tissue samples (n = 4 from women with endometriosis and n = 4 from women without endometriosis for each antibody) and cultured hESFs (n = 4 from women with endometriosis and n = 4 from women without endometriosis). Frozen sections were allowed to air dry briefly, fixed in ice-cold methanol, and processed. Paraffin-embedded biopsies from the endometrium were sectioned to 4 μm and mounted on glass slides. The samples were thereafter deparaffinized in Xylene (Sigma-Aldrich) and washed in decreasing concentrations of ethanol. Cells were plated on cell culture chamber slides (Nalge Nunc International Corp., Naperville, IL). Confluent cells were treated with or without 0.5 mM cAMP for 96 h and thereafter fixed in 4% paraformaldehyde for 10 min and kept in PBS until proceeding. Slides intended for HSD17B1 and HSD17B2 staining were incubated for 15 min in H2O2 (3% in methanol) to block endogenous peroxidase activity after antigen retrieval by boiling slides in citrate buffer (pH 6.0). Thereafter, the slides were blocked with 10% normal goat serum (for HSD3B antibodies) or 3% BSA in PBS (when using HSD17B1 and HSD17B2 antibodies) for 45 min. Sections were then incubated with the primary antibody overnight at 4°C. The primary antibody for HSD3B detection was a polyclonal goat anti-human antibody (Santa Cruz Biotechnology, Santa Cruz, CA); for HSD17B1 it was a rabbit monoclonal antibody (Epitomics, Inc., Burlingame, CA) and for HSD17B2 it was a polyclonal rabbit antibody (Proteintech Group, Inc., Chicago, IL). In negative control slides, the primary antibody was replaced with nonimmune IgG of equivalent concentration from the same species. Slides for HSD3B staining were incubated with secondary fluorescein isothiocyanate (FITC)-conjugated donkey anti-goat antibody (Santa Cruz Biotechnology) for 45 min at room temperature, mounted with Vectashield mounting medium with DAPI (Vector Laboratories, Inc., Burlingame, CA) and viewed under a Leica epifluorescence microscope. The slides for HSD17B1 and HSD17B2 detection were incubated with goat anti-rabbit secondary antibodies (Vector Laboratories) for 45 min at room temperature. After 30 min incubation with ABC complex (Vectastain Elite ABC immunoperoxidase detection kit; Vector Laboratories), freshly prepared diaminobenzidine-hydrogen peroxide solution (DAB kit; Vector Laboratories) was added to the slides, which were thereafter rinsed with distilled water. The slides were counterstained with hematoxylin (Vector Laboratories) and mounted with Clarion mounting medium (Sigma-Aldrich). A Leica microscope was used to visualize the immunostaining and to photograph the results. Sections of 20-wk human placental tissue served as positive controls for all three enzymes studied [34, 35].
CYP19A1 Activity Assay
Aromatase activity of hESF from subjects with and without endometriosis was determined by measuring the amount of [3H]H2O released after conversion of [1β-3H]A4 to E1 [36, 37]. Human ESF from subjects with (n = 4) and without (n = 4) endometriosis were plated in triplicates in 10% fetal calf serum until confluence, as described above. Cells from each subject were then cultured for 96 h and treated with no cAMP, 0.5 mM cAMP, or 1 mM cAMP in low serum-containing medium. Afterwards, 12.5 nM [1β-3H]A4 (SA 23.5Ci/mmol; PerkinElmer, Inc., Boston, MA) was added and cells were incubated for 16 h at 37°C at 5% CO2. Following incubation, 2 ml of medium were transferred to a test tube with 1.0 ml of ice-cold 30% trichloracetic acid, vortexed, and centrifuged. Supernatants were subsequently subjected to chloroform extraction to remove protein and cell debris from tested medium. The aqueous phase was collected and added to 2 ml of 5% activated charcoal (Sigma-Aldrich) with 0.5% dextran (Sigma-Aldrich) in PBS, to bind steroids. After a short incubation on ice and centrifugation, the supernatant (2 ml) was mixed with scintillation fluid (15 ml), vortexed well, and counted in a beta-spectrometer. Cells were harvested with 0.25% trypsin-1 mM EDTA for protein determination. The human granulosa cell line KGN (a kind gift from Dr. T. Yanase, Kyushu University, Japan) was used as a positive control. Results are expressed as picomoles of [3H]H2O formed in 16 h/mg total protein. Statistical analysis was performed using one-way ANOVA with the Tukey-Kramer multiple comparisons test.
RESULTS
Steroidogenic Enzymes in Eutopic Endometrium
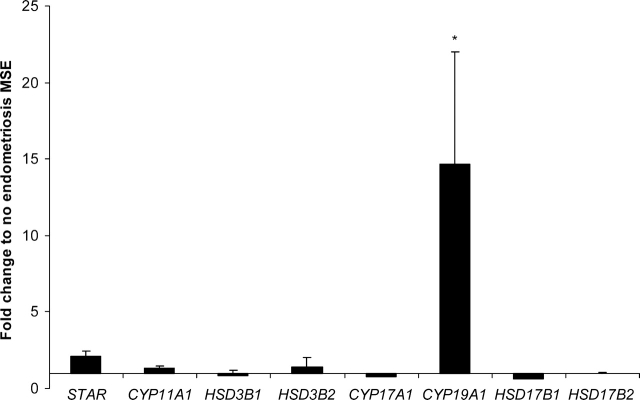
Expression of key steroidogenic enzymes in mid-secretory phase eutopic endometrium in women with vs. those without endometriosis was assessed by quantitative RT-PCR. Most striking was the marked up-regulation of CYP19A1 mRNA in endometrial samples from women with vs. those without disease (14.5-fold; P = 0.0037) (Fig. 1). Expression of HSD17B1, which catalyzes the conversion of E1 to E2 and A4 to testosterone, and HSD17B2, which catalyzes the reverse reaction and is known to be decreased in ectopic endometriotic lesions [21], did not differ between endometrial tissue samples from women with vs. those without disease (Fig. 1). In addition, STAR (rate-limiting step of steroid hormone synthesis) and P450scc (CYP11A1), and HSD3B1 and HSD3B2, which catalyse the conversion of pregnenolone (preg)/17OH-preg to P4/17-OH-P4 and dehydroepiandrosterone to A4, and CYP17A1 were not significantly different in endometrium from women with vs. those without endometriosis.
FIG. 1.
Expression of mRNA for steroidogenic pathway enzymes in tissue biopsies of eutopic MSE from women with endometriosis (n = 5) expressed as fold change to expression in MSE from women without endometriosis (n = 5), as revealed by real-time RT-PCR. *Significance accepted at P ≤ 0.05 (Mann-Whitney test). Error bars represent ± SEM.
PKA Pathway Activation and P4-Treatment of hESF
The finding of marked up-regulation of CYP19A1 mRNA in MSE of women with vs. those without endometriosis suggests a role for P4 in this process, as peak circulating and endometrial tissue levels of P4 are present at this time of the cycle, acting primarily on hESFs [38]. In this cell type, P4 begins the process of hESF decidualization, which is also mediated by the PKA pathway [39, 40]. Thus, we investigated the effects of P4 and cAMP on expression of CYP19A1 and other steroidogenic enzymes in hESFs using an in vitro culture system [31, 41]. Activation of the PKA pathway increased expression of STAR mRNA in hESF from women without and with endometriosis (Fig. 2A). In contrast, no effect of cAMP was observed on the expression of CYP11A1, in hESF from women with or without disease. cAMP significantly decreased the expression of HSD3B1, suggesting decreased capacity to convert preg to P4 in hESF from women with disease. CYP17A1 expression did not differ in hESF from women with vs. those without endometriosis. Furthermore, CYP19A1 mRNA tended to be up-regulated by cAMP, and HSD17B1 mRNA was significantly up-regulated in response to cAMP treatment of hESF from women with vs. those without endometriosis (Fig. 2A). Expression of HSD17B2, which converts E2 to E1, tended to be increased in cells from women without vs. those with disease, although this was not significantly different. Interestingly, and surprisingly, P4 had no effect on the expression of STAR, CYP11A1, CYP17A1, CYP19A1, or HSD17B1 (data not shown).
FIG. 2.

A) Expression of mRNA for steroidogenic pathway enzymes in human endometrial stromal cells from eutopic endometrium from women with (n = 7) and without endometriosis (n = 5) treated with 0.5 mM 8-Br-cAMP for 96 h, expressed as fold change to the expression in 96-h no-treatment controls, as revealed by real-time RT-PCR. *Significance accepted at P ≤ 0.05 (Mann-Whitney test). Error bars represent ± SEM. B) Expression of IGFBP1 and PRL mRNA in endometrial stromal fibroblasts (hESFs) from women with (n = 7) and without endometriosis (n = 5) decidualized in vitro with 0.5 mM 8-Br-cAMP for 96 h, expressed as fold change to the expression in 96-h no-treatment controls (left side of B) and expression of IGFBP1 and PRL mRNA in hESFs from women with (n = 20) and without endometriosis (n = 7) decidualized in vitro for 14 days with P4, expressed as fold change to the expression in 14 days no hormone controls (right side of B), as revealed by real-time RT-PCR. The y-axis is presented as a log scale. *Significance accepted at P ≤ 0.05 (Mann-Whitney test). Error bars represent ± SEM. C) Levels of secreted IGFBP1 and PRL in culture medium from cultured hESFs from women with (n = 7) and without (n = 5) endometriosis decidualized in vitro with 0.5 mM 8-Br-cAMP for 96 h. *Significance accepted at P ≤ 0.05 (two-tailed type 3 Student t-test). Error bars represent ± SEM.
In view of differences in regulation of select steroidogenic enzyme pathway members in hESF in the setting of endometriosis, we investigated responses of the hESF to cAMP and P4 with regard to the decidual biomarkers, IGFBP1 and PRL. Figure 2B (right side) shows that P4 up-regulates IGFBP1 and PRL mRNA in hESFs, independent of coexisting endometriosis. This is in contrast to the observed blunted response to cAMP in the presence vs. absence of disease (Fig. 2B, left side), which was also manifested at the protein level (Fig. 2C). These data suggest a dysregulation in the PKA signaling pathway in hESFs from women with vs. those without endometriosis with regard to these classical markers of decidualization, in addition to dysregulation of select steroidogenic enzymes in these cells, as shown in Figure 2A.
HSD3B, HSD17B2, and HSD17B1 Proteins in Eutopic Endometrium and hESF
Immunohistochemical staining of MSE tissue from women with or without endometriosis revealed minimal stromal cell expression of HSD3B, although glandular cells showed positive fluorescent signals, which were stronger in the samples from women without vs. those with endometriosis (Fig. 3, A and B, and Table 3). HSD3B protein was undetectable in untreated hESF from women with and without disease (Fig. 3, C and E), consistent with a previous report [42] and low mRNA expression in tissue biopsies (data not shown). However, upon treatment with cAMP, HSD3B was increased in hESF from women without disease (Fig. 3D) vs. those with disease (Fig. 3F).
FIG. 3.
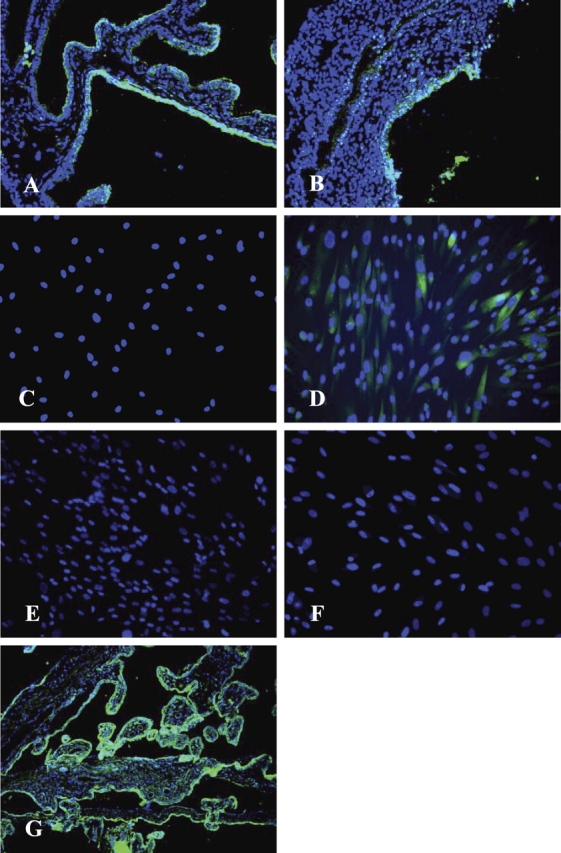
Representative picture of HSD3B immunostaining of MSE from women without endometriosis (n = 4) (A), with endometriosis (n = 4) (B), nondecidualized (C) and decidualized with 8-Br-cAMP (D) hESFs from women without endometriosis (n = 4), as well as nondecidualized (E) and decidualized with 8-Br-cAMP (F) hESFs from women with endometriosis (n = 4). Immunostaining of placental tissue at 20-wk gestational age, which was used as a positive control (G), demonstrates the expression of HSD3B protein in syncytiotrophoblast cells of placental villi. Cytotrophoblasts lying underneath the syncytial layer do not contain detectable levels of this enzyme and serve as an endogenous negative control. Original magnification ×200.
TABLE 3.
Semiquantitative evaluation of steroidogenic enzymes immunostaining in human endometrial tissue sections and hESF.a
Eutopic endometrial tissue from women without, but not with, endometriosis, demonstrated strong epithelial immunostaining for HSD17B2 (Fig. 4, A and B, and Table 3). No stromal staining was observed. Some stromal cells from women with or without endometriosis expressed HSD17B2 in culture (Fig. 4, C and E). After activation of the PKA pathway, hESF from women without endometriosis, demonstrated an increase in HSD17B2 (and change in cell shape) (Fig. 4D), not observed in cells from women with endometriosis (Fig. 4, C–F, and Table 3).
FIG. 4.
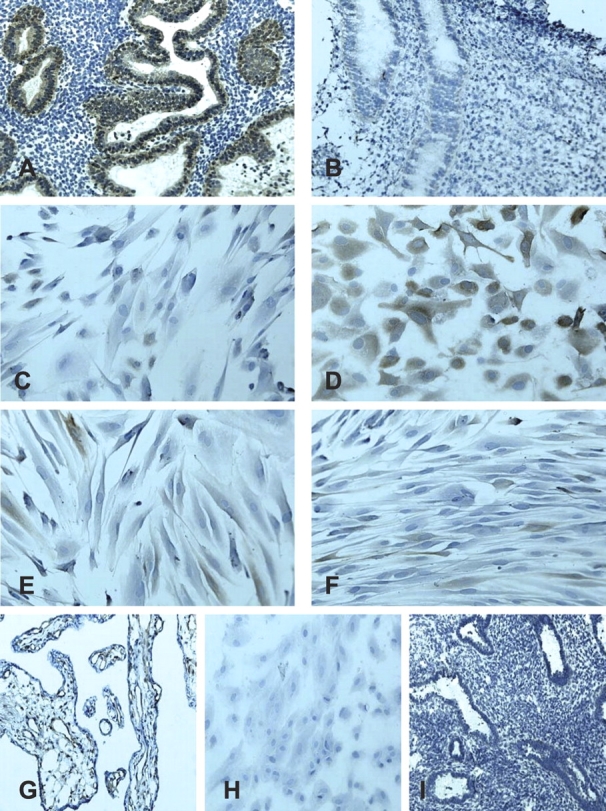
Representative picture of HSD17B2 immunostaining of MSE from women without endometriosis (n = 4) (A), with endometriosis (n = 4) (B), nondecidualized (C) and decidualized with 8-Br-cAMP (D) hESFs from women without endometriosis (n = 4), as well as nondecidualized (E) and decidualized with 8-Br-cAMP (F) hESFs from women with endometriosis (n = 4). HSD17B2 antibody in placental sections cross-reacted with endothelial cells around the perivascular capillary network (G). Negative control samples did not show immunoreactivity: H (hESFs) and I MSE. Original magnification ×200.
With regard to HSD17B1, MSE from women with, but not those without endometriosis, weakly expressed immunoreactive HSD17B1 in the epithelium, and no stromal staining was observed (photomicrographs not shown; Table 3). Human ESF from both groups of women showed faint expression of HSD17B1 protein in both nondecidualized and decidualized states, with no obvious difference after treatment with cAMP, consistent with low mRNA expression in endometrial tissue and stromal cells (data not shown) and in agreement with previous observations [42].
CYP19A1 Enzyme Activity in hESFs
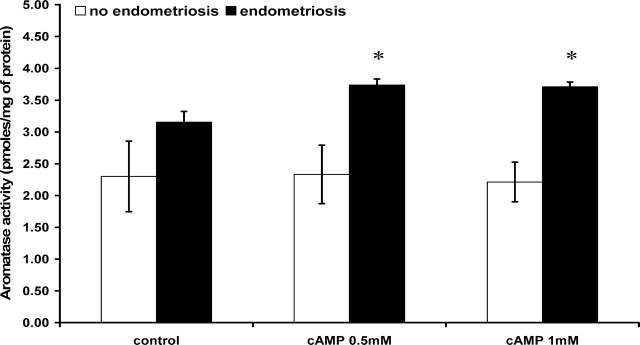
As shown in Figure 5, eutopic hESF from women with endometriosis expressed higher levels of CYP19A1 activity compared with hESF from women without disease. Moreover, hESF from women with endometriosis responded to activation of PKA pathway by an increase in CYP19A1 activity (P < 0.05), which was significantly higher compared with the cAMP response of hESF from women without disease (P < 0.05; Fig. 5). We attempted to measure E2 in the medium conditioned by these cells after preincubation with A4; however, the results were below the limit of detection in the assay.
FIG. 5.
Aromatase (CYP19A1) activity of endometrial stromal fibroblasts (hESFs) from women with (n = 4) and without endometriosis (n = 4) treated in vitro with or without 8-Br-cAMP for 96 h, with subsequent incubation with 12.5 nM of [1β-3H]androstenedione for 16 h. Results are expressed as picomoles of [3H]H2O formed in 16 h/mg total protein. Cells from each patient were run in triplicates. *Significance accepted at P < 0.05 (one-way ANOVA with the Tukey-Kramer Multiple comparisons test). Error bars represent ± SEM.
DISCUSSION
Endometrium as a Steroidogenic Tissue
Endometrium has traditionally been regarded as a target of steroid hormone action and not involved in steroid biosynthesis and metabolism. Transcriptomics and one-by-one gene analysis [22, 23] have demonstrated, however, that endometrial tissue expresses activities of several members of the steroidogenic pathway, such as STAR [43], CYP11A1 [43, 44], HSD3B [23, 42, 44], CYP17A1 [45], HSD17B1 [46, 47], HSD17B2 [21, 48], and CYP19A1 [49]. Thus, endometrium joins the traditional “steroidogenic” organs, along with cardiac and neural tissues and others with recently demonstrated steroidogenic capacity [50–52]. The physiologic relevance of locally produced and local metabolism of steroids within a tissue is not well understood. This is particularly true of tissues that respond to steroids from the circulation, such as the endometrium. One of the most striking observations herein is that human endometrium expresses transcripts for enzymes of the steroidogenic pathway involved in the synthesis and metabolism of estrogens and P4, and that there is dysregulation of some enzymes that favor biosynthesis of E2 locally in eutopic endometrium of women with vs. those without endometriosis. The response to E2 and P4 of eutopic endometrium from women with endometriosis is abnormal in vivo, as evidenced by the persistent footprint of E2-regulated genes in the early secretory phase of women with vs. those without disease [16]. Local E2, present at the time at which systemic P4 is to act on the tissue, may alter P4 actions within the endometrium, leading to the observed molecular phenotype.
CYP19A1 in Endometrium in the Setting of Endometriosis
There is a consensus about the expression of CYP19A1 mRNA, protein, and activity in ectopic lesions of endometriosis [53–55], which supports autonomous E2 production by and survival of endometriotic lesions, even in postmenopausal women with a history of endometriosis and not on hormonal therapies [56, 57]. Indeed, this finding has had a profound impact on medical management of endometriosis, with the introduction of CYP19A1 inhibitors as an alternative treatment for endometriosis-related pelvic pain [57–59]. There are no consistent data, however, regarding CYP19A1 protein, determined by immunohistochemistry, in eutopic endometrium in women with symptomatic endometriosis or in disease-free women. Some studies show low and others high immunoreactivity, likely due to different antibodies and experimental protocols [60–65]. An additional confounder is that CYP19A1 is expressed in endometrium of women with uterine fibroids [66–68]. Regarding the cell type of the enzyme expression, CYP19A1 mRNA (higher in women with vs. those without endometriosis) and protein (absent in some women without endometriosis) expression was shown in both epithelial and endometrial stromal cells, with higher expression in the epithelium [61, 69, 70]. The absence of CYP19A1 gene expression in eutopic endometrium of women without endometriosis has been reported [43, 55, 71], and was confirmed in the current study using sensitive quantitative RT-PCR and endometrial samples from women without disease who had indications for surgical procedures that included uterine prolapse, pelvic pain, and a normal volunteer, but not uterine fibroids.
CYP19A1 in hESFs
Cultured hESF from women with endometriosis demonstrated significantly higher CYP19A1 activity that increased further upon activation of the PKA pathway with tritiated A4 (3H-A4) in the culture medium. These results are consistent with the data of Noble et al. [54], who compared hESF from eutopic endometrium from women with endometriosis with ectopic stromal cells, rather that comparing with eutopic hESF from women without disease (as performed in the present study). Differences in magnitude of enzyme activity observed by Noble et al. [54] and our results are likely due to experimental conditions (substrate concentration, time of reaction). Interestingly, hESF isolated from women without endometriosis also expressed CYP19A1 mRNA and activity, although at extremely low levels and with no significant change in expression after treatment with either cAMP or P4. While several subjects had uterine fibroids (Table 1), which may account for this low constitutive activity, a recent study demonstrated that A4 increased CYP19A1 expression in hESF [72]. This was attributed to E2 (converted from A4), and addition of 3H-A4 to the cultures herein could account for this constitutive activity shown in Figure 5.
Other Steroidogenic Enzymes in Endometrium
Elevated expression of STAR, but not Cyp11A1 or HSD3B1, has been found in ectopic implants, compared to normal endometrium, and has been linked to survival and growth of endometriosis [43], presumably by enabling cholesterol transport into ectopic lesions for biosynthesis of steroids. Herein, we did not observe significant differences in STAR mRNA expression in eutopic endometrium from women with vs. those without endometriosis. However, STAR was up-regulated in hESF from women with and without endometriosis in response to cAMP and P4. STAR is one of the genes that is up-regulated in secretory endometrium [22], and the precise mechanisms underlying its regulation by cAMP in endometrium are unclear at this time and warrant further investigation (and see below).
HSD17B2 mRNA increases dramatically in early and mid-secretory eutopic endometrium in women without [21, 22], but not those with endometriosis [16, 21, 69], although studies showing no cycle variation has been reported [69, 70]. In the current study, HSD17B2 was found to be highly expressed in MSE epithelium in women without vs. those with disease. In contrast, stromal fibroblast immunostaining in eutopic endometrium was low in both groups. HSD17B2 has been reported by others in both epithelium and stroma with high expression in the former [69, 70]. Human ESF from women without endometriosis demonstrated high responsiveness to cAMP treatment with increased HSD17B2 immunoexpression, while in hESF from women with disease, HSD17B2 protein expression did not respond to cAMP. Since HSD17B2 converts E2 into the weaker estrogen, E1, its lower expression in hESF may contribute to an E2-predominant environment in eutopic endometrium of women with disease. Furthermore, this abnormality is present in cells after at least two passages, suggesting perhaps involvement of epigenetic mechanisms that preserve this abnormal phenotype favoring decreased E2 metabolism.
Another enzyme, HSD17B1, which is responsible for the opposite process (i.e., conversion of E1 to E2) was significantly elevated in cAMP-treated hESF from women with vs. those without endometriosis. This may also contribute to accumulation of E2 in endometrial tissue, promoting progression of the disease. However, those changes were not reflected at the protein level, most likely due to low levels of the translated product in the endometrium and/or sensitivity of the antibody used.
Activation of the PKA Pathway and P4 Signaling
Endometrium of women with endometriosis exhibits an abnormal proliferative-to-secretory transition, with persistence of genes and biological processes (e.g., cellular mitoses, DNA replication) that are normally down-regulated after the LH surge and P4 action in the tissue [16]. Furthermore, many P4-regulated genes are dysregulated in the early and mid-secretory phases of the cycle [16, 23]. These observations, taken together, suggest an element of resistance to progesterone action in endometrium of women with endometriosis. However, the data here do not support resistance to P4 action, per se, of hESF from women with endometriosis, but rather a blunted response to cAMP, as demonstrated by significantly reduced IGFBP1 and PRL mRNA and protein in hESF from women with vs. those without disease (Fig. 2), consistent with a previous report [17], and a dysregulation of select steroidogenic enzymes. While the absence of HSD3B1 up-regulation by cAMP in hESF in the setting of endometriosis (Fig. 2A) is consistent with a blunted response, in contrast, some steroidogenic enzymes (e.g., HSD17B1 and CYP19A1) were up-regulated by cAMP treatment of hESF from women with endometriosis compared with those without disease (Figs. 2 and 5). The dichotomy of hESF responsiveness to cAMP and P4 of select steroidogenic enzymes and decidual biomarkers, IGFBP1 and PRL, may lie in one or more of a multiplicity of pathways intersecting with the PKA pathway and/or P4 signaling in this cell type. Of note in the current study, the phenotype observed here persists in hESF after isolation, culture, passaging, and further culture, suggesting an intrinsic abnormality in this cell type in endometrium from women with disease. While it is possible that peritoneal fluid, which is rich in inflammatory cytokines, in women with endometriosis may exert a pathological effect on the eutopic endometrium, leading to its alterations and impaired decidualization, persistence of the phenotype in multiple passages would suggest an intrinsic abnormality in the shed endometrium. This may be due to epigenetic mechanisms that are retained and transmitted in expanded culture, in parallel with the observed hypomethylation of NR5A1 (also known as SF-1) and ESR2 in endometrium from women with vs. those without disease, invoked as part of the pathogenesis of elevated CYP19A1 expression [73, 74].
In summary, herein, we have demonstrated that endometrium is a steroidogenic tissue, which, in the setting of endometriosis, exhibits dysregulation of steroidogenic enzyme pathway genes that results in a shift to an E2-enriched milieu. This can influence proliferation and survival of endometrial cells, up-regulation of other processes (e.g., neurotrophins and their nociceptors, important in the pathogenesis of this disorder). Whether epigenetic mechanisms are involved is not clear at this time, but the shift in local hormonal milieu may be responsible for the impaired in vivo decidualization of hESF from women with disease, leading to impaired implantation in the setting of endometriosis. Most intriguing is the observed dichotomy of altered PKA and P4 signaling pathway regulation of select steroidogenic enzymes and the blunted responsiveness of hESF of the decidual biomarkers, IGFBP1 and PRL, in the setting of endometriosis. Mechanisms underlying these observations are currently under investigation in our laboratory.
Acknowledgments
We thank Dr. James L. Weeks II at Center for Reproductive Sciences, University of California, San Francisco, for his help with aromatase activity assay.
Footnotes
1Supported by the National Institute of Child Health and Human Development/ National Institutes of Health through cooperative agreement 1U54HD055764-01 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
REFERENCES
- Simoens S, Hummelshoj L, D'Hooghe T.Endometriosis: cost estimates and methodological perspective. Hum Reprod Update 2007; 13: 395–404. [DOI] [PubMed] [Google Scholar]
- Minici F, Tiberi F, Tropea A, Fiorella M, Orlando M, Gangale MF, Romani F, Catino S, Campo S, Lanzone A, Apa R.Paracrine regulation of endometriotic tissue. Gynecol Endocrinol 2007; 23: 574–580. [DOI] [PubMed] [Google Scholar]
- Selam B, Arici A.Implantation defect in endometriosis: endometrium or peritoneal fluid. J Reprod Fertil Suppl 2000; 55: 121–128. [PubMed] [Google Scholar]
- Mazella J, Tang M, Tseng L.Disparate effects of relaxin and TGFbeta1: relaxin increases, but TGFbeta1 inhibits, the relaxin receptor and the production of IGFBP-1 in human endometrial stromal/decidual cells. Hum Reprod 2004; 19: 1513–1518. [DOI] [PubMed] [Google Scholar]
- Tierney EP, Tulac S, Huang ST, Giudice LC.Activation of the protein kinase A pathway in human endometrial stromal cells reveals sequential categorical gene regulation. Physiol Genomics 2003; 16: 47–66. [DOI] [PubMed] [Google Scholar]
- Tierney EP, Giudice LC.Role of activin A as a mediator of in vitro endometrial stromal cell decidualization via the cyclic adenosine monophosphate pathway. Fertil Steril 2004; 81(suppl) 1:899–903. [DOI] [PubMed] [Google Scholar]
- Pohnke Y, Schneider-Merck T, Fahnenstich J, Kempf R, Christian M, Milde-Langosch K, Brosens JJ, Gellersen B.Wild-type p53 protein is up-regulated upon cyclic adenosine monophosphate-induced differentiation of human endometrial stromal cells. J Clin Endocrinol Metab 2004; 89: 5233–5244. [DOI] [PubMed] [Google Scholar]
- Schneider-Merck T, Pohnke Y, Kempf R, Christian M, Brosens JJ, Gellersen B.Physical interaction and mutual transrepression between CCAAT/enhancer-binding protein beta and the p53 tumor suppressor. J Biol Chem 2006; 281: 269–278. [DOI] [PubMed] [Google Scholar]
- Strakova Z, Srisuparp S, Fazleabas AT.Interleukin-1beta induces the expression of insulin-like growth factor binding protein-1 during decidualization in the primate. Endocrinology 2000; 141: 4664–4670. [DOI] [PubMed] [Google Scholar]
- Strakova Z, Mavrogianis P, Meng X, Hastings JM, Jackson KS, Cameo P, Brudney A, Knight O, Fazleabas AT.In vivo infusion of interleukin-1beta and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinology 2005; 146: 4097–4104. [DOI] [PubMed] [Google Scholar]
- Bell SC, Jackson JA, Ashmore J, Zhu HH, Tseng L.Regulation of insulin-like growth factor-binding protein-1 synthesis and secretion by progestin and relaxin in long term cultures of human endometrial stromal cells. J Clin Endocrinol Metab 1991; 72: 1014–1024. [DOI] [PubMed] [Google Scholar]
- Zhu HH, Huang JR, Mazella J, Rosenberg M, Tseng L.Differential effects of progestin and relaxin on the synthesis and secretion of immunoreactive prolactin in long term culture of human endometrial stromal cells. J Clin Endocrinol Metab 1990; 71: 889–899. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens J.Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 2003; 178: 357–372. [DOI] [PubMed] [Google Scholar]
- Brar AK, Handwerger S, Kessler CA, Aronow BJ.Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics 2001; 7: 135–148. [DOI] [PubMed] [Google Scholar]
- Popovici RM, Kao LC, Giudice LC.Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology 2000; 141: 3510–3513. [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC.Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007; 148: 3814–3826. [DOI] [PubMed] [Google Scholar]
- Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ.Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril 2006; 85: 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J.Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol 2006; 248: 94–103. [DOI] [PubMed] [Google Scholar]
- Vierikko P, Kauppila A, Ronnberg L, Vihko R.Steroidal regulation of endometriosis tissue: lack of induction of 17 beta-hydroxysteroid dehydrogenase activity by progesterone, medroxyprogesterone acetate, or danazol. Fertil Steril 1985; 43: 218–224. [PubMed] [Google Scholar]
- Utsunomiya H, Cheng YH, Lin Z, Reierstad S, Yin P, Attar E, Xue Q, Imir G, Thung S, Trukhacheva E, Suzuki T, Sasano H, et al. Upstream stimulatory factor-2 regulate steroidogenic factor-1 expression in endometriosis. Mol Endocrinol 2008; 22: 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, Johns A, Meng L, Putman M, Carr B, Bulun SE.Deficient 17beta-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17beta-estradiol. J Clin Endocrinol Metab 1998; 83: 4474–4480. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC.Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006; 147: 1097–1121. [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC.Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 2003; 144: 2870–2881. [DOI] [PubMed] [Google Scholar]
- Fang Z, Yang S, Gurates B, Tamura M, Simpson E, Evans D, Bulun SE.Genetic or enzymatic disruption of aromatase inhibits the growth of ectopic uterine tissue. J Clin Endocrinol Metab 2002; 87: 3460–3466. [DOI] [PubMed] [Google Scholar]
- Casey ML, MacDonald PC, Andersson S.17 beta-Hydroxysteroid dehydrogenase type 2: chromosomal assignment and progestin regulation of gene expression in human endometrium. J Clin Invest 1994; 94: 2135–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitoun K, Takayama K, Michael MD, Bulun SE.Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of steroidogenic factor-1 and chicken ovalbumin upstream promoter transcription factor to the same cis-acting element. Mol Endocrinol 1999; 13: 239–253. [DOI] [PubMed] [Google Scholar]
- Kitawaki J, Noguchi T, Amatsu T, Maeda K, Tsukamoto K, Yamamoto T, Fushiki S, Osawa Y, Honjo H.Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod 1997; 57: 514–519. [DOI] [PubMed] [Google Scholar]
- Yang S, Fang Z, Suzuki T, Sasano H, Zhou J, Gurates B, Tamura M, Ferrer K, Bulun S.Regulation of aromatase P450 expression in endometriotic and endometrial stromal cells by CCAAT/enhancer binding proteins (C/EBPs): decreased C/EBPbeta in endometriosis is associated with overexpression of aromatase. J Clin Endocrinol Metab 2002; 87: 2336–2345. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB.Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 2004; 25: 947–970. [DOI] [PubMed] [Google Scholar]
- The American Fertility Society. Revised American Fertility Society classification of endometriosis. Fertil Steril 1985; 43: 351–352. [DOI] [PubMed] [Google Scholar]
- Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC.Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab 2006; 91: 1453–1461. [DOI] [PubMed] [Google Scholar]
- Kayisli UA, Aksu CA, Berkkanoglu M, Arici A.Estrogenicity of isoflavones on human endometrial stromal and glandular cells. J Clin Endocrinol Metab 2002; 87: 5539–5544. [DOI] [PubMed] [Google Scholar]
- Irwin JC, Kirk D, King RJ, Quigley MM, Gwatkin RB.Hormonal regulation of human endometrial stromal cells in culture: an in vitro model for decidualization. Fertil Steril 1989; 52: 761–768. [DOI] [PubMed] [Google Scholar]
- Drolet R, Simard M, Plante J, Laberge P, Tremblay Y.Human type 2 17 beta-hydroxysteroid dehydrogenase mRNA and protein distribution in placental villi at mid and term pregnancy. Reprod Biol Endocrinol 2007; 5: 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonenfant M, Provost PR, Drolet R, Tremblay Y.Localization of type 1 17beta-hydroxysteroid dehydrogenase mRNA and protein in syncytiotrophoblasts and invasive cytotrophoblasts in the human term villi. J Endocrinol 2000; 165: 217–222. [DOI] [PubMed] [Google Scholar]
- Ackerman GE, Smith ME, Mendelson CR, MacDonald PC, Simpson ER.Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J Clin Endocrinol Metab 1981; 53: 412–417. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K, Takayanagi R, Kashimura Y, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 2001; 142: 437–445. [DOI] [PubMed] [Google Scholar]
- Graham JD, Clarke CL.Physiological action of progesterone in target tissues. Endocr Rev 1997; 18: 502–519. [DOI] [PubMed] [Google Scholar]
- Jones MC, Fusi L, Higham JH, Abdel-Hafiz H, Horwitz KB, Lam EW, Brosens JJ.Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc Natl Acad Sci U S A 2006; 103: 16272–16277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CL, Kelly RW, Critchley HO.Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online 2003; 7: 151–161. [DOI] [PubMed] [Google Scholar]
- Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, Fazleabas AT, Fisher SJ, et al. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod 2007; 76: 102–117. [DOI] [PubMed] [Google Scholar]
- Vani S, McDonald SE, Williams AR, Mason JI, Thong KJ, Critchley HO.Mid-luteal endometrial intracrinology following controlled ovarian hyperstimulation involving use of a gonadotrophin releasing hormone antagonist. Hum Reprod 2007; 22: 2981–2991. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Wu MH, Lin CC, Sun HS, Chen HM.Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J Clin Endocrinol Metab 2001; 86: 5765–5773. [DOI] [PubMed] [Google Scholar]
- Rhee HS, Oh SH, Ko BJ, Han DM, Jeon BH, Park H, Moon HB, Kim WS.Expression of 3beta-hydroxysteroid dehydrogenase and P450 side chain cleavage enzyme in the human uterine endometrium. Exp Mol Med 2003; 35: 160–166. [DOI] [PubMed] [Google Scholar]
- Berstein LM, Imyanitov EN, Gamajunova VB, Kovalevskij AJ, Kuligina E, Belogubova EV, Buslov KG, Karpova MB, Togo AV, Volkov ON, Kovalenko IG.CYP17 genetic polymorphism in endometrial cancer: are only steroids involved? Cancer Lett 2002; 180: 47–53. [DOI] [PubMed] [Google Scholar]
- Peltoketo H, Luu-The V, Simard J, Adamski J.17beta-hydroxysteroid dehydrogenase (HSD)/17-ketosteroid reductase (KSR) family; nomenclature and main characteristics of the 17HSD/KSR enzymes. J Mol Endocrinol 1999; 23: 1–11. [DOI] [PubMed] [Google Scholar]
- Peltoketo H, Nokelainen P, Piao YS, Vihko R, Vihko P.Two 17beta-hydroxysteroid dehydrogenases (17HSDs) of estradiol biosynthesis: 17HSD type 1 and type 7. J Steroid Biochem Mol Biol 1999; 69: 431–439. [DOI] [PubMed] [Google Scholar]
- Cheng YH, Imir A, Suzuki T, Fenkci V, Yilmaz B, Sasano H, Bulun SE.SP1 and SP3 mediate progesterone-dependent induction of the 17beta hydroxysteroid dehydrogenase type 2 gene in human endometrium. Biol Reprod 2006; 75: 605–614. [DOI] [PubMed] [Google Scholar]
- Tseng L, Mazella J, Mann WJ, Chumas J.Estrogen synthesis in normal and malignant human endometrium. J Clin Endocrinol Metab 1982; 55: 1029–1031. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD.Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab 2002; 13: 35–43. [DOI] [PubMed] [Google Scholar]
- Simpton NB, Cunliffe WJ, Hodgins MB.The relationship between the vitro activity of 3 beta-hydroxysteroid dehydrogenase delta 4–5-isomerase in human sebaceous glands and their secretory activity in vivo. J Invest Dermatol 1983; 81: 139–144. [DOI] [PubMed] [Google Scholar]
- Kayes-Wandover KM, White PC.Steroidogenic enzyme gene expression in the human heart. J Clin Endocrinol Metab 2000; 85: 2519–2525. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Clyne C, Speed C, Rubin G, Bulun S.Tissue-specific estrogen biosynthesis and metabolism. Ann N Y Acad Sci 2001; 949: 58–67. [DOI] [PubMed] [Google Scholar]
- Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE.Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab 1997; 82: 600–606. [DOI] [PubMed] [Google Scholar]
- Noble LS, Simpson ER, Johns A, Bulun SE.Aromatase expression in endometriosis. J Clin Endocrinol Metab 1996; 81: 174–179. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Zeitoun K, Sasano H, Simpson ER.Aromatase in aging women. Semin Reprod Endocrinol 1999; 17: 349–358. [DOI] [PubMed] [Google Scholar]
- Takayama K, Zeitoun K, Gunby RT, Sasano H, Carr BR, Bulun SE.Treatment of severe postmenopausal endometriosis with an aromatase inhibitor. Fertil Steril 1998; 69: 709–713. [DOI] [PubMed] [Google Scholar]
- Attar E, Bulun SE.Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil Steril 2006; 85: 1307–1318. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Zeitoun K, Takayama K, Noble L, Michael D, Simpson E, Johns A, Putman M, Sasano H.Estrogen production in endometriosis and use of aromatase inhibitors to treat endometriosis. Endocr Relat Cancer 1999; 6: 293–301. [DOI] [PubMed] [Google Scholar]
- Maia H, Jr, Casoy J, Correia T, Freitas LA, Pimentel K, Athayde C.The effect of oral contraceptives on aromatase expression in the eutopic endometrium of patients with endometriosis. Gynecol Endocrinol 2008; 24: 123–128. [DOI] [PubMed] [Google Scholar]
- Hudelist G, Czerwenka K, Keckstein J, Haas C, Fink-Retter A, Gschwantler-Kaulich D, Kubista E, Singer CF.Expression of aromatase and estrogen sulfotransferase in eutopic and ectopic endometrium: evidence for unbalanced estradiol production in endometriosis. Reprod Sci 2007; 14: 798–805. [DOI] [PubMed] [Google Scholar]
- Wolfler MM, Nagele F, Kolbus A, Seidl S, Schneider B, Huber JC, Tschugguel W.A predictive model for endometriosis. Hum Reprod 2005; 20: 1702–1708. [DOI] [PubMed] [Google Scholar]
- Holloway AC, Stys KA, Foster WG.DDE-induced changes in aromatase activity in endometrial stromal cells in culture. Endocrine 2005; 27: 45–50. [DOI] [PubMed] [Google Scholar]
- Kitawaki J, Kusuki I, Koshiba H, Tsukamoto K, Honjo H.Expression of aromatase cytochrome P450 in eutopic endometrium and its application as a diagnostic test for endometriosis. Gynecol Obstet Invest 1999; 48(suppl 1):21–28. [DOI] [PubMed] [Google Scholar]
- Velasco I, Rueda J, Acien P.Aromatase expression in endometriotic tissues and cell cultures of patients with endometriosis. Mol Hum Reprod 2006; 12: 377–381. [DOI] [PubMed] [Google Scholar]
- Sumitani H, Shozu M, Segawa T, Murakami K, Yang HJ, Shimada K, Inoue M.In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology 2000; 141: 3852–3861. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Simpson ER, Word RA.Expression of the CYP19 gene and its product aromatase cytochrome P450 in human uterine leiomyoma tissues and cells in culture. J Clin Endocrinol Metab 1994; 78: 736–743. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Imir G, Utsunomiya H, Thung S, Gurates B, Tamura M, Lin Z.Aromatase in endometriosis and uterine leiomyomata. J Steroid Biochem Mol Biol 2005; 95: 57–62. [DOI] [PubMed] [Google Scholar]
- Dassen H, Punyadeera C, Kamps R, Delvoux B, Van Langendonckt A, Donnez J, Husen B, Thole H, Dunselman G, Groothuis P.Estrogen metabolizing enzymes in endometrium and endometriosis. Hum Reprod 2007; 22: 3148–3158. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Canis M, Pouly JL, Dechelotte PJ, Mage G.Analysis of aromatase and 17beta-hydroxysteroid dehydrogenase type 2 messenger ribonucleic acid expression in deep endometriosis and eutopic endometrium using laser capture microdissection. Fertil Steril 2006; 85: 308–313. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Mahendroo MS, Simpson ER.Polymerase chain reaction amplification fails to detect aromatase cytochrome P450 transcripts in normal human endometrium or decidua. J Clin Endocrinol Metab 1993; 76: 1458–1463. [DOI] [PubMed] [Google Scholar]
- Bukulmez O, Hardy DB, Carr BR, Auchus RJ, Toloubeydokhti T, Word RA, Mendelson CR.Androstenedione up-regulation of endometrial aromatase expression via local conversion to estrogen: potential relevance to the pathogenesis of endometriosis. J Clin Endocrinol Metab 2008; 93: 3471–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE.Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod 2007; 77: 681–687. [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE.Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab 2007; 92: 3261–3267. [DOI] [PubMed] [Google Scholar]