Abstract
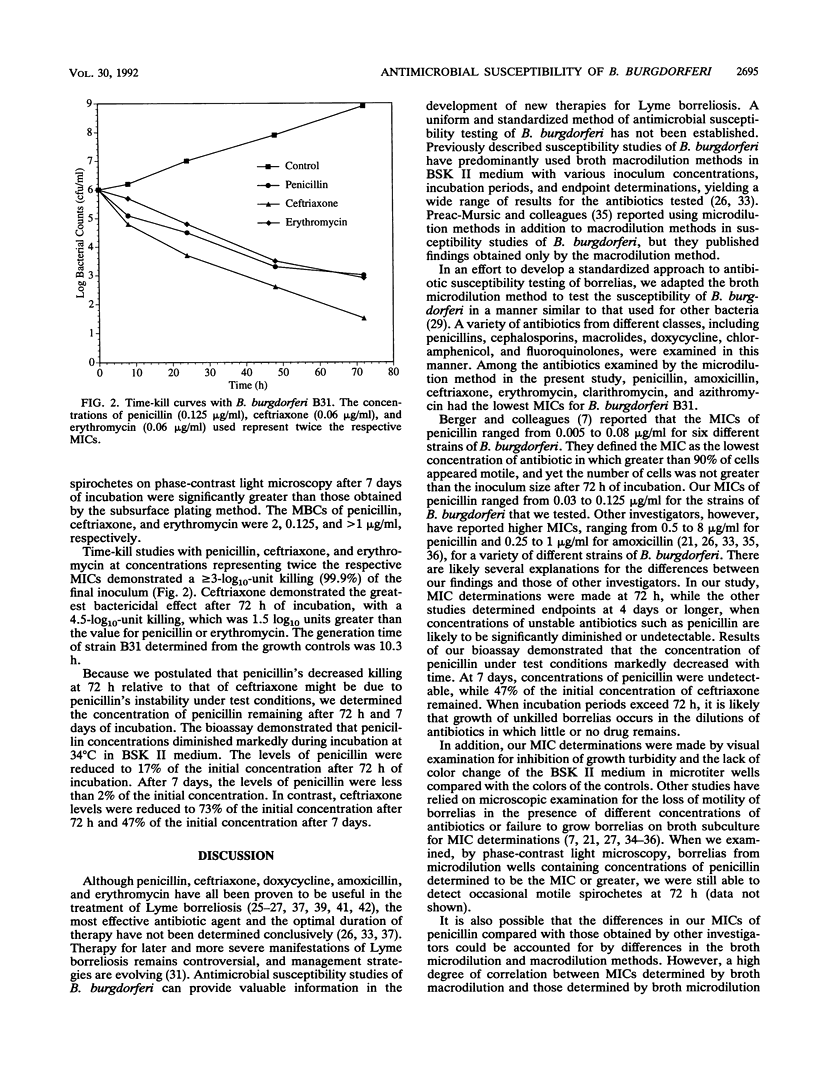
The susceptibility of Borrelia burgdorferi, the causative agent of Lyme borreliosis, to various antimicrobial agents varies widely among published studies. These differences are probably due in part to variations in susceptibility testing techniques and growth endpoint determinations. We developed a microdilution method for determining the MICs of antibiotics against B. burgdorferi. The method incorporated BSK II medium, a final inoculum of 10(6) cells per ml, and a 72-h incubation period and was found to be simple and highly reproducible. A variety of antibiotics and strains of B. burgdorferi and one strain of Borrelia hermsii were examined by this method. MICs of penicillin, ceftriaxone, and erythromycin for the B31 strain of B. burgdorferi were 0.06, 0.03, and 0.03 microgram/ml, respectively. We compared the MICs obtained by the microdilution method with those obtained by a macrodilution method using similar criteria for endpoint determinations and found the values obtained by both methods to be in close agreement. To further investigate the bactericidal activities of penicillin, ceftriaxone, and erythromycin against strain B31, we used subsurface plating to determine MBCs and we also performed time-kill studies. The MBCs of penicillin, ceftriaxone, and erythromycin were 0.125, 0.03, and 0.06 micrograms/ml, respectively. Time-kill curves demonstrated a greater than or equal to 3-log10-unit killing after 72 h with penicillin, ceftriaxone, and erythromycin; ceftriaxone provided the greatest reduction in CFU. The described methods offer a more standardized and objective approach to susceptibility testing of B. burgdorferi.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Todd W. J., Stoenner H. G. Action of penicillin on Borrelia hermsii. Antimicrob Agents Chemother. 1982 May;21(5):823–829. doi: 10.1128/aac.21.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Moody K. D., Beck D. S. Susceptibility of laboratory rats to isolates of Borrelia burgdorferi from different geographic areas. Am J Trop Med Hyg. 1990 Jun;42(6):596–600. doi: 10.4269/ajtmh.1990.42.596. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Lam K. Efficacy of vancomycin plus rifampin in experimental aortic-valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlations. J Infect Dis. 1985 Jan;151(1):157–165. doi: 10.1093/infdis/151.1.157. [DOI] [PubMed] [Google Scholar]
- Berger B. W., Kaplan M. H., Rothenberg I. R., Barbour A. G. Isolation and characterization of the Lyme disease spirochete from the skin of patients with erythema chronicum migrans. J Am Acad Dermatol. 1985 Sep;13(3):444–449. doi: 10.1016/s0190-9622(85)70187-9. [DOI] [PubMed] [Google Scholar]
- Bundoc V. G., Barbour A. G. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect Immun. 1989 Sep;57(9):2733–2741. doi: 10.1128/iai.57.9.2733-2741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Chandrasekar P. H., Crane L. R., Bailey E. J. Comparison of the activity of antibiotic combinations in vitro with clinical outcome and resistance emergence in serious infection by Pseudomonas aeruginosa in non-neutropenic patients. J Antimicrob Chemother. 1987 Mar;19(3):321–329. doi: 10.1093/jac/19.3.321. [DOI] [PubMed] [Google Scholar]
- Jackson G. G., Riff L. J. Pseudomonas bacteremia: pharmacologic and other bases for failure of treatment with gentamicin. J Infect Dis. 1971 Dec;124 (Suppl):S185–S191. doi: 10.1093/infdis/124.supplement_1.s185. [DOI] [PubMed] [Google Scholar]
- Johnson R. C. Isolation techniques for spirochetes and their sensitivity to antibiotics in vitro and in vivo. Rev Infect Dis. 1989 Sep-Oct;11 (Suppl 6):S1505–S1510. doi: 10.1093/clinids/11.supplement_6.s1505. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Kodner C., Russell M., Girard D. In-vitro and in-vivo susceptibility of Borrelia burgdorferi to azithromycin. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):33–38. doi: 10.1093/jac/25.suppl_a.33. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Kodner C., Russell M. In vitro and in vivo susceptibility of the Lyme disease spirochete, Borrelia burgdorferi, to four antimicrobial agents. Antimicrob Agents Chemother. 1987 Feb;31(2):164–167. doi: 10.1128/aac.31.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. E., Klein G. C., Schmid G. P., Feeley J. C. Susceptibility of the Lyme disease spirochete to seven antimicrobial agents. Yale J Biol Med. 1984 Jul-Aug;57(4):549–553. [PMC free article] [PubMed] [Google Scholar]
- Kriuchechnikov V. N., Korenberg E. I., Shcherbakov S. V., Kovalevskii Iu V., Levin M. L. Identifikatsiia borrelii, izolirovannykh v SSSR ot kleshchei Ixodes persulcatus Schulze. Zh Mikrobiol Epidemiol Immunobiol. 1988 Dec;(12):41–44. [PubMed] [Google Scholar]
- Kurtti T. J., Munderloh U. G., Johnson R. C., Ahlstrand G. G. Colony formation and morphology in Borrelia burgdorferi. J Clin Microbiol. 1987 Nov;25(11):2054–2058. doi: 10.1128/jcm.25.11.2054-2058.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft B. J., Gorevic P. D., Halperin J. J., Volkman D. J., Dattwyler R. J. A perspective on the treatment of Lyme borreliosis. Rev Infect Dis. 1989 Sep-Oct;11 (Suppl 6):S1518–S1525. doi: 10.1093/clinids/11.supplement_6.s1518. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Volkman D. J., Halperin J. J., Dattwyler R. J. New chemotherapeutic approaches in the treatment of Lyme borreliosis. Ann N Y Acad Sci. 1988;539:352–361. doi: 10.1111/j.1749-6632.1988.tb31869.x. [DOI] [PubMed] [Google Scholar]
- Mursic V. P., Wilske B., Schierz G., Holmburger M., Süss E. In vitro and in vivo susceptibility of Borrelia burgdorferi. Eur J Clin Microbiol. 1987 Aug;6(4):424–426. doi: 10.1007/BF02013102. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson A. Antibiotic treatment in Lyme borreliosis. Scand J Infect Dis Suppl. 1991;77:145–150. [PubMed] [Google Scholar]
- Preac-Mursic V., Weber K., Pfister H. W., Wilske B., Gross B., Baumann A., Prokop J. Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection. 1989 Nov-Dec;17(6):355–359. doi: 10.1007/BF01645543. [DOI] [PubMed] [Google Scholar]
- Preac-Mursic V., Wilske B., Schierz G. European Borrelia burgdorferi isolated from humans and ticks culture conditions and antibiotic susceptibility. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):112–118. doi: 10.1016/s0176-6724(86)80110-9. [DOI] [PubMed] [Google Scholar]
- Preac-Mursic V., Wilske B., Schierz G., Süss E., Gross B. Comparative antimicrobial activity of the new macrolides against Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis. 1989 Jul;8(7):651–653. doi: 10.1007/BF01968150. [DOI] [PubMed] [Google Scholar]
- Rahn D. W., Malawista S. E. Lyme disease: recommendations for diagnosis and treatment. Ann Intern Med. 1991 Mar 15;114(6):472–481. doi: 10.7326/0003-4819-114-6-472. [DOI] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T. G. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J Infect Dis. 1989 Dec;160(6):1018–1029. doi: 10.1093/infdis/160.6.1018. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Green J., Schoen R. T., Taylor E., Hutchinson G. J., Rahn D. W., Malawista S. E. Successful parenteral penicillin therapy of established Lyme arthritis. N Engl J Med. 1985 Apr 4;312(14):869–874. doi: 10.1056/NEJM198504043121401. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Malawista S. E., Newman J. H., Spieler P. N., Bartenhagen N. H. Antibiotic therapy in Lyme disease. Ann Intern Med. 1980 Jul;93(1):1–8. doi: 10.7326/0003-4819-93-1-1. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Pachner A. R., Malawista S. E. Neurologic abnormalities of Lyme disease: successful treatment with high-dose intravenous penicillin. Ann Intern Med. 1983 Dec;99(6):767–772. doi: 10.7326/0003-4819-99-6-767. [DOI] [PubMed] [Google Scholar]
- Stoenner H. G. Biology of Borrelia hermsii in Kelly medium. Appl Microbiol. 1974 Oct;28(4):540–543. doi: 10.1128/am.28.4.540-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. S., Burgdorfer W., Russell R., Francis B. J. Outbreak of tick-borne relapsing fever in Spokane County, Washington. JAMA. 1969 Nov 10;210(6):1045–1050. [PubMed] [Google Scholar]
- Wretlind B., Johnson R. C., Hansen K., Preac-Mursic V. Antibiotic susceptibility of Borrelia burgdorferi in vitro and in animal models. Scand J Infect Dis Suppl. 1991;77:143–144. [PubMed] [Google Scholar]