Abstract
Objective
The clearance rate of human papillomavirus (HPV) after conization is generally high, although some HPV infections persist. We investigated the factors that affect the clearance of HPV after conization in patients with negative margins.
Methods
We retrospectively analyzed 77 patients (mean age 39.9 years, range 25 to 51 years) with CIN 2/3 who underwent loop electrosurgical excision procedure (LEEP) conization with negative margins. All patients had a Pap smear and high-risk (HR) HPV testing using Hybrid Capture II system and HPV DNA chip before conization. We used≥1 relative light units (RLUs) as the cutoff for persistence of HPV after conization.
Results
High-risk HPV was detected in 73 of 77 (94.8%) patients before conization. At the 6-months follow-up, the high-risk HPV was eliminated in 60 of 73 (82.2%) patients. The HPV persistence rate after conization was 17.8% (13/73). Univariate analysis showed that persistent HPV infection after conization with negative margins was more likely to occur when the pretreatment viral load was high (RLU/positive control >100 (p=0.027) and the HPV was type 16 (p=0.021). Logistic regression analysis showed that preoperative HPV type 16 infection was the only significant independent factor (p=0.021) for HPV persistence out of age, cytology, punch biopsy histology, HPV viral load, and conization histology.
Conclusion
Conization effectively removes HR-HPV infection. HPV type 16 infection before conization was significantly related to HR-HPV persistence after conization with negative margins. Therefore, patients with HPV 16 infection before conization need to be followed closely.
Keywords: Conization, Negative margins, Persistent HPV infection, HPV type 16
INTRODUCTION
Persistent human papillomavirus (HPV) infection is a necessary event in cervical cancer tumorigenesis. Virtually all tumor cells in a cervical cancer contain the sequences of HPV types.1 Due to the universal presence of HPV in cervical tumor cells, HPV DNA tests are very useful for primary screening, the triage of equivocal cervical cytology, and posttreatment surveillance after conization or trachelectomy.2
Cervical intraepithelial neoplasia (CIN) is a well-known precursor of invasive cervical cancer. Women with untreated high-grade CIN are at risk of cervical cancer, whereas the risk is very low in treated women.3,4 The loop electrosurgical excision procedure (LEEP) has been a popular modality for the local treatment of CIN since the early 1990s because it has many advantages over cryosurgery or laser vaporization.5,6
Following the excision of CIN using LEEP, posttreatment CIN rates of 5-15% have been reported.7 Long-term follow-up after local treatment of CIN is mandatory due to the late occurrence of cervical cancer over a period of 20 years.8-12 Therefore, early detection of treatment failure is important.
Several studies have suggested risk factors for the prediction of residual/recurrent disease CIN after LEEP, although the results have been somewhat inconsistent.13-15 Age, cytology grade, menopause status, margin involvement, and HPV viral load have all been observed as risk factors for residual/recurrent disease in CIN treatment. Positive margins in the excised specimen was the greatest risk factors for the development of recurrent CIN in many studies,13,14 while a few recent studies identified the HPV viral load before LEEP as a predictor of persistence/recurrent disease. Only Gok et al.16 have studied the HPV genotype as a predictor for recurrence of CIN after local treatment. Most previous studies analyzed risk factors in a heterogenous group, irrespective of margin involvement, although margin status affects the persistence/recurrence of CIN.
In this retrospective study we assessed the risk factors of HPV persistence after LEEP of CIN 2/3 in the homogenous group of patients with negative margins, incorporating clinical factors including HPV viral loads and DNA types.
PATIENTS AND METHODS
1. Study population
A retrospective analysis was used to examine women who underwent LEEP for CIN at Soonchunhyang University Hospital, Korea, between January 2004 and September 2006. A total of 239 patients underwent LEEP conization due to CIN during the study period. The patients with margin positive (66 cases: 27.6%), unclear margin (16 cases: 6.7%), low grade lesion or less than CIN 1 in LEEP specimen (35 cases: 14.6%) and hysterectomy after conization (10 cases: 4.2%) were excluded. The inclusion criteria consisted of histologically verified CIN 2 and CIN 3 based on LEEP conization, and negative margins on pathological examinations in patients for whom both pre- and post-LEEP high-risk (HR) HPV test results (HCII) and pre-LEEP HR-HPV tests (HPV DNA chip assay) were available. Among 112 patients with negative margins in conization, 35 patients were excluded because of inadequate follow-up. Remaining 77 patients with negative margins were satisfactory for study inclusion criteria. Epidemiological data, HR-HPV test data, pathology reports, and follow-up data were reviewed from medical records.
All patients undergoing conization were followed-up before and after 6 months. HPV DNA type were determined before conization only. Multiple infections including HPV type 16 was allocated to HPV type 16 category. At the follow-up visit, a Pap test and HPV HCII tests were performed, and if indicated, colposcopy was done.
2. Cytology
A cervical smear was performed with a modified plastic spatula and an endocervical cytobrush (Mediland, Seoul, Korea). All of the cervical cytology in this study was liquid-based cytology (ThinPrep; Cytyc Corp., Boxborough, MA, USA). All specimens were stained using the Papanicolaou method and were evaluated using the Bethesda III system (2001). For the pathological diagnosis, colposcopy with direct biopsy was performed as indicated. Cytology was divided into two groups: the low-grade group included normal, atypical squamous cells of undetermined significance (ASCUS), atypical squamous cells-high grade (ASC-H), and low-grade squamous intraepithelial lesions (LSILs), while the high-grade group consisted of high-grade squamous intraepithelial lesions (HSILs). The results of the colposcopic-directed biopsy were also divided into two groups: low (chronic cervicitis, mild dysplasia) and high [moderate dysplasia to carcinoma in situ (CIS)] grade.
3. Detection of HPV
Samples for the HPV test were obtained after cytology sampling. In our study, Hybrid Capture II (HCII) and a HPV DNA chip assay were used to detect HPV. First, samples for HCII were obtained using a cytobrush (Digene cervical sampler; Digene, Gaithersburg, MD, USA) with a second swab, and transferred to a vial containing specimen transport medium. Then, a third swab specimen was obtained for the HPV DNA chip test using a similar cytobrush and transport medium for HPV genotyping. The HCII system (Digene®) for HPV detection is a signal-amplified hybridization antibody capture assay that uses chemiluminescent detection with a specific HPV RNA probe for carcinogenic high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). The relative light units (RLU)/positive control (PC) ratios were calculated as the ratio of the specimen luminescence to the luminescence of the 1.0 pg/ml HPV 16 cutoff standard, which represents a semiquantitative value for the cumulative viral burden of one or more of the above 13 oncogenic HPV types. The RLU/PC ratio ≥1 was considered a positive result, as proposed by the manufacturer. For HPV genotyping, a commercial HPV DNA chip (MyHPV Chip) was used. The HPV chip can detect 24 type-specific HPVs: 16 of the high-risk group (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 54, 56, 58, 59, 66, and 68) and eight of the low-risk group (6, 11, 34, 40, 42, 43, 44, and 70). Target HPV DNA was amplified using the polymerase chain reaction (PCR) with primers (HPV and human B-globulin) and the conditions provided by Mygene® (Seoul, Korea) and labeled using Cy5-deoxyuridine triphosphate (NEM Life Science Products, Boston, MA, USA). The PCR product was hybridized on the chip at 40℃ for 2 h and washed with 3 × SSPE (3.0 M sodium chloride, 0.2 M sodium hydrogen phosphate, 0.02 M EDTA, pH 7.4). Hybridized signals were visualized with a DNA chip scanner (GSI Lumonics; ScanArray Lite, Ottawa, Canada). After LEEP conization, HCII for HPV detection was performed at every follow-up visit. Persistent infection was defined as positive if RLU/PC≥1.
4. Statistical analysis
The data were computerized and analyzed using the SPSS ver.12.0K. Statistical analyses were performed using Student's t test, Fisher's Exact Tests, and Logistic regression analysis. Comparison of means was assessed using Student's t test. The Fisher's Exact Tests were used to evaluate association of age, cytology, HPV load, biopsy histology, HPV type 16, and conization histology with HPV clearance/persistence after conization with negative margins.
A logistic regression analysis was performed to determine the risk factors for HPV persistence with independent variables such as age, HPV load, biopsy histology, HPV type 16, and conization histology. All p-values presented are 2-sided and considered as significant when p≤0.05.
RESULTS
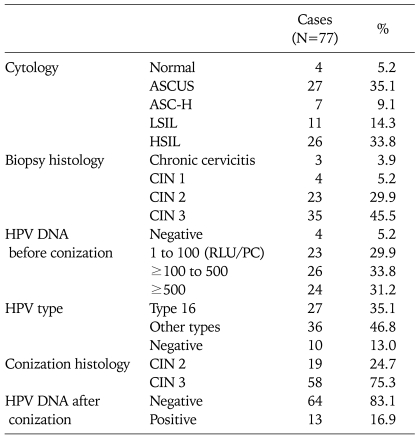
Seventy-seven patients who underwent loop conization with negative margins were reviewed in this study. The median age at diagnosis was 39 years (range, 25 to 51 years). Patient characteristics are depicted in Table 1. The cytology before conization included four normal findings (5.2%), 27 ASCUS (35.1%), 7 ASC-H (9.1%), 11 LSILs (14.3%), and 26 HSILs (33.8%). The colposcopy-directed biopsy before conization showed 3 chronic cervicitis (3.9%), 4 CIN 1 (5.2%), 23 CIN 2 (29.9%), and 35 CIN 3 (45.5%). HR HPV was identified in 73 of 77 patients (94.8%) before conization using the HCII assay. The baseline viral load included 4 (5.2%) women with negative results, 23 (29.9%) women with low loads (RLU/PC = 1 to 100), 26 (33.8%) women with intermediate loads (100 ≤ RLU/PC < 500), and 24 (31.2%) women with high loads (RLU/PC≥500). The HPV genotyping test identified 27 (35.1%) women with HPV 16 infection, 36 (46.8%) women with other type infections, and 10 (13.0%) women without HPV infection. The histology of the conization specimen showed 19 (24.7%) women with CIN 2 and 58 (75.3%) women with CIN 3. After conization, 64 patients had no detectable high-risk HPV, while 13 of 77 patients had persistent high-risk HPV according to the HCII. The four women with negative HPV tests before conization were still negative for high-risk HPV during follow-up. Follow-up 6 months after conization showed that the high-risk HPV had become negative in 60 of 73 patients (82.19%).
Table 1.
Patient characteristics
ASCUS: atypical squamous cells of undetermined significance, ASC-H: atypical squamous cells-high grade, LSIL: low-grade squamous intraepithelial lesion, HSIL: high-grade squamous intraepithelial lesions, CIN: cervical intraepithelial neoplasia, HPV: human papillomavirus, RLU/PC: relative light units/positive control
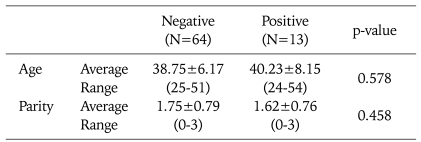
Table 2 shows that the mean age of the patients with negative and positive HPV tests after conization was 38.75±6.17 (25-51) and 40.23±8.15 (24-54) years, respectively. The median parity of the patients with negative and positive HPV tests after conization was 1.75±0.79 and 1.62±0.76, respectively. Neither age nor parity differed significantly between the two groups.
Table 2.
Age and parity of the patients with negative and positive HPV tests after conization with negative margins
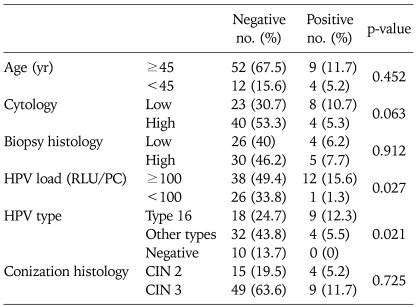
Fisher's exact univariate analysis showed a significantly higher proportion of persistent HPV infection with negative margins in the women with high viral loads (RLU/PC≥100) (p=0.027). HPV infection after conization with negative margins was persistent in 24.0% (12/50) of the women with high viral loads (RLU/PC≥100) and 3.7% (1/27) of the women with low viral loads (RLU/PC<100). The HPV genotype analysis showed a significantly higher proportion of persistent HPV infection with HPV 16 infection compared to other types (p=0.021). In terms of the HPV genotype, in the patients with negative conization specimen margins, HPV infection was persistent in 33.3% (9/27) of the patients with HPV 16 infection before conization and 11.1% (4/36) of the patients with other type infections. Age, cytologic grade before conization, colposcopic-guided biopsy grade, and lesion grade in the cone were not associated with HPV clearance/persistence after conization with negative margins (Table 3).
Table 3.
Characteristics of the patients with negative and positive HPV tests after conization with negative margins
HPV: human papillomavirus, RLU/PC: relative light units/positive control
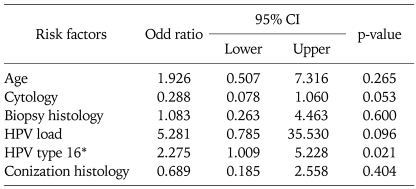
In a logistic regression analysis, preoperative HPV 16 infection was the only significant factor for persistent HPV infection after conization with negative margins (p=0.021). Age, cytology, lesion grade in the colposcopy biopsy, HPV load and conization histology were not associated with HPV persistence after conization with negative margins (Table 4).
Table 4.
Logistic regression analysis of the risk factors predicting HPV persistence in cervical intraepithelial neoplasia after conization with negative margins.
HPV: human papillomavirus
*HPV type 16 versus Other types including negative cases
DISCUSSION
Several studies have reported on the clearance of HPV infection after CIN treatment, but the results have been variable. The well-known risk factors for persistence/recurrence of CIN after LEEP are age, parity, cytology grade, lesion grade, glandular involvement, cytology or curettage specimen immediately after conization, and margin status.
Sarian et al.17 reported that women older than 35 years had a significantly higher risk of persistent infection following LEEP, while Costa et al.18 reported that age and high-grade Pap smear were both significant predictors of HPV clearance after conization. Some studies, however, have shown that age is not related to the persistence of HPV after treatment, which is consistent with our results.19,20 Cytology grade or histologic degree before LEEP were also not significant factors for the persistence of HPV, which are consistent with previous reports.21
Our study assessed the pre-conization HR-HPV load and HPV genotype as risk factors for predicting the persistence of HPV in CIN after LEEP. HPV genotype as a risk factor for the persistence of HPV has not been studied previously.
Our findings suggest that a high pre-conization HR-HPV load results in persistence/recurrence of the disease. Few studies have examined recurrence/persistence and direct HPV viral load. Song et al.15 reported that the pre-LEEP HR-HPV load was related to a higher risk of persistence of HPV in patients with negative margins after LEEP using multiple regression analysis. They used RLU/PC>500 as a cutoff value for a high viral load determined by HCII and reported that among the possible risk factors, including age, parity, conization grade, histology of colposcopic biopsy, and HPV load, HPV load was the only significant factor. Consistent with these results, we found that the pre-LEEP HPV viral load was a meaningful predictor of persistent HPV after LEEP in the univariate analysis.
Alonso et al.14 reported similar results, but they used RLU/PC>1,000 as the cutoff value for a high viral load. Recently, Park et al.21 reported that a high HR-HPV load had higher rates of persistent HR-HPV infection and persistence/recurrence abnormalities after conization using RLU/PC>100 as the cutoff value for a high viral load measured using HCII. Although Bae et al.22 used the same cutoff value of RLU/PC>100, no relationship between recurrent disease and HPV viral load before treatment was found. A review of the results shows that the definition of a high HR-HPV viral load is arbitrary and needs to be standardized in the near future.
In our series, HPV genotype 16 was a predictor for persistent HPV after LEEP in patients with a negative margin. This finding somewhat contradicts the results of Kreimer et al.,23 who recently reported that persistence was significantly greater according to alpha3 HPV type (all are noncarcinogenic; 40.9% compared to 17.6% for alpha9 (HPV 16 and related types) and 17.9% for alpha7 (HPV18 and related types) species; both p<0.001). Persistent HPV 16 can progress to CIN and recurrent disease after LEEP. The 2-year risk associated with HPV 16 positivity after LEEP was 37.0%, which was significantly higher than for other carcinogenic HPV types (10.8%, p<0.001), noncarcinogenic types (1.5%, p<0.001), or testing HPV negative (0%) in Kreimer et al.24 Our drawback was that HPV DNA type test was not done at follow-up after conization. It was unclear whether or not HR-HPV persistence after LEEP was the persistence of the same HPV type before conization or a new HPV infection. In order to elucidate this point, another study incorporating a serial HPV DNA type testing after conization is needed.
Despite the relatively small number of patients in this retrospective study, our data suggest that women who have a high-grade lesion containing HPV 16 should be followed closely after treatment, even with negative margin, given their increased risk of persistent HPV infection resulting in recurrence.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.von Keyserling H, Kaufmann AM, Schneider A. HPV testing in the follow-up after treatment of women with CIN. Gynecol Oncol. 2007;107(1) Suppl 1:S5–S7. doi: 10.1016/j.ygyno.2007.07.048. [DOI] [PubMed] [Google Scholar]
- 3.McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9:425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 4.Pinto AP, Crum CP. Natural history of cervical neoplasia: defining progression and its consequence. Clin Obstet Gynecol. 2000;43:352–362. doi: 10.1097/00003081-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Prendiville W, Cullimore J, Norman S. Large loop excision of the transformation zone (LLETZ): a new method of management for women with cervical intraepithelial neoplasia. Br J Obstet Gynaecol. 1989;96:1054–1060. doi: 10.1111/j.1471-0528.1989.tb03380.x. [DOI] [PubMed] [Google Scholar]
- 6.Lindeque BG. Management of cervical premalignant lesions. Best Pract Res Clin Obstet Gynaecol. 2005;19:545–561. doi: 10.1016/j.bpobgyn.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell MF, Tortolero-Luna G, Cook E, Whittaker L, Rhodes-Morris H, Silva E. A randomized clinical trial of cryotherapy, laser vaporization, and loop electrosurgical excision for treatment of squamous intraepithelial lesions of the cervix. Obstet Gynecol. 1998;92:737–744. [PubMed] [Google Scholar]
- 8.Ghaem-Maghami S, Sagi S, Majeed G, Soutter WP. Incomplete excision of cervical intraepithelial neoplasia and risk of treatment failure: a meta-analysis. Lancet Oncol. 2007;8:985–993. doi: 10.1016/S1470-2045(07)70283-8. [DOI] [PubMed] [Google Scholar]
- 9.Soutter WP, Sasieni P, Panoskaltsis T. Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int J Cancer. 2006;118:2048–2055. doi: 10.1002/ijc.21604. [DOI] [PubMed] [Google Scholar]
- 10.Soutter WP. Invasive cancer after treatment of cervical intraepithelial neoplasia. Ann Acad Med Singapore. 1998;27:722–724. [PubMed] [Google Scholar]
- 11.Soutter WP, de Barros Lopes A, Fletcher A, Monaghan JM, Duncan ID, Paraskevaidis E, et al. Invasive cervical cancer after conservative therapy for cervical intraepithelial neoplasia. Lancet. 1997;349:978–980. doi: 10.1016/s0140-6736(96)08295-5. [DOI] [PubMed] [Google Scholar]
- 12.Strander B, Andersson-Ellstrom A, Milsom I, Sparen P. Long term risk of invasive cancer after treatment for cervical intraepithelial neoplasia grade 3: population based cohort study. BMJ. 2007;335:1077. doi: 10.1136/bmj.39363.471806.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuovo J, Melnikow J, Willan AR, Chan BK. Treatment outcomes for squamous intraepithelial lesions. Int J Gynaecol Obstet. 2000;68:25–33. doi: 10.1016/s0020-7292(99)00162-9. [DOI] [PubMed] [Google Scholar]
- 14.Alonso I, Torne A, Puig-Tintore LM, Esteve R, Quinto L, Campo E, et al. Pre- and post-conization high-risk HPV testing predicts residual/recurrent disease in patients treated for CIN 2-3. Gynecol Oncol. 2006;103:631–636. doi: 10.1016/j.ygyno.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Song SH, Lee JK, Oh MJ, Hur JY, Na JY, Park YK, et al. Persistent HPV infection after conization in patients with negative margins. Gynecol Oncol. 2006;101:418–422. doi: 10.1016/j.ygyno.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Gok M, Coupe VM, Berkhof J, Verheijen RH, Helmerhorst TJ, Hogewoning CJ, et al. HPV 16 and increased risk of recurrence after treatment for CIN. Gynecol Oncol. 2007;104:273–275. doi: 10.1016/j.ygyno.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Sarian LO, Derchain SF, Pitta Dda R, Morais SS, Rabelo-Santos SH. Factors associated with HPV persistence after treatment for high-grade cervical intra-epithelial neoplasia with large loop excision of the transformation zone (LLETZ) J Clin Virol. 2004;31:270–274. doi: 10.1016/j.jcv.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Costa S, De Simone P, Venturoli S, Cricca M, Zerbini ML, Musiani M, et al. Factors predicting human papillomavirus clearance in cervical intraepithelial neoplasia lesions treated by conization. Gynecol Oncol. 2003;90:358–365. doi: 10.1016/s0090-8258(03)00268-3. [DOI] [PubMed] [Google Scholar]
- 19.Dalstein V, Riethmuller D, Pretet JL, Le Bail Carval K, Sautiere JL, Carbillet JP, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer. 2003;106:396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 20.Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau MC, Desy M, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999;180:1415–1423. doi: 10.1086/315086. [DOI] [PubMed] [Google Scholar]
- 21.Park JY, Lee KH, Dong SM, Kang S, Park SY, Seo SS. The association of pre-conization high-risk HPV load and the persistence of HPV infection and persistence/recurrence of cervical intraepithelial neoplasia after conization. Gynecol Oncol. 2008;108:549–554. doi: 10.1016/j.ygyno.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Bae JH, Kim CJ, Park TC, Namkoong SE, Park JS. Persistence of human papillomavirus as a predictor for treatment failure after loop electrosurgical excision procedure. Int J Gynecol Cancer. 2007;17:1271–1277. doi: 10.1111/j.1525-1438.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 23.Kreimer AR, Katki HA, Schiffman M, Wheeler CM, Castle PE. Viral determinants of human papillomavirus persistence following loop electrical excision procedure treatment for cervical intraepithelial neoplasia grade 2 or 3. Cancer Epidemiol Biomarkers Prev. 2007;16:1–6. doi: 10.1158/1055-9965.EPI-06-0710. [DOI] [PubMed] [Google Scholar]
- 24.Kreimer AR, Guido RS, Solomon D, Schiffman M, Wacholder S, Jeronimo J, et al. Human papillomavirus testing following loop electrosurgical excision procedure identifies women at risk for posttreatment cervical intraepithelial neoplasia grade 2 or 3 disease. Cancer Epidemiol Biomarkers Prev. 2006;15:908–914. doi: 10.1158/1055-9965.EPI-05-0845. [DOI] [PubMed] [Google Scholar]