Abstract
Although antiretroviral therapies have been effective in decreasing active viral loads in AIDS patients, the persistence of latent viral reservoirs prevents eradication of the virus. Prostratin and DPP (12-deoxyphorbol-13-phenylacetate) activate the latent virus and thus represent promising adjuvants for antiviral therapy. Their limited supply and the challenges of accessing related structures have, however, impeded therapeutic development and the search for clinically superior analogs. Here we report a practical synthesis of prostratin and DPP starting from phorbol or crotophorbolone, agents readily available from renewable sources, including a biodiesel candidate. This synthesis reliably supplies gram quantities of the therapeutically promising natural products, hitherto available only in low and variable amounts from natural sources, and opens access to a variety of new analogs.
AIDS is a pandemic disease caused by HIV. In a recent report, the Joint United Nations Programme on HIV/AIDS (UNAIDS) estimated that 33.2 million people were living with HIV and that 2.1 million people lost their lives to AIDS in the year 2007 (1).
Highly active antiretroviral therapy (HAART) has been successful in reducing HIV-1 levels in the plasma of many treated patients to undetectable levels. However, latent virus reservoirs remain in patients even after HAART (2). Such reservoirs are not targeted by current drug treatments, and as a consequence viral rebound often occurs if therapy is interrupted.
These latent viral reservoirs decrease only slowly in patients undergoing HAART. It is estimated that decades of treatment would be required to completely eliminate the latent virus. Such chronic treatment is undesirable because of the increased risk of side effects over time; the emergence of resistance through viral mutation; the increased demand on patients to maintain a long-term treatment regimen; and the cumulative financial burden of prolonged therapy, a particularly problematic issue in less-developed countries. Therefore, agents that can controllably flush the latent virus from its reservoirs could, in principle, provide a means to eradicate the virus when used as adjuvants in combination with HAART (3).
Although agents such as interleukin-2 and valproic acid have been tested as adjuvants in HAART, they cause toxicity or efficacy problems (4). Phorbol-13-myrisitate-12-acetate (PMA), a phorbol diester, is also reported to induce HIV-1 activation, but its potent tumor-promoting activity raises concerns about its therapeutic use (5, 6).
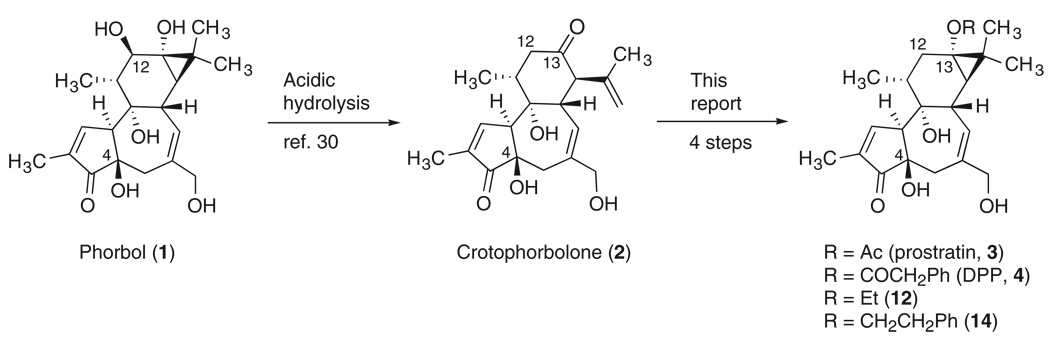
Prostratin (3, 12-deoxyphorbol-13-acetate) and DPP (4, 12-deoxyphorbol-13-phenylacetate) are non–tumor-promoting 12-deoxytigliane diterpenes that exhibit potent in vitro activity in inducing HIV expression in latently infected cell lines and primary cells (7–11). Prostratin and DPP also inhibit HIV entry into target cells by down-regulating CD4 and CXCR4 receptors (12–14). The mechanism of action of prostratin has not yet been completely elucidated, but the activation of protein kinase C (PKC) and nuclear factor κB (NF-κB) by prostratin have been proposed as key events (15–17). Prostratin has been advanced into preclinical development (18).
Unfortunately, a major obstacle to further development of prostratin, DPP, or related analogs as therapeutic agents has been their limited availability (19). Prostratin itself was first isolated from Pimelea prostrata and characterized by Hecker in 1976 (20). It was subsequently isolated from Euphorbia cornigera by Evans (21). The levels of prostratin in these source plants have, however, not been reported. More recently, impressive and seminal work by Cox in collaboration with Samoan healers and scientists at the U.S. National Institutes of Health identified prostratin as the active constituent in a traditional Samoan medicinal regimen (22). However, the Samoan source plant, Homalanthus nutans, affords prostratin only in low and variable isolated yields (0.2 to 52 µg/g by weight from the tree stem) (23, 24). It is note-worthy for projected clinical use of prostratin that these plant extracts do not produce acute side effects in humans, having already been used by the Samoan healers to treat individuals with certain (non-AIDS) medical conditions such as hepatitis.
The limited and varied availability of prostratin has hampered studies on its mode of action and the identification of clinically superior analogs. Only a handful of C12-deoxytiglianes have been investigated for HIV induction activity (7, 11), and little is known about the structural basis of prostratin’s biological activity. Although efforts to address this supply problem through microbial engineering have been initiated (26), the only known source of prostratin at present is plants.
Here we report practical and step economical syntheses of prostratin and DPP that can deliver research quantities (hundreds of milligrams to grams) of the targets and can serve as viable routes for addressing potential clinical needs (Scheme 1). We also show that this synthesis is sufficiently flexible to access new analogs, thereby providing an opportunity to comprehensively investigate this therapeutic lead and identify superior candidates. The step economy and flexibility of these syntheses allow facile and general access to a variety of 12-deoxytiglianes. The impact of this study may reach beyond the realm of anti-HIV therapy, because non–tumor-promoting PKC modulators such as bryostatin and its analogs represent candidates for the treatment of cancer and Alzheimer’s disease (25, 27). Prostratin also inhibits the tumor-promoting effects of PMA on mouse skin (28).
Scheme 1.
Available from croton oil (ref. 29) or by total synthesis (refs. 31–33)
Available from 1 or Jatropha curcas seed oil (refs. 34–36)
Overall yield 7–16% from 1
Our synthesis begins with the acidic hydrolysis of phorbol (1, Scheme 1), a tigliane diterpene isolated from croton oil (29), to produce crotophorbolone (2) (30). Croton oil is obtained from the seeds of Croton tiglium, a renewable source, and it is readily available in kilogram quantities in commercially long-established procedures. Phorbol itself is also commercially available, and its total synthesis has been accomplished in our laboratory (31–33). Alternatively, crotophorbolone could be obtained by the hydrolysis of 12-deoxy-16-hydroxyphorbol esters (34, 35), which are available from Jatropha curcas seed oil, an abundant renewable feedstock being developed as biodiesel (36, 37).
Although the synthetic conversion of phorbol to 12-deoxyphorbol (desacetylprostratin) involves the seemingly simple removal of the C12 oxygen, the proximity of this group to a strained cyclopropane ring and the sensitivity of the molecule to acid, base, heat, air, and light makes a selective deoxygenation difficult. For example, attempts to deoxygenate the C12 position of alcohol 5, derived in two steps from phorbol, by conversion to and subsequent reduction of xanthate 6 provided only enol acetate 7, arising from cleavage of the cyclopropane ring initiated by a C12 radical intermediate (Scheme 2). This was largely an anticipated result, given the known reactivity of cyclopropyl methyl radicals to undergo ring cleavage at near diffusion-controlled rates (108 s−1) (38). Thus, intramolecular ring cleavage occurs faster than the intermediate C12 radical can be trapped intermolecularly by an H-atom donor, despite using 10 equivalents of Bu3SnH. Intramolecular H-atom abstraction could potentially be used to outcompete the ring opening, but even if successful, this approach would suffer from the required use of additional synthetic steps to protect the complex array of oxygens in these molecules. Like radical-based deoxygenation procedures, reductions involving conversion of the C12 hydroxyl into a good leaving group are also known to fragment the cyclopropane ring (39).
Scheme 2.
(a) thiocarbonyl diimidazole (3 equiv.), DMAP (3 equiv.), CH2Cl2, 25 °C, 9 h, 87%; (b) AIBN (1 equiv.), Bu3SnH (10 equiv.), PhCH3, 90 °C, 15 min, 75% (α:β = 1:2)
We therefore adopted a C12 deoxygenation strategy that involves first cleaving and then reestablishing the cyclopropane ring. This approach favorably moves our synthetic starting point to readily available and unprotected phorbol itself or to phorbol-derived crotophorbolone (2). With the C12 oxygen eliminated in the formation of crotophorbolone, we reestablished the cyclopropane ring in four steps. (40) First, treatment of crotophorbolone with hydrazine in the presence of acetic acid selectively affords the C13 hydrazone (not shown in Scheme 3), which without isolation is cyclized to pyrazoline 8 when heated in the presence of base (Scheme 3). Oxidation of pyrazoline 8 with lead (IV) tetraacetate gives cyclic diazene 9, allowing for concomitant direct introduction of a C13 acetate group and a diazene bridge between C13 and C15. Other C13 esters can also be directly introduced with this procedure by using the corresponding lead (IV) carboxylate or related oxidants. Photolysis of cyclic diazene 9 results in the extrusion of nitrogen and reestablishment of the C13–C15 cyclopropane bond (41, 42), providing prostratin (3) in high yield and in a remarkably concise four-step sequence from 2 (or five steps in 12 to 16% overall yield from 1, producing over 100 mg of 3 in a single run). The synthetic sample of prostratin so obtained was identical in all standard analytical tests to a sample of natural prostratin. This sequence can be readily conducted on a gram scale and would allow for the production of larger quantities in a proper scaleup facility (43). Moreover, this procedure is shorter than the originally considered direct C12 deoxygenation strategy (Scheme 2) because it avoids the need to introduce or remove protecting groups in these densely functionalized molecules.
Scheme 3.
(a) H4N2 H2O (2 equiv.), AcOH (5 equiv.), MeOH, 25 °C, 45 min; (b) pyridine/DIPEA (9:1), 150 °C, 48 h; (c) Pb(OAc)4 (1.1 equiv.), CH2CI2, 0 °C, 30 min (43% of 9 from 2); (c') Pb(OAc)4 (1.2 equiv.), PhCH2COOH (50 equiv.) (premixed), CH2CI2, 0 °C, 30 min. (36% of 10 from 2); (d) hv (300 nm), EtOAc/benzene (1:1) or MeOH, 25 °C (67–92% for 3, 90% for 4)
An additionally attractive aspect of this synthetic strategy is that it allows access to a wide variety of analogs as exemplified below. For example, the lead (IV)–mediated pyrazoline oxidation can be easily modified to accommodate the direct introduction of other esters at the C13 position. Toward this end, when the acetate ligands of lead tetraacetate are exchanged by premixing with an excess of phenylacetic acid (44), the resulting salt induces the oxidative conversion of pyrazoline 8 to diazene 10 (in 36% yield for three steps from 2). Subsequent photolysis affords the natural product and therapeutic lead DPP (4) in 90% yield, or 13% overall yield from 1.
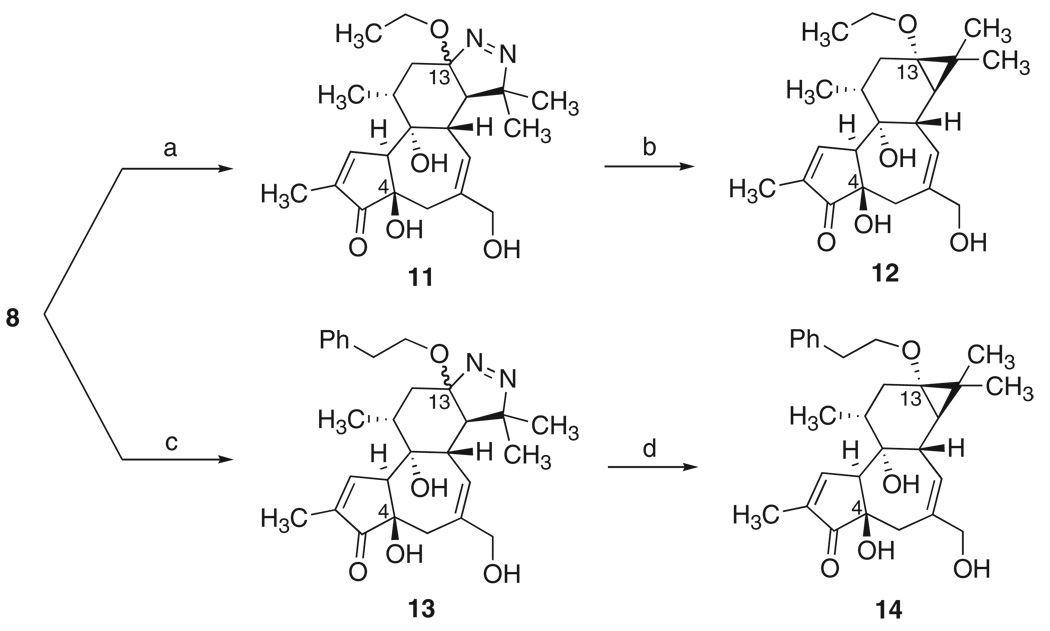
We have also found that this procedure can be used to access previously unknown ether analogs of prostratin (Scheme 4). Ether analogs 12 and 14 were selected as initial targets on the basis of their structural similarity to prostratin and DPP. They would also be more stable against hydrolytic decomposition than prostratin itself, which contains a hydrolytically labile ester group. The use of a seemingly straightforward, classical Williamson etherification to make such ethers from a C13 alcohol would be complicated because of the well-known facile epimerization of the C4 center in phorbol derivatives under mildly basic reaction conditions (45). Selective etherification of the C13 alcohol would also require extensive use of protecting groups to suppress reaction of the other alcohol functionalities. Instead, the ethyl ether analog 12 was readily prepared from pyrazoline 8 by treatment with PhI(OAc)2 in ethanol to afford diazenes 11, which were subsequently photolyzed. Intermediate 11 is a 3:2 mixture of C13 epimers, but photolysis of either isomer gives the desired product 12 in up to 90% yield. The phenylethyl ether analog 14 was analogously synthesized in a straightforward fashion from 8 via intermediate 13. This procedure thus provides a facile route to non-natural analogs of prostratin, based on a simple variation in our original plan.
Scheme 4.
(a) Phl(OAc)2 (1.2 equiv.), EtOH, 0 °C, 30 min, 24% (3:2 mixture of diastereomers, 3 steps from 2); (b) hv (300 nm), EtOAc, 25 °C, 72–90%; (c) Phl(OAc)2 (5 equiv.), PhCH2CH2OH, 0 °C to 25 °C, 4 h, 18% (2:1 mixture of diastereomers, 3 steps from 2); (d) hv(300 nm), EtOAc,25 °C, 87–91%.
This practical synthesis of prostratin, DPP, and other 12-deoxyphorbol analogs enables the fuller investigation of their mode of action and the identification of potentially superior clinical candidates that could be used in the treatment of HIV.
Footnotes
Information about obtaining reprints of this article or about obtaining permission to reproduce this article in whole or in part can be found at: http://www.sciencemag.org/about/permissions.dtl
Supporting Online Material
www.sciencemag.org/cgi/content/full/320/5876/649/DC1
Materials and Methods
References and Notes
- 1.UNAIDS/WHO. AIDS Epidemic Update: December 2007. available at http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf.
- 2.Chun T-W, et al. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13193. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang QE. Med. Sci. Monit. 2004;10:155. [PubMed] [Google Scholar]
- 4.Siliciano JD, et al. J. Infect. Dis. 2007;195:833. doi: 10.1086/511823. [DOI] [PubMed] [Google Scholar]
- 5.Laurence J, Sikder SK, Jhaveri S, Salmon JE. Biochem. Biophys. Res. Commun. 1990;166:349. doi: 10.1016/0006-291x(90)91952-o. [DOI] [PubMed] [Google Scholar]
- 6.Roebuck KA, Gu DS, Kagnoff MF. AIDS. 1996;10:819. doi: 10.1097/00002030-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Bocklandt S, Blumberg PM, Hamer DH. Antiviral Res. 2003;59:89. doi: 10.1016/s0166-3542(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 8.Biancotto A, et al. J. Virol. 2004;78:10507. doi: 10.1128/JVI.78.19.10507-10515.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulkosky J, et al. Blood. 2001;98:3006. doi: 10.1182/blood.v98.10.3006. [DOI] [PubMed] [Google Scholar]
- 10.Korin YD, Brooks DG, Brown S, Korotzer A, Zack JA. J. Virol. 2002;76:8118. doi: 10.1128/JVI.76.16.8118-8123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulkosky J, et al. AIDS Res. Hum. Retroviruses. 2004;20:497. doi: 10.1089/088922204323087741. [DOI] [PubMed] [Google Scholar]
- 12.Gulakowski RJ, McMahon JB, Buckheit RW, Jr, Gustafson KR, Boyd MR. Antiviral Res. 1997;33:87. doi: 10.1016/s0166-3542(96)01004-2. [DOI] [PubMed] [Google Scholar]
- 13.Witvrouw M, et al. Antiviral Chem. Chemother. 2003;14:321. doi: 10.1177/095632020301400604. [DOI] [PubMed] [Google Scholar]
- 14.Rullas J, et al. Antiviral Ther. 2004;9:545. [PubMed] [Google Scholar]
- 15.Hezareh M, et al. Antiviral Chem. Chemother. 2004;15:207. doi: 10.1177/095632020401500404. [DOI] [PubMed] [Google Scholar]
- 16.Trushin SA, et al. J. Virol. 2005;79:9821. doi: 10.1128/JVI.79.15.9821-9830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams SA, et al. J. Biol. Chem. 2004;279:42008. doi: 10.1074/jbc.M402124200. [DOI] [PubMed] [Google Scholar]
- 18.Brown SJ, et al. paper presented at the 15th International AIDS Conference; 11 to 16 June 2004; Bangkok, Thailand. (abstract no. TuPeB4490), available at http://gateway.nlm.nih.gov/MeetingAbstracts/102282312.html. [Google Scholar]
- 19.Evans FJ, Schmidt RG. Acta Pharmacol. Toxicol. (Copenhagen) 1979;45:181. doi: 10.1111/j.1600-0773.1979.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 20.Cashmore AR, et al. Tetrahedron Lett. 1976;17:1737. [Google Scholar]
- 21.Miana GA, Bashir M, Evans FJ. Planta Med. 1985;51:353. doi: 10.1055/s-2007-969515. [DOI] [PubMed] [Google Scholar]
- 22.Cox PA. Pharm. Biol. 2001;39:33. doi: 10.1076/phbi.39.s1.33.0001. [DOI] [PubMed] [Google Scholar]
- 23.Gustafson KR, et al. J. Med. Chem. 1992;35:1978. doi: 10.1021/jm00089a006. [DOI] [PubMed] [Google Scholar]
- 24.Johnson H, Banack SA, Cox PA. paper presented at the 48th Annual Meeting of Society for Economic; 4 to 7 June 2007; Botany, Chicago, IL. available at www.econbot.org/_organization_/07_annual_meetings/meeting_abstracts/2007.php. [Google Scholar]
- 25.Hongpaisan J, Alkon DL. Proc. Natl. Acad. Sci. U.S.A. 2007;104:19571. doi: 10.1073/pnas.0709311104. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black H. Scientist. 2004;18:59. [Google Scholar]
- 27.Wender PA, et al. Curr. Drug Discov. Technol. 2004;1:1. doi: 10.2174/1570163043484888. [DOI] [PubMed] [Google Scholar]
- 28.Szallasi Z, Krsmanovic L, Blumberg PM. Cancer Res. 1993;53:2507. [PubMed] [Google Scholar]
- 29.Cairnes DA, Mirvish SS, Wallcave L, Nagel DL, Smith JW. Cancer Lett. 1981;14:85. doi: 10.1016/0304-3835(81)90013-6. [DOI] [PubMed] [Google Scholar]
- 30.Thielmann HW, Hecker E. Liebigs Ann. Chem. 1969;728:158. [Google Scholar]
- 31.Wender PA, et al. J. Am. Chem. Soc. 1989;111:8957. [Google Scholar]
- 32.Wender PA, McDonald FE. J. Am. Chem. Soc. 1990;112:4956. [Google Scholar]
- 33.Wender PA, Rice KD, Schnute ME. J. Am. Chem. Soc. 1997;119:7897. [Google Scholar]
- 34.Gschwendt M, Hecker E. Tetrahedron Lett. 1970;11:567. doi: 10.1016/s0040-4039(01)97771-8. [DOI] [PubMed] [Google Scholar]
- 35.Hirota M, et al. Cancer Res. 1988;48:5800. [PubMed] [Google Scholar]
- 36.Haas W, Sterk H, Mittelbach M. J. Nat. Prod. 2002;65:1434. doi: 10.1021/np020060d. [DOI] [PubMed] [Google Scholar]
- 37.Fairless D. Nature. 2007;449:652. doi: 10.1038/449652a. [DOI] [PubMed] [Google Scholar]
- 38.Bowry VW, Ingold KU. J. Am. Chem. Soc. 1992;114:4992. [Google Scholar]
- 39.Bartsch H, Hecker E, Naturforsch Z. Teil B. 1969;24:91. [Google Scholar]
- 40.Materials and methods are detailed in supporting material available on Science Online.
- 41.Engel PS, Shen L. Can. J. Chem. 1974;52:4040. [Google Scholar]
- 42.Freeman JP. J. Org. Chem. 1964;29:1379. [Google Scholar]
- 43.For an example of demonstrated technology for large-scale organic photolysis, see (46).
- 44.Buston JEH, Claridge TDW, Moloney MG. J. Chem. Soc. Perkin Trans.2. 1995;1995:639. [Google Scholar]
- 45.Tseng S-S, Van Duuren BL, Solomon JJ. J. Org. Chem. 1977;42:3645. doi: 10.1021/jo00443a002. [DOI] [PubMed] [Google Scholar]
- 46.Hook BDA, et al. J. Org. Chem. 2005;70:7558. doi: 10.1021/jo050705p. [DOI] [PubMed] [Google Scholar]
- 47.We thank T. Benvegnu T. Storz-Eckerlin (Stanford University) for exploratory studies on this project. This work was supported by grants from NIH to P.A.W. (CA31841 and CA31845).