Summary
Myristoyl-CoA:protein N-myristoyltransferase (NMT), an essential protein in Trypanosoma brucei and Leishmania major, catalyses the covalent attachment of the fatty acid myristate to the N-terminus of a range of target proteins. In order to define the essential targets contributing to lethality in the absence of NMT activity, we have focused on the ADP-ribosylation factor (Arf) family of GTP-binding proteins, as growth arrest in Saccharomyces cerevisiae mutants with reduced NMT activity correlates with a decrease in N-myristoylated Arf proteins. We have identified nine Arf/Arls in the T. brucei and T. cruzi genomes and ten in L. major. Characterization of the T. brucei ARL1 homologue has revealed that the protein is localized in the Golgi apparatus and is expressed only in the mammalian bloodstream form of the parasite and not in the insect procyclic stage. This is the only reported example to date of a differentially expressed ARL1 homologue in any species. We have used RNA interference to demonstrate that ARL1 is essential for viability in T. brucei bloodstream parasites. Prior to cell death, depletion of ARL1 protein in bloodstream parasites results in abnormal morphology, including disintegration of the Golgi structure, multiple flagella and nuclei, and the presence of large numbers of vesicles. The cells have only a minor apparent defect in endocytosis but exocytosis of variant surface glycoprotein to the parasite surface is significantly delayed. RNA interference of ARL1 in procyclic cells has no effect on parasite growth or morphology. Our results suggest that there may be different pathways regulating Golgi structure and function in the two major life cycle stages of T. brucei.
Keywords: Trypanosoma, ADP-ribosylation factor, RNA interference, Golgi proteins
Introduction
Trypanosomatid protozoans are the causative agents of several of the most important human parasitic infections, including visceral and cutaneous leishmaniases (Leishmania spp.), African sleeping sickness (Trypanosoma brucei) and Chagas' disease (T. cruzi). No vaccines are currently available to control these diseases and treatment is largely dependent on the use of costly and toxic drugs. There is an urgent requirement for the development of novel chemotherapeutic agents to combat these recurrent infections.
The enzyme N-myristoyl transferase (NMT) catalyses the attachment of the fatty acid myristate to the N-terminal glycine of a number of target proteins. NMT is essential for viability in many eukaryotic species including T. brucei and Leishmania major (Price et al., 2003). Myristate analogues are toxic to both T. brucei and L. major in vitro (Doering et al., 1994; Price et al., 2003), suggesting that evaluation of specific inhibitors of parasite NMTs may lead to the development of effective drugs (Gelb et al., 2003). In parallel, identification of the downstream targets of NMT that are essential for parasite viability may provide new insights into cell signalling and protein trafficking in these divergent eukaryotes.
The most extensively characterized N-myristoylated proteins in yeast and mammals are the ADP-ribosylation factors (Arfs), a family of small GTP-binding proteins of the Ras superfamily involved in intracellular protein transport and secretion. Although the Rab family of small GTPases has been extensively studied in trypanosomatids (Morgan et al., 2002), little is known of the Arf family members in these species.
Arf proteins are characterized by their ability to enhance ADP-ribosyl transferase activity of cholera toxin A (Kahn and Gilman, 1984; Boman and Kahn, 1995) and to activate phospholipase D (Cockcroft et al., 1994; Brown et al., 1995). Members of the Arf-like protein subfamily (Arls) share 30-60% sequence identity with Arfs but have no functional Arf activity with the exception of ARL1, which can activate phospholipase D under certain conditions (Hong et al., 1998). The Arl proteins are also unable to rescue lethality in Saccharomyces cerevisiae mutants without functional arf1 and arf2 genes (Tamkun et al., 1991). The mammalian Arls show a range of cell and tissue specific expression patterns but remain largely uncharacterized with respect to function. ARL1, the best studied of these proteins and the only member of the subfamily to be found in the trans-Golgi network (TGN), has a role in the maintenance of Golgi structural integrity and function (Lowe et al., 1996; Lu et al., 2001; Van Valkenburgh et al., 2001).
Both Arfs and Arls cycle between an inactive GDP-bound form in the cytosol and a functionally active GTP-bound protein associated with phospholipid membranes (reviewed by Pasqualato et al., 2002). All known Arfs are N-myristoylated, as are many of the human Arls, including ARL1, ARL4 and ARL5 (Lee et al., 1997; Lin et al., 2000; Lin et al., 2002). Presence of an N-terminal myristate group is essential for interaction with membranes (Haun et al., 1993) and provides a hydrophobic environment in which the N-terminus can adopt an α-helical structure, often necessary for function (Kahn et al., 1992). In GDP-bound human ARF1, a region termed the interswitch domain forms a pocket to which the N-myristoylated N-terminus binds, stabilizing the inactive conformation. During exchange of GDP for GTP through interaction with a specific guanine nucleotide exchange factor (Gef), conformational changes occur within the Switch 1, interswitch and Switch 2 effector domains of ARF1, causing the myristate group on the N-terminus to be exposed and thus allowing the protein and its effectors to associate with membranes (Antonny et al., 1997). Reduction of N-myristoylated Arf to less than 50% of the total cellular Arf in S. cerevisiae is associated with growth arrest and loss of viability (Lodge et al., 1997) and a non-myristoylated [G2A]Arf1p mutant has no function in vivo, although the protein is functional in in vitro assays (Kahn, 1991).
Here, we identify the Arf and Arl genes present in the genomes of three trypanosomatid species, L. major, T. brucei and T. cruzi, and describe the functional characterization of one of the proteins, the T. brucei homologue of ARL1. Unexpectedly, TbARL1 is expressed only in the bloodstream form (BSF) of the parasite, in which it localizes to the Golgi apparatus. RNA interference analysis shows that TbARL1 is essential in BSF T. brucei and depletion of this protein results in abnormal morphology, including disintegration of the Golgi structure, and defects in exocytic flux.
Materials and Methods
Bioinformatics
Putative Arf/Arl genes were identified by BLAST searching L. major, T. brucei and T. cruzi databases available on the GeneDB and EBI websites (http://www.genedb.org and http://www.ebi.ac.uk/blast2/parasites) using human and S. cerevisiae Arf protein and nucleotide sequences. Sequences were aligned using CLUSTALW software and the program ScanProsite was used to identity motifs.
Parasite culture
The BSF T. brucei strains 90-13 and s427 (‘SMB’) (Wirtz et al., 1999) were maintained in vitro at 37°C with 5% CO2 in HMI-9 medium supplemented with 10% FCS, 5 μg/ml hygromycin and 2.5 μg/ml G418 (strain 90-13) or G418 alone (strain s427). The procyclic form (PCF) T. brucei strain 29-13 (Wirtz et al., 1999) was maintained in vitro at 27°C in SDM-79 medium supplemented with 10% FCS, 25 μg/ml hygromycin and 25 μg/ml G418. All of these parasitic strains contain the genes expressing T7 RNA polymerase and tetracycline repressor.
DNA constructs
In all primer sequences listed here, the T. brucei sequence is shown in upper case and cloning sites are underlined. The T. brucei ARL1 open reading frame was amplified from genomic DNA using the following primers: TbARL1F1, 5′-aatatCCATGGGGGCTTTGGTTTCGCAG-3′ and TbARL1R1, 5′-ATATTAgcggccgcGCCGAGTCTGCAGTGA-3′. After digestion with NcoI/NotI, the amplified fragment was cloned into the expression vector pET28a (Novagen) to produce the construct pETARL1. The open reading frame of T. brucei NMT was cloned into the expression vector pET15b to produce the construct pETTbNMT (C.P., unpublished data). The T. brucei ARL1 open reading frame was amplified from genomic DNA using the following primers: TbARL1F2, 5′-ATTTaAGcttCCATGGGGGCTTTGGTTT-3′ and TbARL1R2, 5′-ACAAGCTTtctagaCGAGTCTGCAGTGA-3′. A non-myristoylated G2A mutant form of ARL1 was amplified using TbARL1R2 as above and the primer TbARL1F2a, 5′-ATTTaAGcttCCATGGCGGCTTTGGTTT-3′. After digestion with HindIII/XbaI, the amplified products were cloned into the plasmid vector pM2cC (Sam Alsford and David Horn, unpublished). The resulting constructs pM2cARL1 and pM2cARL1G2A encode the target gene with a C-terminal myc tag epitope under the control of a tetracycline-inducible T7 promoter. A non-conserved region of the T. brucei ARL1 open reading frame was selected for RNAi using the program RNAit (http://www.trypanofan.org/software/RNAit). A 343 bp fragment was amplified from T. brucei genomic DNA using the following primers: TbARL1F3, 5′-AAAATCCCTTCTaGaAATCCTACCG-3′ and TbARL1R3, 5′-TTCGTCTAgaAGGTTGCACAACT-3′. The PCR fragment was digested with XbaI and cloned into the RNAi vector, p2T7Ti, which supports expression of double-stranded RNA from tetracycline-inducible T7 promoters (LaCount et al., 2000), generating the construct p2T7ARL1.
DNA and RNA hybridization analysis
Genomic DNA was used to prepare DNA blots using methods described previously (Price et al., 2003). Total RNA was extracted from exponentially growing parasites using Trizol Reagent (Invitrogen) following the manufacturer's instructions. After treatment with DNase I (Ambion), RNA blots were prepared as described (McKean et al., 2001). DNA and RNA membranes were probed with a 320 bp DNA fragment from XbaI-digested construct p2T7ARL1. RNA blots were re-probed with β-tubulin as a loading control. For RT-PCR, DNase-treated RNA samples were reverse transcribed using Omniscript RT (Qiagen) and oligo-dT (Promega). PCR was performed using TbARL1F3 and TbARL1R3 (see above). The constitutively expressed gene Rab4 (Field et al., 1998) was amplified using the following primers: TbRAB4F, 5′-ACTTGCAGGACCGGATGTAG-3′ and TbRAB4R, 5′-GTGCCCAAACTCAAACCAGT-3′.
NMT coexpression assay
The N-myristoylation assay was performed as described (Duronio et al., 1990; Price et al., 2003). Briefly, E. coli BL21(DE3)pLysS cells were co-transformed with constructs pETARL1 and pETTbNMT. Expression of recombinant T. brucei ARL1 and NMT proteins was induced by the addition of IPTG in the presence of [3H]myristate (Amersham Biosciences) and, following SDS-PAGE, radiolabelled proteins were detected by autoradiography.
Immunological procedures
Log-phase BSF parasites were fixed and indirect immunofluorescence assays performed as described (Allen et al., 2003). The primary rabbit anti-TbBiP and mouse anti-p67 antibodies, used at 1:1500 and 1:500 dilution respectively, were gifts from James Bangs (Madison, USA). Rabbit primary antibodies against TbRAB1 (Dhir et al., 2004) and clathrin heavy chain (TbCLH) (Morgan et al., 2001), used at 1:100 and 1:500 dilution respectively, were gifts from Mark Field (University of Cambridge). The mouse monoclonal anti-myc antibody (Invitrogen) was used at a 1:250 dilution. p67 immunoprecipitation was performed as described (Bangs et al., 1996; Alexander et al., 2002).
Subcellular localization
Mid-log phase T. brucei BSF strain s427 parasites were electroporated with 10 μg NotI-digested pM2cARL1 or pM2cARL1G2A as described (Biebinger et al., 1997; Price et al., 2003) and transfectants selected with 5 μg/ml hygromycin. Expression of myc-tagged proteins was induced in stable cell lines by incubating parasites in 1 μg/ml tetracycline for 48 hours before analysis by indirect immunofluorescence assays as described above. For subcellular fractionation, BSF cells were subjected to hypotonic lysis in 10 mM Tris-HCl (pH 7.5) on ice for 1 hour. Following centrifugation, immunoblots were prepared using soluble and insoluble fractions.
RNA interference in T. brucei BSF
Mid-log phase parasites of the T. brucei BSF strain 90-13 were electroporated with 10 μg NotI-linearized p2T7-ARL1 and transfectants selected with 2.5 μg/ml phleomycin. Once stable cell lines (Bp2T7/ARL1) had been established, expression of double-stranded RNA was induced by addition of 1 μg/ml tetracycline to parasites diluted to 1×105 cells/ml in HMI-9 medium. Cell numbers were monitored using a Beckman Coulter counter. Total RNA samples were prepared at 24 hour intervals and 10 μg samples were analysed by RNA blotting as described above. Electron microscopy was performed as described (Price et al., 2003).
RNA interference in T. brucei PCF
Mid-log phase parasites of the PCF T. brucei strain 29-13 were electroporated with 20 μg NotI-linearized p2T7-ARL1 DNA, as described (Hill et al., 1999) and transfectants selected using 2.5 μg/ml phleomycin. The expression of double-stranded RNA was induced by addition of 1 μg/ml tetracycline to parasites (cell line Pp2T7/ARL1) diluted to 1×106 cells/ml in SDM-79 medium. Cell numbers were monitored as above.
Endocytosis and exocytosis assays
Receptor-mediated endocytosis of FITC-labelled lectin concanavalin A (ConA) was monitored in mid-log phase uninduced and tetracycline-induced BSF cells using methods described previously (Allen et al., 2003). Exocytosis of VSG was monitored in mid-log phase uninduced and tetracycline-induced BSF cells as described (Bangs et al., 1986; Allen et al., 2003). Following SDS-PAGE, radiolabelled proteins were detected by autoradiography. Gels were analysed using the program ImageJ version 1.24 (NIH).
Results
Sequence analysis
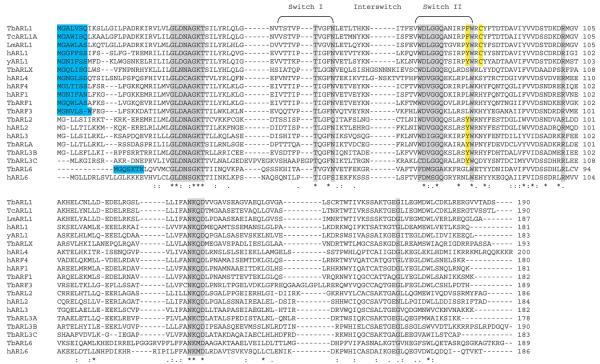
Based on identity with previously characterized sequences, we have identified genes encoding nine putative Arf/Arls in T. brucei and T. cruzi, and ten in L. major (see Table S1 in supplementary material). This is considerably more than in S. cerevisiae, which has only five members of the Arf/Arl family (yARF1, 2 and 3, yARL1 and yARL3), whereas there are six Arfs and at least eight Arls in humans, most of unknown function (reviewed by Pasqualato et al., 2002). The trypanosomatid Arf and Arl proteins share 40-76% identity with their closest human homologues. Three of the L. major and two of the Trypanosoma spp. sequences have been designated as Arfs owing to a high degree of amino acid identity with human ARF1 and ARF4 in the regions of the Switch I and II effector domains (Fig. 1). TbARF1 and human ARF1 Switch I and II domains share 100% and 84% identity, respectively. The remaining sequences have been designated as putative Arls, a group that are characteristically more divergent than the Arf proteins.
Fig. 1.
Alignment of the T. brucei ARF protein family with related sequences. Residues and motifs conserved in ARFs are highlighted in dark grey. These include the GDP/GTP α- and β-phosphate binding site GLDXAGKT, of which the threonine residue is essential for binding to guanine nucleotide exchange factors; WDXGGQ, which interacts with the purine ring of GTP; and NKXD, which binds magnesium and the β phosphate of GTP. Several Arf-specific residues are found in the majority of the parasite proteins, including N48, W79, R100 and G165. N-myristoylation motifs are indicated by blue shading. Residues essential for binding to GRIP domain proteins are highlighted in yellow.
The identified trypanosomatid ORFs contain many of the conserved domains and residues characteristically found in GTP-binding proteins (Fig. 1) (Kjeldgaard et al., 1996). A potential N-myristoylation motif (G-{EDRKHPFYW}-x(2)-[STAGCN]-{P}) is present in the trypanosomatid ARF, ARL1, ARLX and ARL6 proteins but not in the ARL2 or ARL3 sequences. Interestingly, T. cruzi contains two putative ARL1 homologues, TcARL1A (Fig. 1) and TcARL1B (Table S1, in supplementary material), which are identical except for an extended N-terminal region in TcARL1B that contains a putative signal peptide and a transmembrane domain. This suggests that TcARL1B may function as a non-myristoylated form of ARL1 or may be an unusual example of a protein undergoing post-translational rather than co-translational N-myristoylation, similar to the human pro-apoptotic protein BID and cytoskeletal actin, which are both N-myristoylated following caspase cleavage (Zha et al., 2000; Utsumi et al., 2003).
ARL1 and ARLX sequences
All three trypanosomatid species contain a putative ARL1 homologue sharing over 50% identity with human ARL1 at the amino acid level (Fig. 1). Critically, these sequences contain a residue found only in ARL1 homologues, Cys80, which is essential for binding to GRIP domain proteins (Wu et al., 2004). The sequences designated here as ARLX also share significant identity (45%) with human ARL1 at the amino acid level but are divergent in the Switch I and II effector domains and are therefore unlikely to bind to ARL1 effectors. A unique feature of the ARLX sequences is the presence of an extended interswitch region similar to those in the human nuclear localizing proteins ARL4 and ARL7 but not seen in the other parasite or human Arf/Arls. Long interswitch regions may not be able to retract fully when the protein is in the inactive GDP-bound form, an indication that these Arls undergo the classical GDP/GTP cycle of other small GTPases such as Ras (Pasqualato et al., 2002).
TbARL1 is encoded by a single-copy gene that is differentially expressed
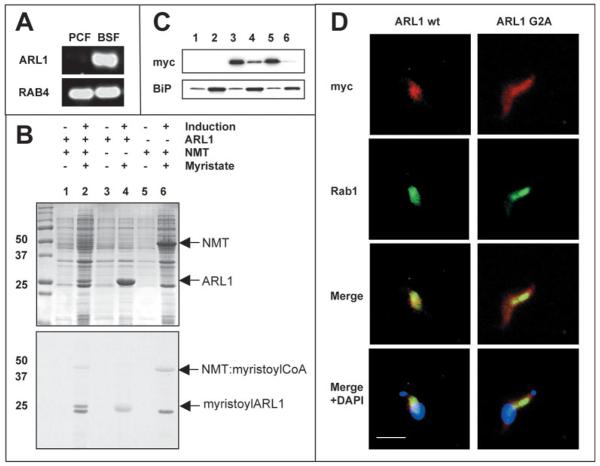
The T. brucei ARL1 homologue (TbARL1) was selected for functional analysis because of its unusual expression pattern during the parasite life cycle. Southern hybridization of genomic DNA confirmed that TbARL1 is present in the genome as a single-copy gene (data not shown). RT-PCR (Fig. 2A) and northern blotting (Fig. 3B, lower panel) detected a single ARL1 transcript only in the mammalian BSF and not in the insect PCF of the parasite. At the present time this is the only reported example of a differentially expressed ARL1 homologue in any cell type.
Fig. 2.
Characterization of TbARL1. (A) RT-PCR showing differential expression of ARL1 in procyclic (PCF) and bloodstream (BSF) life cycle stages of T. brucei. Amplification of constitutively expressed Rab4 was used to show equal amounts of cDNA in the reactions. (B) N-myristoylation assay. TbARL1 and TbNMT were expressed as recombinant proteins of 22 kDa and 48.5 kDa respectively in the presence of [3H]myristate, following induction with IPTG (upper panel, Coomassie Blue-stained gel). The radiolabelled products (the NMTmyristoyl CoA binary complex and myristoylated ARL1) were detected by autoradiography (lower panel). The data shown represent one of three independent experiments. (C) Subcellular fractionation of myc-epitope tagged ARL1 wild type (wt) and G2A mutant proteins in BSF T. brucei. Immunoblots were probed with anti-myc antibody to detect ARL1 proteins and anti-BiP antibody to control for equal sample loading. Lanes 1, 2, BSF s427 parental line, cytosolic and membrane fractions respectively; lanes 3, 4, BSF transfected line 427/pM2cCARL1WT grown in tetracycline for 48 hours, cytosolic and membrane fractions, respectively; lanes 5, 6, BSF line 427/pM2cCARL1G2A grown in tetracycline as above, cytosolic and membrane fractions respectively. (D) Immunofluorescence of parasites described in C using anti-myc (red) and anti-Rab1 (green) antibodies. In the bottom panel, cells are co-stained with DAPI (shown in blue). Bar, 10 μm.
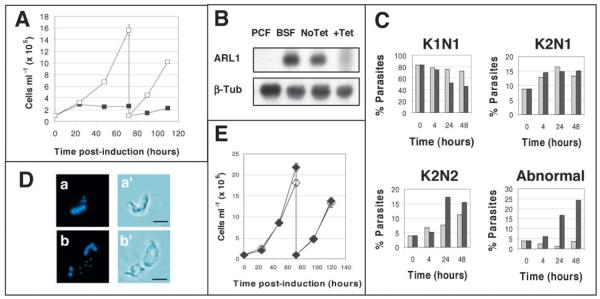
Fig. 3.
RNA interference of TbARL1 expression. (A) Growth of the T. brucei bloodstream form (BSF) transfected line Bp2T7/ARL1 in the absence (open squares) and presence (filled squares) of tetracycline, monitored over a 5 day time course. (B) Northern blots of RNA (10 μg) from procyclic strain 29-13 (PCF), bloodstream parental line 90-13 (BSF) and transfected line Bp2T7/ARL1 grown in the absence (No Tet) and presence (+Tet) of tetracycline for 24 hours. The blot was hybridised with an ARL1-specific probe, and with β-tubulin to monitor equal sample loading. (C) Nuclei and kinetoplasts were counted in parasitic line Bp2T7/ARL1 in the absence (light shading) and presence (dark shading) of tetracycline, over a 48 hour time course. Any configurations other than K1N1, K2N1 and K2N2 were classified as abnormal. 250 parasites were counted per experimental group. (D) Representative images of Bp2T7/ARL1 parasites with abnormal nucleus/kinetoplast configurations after induction with tetracycline for 36 hours. DAPI-stained cells are shown (a,b) with corresponding phase contrast images (a′,b′). (E) Growth of the procyclic transfected line Pp2T7/ARL1 in the absence (open symbols) and presence (filled symbols) of tetracycline over a 5 day time course. Bar, 10 μm.
TbARL1 is a target for NMT
A bacterial coexpression assay was used to assess whether TbARL1 protein can be N-myristoylated in vivo by TbNMT. The protein was overexpressed with a C-terminal His tag in the presence of [3H]myristate in E. coli that were also expressing T. brucei N-myristoyltransferase (C.P., unpublished data). Following labelling, total cell lysates were separated by SDS-PAGE and analysed by autoradiography (Fig. 2B). When only TbNMT was expressed, two proteins incorporated [3H]myristate: the 50 kDa NMT-myristoyl-CoA binary complex and a 20 kDa N-myristoylated endogenous E. coli protein, as described in previous bacterial coexpression assay studies (Duronio et al., 1991a; Duronio et al., 1991b; Price et al., 2003). In cells coexpressing TbNMT and TbARL1, an additional band of 22 kDa was detected by autoradiography, corresponding to N-myristoylated TbARL1. The identity of this protein was confirmed by immunoblotting using an anti- His tag antibody (data not shown). A faint 22 kDa band is also visible when TbARL1 is expressed in the absence of NMT, probably owing to non-specific myristate binding, as TbARL1 is overexpressed at a higher level in these cells.
TbARL1 is localized in the Golgi apparatus
A C-terminal myc-tagged ARL1 protein was expressed in T. brucei BSF and detected in both the cytosolic and membrane fractions of lysed cells (Fig. 2C). Quantification by densitometry indicates that approximately 20% of the protein is in the membrane fraction. Immunofluorescence (Fig. 2D, top left panel) showed faint diffuse staining of the cytosol, together with dense staining of a small region close to the nucleus in a position similar to that of the Golgi matrix protein, GRASP55 (He et al., 2004). The protein colocalized with TbRab1 (Dhir et al., 2004), confirming that TbARL1 is found in the Golgi apparatus (Fig. 2D). By contrast, a non-myristoylated G2A mutant form of ARL1 was detected predominantly (98%) in the cytosolic fraction of cell lysates (Fig. 2C). Immunofluorescence showed the protein to be localized to the cytosol (Fig. 2D, top right panel). These observations suggest that N-myristoylation is essential for the correct subcellular localization of TbARL1.
RNA interference analysis of ARL1 expression in T. brucei
Depletion of ARL1 by RNA interference in T. brucei resulted in a lethal phenotype in the bloodstream stage, with a cessation in cell division and motility occurring between 24 and 48 hours after induction of ARL1-specific dsRNA production by the addition of tetracycline (Fig. 3A). Northern blotting showed significant depletion of ARL1 transcript at 24 hours post-induction compared to untreated cells (Fig. 3B). Tetracycline had no effect on the growth or morphology of the parental parasite line 90-13 (data not shown).
Trypanosomes in the G1 phase of the cell cycle contain one nucleus and one kinetoplast (K1N1). Parasites undergo kinetoplast replication, forming K2N1 cells in early G2 phase. Nuclear replication results in the formation of K2N2 cells at mitosis, which is then followed by cytokinesis (Woodward and Gull, 1990). ARL1 depletion resulted in a steady accumulation of bloodstream parasites displaying abnormal numbers of nuclei and kinetoplasts (Fig. 3C), affecting 24% of cells by 48 hours post-induction compared to only 3% of cells in the absence of tetracycline at this time point. The most common abnormal configurations seen were K1N2 and K2N3. The proportion of K2N2 cells was also greater in induced compared to uninduced cells, suggesting that there is a defect in cytokinesis. Examples of abnormal cells are shown in Fig. 3D.
As a control for the specificity of these effects, RNA interference of ARL1 expression was also performed in PCF T. brucei, which do not express detectable levels of ARL1. As predicted, the production of ARL1-specific dsRNA had no effect on PCF cell growth (Fig. 3E) or morphology (data not shown).
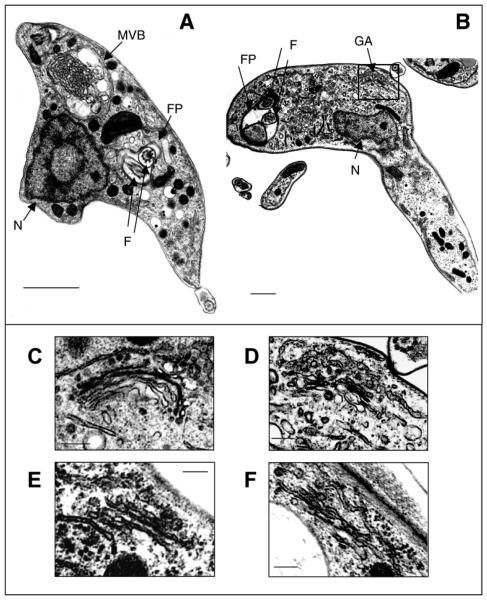
High resolution electron micrographs of BSF cells at 36 hours post-induction are shown in Fig. 4A,B. The ARL1-deficient BSF cells contain multiple flagella and nuclei and have an accumulation of both round and flattened coated vesicles, either unbound or within dilated multi-vesicular bodies in the regions of the nucleus, Golgi apparatus or flagellar pocket. In wild-type T. brucei parasites, the Golgi apparatus consists of 4-6 well-aligned stacked cisternae, with a transitional ER cisterna close to the cis face (Fig. 4C) (Duszenko et al., 1988). In contrast to this, ARL1-deficient cells consistently display disorganisation of Golgi structure (Fig. 4D-F) with misaligned swollen cisternae and extensive vesiculisation at one face of the organelle. Using the position of the transitional ER cisterna as a guide, this is predicted to be the trans-face of the Golgi.
Fig. 4.
Electron micrographs of BSF line Bp2T7/ARL1. (A,B) Cells grown in the presence of tetracycline for 36 hours. F, flagellum; FP, flagellar pocket; GA, Golgi apparatus; MVB, multi-vesicular body; N, nucleus. The Golgi apparatus from cells grown in the absence (C) and presence (D-F) of tetracycline. Image D is an enlarged view (×4.5) of the boxed area in B. Bar, 1 μm (A,B); 0.2 μm (C-F).
The effect of ARL1-depletion on intracellular structure was further investigated using immunofluorescence. There are minor changes in the localization of the ER-resident molecular chaperone, BiP (Bangs et al., 1993) at 24 hours, with staining of fine thread-like structures. By 36 hours, these effects are more apparent as BiP localization becomes more constricted towards the cell membrane. Therefore, ARL1 depletion may have an effect on ER structure, possibly as a secondary effect of disturbance to Golgi function, thereby disrupting protein trafficking and causing a large accumulation of stored material, and vesicles building up inside the cell.
Immunofluorescence with an antibody against p67, which localizes to the lysosome (Bangs, 1999), suggests that the lysosome remains intact in tetracycline-induced cells (Fig. 5B). Moreover, densitometric analysis of 20 cell images from each experimental group shows that p67 staining is approximately twice as bright and covers an area four times as large in cells induced for 24 or 36 hours than in uninduced control cells. This indicates an upregulation in the expression of p67 and probably enlargement of the lysosome in ARL1-depleted cells. Alternatively, as there is a cytokinesis defect in the induced cells, the observed increase in p67 may be due to accumulation of the protein in duplicated but undivided lysosomes.
Fig. 5.
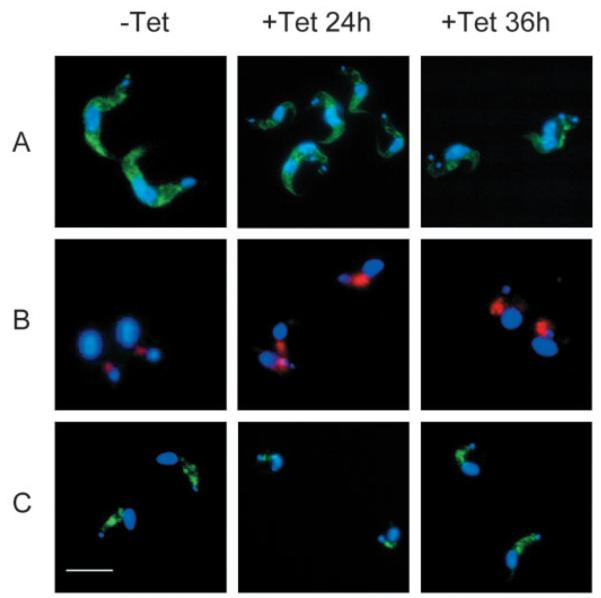
Immunofluorescence of BSF line Bp2T7/ARL1 grown in the absence (−Tet) and presence (+Tet) of tetracycline for 24 and 36 hours. All cells are co-stained with DAPI (shown in blue). (A) ER-localized BiP staining (green). (B) Lysosomal marker, p67 (red). (C) Heavy chain of T. brucei clathrin (green). Bar, 10 μm.
Localization and intensity of the membrane coat protein clathrin was not significantly altered in tetracycline-induced cells (Fig. 5C), suggesting that TbARL1 is not involved in clathrin recruitment.
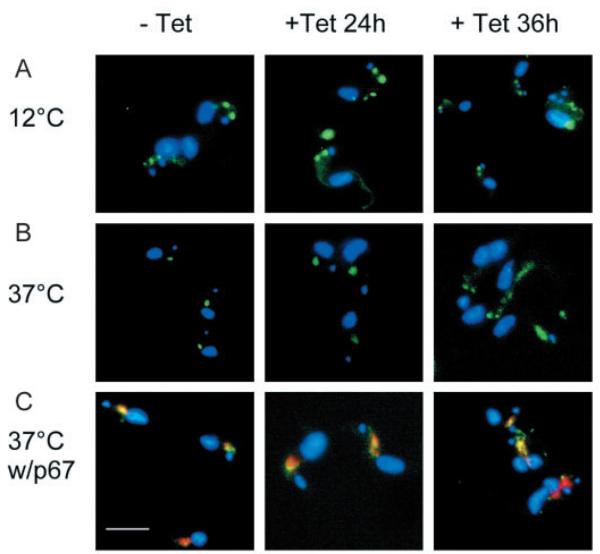
Concanavalin A (ConA) is a mannose-binding lectin used as a marker for receptor-mediated endocytosis in T. brucei. An assay to analyse uptake of fluorescently labelled ConA by BSF parasites revealed minor differences in staining between uninduced and induced cells (Fig. 6). In all experimental groups, ConA is restricted to the flagellar pocket in cells incubated at 4°C (data not shown). At 12°C, the lectin localizes to the TbRab5A early endosomes or collecting tubules (Jeffries et al., 2001); this was seen in all experimental groups here (Fig. 6A). In uninduced cells incubated at 37°C, ConA is able to reach the lysosome (Fig. 6B), as confirmed by colocalization with p67 (Fig. 6C). This was also observed in cells induced for 24 hours, but in parasites induced for 36 hours, a significant quantity of ConA was maintained in the endosomes, suggesting that ARL1 depletion results in a minor decrease in the rate of receptor-mediated endocytosis between the Rab5A endosomes and the lysosome. As this defect occurs only at later time points post induction, we predict that this is a secondary effect of ARL1 loss from the cell.
Fig. 6.
Effects of ARL1 depletion on endocytosis. Receptor-mediated endocytosis was analysed by monitoring the uptake of FITC-labelled ConA in BSF line Bp2T7/ARL1 grown in the absence (−Tet) and presence (+Tet) of tetracycline for 24 and 36 hours. All cells are co-stained with DAPI (blue). (A) In cells incubated for 30 minutes at 12°C, ConA (green) is present in the endosomes. (B) In cells incubated for 30 minutes at 37°C, all ConA has reached the lysosome, except in parasites induced for 36 hours, which still contain a significant quantity of ConA in the endosomes. (C) Colocalisation of FITC-ConA (green) with the lysosomal marker p67 (red) in cells as in B. Bar, 10 μm.
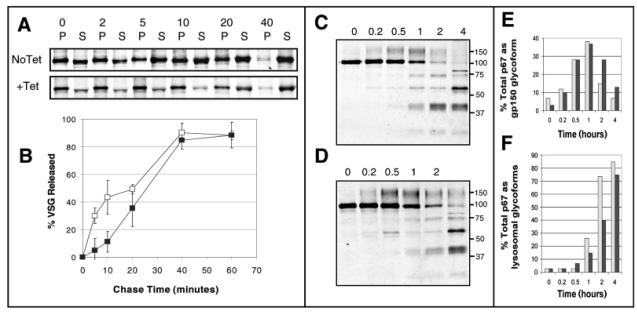
Exocytosis of VSG was monitored using a quantitative assay which tracks the movement of newly synthesized radiolabelled VSG on to the cell surface. Following a pulse-chase using an [35S]-labelled mix of methionine and cysteine, parasite lysates were incubated at 37°C to enable endogenous GPI-phospholipase C (GPI-PLC) to cleave VSG from the plasma membrane, producing soluble VSG (sVSG). By contrast, VSG within the endomembrane system is protected from the action of GPI-PLC (Bangs et al., 1986) and so remains in a membrane-bound form (mfVSG). Therefore, as exocytosis proceeds, the majority of VSG shifts from the insoluble mfVSG form to the soluble form. Results of this assay with ARL1 mutant parasites are shown in Fig. 7A,B. For each time point, the percentage of radiolabelled VSG found at the cell surface was calculated and plotted relative to the percentage of surface VSG at the start of the chase, the latter corrected for VSG transport during the pulse period (as described by Dhir et al., 2004). In comparison to the control group, cells with depleted levels of ARL1 mRNA (24 hours post-induction) consistently showed a reduced rate of VSG movement to the cell surface during the first 10 minutes of the time course. This may be highly significant considering the extremely rapid rate of VSG recycling in BSF parasites (Engstler et al., 2004). By 20 minutes, the rate of VSG transport had recovered and by 40 minutes, the majority of radiolabelled VSG had shifted to the soluble fraction in both ARL1-depleted and uninduced cells. Parasites induced with tetracycline for 36 hours showed a similar initial delay in exocytosis of VSG (data not shown), although the levels of radioactively labelled VSG detected were relatively low, probably owing to the onset of widespread cell death by this time point. Uninduced cells showed similar rates of VSG exocytosis as the parental cell line (data not shown).
Fig. 7.
Effects of ARL1 depletion on exocytosis and Golgi function. (A) VSG exocytosis assay in BSF line Bp2T7/ARL1 grown in the absence (No Tet) and presence (+Tet) of tetracycline for 24 hours. Parasites were labelled by pulse-chase using [35S]-labelled methionine and cysteine over a time course of 40 minutes and VSG isolated from cell lysates using ConA sepharose. Radiolabelled products were separated by SDS-PAGE and detected by autoradiography. Numbers represent length of pulse-chase (minutes). Insoluble pellet fractions (P) represent newly synthesized membrane-bound VSG in the endomembrane system, whereas soluble fractions (S) represent newly synthesized VSG delivered to the plasma membrane, where it is susceptible to the action of endogenous GPI-phospholipase C. The data shown represent one of three independent experiments. (B) Data from three assays as described in (A) were quantified by densitometry and mean values (±s.d.) are shown here as % VSG released on to the plasma membrane in BSF line Bp2T7/ARL1 grown in the absence (open squares) and presence (filled squares) of tetracycline for 24 hours. 0% is the ratio obtained at time 0 and 100% is the point at which all VSG is in the soluble fraction. (C,D) Immunoprecipitation of p67, following radioactive labelling of parasites by pulse chase with [35S]methionine and [35S]cysteine over a 4-hour time course. BSF line Bp2T7/ARL1 cells were grown in the absence (C) and presence (D) of tetracycline for 36 hours. Proteins were separated by SDS-PAGE and radiolabelled products detected by autoradiography. P67 is synthesized as a 100 kDa protein, which is modified by N-glycans to form a 150 kDa molecule, clearly visible in both samples by 30 minutes. After delivery to the lysosome, the protein is proteolytically cleaved into fragments of 75, 42, 32 and 28 kDa, as observed after 1 hour. Note that the lower fragment is not seen in this figure and that there is an unidentified band in samples from later time points with a molecular weight of approximately 55 kDa. (E,F) p67 immunoprecipitation results were quantified by densitometry in cells grown in the absence (light shading) and presence (dark shading) of tetracycline for 36 hours. (E) The percentage of total [35S]p67 present as the N-glycan modified glycoform (gp150) during the 4 hour pulse-chase time course. (F) Percentage of total [35S]p67 present as proteolytically cleaved fragments (gp75, 42 and 32 glycoforms) in the lysosome. Data shown represent one of two independent experiments.
These results demonstrate a marked effect of ARL1 depletion on the initial stages of transport of newly synthesized VSG to the plasma membrane in T. brucei bloodstream parasites. However, exocytosis is not fully blocked, concomitant with a requirement for ARL1 early in this process, followed by rapid movement of radioactively labelled VSG to the cell surface at later time points.
In order to study Golgi function in the absence of ARL1, the lysosomal protein p67 was immunoprecipitated from parasites following a pulse-chase using [35S]-labelled methionine and cysteine. In bloodstream parasites, ER-associated p67 is synthesised as a gp100 glycoform, which is delivered to the Golgi for N-glycan processing to produce a gp150 glycoform. This is transported to the lysosome, where it is proteolytically cleaved into four fragments: gp75, gp42, gp32 and gp28 (Alexander et al., 2002). Here we show that even 36 hours after induction, ARL1-depleted cells are able to produce the N-glycan modified glycoform, gp150, of p67, which is clearly visible by 30 minutes (Fig. 7C,D). Quantification by densitometry (Fig. 7E) shows that approximately equal proportions of gp150 are present in uninduced and induced cells at the 30 minute and 1 hour time points of the pulse-chase assay, relative to total amounts of [35S]p67. This implies that the compartment in which this modification occurs is not affected by the absence of ARL1 and its consequential effects on Golgi structure. However, there is a marked increase in the levels of uncleaved gp100 and gp150 glycoforms at later stages of the pulse-chase assay, compared to uninduced cells. The proportion of p67 present as proteolytic products in the lysosome is significantly lower in induced cells at the 1 and 2 hour time points of the assay (Fig. 7F), which may be due to a decrease in the rate of p67 transport from the Golgi to the lysosome. However, as p67 is clearly upregulated in ARL1-depleted cells, it is possible that the effects seen here are due to saturation in p67 processing mechanisms rather than a defect in transport.
Discussion
In the present study we have identified a number of uncharacterized members of the Arf family of proteins in three trypanosomatid species. The only other studies on Arf proteins in these organisms to date have identified ARF1 in T. cruzi (de Sa-Freire et al., 2003) and characterized ARL3A in Leishmania spp. (Sturm et al., 1998; Cuvillier et al., 2000; Cuvillier et al., 2003). ARL3A is believed to have a role in flagellum biosynthesis as overexpression of a constitutively active Q70L mutant form of the L. donovani protein leads to flagellar disappearance in promastigotes (Cuvillier et al., 2000). Parasites expressing the mutant protein have no defects in cell division or differentiation, can infect and survive within macrophages but are unable to survive in the insect vector (Cuvillier et al., 2003). As the known trypanosomatid ARL3A homologues share a high degree of identity at the amino acid level (79-98%), it is likely that they have similar roles in flagellum development in these parasite species.
ARL1 has been implicated in the mediation of membrane traffic in yeast, Drosophila and humans. Previous studies show that human ARL1 binds to a number of effectors, including POR1/Arfaptin2, pericentrin, phosphodiesterase (PDE) δ, mitotic kinesis-like protein 1 (MKLP1), short coiled-coil protein (SCOCO) and two of the four human GRIP domain proteins, Golgin 97 and Golgin 245 (Lu et al., 2001; Van Valkenburgh et al., 2001; Lu and Hong, 2003). The GRIP domain, which has the ability to target reporter genes to the Golgi membrane, is a motif of approximately 50 amino acids found near the protein C-terminus, which binds directly to the Switch II region of GTP-bound ARL1 but not to the other Arf family members (Kjer-Nielsen et al., 1999; Munro and Nichols, 1999). GRIP domains are present in many of the golgins, which have roles in the tethering of vesicles to the Golgi membrane before fusion and in the maintenance of stacking cisternal structure. Yeast Arl1p also binds to and directs the Golgi localization of the only GRIP domain protein in yeast, lmh1p, under the direction of Arl3p (Setty et al., 2003). As a GRIP-containing protein has been isolated in T. brucei (McConville et al., 2002), a similar pathway is probably operating in trypanosomatid species.
The results presented here strongly suggest that ARL1 is essential for viability in T. brucei BSF parasites. Previous studies have shown that ARL1 is an essential gene for development in Drosophila (Tamkun et al., 1991) but the protein is not required for normal growth in yeast, with a knockout strain showing only minor defects in protein sorting (Lee et al., 1997). By contrast, ARL1 expression has not been detected in the T. brucei insect PCF stage and ARL1 RNAi in this lifecycle stage has no effect on parasite growth. These observations are consistent with the higher rates of protein trafficking seen in BSF stage parasites as compared to PCF stages, characterized by upregulated expression of specific proteins including the endosomal GTPase Rab11 which has a role in the exocytosis of recycling VSG (Jeffries et al., 2001) and the lysosomal membrane protein, p67 (Bangs, 1999). Ongoing studies will establish whether ARL1 protein is absent or downregulated in this lifecycle stage.
Our results show that TbARL1 is present in both the Golgi apparatus and the cytosol, and that Golgi localization is dependent on N-myristoylation of the protein. GTP-bound ARL1 in yeast and mammalian cells is localized in the trans-Golgi network and localization of human ARL1 is also dependent on N-myristoylation, with a G2A non-myristoylated mutant mistargeted to the cytosol (Lu et al., 2001). Knockout strains of yeast have shown that the Golgi localization of yArl1 is dependent on the presence of another Arf family member, yArl3, the homologue of human ARFRP1, neither of which are N-myristoylated (Panic et al., 2003; Setty et al., 2003). Instead, Arl3p must be N-acetylated by the NatC complex in order to be localized to the Golgi, and also requires interaction with a Golgi membrane protein of unknown function, Sys1p. The human proteins ARFRP1 and Sys1 can also bind to form a complex, suggesting that this mechanism may be conserved in eukaryotes (Behnia et al., 2004).
VSG is highly expressed in BSF T. brucei, forming a dense protective coat on the parasite surface. Newly synthesized GPI-anchored VSG passes from the ER through the Golgi apparatus and reaches the cell surface via the flagellar pocket membrane, an invagination in the plasma membrane through which all nutritional uptake and exocytosis occurs (reviewed by Landfear and Ignatushchenko, 2001). The protein is rapidly recycled, entering the cell through the flagellar pocket in Class I clathrin-coated vesicles. These are transported to the lysosome, before fusing with endosomes, where the protein is concentrated by both negative and positive selection because of the budding off of Class II VSG-depleted clathrin-coated vesicles. Once VSG has been concentrated in the endosome, it is packaged into TbRab11-positive exocytic carriers that fuse with the flagellar pocket membrane (Overath and Engstler, 2004). It is estimated that the entire pool of surface VSG is internalized and recycled in approximately 12.5 minutes (Engstler et al., 2004).
In the present study, we demonstrate that the depletion of ARL1 from the Golgi apparatus causes a delay in the exocytosis of VSG to the cell surface but has only a minor effect on endocytosis of ConA and does not appear to change the cellular distribution of clathrin. This implies that ARL1 has a role in the exocytosis of newly synthesized VSG: it may be involved in the recruitment of coatomers for the production of non-clathrin vesicles at the TGN for export of material to the plasma membrane and outer cell membrane. ARL1 is unlikely to mediate VSG recycling as there is no evidence that internalized protein passes through the Golgi during export back to the cell surface. In the absence of ARL1, inhibition of exocytosis from the TGN to the plasma membrane would result in the accumulation of proteins intended for export in the Golgi cisternae. Our results show that ARL1-depleted cells have a wide distribution of vesicular material in the cytoplasm, which could be caused by this blockage.
A striking feature of ARL1-depleted bloodstream cells is the complete disorganisation of the Golgi apparatus. This could be due to disruption of the recruitment of ARL1-specific effectors including GRIP-domain proteins. A similar phenotype has been observed by the overexpression of a dominant-negative T31N mutant form of ARL1 in CHO cells (Lu et al., 2001) and also by the treatment of mammalian cells with the fungal metabolite Brefeldin-A, which inhibits Arf-specific GEFs (Donaldson et al., 1991; Randazzo et al., 1993). Both of these disrupt Golgi localization of γ-adaptin, a component of the AP-1 complex but leave the Golgi matrix relatively intact (Lu et al., 2001). As ARL1 appears to be essential for maintaining Golgi structure in bloodstream form parasites but is not expressed in PCF cells, these results bring into question whether different mechanisms are in place to conserve structure and organisation of the organelle in the two life cycle stages of the parasite.
It is interesting to note that ARL1-depleted bloodstream form parasites do not show an obvious defect in N-linked glycosylation of the lysosomal marker p67 as a protein corresponding to the molecular weight of the N-glycan modified molecule (gp150) is clearly produced in the cells. However, this does not rule out the possibility of differences in N-glycan composition that are not detectable using this assay. In eukaryotes, N-glycan biosynthesis is initiated in the ER where a core structure is attached to newly synthesized proteins. The resulting glycoproteins are delivered to the medial Golgi for mannose to GlcNAc substitution mediated by glycosyltransferases, followed by trimming and then elongation of branches in the TGN to form mature N-glycans (reviewed by Dennis et al., 1999). If loss of ARL1 affects cisternal organisation predominantly in the TGN as indicated above, some inhibition of correct branch elongation would be expected. Alternatively, it is possible that the enzymes involved in Golgi processing of p67 are translocated to another subcellular compartment in ARL1-depleted cells, enabling N-glycan modification to continue in a different location.
Overall, there are considerable similarities between the phenotype described here and that of parasites treated with the ionophore monensin, which causes H+/Na+ ion exchange across membranes accompanied by osmotic swelling (reviewed by Dinter and Berger, 1998). Treatment of T. brucei BSF cells with monensin causes swelling of the Golgi cisternae, particularly at the trans face, together with expansion of the lysosome and the appearance of multi-vesicular bodies (Duszenko et al., 1988). All of these effects are evident in ARL1-depleted cells, suggesting a possible association between the observed phenotype and changes in ion exchange. In support of this, a recent paper postulates a novel role for ARL1 in controlling potassium ion homeostasis in yeast, by positive regulation of the Ser/Thr protein kinases HAL4/HAL5, which in turn regulate the plasma membrane K+ transporters Trk1p and Trk2p (Munson et al., 2004). In this context, further research is in progress to establish more fully the function of ARL1 in bloodstream trypanosomes. Our data clearly indicate, however, that ARL1 depletion can only contribute in part to the phenotypic effects resulting from NMT depletion, which are observed throughout the parasite life cycle and not confined to just mammalian stage T. brucei.
Acknowledgments
We gratefully acknowledge contributions from the following colleagues: Sam Alsford and David Horn for the pM2cC vector construct; George Cross and Doug LaCount for parasite strains and the p2T7Ti vector construct; Jay Bangs and Mark Field for antibodies, and the L. major, T. brucei and T. cruzi genome sequencing teams at the Wellcome Trust Sanger Institute Pathogen Sequencing Unit, the Institute of Genome Research, Seattle Biomedical Research Institute and the Karolinska Institue for access to sequence data. We would also like to thank Clare Allen, Belinda Hall, Mark Field, Cristina Guerra, Paul Denny and members of the Smith group for technical advice and helpful discussions. This work was funded by the Wellcome Trust (061343/Z/00/Z). C.P. is the recipient of a research studentship from the UK Biological and Biotechnological Scientific Research Council.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/118/4/831/DC1
References
- Alexander DL, Schwartz KJ, Balber AE, Bangs JD. Developmentally regulated trafficking of the lysosomal membrane protein p67 in Trypanosoma brucei. J. Cell Sci. 2002;115:3253–3263. doi: 10.1242/jcs.115.16.3253. [DOI] [PubMed] [Google Scholar]
- Allen CL, Goulding D, Field MC. Clathrin-mediated endocytosis is essential in Trypanosoma brucei. EMBO J. 2003;22:4991–5002. doi: 10.1093/emboj/cdg481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B, Huber I, Paris S, Chabre M, Cassel D. Role of protein-phospholipid interactions in the activation of ARF1 by the guanine nucleotide exchange factor Arno. J. Biol. Chem. 1997;272:22221–22226. doi: 10.1074/jbc.272.35.22221. [DOI] [PubMed] [Google Scholar]
- Bangs JD. Molecular cloning of p67, a lysosomal membrane glycoprotein from Trypanosoma brucei. Curr. Opin. Microbiol. 1999;98:17–28. doi: 10.1016/s0166-6851(98)00155-8. [DOI] [PubMed] [Google Scholar]
- Bangs JD, Brouch EM, Ransom DM, Roggy JL. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J. Biol. Chem. 1993;105:1101–1113. doi: 10.1242/jcs.105.4.1101. [DOI] [PubMed] [Google Scholar]
- Bangs JD, Brouch EM, Ransom DM, Roggy JL. A soluble secretory reporter system in Trypanosoma brucei. Studies on endoplasmic reticulum targeting. J. Biol. Chem. 1996;271:18387–18393. doi: 10.1074/jbc.271.31.18387. [DOI] [PubMed] [Google Scholar]
- Bangs JD, Doering TL, Englund PT, Hart GW. A phospholipase C from Trypanosoma brucei which selectively cleaves the glycolipid on the variant surface glycoprotein. J. Biol. Chem. 1986;261:13813–13819. [PubMed] [Google Scholar]
- Behnia R, Panic B, Whyte JR, Munro S. Targeting of the Arf-like GTPase Arl3p to the Golgi requires N-terminal acetylation and the membrane protein Sys1p. Nat. Cell Biol. 2004;6:405–413. doi: 10.1038/ncb1120. [DOI] [PubMed] [Google Scholar]
- Biebinger S, Wirtz LE, Lorenz P, Clayton C. Vectors for inducible expression of toxic gene products in bloodstream and procyclic Trypanosoma brucei. Mol. Biochem. Parasitol. 1997;85:99–112. doi: 10.1016/s0166-6851(96)02815-0. [DOI] [PubMed] [Google Scholar]
- Boman AL, Kahn RA. Arf proteins: the membrane traffic police? Trends Biochem. Sci. 1995;20:147–150. doi: 10.1016/s0968-0004(00)88991-4. [DOI] [PubMed] [Google Scholar]
- Brown HA, Gutowski S, Kahn RA, Sternweis PC. Phospholipase D is present on Golgi-enriched membranes and its activation by ADP ribosylation factor is sensitive to brefeldin A. J. Biol. Chem. 1995;92:4952–4956. doi: 10.1073/pnas.92.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S, Thomas GM, Fensome A, Geny B, Cunningham E, Gout I, Hiles I, Totty NF, Truong O, Hsuan JJ. Purification of phosphatidylinositol transfer protein from brain cytosol for reconstituting G-protein-regulated phosphoinositide-specific phospholipase C-beta isozymes. Science. 1994;238:168–181. doi: 10.1016/0076-6879(94)38015-5. [DOI] [PubMed] [Google Scholar]
- Cuvillier A, Miranda JC, Ambit A, Barral A, Merlin G. Abortive infection of Lutzomyia longipalpis insect vectors by aflagellated LdARL-3A-Q70L overexpressing Leishmania amazonensis parasites. Cell Microbiol. 2003;5:717–728. doi: 10.1046/j.1462-5822.2003.00316.x. [DOI] [PubMed] [Google Scholar]
- Cuvillier A, Redon F, Antoine JC, Chardin P, DeVos T, Merlin G. LdARL-3A, a Leishmania promastigote-specific ADP-ribosylation factor-like protein, is essential for flagellum integrity. J. Cell Sci. 2000;113:2065–2074. doi: 10.1242/jcs.113.11.2065. [DOI] [PubMed] [Google Scholar]
- de Sa-Freire A, Nepomuceno-Silva JL, da Paixao JC, de Mendonca SM, de Melo LD, Lopes UG. TcArf1: a Trypanosoma cruzi ADP-ribosylation factor. Parasitol. Res. 2003;91:166–170. doi: 10.1007/s00436-003-0952-0. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Granovsky M, Warren CE. Protein glycosylation in development and disease. BioEssays. 1999;21:412–421. doi: 10.1002/(SICI)1521-1878(199905)21:5<412::AID-BIES8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Dhir V, Goulding D, Field MC. TbRAB1 and TbRAB2 mediate trafficking through the early secretory pathway of Trypanosoma brucei. Mol. Biochem. Parasitol. 2004;137:253–265. doi: 10.1016/j.molbiopara.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Dinter A, Berger EG. Golgi-disturbing agents. Histochem. Cell Biol. 1998;109:571–590. doi: 10.1007/s004180050256. [DOI] [PubMed] [Google Scholar]
- Doering TL, Lu T, Werbovetz KA, Gokel GW, Hart GW, Gordon JI, Englund PT. Toxicity of myristic acid analogs toward African trypanosomes. Proc. Natl. Acad. Sci. USA. 1994;91:9735–9739. doi: 10.1073/pnas.91.21.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Kahn RA, Lippincott-Schwartz J, Klausner RD. Binding of ARF and beta-COP to Golgi membranes: possible regulation by a trimeric G protein. Science. 1991;254:1197–1199. doi: 10.1126/science.1957170. [DOI] [PubMed] [Google Scholar]
- Duronio RJ, Jackson-Machelski E, Heuckeroth RO, Olins PO, Devine CS, Yonemoto W, Slice LW, Taylor SS, Gordon JI. Protein N-myristoylation in Escherichia coli: reconstitution of a eukaryotic protein modification in bacteria. Proc. Natl. Acad. Sci. USA. 1990;87:1506–1510. doi: 10.1073/pnas.87.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio RJ, Rudnick DA, Adams SP, Towler DA, Gordon JI. Analyzing the substrate specificity of Saccharomyces cerevisiae myristoyl-CoA:protein N-myristoyltransferase by co-expressing it with mammalian G protein alpha subunits in Escherichia coli. J. Biol. Chem. 1991a;266:10498–10504. [PubMed] [Google Scholar]
- Duronio RJ, Rudnick DA, Johnson RL, Johnson DR, Gordon JI. Myristic acid auxotrophy caused by mutation of S. cerevisiae myristoyl-CoA:protein N-myristoyltransferase. J. Cell Biol. 1991b;113:1313–1330. doi: 10.1083/jcb.113.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duszenko M, Ivanov IE, Ferguson MA, Plesken H, Cross GA. Intracellular transport of a variant surface glycoprotein in Trypanosoma brucei. J. Cell Biol. 1988;106:77–86. doi: 10.1083/jcb.106.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstler M, Thilo L, Weise F, Grunfelder CG, Schwarz H, Boshart M, Overath P. Kinetics of endocytosis and recycling of the GPI-anchored variant surface glycoprotein in Trypanosoma brucei. J. Cell Sci. 2004;117:1105–1115. doi: 10.1242/jcs.00938. [DOI] [PubMed] [Google Scholar]
- Field H, Farjah M, Pal A, Gull K, Field MC. Complexity of trypanosomatid endocytosis pathways revealed by Rab4 and Rab5 isoforms in Trypanosoma brucei. J. Biol. Chem. 1998;273:32102–32110. doi: 10.1074/jbc.273.48.32102. [DOI] [PubMed] [Google Scholar]
- Gelb MH, van Voorhis WC, Buckner FS, Yokoyama K, Eastman R, Carpenter EP, Panethymitaki C, Brown KA, Smith DF. Protein farnesyl and N-myristoyl transferases: piggy-back medicinal chemistry targets for the development of antitrypanosomatid and antimalarial therapeutics. Mol. Biochem. Parasitol. 2003;126:155–163. doi: 10.1016/s0166-6851(02)00282-7. [DOI] [PubMed] [Google Scholar]
- Haun RS, Moss J, Vaughan M. Effect of myristoylation on GTP-dependent binding of ADP-ribosylation factor to Golgi. J. Biol. Chem. 1993;268:7064–7068. [PubMed] [Google Scholar]
- He CY, Ho HH, Malsam J, Chalouni C, West CM, Ullu E, Toomre D, Warren G. Golgi duplication in Trypanosoma brucei. J. Cell Biol. 2004;165:313–321. doi: 10.1083/jcb.200311076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KL, Hutchings NR, Russell DG, Donelson JE. Expression of a marker for intracellular Trypanosoma cruzi amastigotes in extracellular spheromastigotes. J. Cell Sci. 1999;98:265–270. doi: 10.1016/s0166-6851(98)00158-3. [DOI] [PubMed] [Google Scholar]
- Hong JX, Lee FJ, Patton WA, Lin CY, Moss J, Vaughan M. Phospholipid- and GTP-dependent activation of cholera toxin and phospholipase D by human ADP-ribosylation factor-like protein 1 (HARL1) J. Biol. Chem. 1998;273:15872–15876. doi: 10.1074/jbc.273.25.15872. [DOI] [PubMed] [Google Scholar]
- Jeffries TR, Morgan GW, Field MC. A developmentally regulated rab11 homologue in Trypanosoma brucei is involved in recycling processes. J. Cell Sci. 2001;114:2617–2626. doi: 10.1242/jcs.114.14.2617. [DOI] [PubMed] [Google Scholar]
- Kahn RA. Quantitation and purification of ADP-ribosylation factor. Methods Enzymol. 1991;195:233–242. doi: 10.1016/0076-6879(91)95169-k. [DOI] [PubMed] [Google Scholar]
- Kahn RA, Gilman AG. Purification of a protein cofactor required for ADP-ribosylation of the stimulatory regulatory component of adenylate cyclase by cholera toxin. J. Biol. Chem. 1984;259:6228–6234. [PubMed] [Google Scholar]
- Kahn RA, Randazzo P, Serafini T, Weiss O, Rulka C, Clark J, Amherdt M, Roller P, Orci L, Rothman JE. The amino terminus of ADP-ribosylation factor (ARF) is a critical determinant of ARF activities and is a potent and specific inhibitor of protein transport. J. Biol. Chem. 1992;267:13039–13046. [PubMed] [Google Scholar]
- Kjeldgaard M, Nyborg J, Clark BF. The GTP binding motif: variations on a theme. FASEB J. 1996;10:1347–1368. [PubMed] [Google Scholar]
- Kjer-Nielsen L, Teasdale RD, van Vliet C, Gleeson PA. A novel Golgi-localisation domain shared by a class of coiled-coil peripheral membrane proteins. Curr. Biol. 1999;9:385–388. doi: 10.1016/s0960-9822(99)80168-7. [DOI] [PubMed] [Google Scholar]
- LaCount DJ, El-Sayed NM, Kaul S, Wanless D, Turner CM, Donelson JE. Double-stranded RNA interference in Trypanosoma brucei using head-to-head promoters. Nucleic Acids Res. 2000;111:67–76. doi: 10.1016/s0166-6851(00)00300-5. [DOI] [PubMed] [Google Scholar]
- Landfear SM, Ignatushchenko M. The flagellum and flagellar pocket of trypanosomatids. Mol. Biochem. Parasitol. 2001;115:1–17. doi: 10.1016/s0166-6851(01)00262-6. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Huang CF, Yu WL, Buu LM, Lin CY, Huang MC, Moss J, Vaughan M. Characterization of an ADP-ribosylation factor-like 1 protein in Saccharomyces cerevisiae. J. Biol. Chem. 1997;272:30998–31005. doi: 10.1074/jbc.272.49.30998. [DOI] [PubMed] [Google Scholar]
- Lin CY, Huang PH, Liao WL, Cheng HJ, Huang CF, Kuo JC, Patton WA, Massenburg D, Moss J, Lee FJ. ARL4, an ARF-like protein that is developmentally regulated and localized to nuclei and nucleoli. J. Biol. Chem. 2000;275:37815–37823. doi: 10.1074/jbc.M002470200. [DOI] [PubMed] [Google Scholar]
- Lin CY, Li CC, Huang PH, Lee FJ. A developmentally regulated ARF-like 5 protein (ARL5), localized to nuclei and nucleoli, interacts with heterochromatin protein 1. J. Cell Sci. 2002;115:4433–4445. doi: 10.1242/jcs.00123. [DOI] [PubMed] [Google Scholar]
- Lodge JK, Jackson-Machelski E, Devadas B, Zupec ME, Getman DP, Kishore N, Freeman SK, McWherter CA, Sikorski JA, Gordon JI. N-myristoylation of Arf proteins in Candida albicans: an in vivo assay for evaluating antifungal inhibitors of myristoyl-CoA: protein N-myristoyltransferase. Microbiology. 1997;143:357–366. doi: 10.1099/00221287-143-2-357. [DOI] [PubMed] [Google Scholar]
- Lowe SL, Wong SH, Hong W. The mammalian ARF-like protein 1 (Arl1) is associated with the Golgi complex. J. Cell Sci. 1996;109:209–220. doi: 10.1242/jcs.109.1.209. [DOI] [PubMed] [Google Scholar]
- Lu L, Hong W. Interaction of Arl1-GTP with GRIP domains recruits autoantigens Golgin-97 and Golgin-245/p230 onto the Golgi. Mol. Biol. Cell. 2003;14:3767–3781. doi: 10.1091/mbc.E03-01-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Horstmann H, Ng C, Hong W. Regulation of Golgi structure and function by ARF-like protein 1 (Arl1) J. Cell Sci. 2001;114:4543–4555. doi: 10.1242/jcs.114.24.4543. [DOI] [PubMed] [Google Scholar]
- McConville MJ, Ilgoutz SC, Teasdale RD, Foth BJ, Matthews A, Mullin KA, Gleeson PA. Targeting of the GRIP domain to the trans-Golgi network is conserved from protists to animals. Eur. J. Cell Biol. 2002;81:485–495. doi: 10.1078/0171-9335-00268. [DOI] [PubMed] [Google Scholar]
- McKean PG, Denny PW, Knuepfer E, Keen JK, Smith DF. Phenotypic changes associated with deletion and overexpression of a stage-regulated gene family in Leishmania. Cell Microbiol. 2001;3:511–523. doi: 10.1046/j.1462-5822.2001.00135.x. [DOI] [PubMed] [Google Scholar]
- Morgan GW, Allen CL, Jeffries TR, Hollinshead M, Field MC. Developmental and morphological regulation of clathrin-mediated endocytosis in Trypanosoma brucei. J. Cell Sci. 2001;114:2605–2615. doi: 10.1242/jcs.114.14.2605. [DOI] [PubMed] [Google Scholar]
- Morgan GW, Hall BS, Denny PW, Field MC, Carrington M. The endocytic apparatus of the kinetoplastida. Part II: machinery and components of the system. Trends Parasitol. 2002;18:540–546. doi: 10.1016/s1471-4922(02)02392-9. [DOI] [PubMed] [Google Scholar]
- Munro S, Nichols BJ. The GRIP domain – a novel Golgi-targeting domain found in several coiled-coil proteins. Curr. Biol. 1999;9:377–380. doi: 10.1016/s0960-9822(99)80166-3. [DOI] [PubMed] [Google Scholar]
- Munson AM, Haydon DH, Love SL, Fell GL, Palanivel VR, Rosenwald AG. Yeast ARL1 encodes a regulator of K+ influx. J. Cell Sci. 2004;117:2309–2320. doi: 10.1242/jcs.01050. [DOI] [PubMed] [Google Scholar]
- Overath P, Engstler M. Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol. Microbiol. 2004;53:735–744. doi: 10.1111/j.1365-2958.2004.04224.x. [DOI] [PubMed] [Google Scholar]
- Panic B, Whyte JR, Munro S. The ARF-like GTPases Arl1p and Arl3p act in a pathway that interacts with vesicle-tethering factors at the Golgi apparatus. Curr. Biol. 2003;13:405–410. doi: 10.1016/s0960-9822(03)00091-5. [DOI] [PubMed] [Google Scholar]
- Pasqualato S, Renault L, Cherfils J. Arf, Arl, Arp and Sar proteins: a family of GTP-binding proteins with a structural device for ‘front-back’ communication. EMBO Rep. 2002;3:1035–1041. doi: 10.1093/embo-reports/kvf221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price HP, Menon MR, Panethymitaki C, Goulding D, McKean PG, Smith DF. Myristoyl-CoA:protein N-myristoyltransferase, an essential enzyme and potential drug target in kinetoplastid parasites. J. Biol. Chem. 2003;278:7206–7214. doi: 10.1074/jbc.M211391200. [DOI] [PubMed] [Google Scholar]
- Randazzo PA, Yang YC, Rulka C, Kahn RA. Activation of ADP-ribosylation factor by Golgi membranes. Evidence for a brefeldin A- and protease-sensitive activating factor on Golgi membranes. J. Biol. Chem. 1993;268:9555–9563. [PubMed] [Google Scholar]
- Setty SR, Shin ME, Yoshino A, Marks MS, Burd CG. Golgi recruitment of GRIP domain proteins by Arf-like GTPase 1 is regulated by Arf-like GTPase 3. Curr. Biol. 2003;13:401–404. doi: 10.1016/s0960-9822(03)00089-7. [DOI] [PubMed] [Google Scholar]
- Sturm NR, Yu MC, Campbell DA. Characterization of a GTP-binding protein in the ADP-ribosylation factor subfamily from Leishmania tarentolae. Mol. Cell Biol. 1998;1442:347–352. doi: 10.1016/s0167-4781(98)00150-x. [DOI] [PubMed] [Google Scholar]
- Tamkun JW, Kahn RA, Kissinger M, Brizuela BJ, Rulka C, Scott MP, Kennison JA. The arflike gene encodes an essential GTP-binding protein in Drosophila. Proc. Natl. Acad. Sci. USA. 1991;88:3120–3124. doi: 10.1073/pnas.88.8.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi T, Sakurai N, Nakano K, Ishisaka R. C-terminal 15 kDa fragment of cytoskeletal actin is posttranslationally N-myristoylated upon caspase-mediated cleavage and targeted to mitochondria. FEBS Lett. 2003;539:37–44. doi: 10.1016/s0014-5793(03)00180-7. [DOI] [PubMed] [Google Scholar]
- Van Valkenburgh H, Shern JF, Sharer JD, Zhu X, Kahn RA. ADP-ribosylation factors (ARFs) and ARF-like 1 (ARL1) have both specific and shared effectors: characterizing ARL1-binding proteins. J. Biol. Chem. 2001;276:22826–22837. doi: 10.1074/jbc.M102359200. [DOI] [PubMed] [Google Scholar]
- Wirtz E, Leal S, Ochatt C, Cross GA. Trypanosoma brucei variant surface glycoprotein regulation involves coupled activation/inactivation and chromatin remodeling of expression sites. Mol. Biochem. Parasitol. 1999;18:2265–2272. doi: 10.1093/emboj/18.8.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward R, Gull K. Timing of nuclear and kinetoplast DNA replication and early morphological events in the cell cycle of Trypanosoma brucei. J. Cell Sci. 1990;95:49–57. doi: 10.1242/jcs.95.1.49. [DOI] [PubMed] [Google Scholar]
- Wu M, Lu L, Hong W, Song H. Structural basis for recruitment of GRIP domain golgin-245 by small GTPase Arl1. Nat. Struct. Mol. Biol. 2004;11:86–94. doi: 10.1038/nsmb714. [DOI] [PubMed] [Google Scholar]
- Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]