Abstract
Objectives
To examine whether indomethacin, gender, neonatal and sociodemographic factors predict patterns of receptive language development from 3–12 years of age in preterm children.
Methods
355 children born in 1989–1992 with birth weight 600–1250g were evaluated at 3, 4.5, 6, 8 and 12 years with the Peabody Picture Vocabulary Test - Revised (PPVT-R) as a measure of receptive language. Hierarchical growth-curve modeling was used to explore differences in language trajectories.
Results
From 3 to 12 years corrected ages, preterm children displayed catch-up gains on the PPVT-R. Preterm children started with an average standard score of 84.1 at 3 years and gained 1.2 points per year across the age period studied. Growth-curve analyses on PPVT-R raw scores revealed an indomethacin-by-gender effect on initial scores at 3 years with preterm boys randomized to indomethacin scoring, on average, 4.2 points higher than placebo control boys. However, velocity of receptive vocabulary development from 3–12 years did not differ by treatment groups. Children with grade 3–4 intraventricular hemorrhage, periventricular leukomalacia or grade 2 and above ventriculomegaly demonstrated slower gains in skills over time than those who did not suffer severe brain injury. Significant differences in language trajectories were predicted by maternal education and minority status. Higher initial scores and faster language development were observed among children whose mothers had higher education levels and who had not identified themselves as a minority ethnic group.
Conclusion
Although indomethacin incurs an initial benefit in preterm boys, this pharmacologic intervention did not alter the developmental trajectory of PPVT-R scores in our study subjects. Severe brain injury leads to long-term sequelae on language development, whereas a socioeconomically advantaged environment supports better language development among preterm children.
Keywords: Very low birth weight, preterm birth, language development, preschool outcome, middle childhood development, indomethacin, intraventricular hemorrhage
Preterm birth increases the risk of neurodevelopmental impairment. Studies have consistently shown poorer cognitive, language, and academic skills among preterm children compared to term peers.(1–4) Whereas some authors have suggested stable neurodevelopmental function with increasing age,(5) some have found a decline in cognitive scores,(3, 6) while others have observed the opposite.(7–9) Studies on longitudinal neurodevelopmental outcomes have generally provided broad descriptions of test findings(7) or compared results across two distinct periods in the child’s life using statistical techniques that may be insufficient to capture change in cognitive function.(3, 5, 6, 9, 10)
One of the main goals of neonatal interventions is to reduce the rates of both neonatal morbidities and neurodevelopmental impairment. Prophylactic indomethacin decreases the incidence and severity of intraventricular hemorrhage (IVH).(11, 12) Furthermore, exposure to indomethacin is associated with reduction in white matter injury on cerebral magnetic resonance imaging (MRI) in extremely preterm infants.(13) As part of the Multicenter Randomized Indomethacin IVH Prevention Trial, preterm children were longitudinally followed from birth to 12 years. A previous study on this cohort showed an increase in median Peabody Picture Vocabulary Test - Revised (PPVT-R) standard scores from 3 to 8 years.(8) Among preterm children with no early brain injury, standard scores were 89, 92, 97, and 99 at 3, 4.5, 6, and 8 years corrected age, respectively. Another report of this cohort also indicated that preterm boys assigned to indomethacin performed better in cognitive and verbal tasks compared to preterm placebo controls across time.(14)
Statistical analyses used in the previous studies on this cohort did not allow examination of patterns of change, at the individual or group level. One way to overcome this limitation is to apply individual growth modeling for analyzing outcome data. One of the advantages of growth modeling is estimation of both average trajectory (change at the group-level) and individual trajectories (change at the person-level), thus permitting exploration of putative factors in variability across subjects as well as within the subject.(15, 16)
Taylor and Hack(17) have used growth modeling to describe the longitudinal neuropsychological findings of very low birth weight (VLBW) children from 7–14 years of age compared to term controls. Their study demonstrated that children with birth weights less than 750 grams (g) made slower cognitive progress than their term counterparts, especially on tasks of visual-motor integration and executive function. Moreover, environmental factors seemed to modulate changes in cognitive function in certain subgroups: among children from lower socio-economic background, those with birth weight between 750–1499 g exhibited some catch-up growth on the vocabulary subtest of the Wechsler Intelligence Scale for Children between 7–14 years of age.
Using growth modeling, the main objective of the current study was to examine the effect of prophylactic indomethacin on receptive language development across 3–12 years corrected ages among preterm children and to determine whether this effect differed by gender. A second objective was to assess the impact of other biological and environmental factors on language trajectories. It was hypothesized that preterm males assigned to indomethacin would demonstrate improved language development compared to male placebo controls, and that preterm children who suffered severe brain injury on neonatal cranial ultrasound would exhibit a slower pattern of language development compared to preterm children without brain injury.
METHODS
Between September 1989 and August 1992, 505 infants with a birth weight of 600–1250 g admitted by 6 hours of age to Women and Infants’ Hospital (Providence, RI), Maine Medical Center (Portland, ME), and Yale New Haven Hospital (New Haven, CT) were enrolled after parental consent in a trial of prophylactic indomethacin to prevent IVH. Details of the newborn protocol have been described previously.(11, 18, 19) Of the 505 infants, 431 did not have IVH by 6 hours and were randomized to indomethacin or placebo. Follow-up at any visit from 3 to 12 years of age was available for 355 (92%) of the 385 known survivors at 3 years.
The institutional review boards of the 3 participating centers approved all protocols related to the trial. Informed consents were obtained for each follow-up visit.
Neonatal and socio-demographic data were retrieved from the study database. Severe brain injury was defined as the presence of either grade 3–4 IVH, periventricular leukomalacia (PVL), or moderate to severe ventriculomegaly on cranial ultrasounds.(20) Bronchopulmonary dysplasia (BPD) was defined as oxygen need at 28 days of age and chest x-ray findings consistent with BPD.
The Peabody Picture Vocabulary Test-Revised (PPVT-R)(21) was administered from 3 to 12 years by school psychologists blinded to the participants’ perinatal histories and trained for high inter-rater reliability. Participants with significant impairments were assigned a raw score of 0 when unable to be tested. The PPVT-R is a multiple-choice test that assesses receptive vocabulary, more specifically word knowledge. The examinee is asked to identify the picture associated with the spoken word. The number of correct answers gives the raw score (i.e. a raw score of 50 indicates that 50 words were well understood). The raw score is then transformed into a standard score (mean 100, standard deviation 15).
STATISTICAL ANALYSIS
The multilevel model approach to individual growth-curve modeling (SAS 9.1 PROC MIXED)(16, 22) was used to delineate the different language trajectories. All subjects were entered in the statistical model. Two main parameters were involved in growth modeling: the “intercept parameter”, which represented initial status and the “slope parameter”, which described the rate of growth. Both were interpreted as regular regression coefficients. To improve the interpretability of the coefficients, the age variable was centered at 3 years (AGE-3) , since this was the starting point of the study period.(22) Therefore, the intercept estimate corresponded to the initial PPVT-R score at 3 years of age. The slope estimate expressed the rate of change in PPVT-R scores from 3 through 12 years. In addition, growth-curve analyses yielded a random effect component (or variance component) which described the residual variability within the individual and between individuals. The variance component allowed computation of the explainable variation accounted by a given predictor.(22)
An initial unconditional growth model was created that did not include any predictor variables to examine the effect of time on change in PPVT-R scores. This model provided a baseline for comparison when predictors were entered in the analysis to explore inter-individual variability. The following predictors were examined for their effect on the intercept, slope, and curvature parameters: indomethacin, male gender, birth weight, antenatal steroids, severe brain injury, BPD, maternal education (< high school, high school graduate, ≥ 1 year of college), and minority status by maternal report. Interaction terms were created between gender and indomethacin, gender and BPD, and gender and minority status. Only significant predictors (significant p-value set at < 0.15) were entered in the final step of the analysis which involved fitting a model that controlled for all variables at all assessment points. Indomethacin and gender were retained by force.
In the first step, age-standardized scores were used to compare preterm children as a group to standard norms and to determine whether catch-up gains were observed over time. The model only involved intercept and slope parameters. In the second step, to compare word knowledge between the indomethacin versus placebo groups, analyses were conducted on raw scores rather than on age-standardized scores since raw scores normally systematically increased over time and therefore were more sensitive to changes. A quadratic term, (AGE-3)2, was added for a better fit to the curvilinear relationship between time and PPVT-R raw scores. This quadratic change trajectory did not have a constant slope, i.e. rate of growth accelerated (positive values), remained constant, or decelerated (negative values) over time as indicated by the “curvature parameter” associated with (AGE-3).2
RESULTS
Table 1 describes neonatal characteristics and demographics of the preterm cohort. At each assessment point, groups were similar for child and maternal factors. Attrition rates remained constant for the five follow-up evaluations. Table 2 displays PPVT-R standardized scores by gender and treatment groups. Based on all subjects across the five time periods, an indomethacin-by-gender effect was observed with preterm boys randomized to indomethacin performing better than preterm boys randomized to placebo (p-value 0.03). This beneficial effect was not demonstrated in girls.
TABLE 1.
Characteristics of the preterm cohort at each assessment from 3 to 12 years (total cohort followed at any point n=355).
| 3 years | 4.5 years | 6 years | 8 years | 12 years | |
|---|---|---|---|---|---|
| Number | 317 | 313 | 313 | 321 | 314 |
| Attrition rate* | 18% | 19% | 19% | 17% | 18% |
| Child characteristics | |||||
| Gestation, mean (SD), wk | 28 (2) | 28 (2) | 28 (2) | 28 (2) | 28 (2) |
| Birth weight, mean (SD), g | 963 (174) | 966 (177) | 965 (174) | 967 (175) | 965 (175) |
| Male sex | 54% | 55% | 53% | 54% | 55% |
| Maternal steroids | 35% | 36% | 36% | 35% | 36% |
| Indomethacin | 50% | 51% | 50% | 50% | 50% |
| Severe brain injury | 6% | 6% | 7% | 7% | 6% |
| Bronchopulmonary dysplasia | 45% | 45% | 43% | 43% | 45% |
| Maternal factors | |||||
| Maternal education | |||||
| - < high school | 14% | 13% | 14% | 13% | 13% |
| - High school | 48% | 48% | 47% | 50% | 48% |
| - > 1 year of college | 38% | 39% | 39% | 37% | 39% |
| Race and ethnicity** | |||||
| - White non-Hispanic | 73% | 73% | 74% | 72% | 72% |
| - African American | 19% | 19% | 18% | 19% | 19% |
| - White Hispanic | 6% | 5% | 6% | 7% | 7% |
| - Others | 2% | 2% | 3% | 2% | 2% |
| Age at assessment, mean (SD), years |
3.2 (0.1) | 4.7 (0.1) | 6.3 (0.2) | 8.3 (0.2) | 12.2 (0.4) |
Attrition rate is calculated with a denominator of 385 eligible subjects at each assessment.
Sum of percentages does not always equal to 100% because of rounding.
TABLE 2.
Mean Peabody Picture Vocabulary Test - Revised standard scores by indomethacin and gender at each assessment time
| 3 years Mean (SD) |
4.5 years Mean (SD) |
6 years Mean (SD) |
8 years Mean (SD) |
12 years Mean (SD) |
|
|---|---|---|---|---|---|
| Males | |||||
| Indomethacin | 87 (21) | 87 (20) | 97 (20) | 95 (23) | 97 (23) |
| Placebo | 78 (25) | 79 (30) | 87 (30) | 90 (30) | 92 (28) |
| Females | |||||
| Indomethacin | 83 (22) | 88 (22) | 93 (25) | 91 (25) | 92 (25) |
| Placebo | 89 (17) | 90 (22) | 92 (22) | 93 (22) | 97 (20) |
Interaction test of indomethacin by male gender, p-value = 0.03 across all age groups
Growth-curve analysis performed on PPVT-R standardized scores from 3 through 12 years suggested catch-up gains over time for the entire preterm cohort compared to the reference population (age standardized mean of 100). Based on the data displayed in figure 1, average preterm children began with a standard score of 84.1 at 3 years (95% confidence interval (CI) 81.8–86.5) and gained 1.2 points per year (95% CI 1.0–1.4) across the study period.
FIGURE 1.
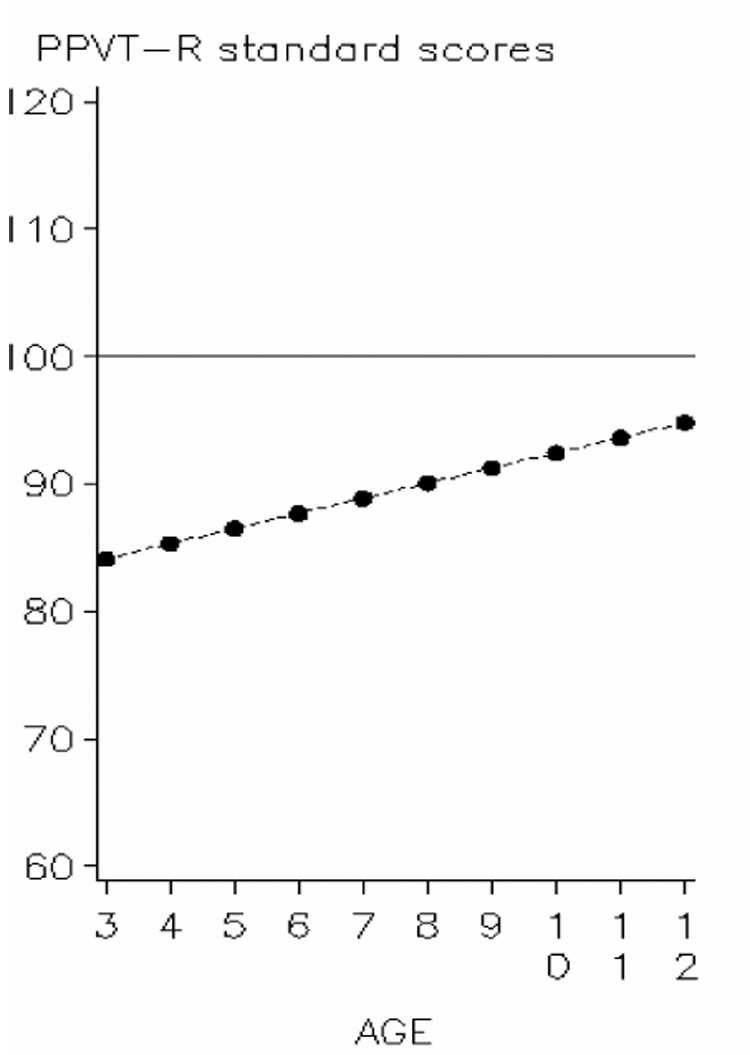
Predicted trajectory of PPVT-R standard scores among preterm children from 3 to 12 years corrected age in comparison to the reference population standard norms.
Preterm children (dotted line) exhibited catch-up in PPVT-R standard scores when compared to the reference population (mean = 100). Average initial score at 3 years for the preterm cohort was 84.1 increased by 1.2 point per year over the studied period.
Analysis on PPVT-R raw scores to delineate developmental trajectories within the preterm cohort indicates that based on the unconditional growth model which explores the relationship between age and outcome (table 3), PPVT-R raw scores increased, as expected, over baseline (AGE estimate = 17.4), although growth rate decelerated as the child got older (AGE2 estimate = −0.7). In other words, the increase in PPVT-R raw scores was more pronounced in the earlier years, but over time the magnitude of growth diminished. The model estimated that preterm children were able to identify, on average, 12.2 pictures on the PPVT-R at age 3, which is lower than the average PPVT-R raw scores of 14 to 38 for the standard population.(21) Raw scores increased by 16.7 points from 3 to 4.5 years (see appendix 1 for calculations). Growth rate then gradually declined over time with an increase in score of 5.5 points from 11 to 12 years.
TABLE 3.
Individual growth models for longitudinal changes in PPVT-R raw scores and effects of indomethacin and gender on receptive language development from 3 through 12 years corrected ages
| Parameters and growth predictors | Unconditional growth model - quadratic change | Unadjusted model-indomethacin and gender effect | Adjusted model ∫- indomethacin and gender effect | |
|---|---|---|---|---|
| Estimate (SE) | Estimate (SE) | Estimate (SE) | ||
| Fixed Effects | ||||
| Initial | Intercept | 12.2 (0.7)* | 13.9 (1.4)* | 11.3 (2.1)* |
| Status | Indomethacin | −1.3 (2.0) | −1.2 (1.8) | |
| Male gender | −5.0 (1.9)** | −4.6 (1.7)** | ||
| Indomethacin × Male | 5.7 (2.7)** | 5.4 (2.4)** | ||
| Rate Of change | AGE (in years) | 17.4 (0.3)* | 17.1 (0.7)* | 15.6 (1.0)* |
| AGE × Indomethacin | 0.4 (1.0) | −0.5 (0.4) | ||
| AGE × Male | −0.1 (1.0) | 0.1 (0.4) | ||
| AGE × Indomethacin × Male | 0.4 (1.4) | 0.4 (0.5) | ||
| AGE2 | −0.7 (0.0)* | −0.7 (0.1)* | −0.5 (0.1)* | |
Adjusted for antenatal steroids, BPD, maternal education, minority status.
p-value < 0.001
p-value<0.05
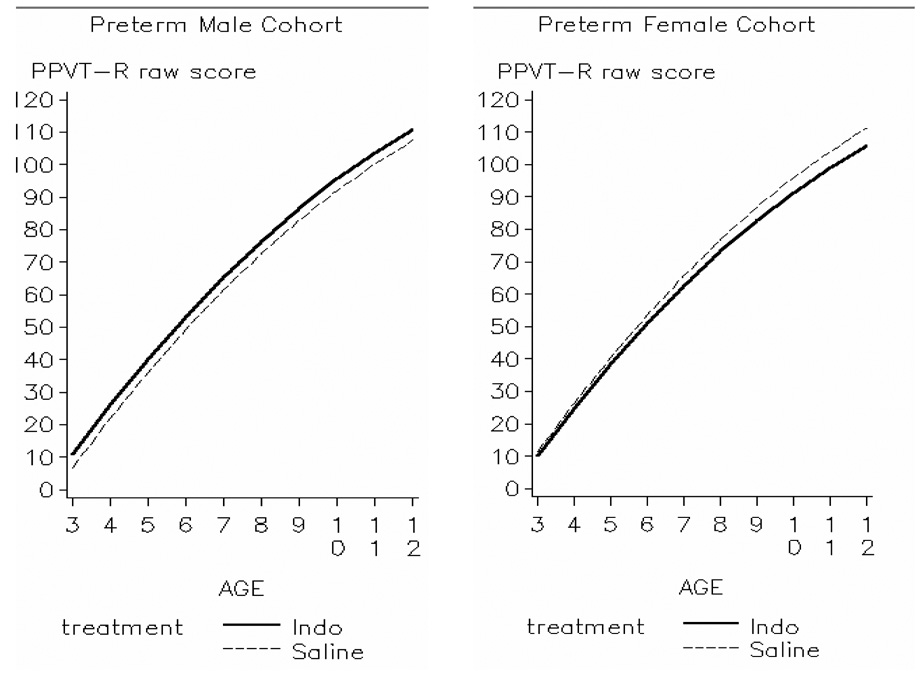
Figure 2 along with results in table 3 display patterns of vocabulary development for each treatment group (indomethacin vs. placebo) stratified by gender. Males randomized to indomethacin had higher scores at 3 years than male placebo controls, whereas both indomethacin and placebo females had comparable initial PPVT-R raw scores. Stated differently, after controlling for confounders, the indomethacin effect was not significant in females (estimate ± standard error: −1.2 ± 1.8), but the significant indomethacin-by-gender effect (5.4 ± 2.4, p-value < 0.05) translated into a 4.2-point advantage in PPVT-R raw scores at 3 years for indomethacin males. Furthermore, preterm boys, as a group, obtained lower 3-year PPVT-R raw scores than preterm girls (−4.6 ± 1.7, p-value < 0.05). As the children grew older, rates of receptive language development were comparable between the two treatment groups irrespective of gender (non significant interactions between Age × Indomethacin and Age × Indomethacin × Male).
FIGURE 2.
Comparison of increase in PPVT-R raw scores from 3 to 12 years corrected age between children randomized to indomethacin or placebo stratified by gender
Preterm males randomized to indomethacin (solid line) started at a slightly higher level than preterm saline males (dashed line), whereas preterm females, regardless of treatment, started at similar levels (p = 0.025 for interaction term indomethacin × male). Over time, growth in PPVT-R raw scores was similar across groups (p = 0.445).
Table 4 shows the effect of selected predictors on PPVT-R raw scores after adjustment for the other covariates. The intercept column indicates the magnitude of the effect of each predictor on initial PPVT-R raw score at 3 years. The slope column depicts the effect of each predictor on annual increase in PPVT-R raw score. Finally, the curvature column describes whether the predictor is associated with an acceleration, stabilization, or deceleration in growth. The intercept was influenced by gender (p-value < 0.05), presence of BPD (p-value < 0.05), level of maternal education (p-value < 0.005), and minority status (p-value < 0.005). The indomethacin-by-gender effect was lost with addition of severe brain injury in the model. When indomethacin, male gender, and indomethacin-by-gender were entered separately (as shown in the unadjusted model in table 3), they accounted for 6% of the variance in initial PPVT-R raw score at 3. The presence of severe brain injury, maternal education (college education vs. no high school degree), and minority status had an impact on annual increase in PPVT-R raw score. All preterm children displayed deceleration in rate of vocabulary development, but greater deceleration was observed among those whose mothers had higher levels of education (vs. no high school degree), whereas less deceleration was found among children with severe brain injury or from minority groups.
TABLE 4.
Effects of child and maternal variables on development in PPVT-R raw scores from 3 to 12 years of corrected ages among preterm children after adjustment for covariates (n=346)
| Predictors | Intercept | Slope (interaction with AGE) Estimate (SE) |
Curvature (interaction with AGE2) Estimate (SE) |
Explainable between subject variation in initial status ∫ | Explainable between subject variation in linear growth ∫ |
|---|---|---|---|---|---|
| Baseline | 11.2 (2.1) | 16.1 (1.0) | −0.6 (0.1) | - | - |
| Indomethacin | −0.7 (1.8) | NS | - |
 6% 6% |
0 |
| Male gender | −3.9 (1.7)** | NS | - | ||
| Indomethacin × Male gender | 4.3 (2.4)*** | NS | - | ||
| BPD | −3.3 (1.2)** | - | - | <1% | - |
| Severe brain injury | −3.9 (2.5) | −5.8 (1.3)* | 0.3 (0.1)** | <1% | 14% |
| Antenatal steroids | 1.7 (1.3) | NS | - | <1% | 0 |
| Maternal education (vs. no high school) | |||||
| High school | 5.1 (1.9)* | 1.8 (1.0)*** | −0.2 (0.1)** | 2% | 0 |
| College education | 8.6 (2.0)* | 3.1 (1.1)** | −0.2 (0.1)** | 22% | 10% |
| Minority status by maternal report (vs.white non-hispanic) | −8.1 (1.4)* | −2.3 (0.8)** | 0.2 (0.1)** | 34% | 16% |
Calculated from the variance component (random effects) when each predictor is entered individually in the growth-curve model.
p-value < 0.001
p-value < 0.05
p-value < 0.15, NS: p-value > 0.15
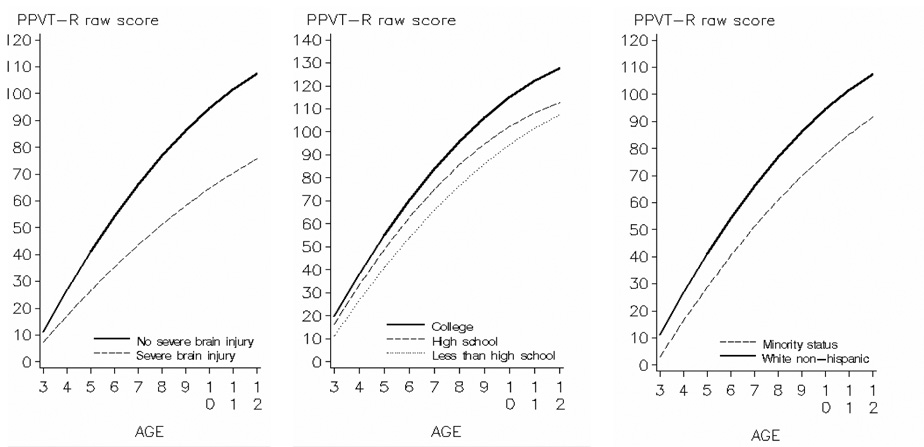
Figure 3 illustrates patterns of receptive language development for each level of predictive factors. Significant differences in language trajectories were predicted by severe neonatal brain injury. Although preterm children either with or without brain injury had similar PPVT-R raw scores at 3 years, rate of vocabulary development was much slower for children who suffered neonatal brain injury. This complication accounted for 14% of the variability in language development observed between subjects in the cohort. Maternal level of education also impacted on initial status. Children whose mothers did not complete high school started with lower scores than children whose mothers had at least a high school degree. By the end of the study period, the predicted gap between children whose mothers had completed at least one year of college and the combined group of those whose mothers had not graduated from high school or had only a high school degree increased significantly. Finally, minority status by maternal report influenced initial PPVT-R raw score at 3 years. White non-Hispanic children exhibited faster rate of receptive language development compared to others. Overall, minority status contributed to 34% of the explainable difference between subjects in initial raw scores and to 16% in growth rate.
FIGURE 3.
Effect of severe brain injury, maternal education, and minority status on development in PPVT-R raw scores from 3 to 12 years corrected age among preterm children
DISCUSSION
This study used a growth-curve modeling approach to determine the contribution of indomethacin and various biological and socio-demographic factors on vocabulary development of preterm children from 3 through 12 years. Deficits in receptive language development are associated with poorer reading skills which negatively affect academic achievement, hence the importance of studying vocabulary knowledge. Indomethacin incurred an advantage in boys, who displayed higher scores at 3 years compared to placebo controls. However, over time, indomethacin was not associated with faster vocabulary development. Children who sustained severe neonatal brain injury showed slower gains over time compared to those who did not have significant cerebral lesions. Finally, maternal education and minority status had the strongest impact on receptive language trajectories.
The benefit of prophylactic indomethacin on vocabulary and IQ test scores in boys during the early years has been demonstrated previously.(14) Preterm boys assigned to indomethacin had higher PPVT-R, verbal IQ and full-scale IQ scores from 3–8 years compared to placebo controls. This effect was not found among girls. It was speculated that indomethacin may have prevented preterm brain injury associated with maternal inflammation which led to pre-oligodendroglia injury and subsequent disturbances in myelination and connectivity.(13, 23) However, at 12 years, significant differences in neuropsychological test scores were no longer observed between the two treatment groups, even after gender stratification (in press). Findings from the current study show that the initial 4-point advantage in PPVT-R raw scores at 3 years among boys who received indomethacin was significant because the average raw score was 12.2 points at that age. Since indomethacin did not alter vocabulary development, this 4-point difference remained unchanged between 3 and 12 years. When preterm children reached 12 years, their mean PPVT-R raw score was 112 (appendix 1). These data suggest that between 3 and 12 years prophylactic indomethacin did not alter the developmental time course for PPVT-R scores in our study subjects.
Severe brain injury had a more sustained influence on receptive language trajectories. High-grade IVH and cystic PVL on neonatal ultrasounds are strong predictors of adverse neurodevelopmental outcomes in preterm children from infancy(24–26) to school-age years.(4, 27–30) This study identified an increasing gap in PPVT-R scores between preterm children with and without severe cerebral injury with increasing age. Koller et al studied developmental changes between birth and 6 years in a cohort of VLBW children(31) and found that higher indices of neonatal morbidity were associated with lower initial developmental scores. Furthermore, neurologically impaired infants at 12 months consistently achieved lower cognitive scores compared to neurologically normal VLBW children. These findings suggest that as cognitive demands increase in complexity with age, the injured brain has limited plasticity to compensate. Volumetric analysis of regional brain development in preterm children and adolescents using MRI has shown correlations between IVH and decreased subcortical gray matter,(32, 33) alteration in cortical white matter volumes,(33) and decreased volumes of the cerebellum and left caudate nucleus.(34) Therefore, severe neonatal brain insults disrupt cerebral development, potentially leading to slower gains in certain neuropsychological functions such as receptive language, thus indicating the vulnerability of the immature brain. Research on children who sustained severe traumatic brain injury at an early age show that these patients displayed lasting cognitive sequelae.(35).
This study highlighted the positive impact of higher levels of maternal education and an advantaged environment (measured by mother’s race and ethnicity) on vocabulary trajectories in preterm children, in keeping with previous longitudinal research.(17, 31, 36) Our data did not allow us to explore whether maternal education and minority status directly affected the outcome or were correlates of other sociodemographic factors associated with better outcomes such as higher socioeconomic status,(17) lower family stressors,(17) sensitive, stimulating and less restrictive home milieu,(37, 38) and living in a suburban rather than an urban community where early intervention and school services may be more widely available.(39) Our findings emphasize the importance of providing continuous developmental and academic supports to children at higher social risk. A systematic review(40) concluded that early childhood development programs were effective in improving school readiness and reducing grade retention and special education requirement among economically disadvantaged children.
One limitation of this study was the absence of a term control group since term subjects were enrolled at 8 years and growth-curve modeling requires at least three sets of data. Therefore, we used the PPVT-R age-standardized norm to evaluate catch-up over time. Although the average rise of 1.2 points per year in PPVT-R standard score is encouraging, it is possible that term controls would also exhibit similar gains. The PPVT-R scores of our study subjects will need to be followed through adolescence and into early adulthood.
CONCLUSION
Longitudinal follow-up of preterm children reveals that although a beneficial effect of prophylactic indomethacin is observed in the early years, this pharmacologic agent does not influence the developmental trajectory of PPVT-R scores in our VLBW preterm study subjects. Whereas severe brain injury during the neonatal period is the most detrimental biological factor on vocabulary development, social risks have the strongest effect on this outcome. Our findings support the need for preschool and school age interventions to support preterm children from economically disadvantaged environments.
Acknowledgments
This work was supported the National Institute of Health NS 27116
Abbreviations
- BPD
Bronchopulmonary dysplasia
- IVH
Intraventricular hemorrhage
- PPVT-R
Peabody Picture Vocabulary Test - Revised
- PVL
Periventricular leukomalacia
- SE
Standard error
- VLBW
Very low birth weight
APPENDIX 1
Calculations for predicted PPVT-R raw scores from the growth-curve model.
Based on the computed estimates from the growth-curve model, the following equation can be derived. Age is centered around 3 (age at the beginning of the studied period) to facilitate interpretability of the parameter estimates.
| PPVT-R raw score at given AGE | Equation from growth model 12.2 + 17.4 (AGE−3) − 0.7 (AGE−3)2 |
Estimated score | |
|---|---|---|---|
| 3 years | = | 12.2 + 17.4 (3−3) − 0.7 (3−3)2 | = 12.2 |
| 4 years | = | 12.2 + 17.4 (4−3) − 0.7 (4−3)2 | = 28.9 |
| PPVT-R raw score increase from 3 to 4 years = 16.7 points | |||
| 11 years | = | 12.2 + 17.4 (11−3) − 0.7 (11−3)2 | = 106.6 |
| 12 years | = | 12.2 + 17.4 (12−3) − 0.7 (12−3)2 | = 112.1 |
| PPVT-R raw score increase from 11 to 12 years = 5.5 points | |||
Footnotes
The authors have no financial relationships relevant to this article to disclose.
References
- 1.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 2.Anderson P, Doyle LW. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289(24):3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- 3.Saigal S, Hoult LA, Streiner DL, Stoskopf BL, Rosenbaum PL. School difficulties at adolescence in a regional cohort of children who were extremely low birth weight. Pediatrics. 2000;105(2):325–331. doi: 10.1542/peds.105.2.325. [DOI] [PubMed] [Google Scholar]
- 4.Taylor HG, Klein N, Drotar D, Schluchter M, Hack M. Consequences and risks of <1000-g birth weight for neuropsychological skills, achievement, and adaptive functioning. J Dev Behav Pediatr. 2006;27(6):459–469. doi: 10.1097/00004703-200612000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson CJ, Blackburn P, Pharoah PO. Longitudinal study of behaviour disorders in low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1999;81(1):F5–F9. doi: 10.1136/fn.81.1.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien F, Roth S, Stewart A, et al. The neurodevelopmental progress of infants less than 33 weeks into adolescence. Arch Dis Child. 2004;89(3):207–211. doi: 10.1136/adc.2002.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Angio CT, Sinkin RA, Stevens TP, et al. Longitudinal, 15-year follow-up of children born at less than 29 weeks' gestation after introduction of surfactant therapy into a region: neurologic, cognitive, and educational outcomes. Pediatrics. 2002;110(6):1094–1102. doi: 10.1542/peds.110.6.1094. [DOI] [PubMed] [Google Scholar]
- 8.Ment LR, Vohr B, Allan W, et al. Change in cognitive function over time in very low-birth-weight infants. JAMA. 2003;289(6):705–711. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]
- 9.Samuelsson S, Finnstrom O, Flodmark O, et al. A longitudinal study of reading skills among very-low-birthweight children: is there a catch-up? J Pediatr Psychol. 2006;31(9):967–977. doi: 10.1093/jpepsy/jsj108. [DOI] [PubMed] [Google Scholar]
- 10.Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116(2):333–341. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 11.Ment LR, Oh W, Ehrenkranz RA, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. 1994;93(4):543–550. [PubMed] [Google Scholar]
- 12.Schmidt B, Davis P, Moddemann D, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344(26):1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 13.Miller SP, Mayer EE, Clyman RI, et al. Prolonged indomethacin exposure is associated with decreased white matter injury detected with magnetic resonance imaging in premature newborns at 24 to 28 weeks' gestation at birth. Pediatrics. 2006;117(5):1626–1631. doi: 10.1542/peds.2005-1767. [DOI] [PubMed] [Google Scholar]
- 14.Ment LR, Vohr BR, Makuch RW, et al. Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. J Pediatr. 2004;145(6):832–834. doi: 10.1016/j.jpeds.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Cohen P. Using individual growth model to analyze the change in quality of life from adolescence to adulthood. Health Qual Life Outcomes. 2006;4:10. doi: 10.1186/1477-7525-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer JD. Using SAS PROC MIXED to Fit Multilevel Models, Hierarchical Models, and Individual Growth Models. J Educ Behav Stat. 1998;24(4):33. [Google Scholar]
- 17.Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight: neuropsychological findings. J Int Neuropsychol Soc. 2004;10(2):149–163. doi: 10.1017/S1355617704102038. [DOI] [PubMed] [Google Scholar]
- 18.Vohr B, Allan WC, Scott DT, et al. Early-onset intraventricular hemorrhage in preterm neonates: incidence of neurodevelopmental handicap. Semin Perinatol. 1999;23(3):212–217. doi: 10.1016/s0146-0005(99)80065-2. [DOI] [PubMed] [Google Scholar]
- 19.Ment LR, Oh W, Ehrenkranz RA, et al. Low-dose indomethacin therapy and extension of intraventricular hemorrhage: a multicenter randomized trial. J Pediatr. 1994;124(6):951–955. doi: 10.1016/s0022-3476(05)83191-9. [DOI] [PubMed] [Google Scholar]
- 20.Ment LR, Vohr B, Allan W, et al. The etiology and outcome of cerebral ventriculomegaly at term in very low birth weight preterm infants. Pediatrics. 1999;104(2 Pt 1):243–248. doi: 10.1542/peds.104.2.243. [DOI] [PubMed] [Google Scholar]
- 21.Dunn L, Dunn L. PPVT-R: Peabody Picture Vocabulary Test - Revised Form. Circle Pines, Minn: 1981. [Google Scholar]
- 22.Singer JD, Willet JB. Applied Longitudinal Data Analysis - Modeling Change and Event Occurence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 23.Volpe JJ, editor. Neurology of the Newborn. Fifth ed. Philadelphia: 2008. [Google Scholar]
- 24.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105(6):1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 25.Hack M, Wilson-Costello D, Friedman H, et al. Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000 g: 1992–1995. Arch Pediatr Adolesc Med. 2000;154(7):725–731. doi: 10.1001/archpedi.154.7.725. [DOI] [PubMed] [Google Scholar]
- 26.Aziz K, Vickar DB, Sauve RS, et al. Province-based study of neurologic disability of children weighing 500 through 1249 grams at birth in relation to neonatal cerebral ultrasound findings. Pediatrics. 1995;95(6):837–844. [PubMed] [Google Scholar]
- 27.Vollmer B, Roth S, Baudin J, et al. Predictors of long-term outcome in very preterm infants: gestational age versus neonatal cranial ultrasound. Pediatrics. 2003;112(5):1108–1114. doi: 10.1542/peds.112.5.1108. [DOI] [PubMed] [Google Scholar]
- 28.Neubauer AP, Voss W, Kattner E. Outcome of extremely low birth weight survivors at school age: the influence of perinatal parameters on neurodevelopment. Eur J Pediatr. 2007 doi: 10.1007/s00431-007-0435-x. [DOI] [PubMed] [Google Scholar]
- 29.Sherlock RL, Anderson PJ, Doyle LW. Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Hum Dev. 2005;81(11):909–916. doi: 10.1016/j.earlhumdev.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Ment LR, Allan WC, Makuch RW, Vohr B. Grade 3 to 4 intraventricular hemorrhage and Bayley scores predict outcome. Pediatrics. 2005;116(6):1597–1598. doi: 10.1542/peds.2005-2020. author reply 1598. [DOI] [PubMed] [Google Scholar]
- 31.Koller H, Lawson K, Rose SA, Wallace I, McCarton C. Patterns of cognitive development in very low birth weight children during the first six years of life. Pediatrics. 1997;99(3):383–389. doi: 10.1542/peds.99.3.383. [DOI] [PubMed] [Google Scholar]
- 32.Kesler SR, Ment LR, Vohr B, et al. Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol. 2004;31(5):318–325. doi: 10.1016/j.pediatrneurol.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nosarti C, Giouroukou E, Healy E, et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2008;131(Pt 1):205–217. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- 34.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284(15):1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 35.Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional Plasticity of Vulnerability After Eary Brain Injury? Pediatrics. 2005;116(6):1374–1382. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
- 36.Liaw FR, Brooks-Gunn J. Patterns of low-birth-weight children's cognitive development. Dev Psychol. 1993;29(6):12. [Google Scholar]
- 37.Landry SH, Smith KE, Miller-Loncar CL, Swank PR. Predicting cognitive-language and social growth curves from early maternal behaviors in children at varying degrees of biological risk. Dev Psychol. 1997;33(6):1040–1053. doi: 10.1037//0012-1649.33.6.1040. [DOI] [PubMed] [Google Scholar]
- 38.Burchinal MR, Roberts JE, Hooper S, Zeisel SA. Cumulative risk and early cognitive development: a comparison of statistical risk models. Dev Psychol. 2000;36(6):793–807. doi: 10.1037//0012-1649.36.6.793. [DOI] [PubMed] [Google Scholar]
- 39.Breslau N, Chilcoat HD, Susser ES, et al. Stability and change in children's intelligence quotient scores: a comparison of two socioeconomically disparate communities. Am J Epidemiol. 2001;154(8):711–717. doi: 10.1093/aje/154.8.711. [DOI] [PubMed] [Google Scholar]
- 40.Anderson LM, Shinn C, Fullilove MT, et al. The effectiveness of early childhood development programs. A systematic review. Am J Prev Med. 2003;24(3 Suppl):32–46. doi: 10.1016/s0749-3797(02)00655-4. [DOI] [PubMed] [Google Scholar]