Abstract
Functional transfer RNA (tRNA) molecules are a prerequisite for protein biosynthesis. Several processing steps are required to generate the mature functional tRNA from precursor molecules. Two of the early processing steps involve cleavage at the tRNA 5′ end and the tRNA 3′ end. While processing at the tRNA 5′ end is performed by RNase P, cleavage at the 3′ end is catalyzed by the endonuclease tRNase Z. In eukaryotes, tRNase Z enzymes are found in two versions: a short form of about 250 to 300 amino acids and a long form of about 700 to 900 amino acids. All eukaryotic genomes analyzed to date encode at least one long tRNase Z protein. Of those, Arabidopsis (Arabidopsis thaliana) is the only organism that encodes four tRNase Z proteins, two short forms and two long forms. We show here that the four proteins are distributed to different subcellular compartments in the plant cell: the nucleus, the cytoplasm, the mitochondrion, and the chloroplast. One tRNase Z is present only in the cytoplasm, one protein is found exclusively in mitochondria, while the third one has dual locations: nucleus and mitochondria. None of these three tRNase Z proteins is essential. The fourth tRNase Z protein is present in chloroplasts, and deletion of its gene results in an embryo-lethal phenotype. In vitro analysis with the recombinant proteins showed that all four tRNase Z enzymes have tRNA 3′ processing activity. In addition, the mitochondrial tRNase Z proteins cleave tRNA-like elements that serve as processing signals in mitochondrial mRNA maturation.
The generation of functional tRNA molecules is essential not only for protein biosynthesis but also for other cellular processes in all organisms (Söll, 1993). Maturation of tRNAs involves the removal of additional sequences from tRNA precursors (Hopper and Phizicky, 2003). Processing at the tRNA 5′ end has been well studied and is catalyzed by RNase P (Evans et al., 2006), but generation of the tRNA 3′ end is not as well understood. The endonuclease involved in this process has recently been identified and is termed tRNase Z (Schiffer et al., 2002). tRNase Z has been found in organisms from all three domains, bacteria, archaea, and eukarya. It exists in two forms: a short version of about 250 to 300 amino acids and a long version of about 700 to 900 amino acids. In bacteria and archaea, only short tRNase Z proteins are present. Eukaryotic organisms generally contain a long tRNase Z, and in some cases they have both forms, long and short tRNase Z proteins (Vogel et al., 2005; Redko et al., 2007).
tRNase Z proteins belong to the superfamily of the metallo-β-lactamases (MBLs), which are characterized by the α-β/β-α fold. All MBLs contain five highly conserved motifs that are involved in the metal coordination in the active site (Aravind, 1999). In addition to the MBL domain, members of this superfamily contain other different domains, resulting in a wide substrate spectrum for proteins of this family (Aravind, 1999; Callebaut et al., 2002; Dominski, 2007). Several enzymes of the MBL family are involved in nucleic acid metabolism. These include the polyadenylation factor CPSF73, the mRNA 3′ endonuclease (Ryan et al., 2004), and the ribonuclease J1 (Mathy et al., 2007).
tRNase Z was isolated and identified by biochemical purification of the enzyme from wheat (Triticum aestivum) embryos (Schiffer et al., 2002). Database searches identified four homologs of the wheat tRNase Z in Arabidopsis (Arabidopsis thaliana): two short tRNase Z proteins of 280 and 374 amino acids (termed AthTrZS1 and AthTrZS2) and two long tRNase Z proteins of 890 and 943 amino acids (termed AthTrZL1 and AthTrZL2; Schiffer et al., 2002; Vogel et al., 2005). Bioinformatic analyses using sorting servers showed that AthTrZS1 has no predicted signal peptide while AthTrzS2 has a potential signal sequence for chloroplasts of about 68 amino acids (Schiffer et al., 2002).
The detection of four different tRNase Z enzymes in Arabidopsis was surprising, since all other organisms contain only one or two tRNase Z proteins (Vogel et al., 2005). Thus, the question arose, why does Arabidopsis need four tRNase Z enzymes? One possible explanation is that the four tRNase Z homologs have different RNA substrates. tRNase Z enzymes from bacteria and archaea have been shown to be able to process other substrates besides tRNA precursors: mRNAs (Perwez and Kushner, 2006) and 5S rRNA (Hölzle et al., 2008). In addition, it is known that the MBL enzymes show a broad substrate spectrum (Daiyasu et al., 2001; Callebaut et al., 2002; Dominski, 2007). Therefore, the four tRNase Z proteins in Arabidopsis might process different RNA substrates. Only a few ribonucleases have been identified in Arabidopsis, and the tRNase Z proteins are good candidates for the missing ribonucleases.
Another explanation for the occurrence of four tRNase Z proteins might be that they are active in different compartments or different tissues or are differentially expressed. The prediction by different sorting servers that three of the proteins are routed to organelles supports the hypothesis of ribonuclease activity in different compartments.
To reveal the specific function of each individual tRNase Z protein, we investigated their functions in detail using in vitro and in vivo approaches.
RESULTS
Arabidopsis is the only organism found to date that encodes four tRNase Z enzymes; all other organisms encode only one or two tRNase Z proteins (Vogel et al., 2005). Searches of the National Center for Biotechnology Information databases (http://www.ncbi.nlm.nih.gov/) revealed that full-length cDNAs corresponding to all four tRNase Z genes are available. Two full-length cDNAs have been reported for AthTrzS1 and AthTrzL1, three for AthTrzL2 (the clone NM_112497 was wrongly assigned to AthTrzL1), and five for AthTrzS2. In general, all of the cDNA clones for one gene code for the same protein; thus, no alternative splicing seems to take place. The expression profile of the tRNase Z genes in Arabidopsis was monitored using the Genevestigator Web site (https://www.genevestigator.ethz.ch.at; Zimmermann et al., 2004, 2005). mRNAs corresponding to the tRNase Z proteins were found in all plant tissues tested. In particular, there was no significant variation between mRNA levels for AthTrzS1, AthTrzL1, and AthTrzL2. Only in root tips was the expression significantly high for all three mRNAs. In contrast, AthTrzS2 was poorly expressed in nongreen tissue like flowers, pollen, and stamen and was abundant in young green tissue (rosette leaves) and in actively dividing tissue (i.e. shoot apex, pedicel, and root tips). All tRNase Z genes were expressed during all growth stages of Arabidopsis, in particular during the first stage of development (seedling) and at the start of flowering.
Four tRNase Z Proteins in Arabidopsis
The short tRNase Z proteins AthTrZS1 and AthTrZS2 have a sequence identity of 44%, and protein sequence alignments show that AthTrZS2 has a longer N terminus, which suggests that this N terminus is a potential signal sequence (Supplemental Fig. S1). Sequence identity between the long tRNase Z enzymes is 69%. The short tRNase Z enzymes have sequence similarity to the C-terminal part of the long tRNase Z enzymes.
In silico analysis of subcellular targeting using different sorting servers (see “Materials and Methods”) suggested that three of the four tRNase Z proteins are routed to organelles (Supplemental Table S1). AthTrZS2 and AthTrZL2 are predicted to be routed to chloroplasts, and AthTrZL1 is predicted to be routed to mitochondria, chloroplasts, and the nucleus. AthTrZS1 seems to contain no signal sequence.
Subcellular Localization of Arabidopsis tRNase Z Proteins
To investigate localization in vivo, the complete coding regions of AthTrZS1 and AthTrZS2 were cloned in frame upstream of the GFP (AthTrZS1) or the red fluorescent protein (RFP; AthTrZS2) genes (Fig. 1A). The fusion constructs were then stably transformed into Arabidopsis wild-type plants. Protoplasts were released from selected plants and analyzed by fluorescence microscopy (Fig. 1, B and C). Analysis of the AthTrZS1:GFP fusion protein showed a cytoplasmic location for this short tRNase Z (Fig. 1B). The red fluorescence emitted by the AthTrZS2:RFP fusion protein is identical to the pattern of chlorophyll fluorescence in the chloroplast, indicating that AthTrZS2 is located in these organelles (Fig. 1C). To investigate the localization of AthTrZL1 and AthTrZL2, the 5′ part of the corresponding cDNAs was cloned in frame upstream of the GFP gene (Fig. 1A). Constructs were transformed into tobacco (Nicotiana tabacum) protoplasts, and localization was investigated using fluorescence microscopy. This analysis revealed the AthTrZL1:GFP fusion proteins to be located in two different cell compartments: mitochondria and nucleus (Fig. 1, D and E). The same result was obtained by fluorescence analysis of AthTrZL1:RFP in tobacco protoplasts (data not shown). The analogous investigation of the AthTrZL2:GFP fusion protein showed this protein to be located in mitochondria, indicating that AthTrZL2 is directed to this compartment (Fig. 1E). In contrast to the prediction by the TargetP server, neither AthTrZL1 nor AthTrZL2 is located in chloroplasts.
Figure 1.
Subcellular localization of tRNase Z proteins in Arabidopsis. A, Schematic drawing of the GFP and RFP constructs used. B to F, The coding sequences of tRNase Z proteins were cloned in frame with the GFP and/or RFP, and the resulting fusion proteins were expressed in plants. The localization of AthTrzS1 and AthTrzS2 was analyzed in protoplasts of transformed Arabidopsis (B and C). The emitted fluorescence of AthTrzL1-GFP and AthTrzL2-GFP was investigated in transformed tobacco protoplast (D–F). Fluorescence emitted by GFP and chlorophyll was detected with a fluorescein isothiocyanate filter. A super-GFP filter was used to detect only the GFP fluorescence (excluding the chlorophyll fluorescence). A MitoTracker filter was used for the detection of the RFP protein fluorescence and the MitoTracker staining.
All Four Recombinant tRNase Z Proteins Process tRNA Precursors
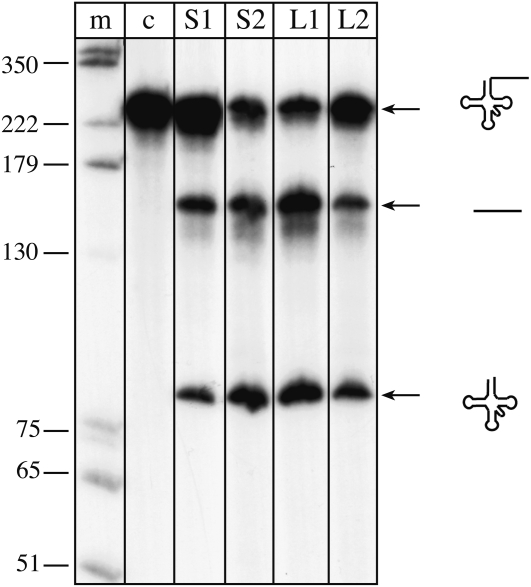
The four tRNase Z proteins in Arabidopsis were identified solely by sequence similarity to the wheat tRNase Z. In vitro tRNA processing activity was only shown for the short tRNase Z AthTrZS1 (Schiffer et al., 2002), confirming that this enzyme is a tRNA 3′ endonuclease. To determine whether the other three homologs also have pre-tRNA processing activity in vitro, the corresponding cDNAs were cloned into pET expression vectors and expressed in Escherichia coli. All proteins were expressed in soluble form; proteins AthTrZS1, AthTrZS2, and AthTrZL1 were obtained as pure recombinant protein fractions, while the purified fraction of recombinant AthTrZL2 contained a few residual E. coli proteins (Supplemental Fig. S2). Incubation with precursor tRNATyr (Fig. 2) showed that all tRNase Z homologs have tRNA 3′-processing activity. They cleave the precursor tRNA efficiently at the tRNA 3′ end, generating two products, the tRNA and the 3′ trailer (Fig. 2). The long tRNase Z AthTrzL2 processes the precursor less efficiently, which might be due to the fact that this enzyme was difficult to express and only low amounts of recombinant protein could be obtained. In addition, the recombinant protein fraction of AthTrzL2 was not as pure as those of the other tRNase Z proteins (Supplemental Fig. S2).
Figure 2.
In vitro processing assay of recombinant tRNase Z proteins. Pre-tRNATyr molecules were incubated with recombinant tRNase Z proteins, and reaction products were separated by denaturing PAGE. DNA size markers are shown on the left (lane m), and the control reaction without the addition of any proteins is shown in lane c. Lanes S1, S2, L1, and L2 indicate incubation with the tRNase Z proteins AthTrzS1, AthTrzS2, AthTrzL1, and AthTrzL2, respectively. Precursor and products are shown schematically on the right. All four tRNase Z proteins process the precursor.
Processing of Mitochondrial tRNA-Like Structures
In plant mitochondria, tRNA-like structures, the so-called t-elements, are located adjacent to 5′ or 3′ mRNA termini (Hanic-Joyce and Gray, 1990; Bellaoui et al., 1997). In some of these transcripts, removal of the t-element by endonucleolytic cleavage was observed and a possible role of tRNase Z in this reaction was suggested (Forner et al., 2007).
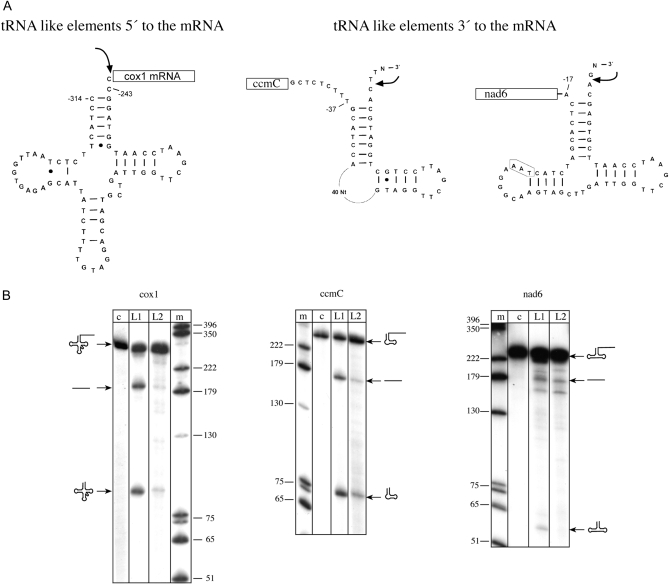
Since AthTrZL1 and AthTrZL2 proteins are routed to mitochondria, we wondered whether recombinant AthTrZL1 and AthTrZL2 can process these mitochondrial tRNA-like structures in vitro. To analyze processing of RNAs containing a t-element, we first generated the cox1 (for cytochrome c oxidase subunit 1) precursor RNA in vitro. The transcript contains the t-element structure and the 5′ part of the cox1 mRNA (Fig. 3A). Both tRNase Z proteins cleaved the cox1 substrate, although AthTrZL2 cleaved the substrate less efficiently (Fig. 3B). As stated above, this may be due to the fact that expression of that protein was not as pure as that of the other proteins. tRNase Z cleavage resulted in two processing products: an 81-nucleotide-long RNA corresponding to the t-element and a 174-nucleotide-long RNA corresponding to the cox1 mRNA (Fig. 3B). To investigate whether t-elements located 3′ to an mRNA can also be processed by tRNase Z proteins, substrates for the ccmC (for cytochrome c maturation subunit C) gene and for the nad6 (for NADH dehydrogenase subunit 6) gene were prepared (Fig. 3A). These substrates contain the t-element downstream of the mRNA 3′ end. The ccmC substrate was processed by both tRNase Z enzymes tested, yielding two processing products of 73 and 159 nucleotides in length, which correspond in size to the t-element and the 3′ trailer (Fig. 3B). Again, processing by AthTrzL2 was less efficient. The nad6 substrate was processed by AthTrZL1, although not very efficiently. Processing generates products of 58, 165, 175, and 180 nucleotides length. The two products of 58 and 175 nucleotides correspond to the t-element and the 3′ trailer. AthTrZL2 also generates the mRNA fragment of the expected size (175 nucleotides), but the shorter t-element is not visible. Again, that could be due to the quality of the recombinant protein preparation.
Figure 3.
A, Schematic representation of the mitochondrial t-element substrates used. The secondary structures of the mitochondrial substrates used are shown. The sequence upstream of the cox1 gene folds into an almost perfect cloverleaf tRNA structure, while the sequences downstream of the ccmC and nad6 genes contain only parts of the tRNA (ccmC, only acceptor stem and T arm; nad6, only acceptor stem, D arm, and T arm). B, In vitro processing of mitochondrial transcripts by tRNase Z proteins. Purified tRNase Z enzymes from Arabidopsis were incubated with mitochondrial transcripts, and reaction products were separated by denaturing PAGE. The names of the transcripts used are indicated above the gels. At the sides, DNA size markers are indicated (lane m) as well as schematic drawings of precursors and products. Lane c, Control reactions without proteins; lane L1, addition of AthTrZL1; lane L2, addition of AthTrZL2.
Identification and Analysis of tRNase Z Knockout Mutants
The physiological function of a protein in Arabidopsis can be analyzed using T-DNA insertion mutants of the respective gene. T-DNA insertion mutants are available for all four tRNase Z genes in different Arabidopsis T-DNA collections (Supplemental Table S2). For all mutants, the presence and the localization of the T-DNA insertion were determined by PCR using genomic DNA and subsequent sequencing analysis. The absence of the corresponding tRNase Z mRNAs was confirmed by northern-blot analyses and reverse transcription-PCR (data not shown). In the T-DNA mutant for the AthTrzS1 protein gene, ΔAthZ1, the T-DNA is inserted between the second and fifth exons (the exact localization could not be determined because of a gene rearrangement probably caused by the insertion itself). In ΔAthZ2, the T-DNA mutant of the AthTrzS2 protein gene, the T-DNA is inserted one nucleotide upstream of the initiation codon. In the T-DNA mutants for the genes of the AthTrzL1 and AthTrzL2 proteins, ΔAthZ3 and ΔAthZ4, the T-DNA is localized in the second and the fifth exon, respectively.
Homozygous plants were isolated for three mutants: ΔAthZ1, ΔAthZ3, and ΔAthZ4. None showed a visible phenotype under standard conditions (see “Materials and Methods”). Since AthTrzS1 was shown to be involved in the processing of small nucleolar RNAs (snoRNAs; Kruszka et al., 2003; Barbezier et al., 2009), defects in snoRNA processing were analyzed in ΔAthZ1 by primer extension and RNase mapping (data not shown). No differences in snoRNA processing were detected between wild-type plants and ΔAthZ1. Northern-blot analyses were performed with RNA from ΔAthZ3 and ΔAthZ4 to investigate whether depletion of the respective tRNase Z protein results in processing defects of nuclear or mitochondrial tRNAs. Northern blots containing total RNA from mutant plants were hybridized with probes against nuclear tRNALeu and with probes against mitochondrial tRNASer, tRNATyr, tRNAPro, and tRNACys. Again, no differences were detected between wild-type and mutant plants (data not shown).
Depletion of AthTrzS2 Results in an Embryo-Lethal Phenotype
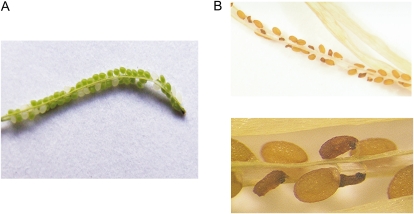
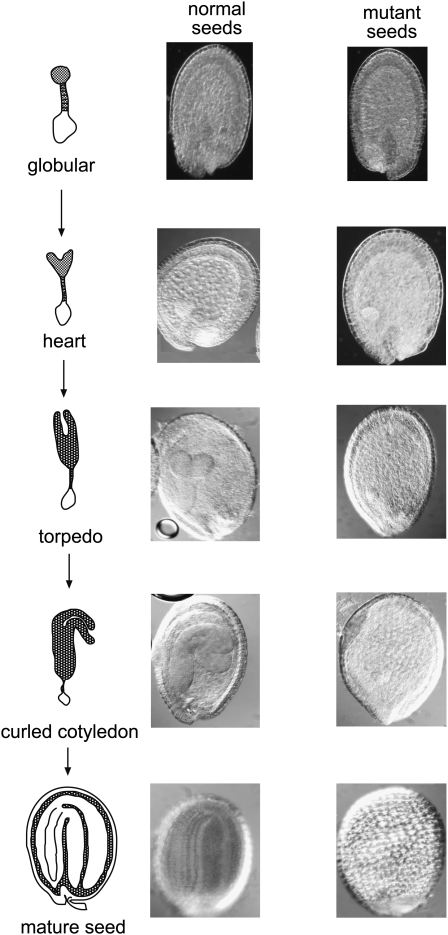
It was impossible to obtain a homozygous plant for ΔAthZ2, since the absence of AthTrzS2 causes an embryo-lethal phenotype (Figs. 4 and 5). In immature siliques, embryo-defective seeds are readily distinguishable as white seeds compared with the green wild-type seeds (Fig. 4A). About 25% of the seeds are white. In mature siliques, the mutant seeds are severely shrunken (Fig. 4B). Mutant seeds are randomly distributed throughout the siliques. Analysis of these seeds with a microscope using Nomarski optics showed that the homozygous seeds did not develop beyond the globular stage of the embryo. In addition, the embryo in ΔAthZ2 was not detectable after the wild-type embryo reached the curled cotyledon stage of development (Fig. 5).
Figure 4.
Seed phenotype of ΔAthTRZ2. Examples of an immature silique and a dry mature silique from a hygromycin-resistant plant heterozygous for the embryo-defective mutation. A, Immature silique containing green (42) and white (15) seeds. B, Mature silique after desiccation. The mutant seeds are severely shrunken.
Figure 5.
Embryo phenotype of ΔAthTRZ2. Seeds at different stages of development were collected from plants heterozygous for the T-DNA insertion in the TRZ2 gene. Seeds were isolated from the siliques, cleared in Hoyer's solution, and analyzed with the microscope at 10× magnification with Nomarski optics. Seed developmental stages are shown schematically on the left.
DISCUSSION
All four tRNase Z enzymes have in vitro tRNA processing activity and are able to generate tRNA 3′ ends ready for CCA addition, showing that they are true tRNase Z enzymes. Generally, the in vitro activity of AthTrzL2 is less efficient than that of the other three tRNase Z proteins, which is probably due to the fact that purification of the recombinant protein was not as efficient as for the other proteins (Fig. 2; Supplemental Fig. S2). Localization studies showed that each cell compartment is provided with at least one tRNase Z protein.
Expression of the Chloroplast tRNase Z Protein in Arabidopsis Is Essential
Our in vivo analyses showed that the tRNase Z AthTrzS2, which is located in chloroplasts, has a unique function that cannot be taken on by the other tRNase Z enzymes. AthTrzS2 is the only tRNase Z present in chloroplasts, and its function cannot be replaced by other nucleases. The localization of AthTrzS2 in chloroplasts is also indicated by the data on the Genevestigator site: the AthTrzS2 transcript is poorly expressed in nongreen tissue and is highly expressed in green and actively dividing tissues, where high amounts of energy are required.
Analysis of heterozygous siliques showed about 25% aborted seeds: embryonic development is arrested at the preglobular/globular stage, which is the most common phase of arrest for embryo-lethal mutations (http://www.seedgenes.org/). Many embryo-defective lethal mutants are known to exhibit a nonrandom distribution of mutant seeds in siliques of heterozygous plants, suggesting that the corresponding genes are expressed prior to fertilization as well as having an essential function during embryogenesis (Meinke, 1991). In the case of the embryo-defective mutation described here, heterozygous mutant plants exhibited a random distribution of mutant seeds, suggesting that expression of TRZ2 in haploid cells that give rise to pollen grains is not necessary for the development of pollen grains and growth of the pollen tube. Moreover, defective embryos that are homozygous for the mutant gene can develop at least to the globular stage, indicating that expression of the TRZ2 gene is not necessary for seed development prior to the globular stage.
The role of the chloroplast in embryogenesis has not yet been clarified, but it is accepted that this organelle plays a significant role in plant embryogenesis (Tsugeki et al., 1996; Uwer et al., 1998; Budziszewski et al., 2001). Indeed, embryo-lethal phenotypes are often the result of a loss of function in chloroplast genes or in nuclear genes encoding proteins predicted to be directed to chloroplasts (Budziszewski et al., 2001). To date, it is not clear whether chloroplast proteins are required because of the need for energy at this developmental stage or whether they might have an additional function in embryogenesis.
The developmental stage of the chloroplast appears to regulate the expression of nuclear genes coding for proteins destined for the chloroplast. Inhibition of plastid protein synthesis, resulting from a lack of tRNAs caused by the missing tRNase Z, is expected to interfere with the complex signal-exchange program between plastids and the nucleus. A similar observation was made for mutants defective in the plastid glycyl-tRNA synthetase, since they also show an embryo-lethal phenotype (Ruppel and Hangarter, 2007). The failure to generate plastid proteins at that point of development seems to be signaled to the nucleus, and further development of the embryo ceases.
Two tRNase Z Proteins for Mitochondria
In contrast to plastids, mitochondria contain two different tRNase Z proteins: AthTrzL1 and AthTrzL2. The observation that the T-DNA mutants of either of these proteins are viable suggests that they can functionally replace each other. Failure to obtain the double mutant AthTrzL1 × AthTrzL2 confirms this hypothesis (G. Canino and A. Marchfelder, unpublished data). In vitro processing analyses showed that recombinant AthTrzL1 and AthTrzL2 can process the mitochondrial t-element structures in precursor RNAs of cox1 and ccmC and to some extent also nad6. Thus, these tRNase Z proteins might be responsible for cleavage at these sites in vivo.
One tRNase for the Nucleus
While AthTrzL2 is confined to mitochondria, AthTrzL1 is located in mitochondria and the nucleus. According to the fluorescence experiments, AthTrzL1 is the only tRNase Z protein located in the nucleus. Processing of tRNAs engaged in cytoplasmic protein biosynthesis occurs in the nucleus (Hopper and Phizicky, 2003). Thus, AthTrzL1 is the only tRNase Z responsible for the maturation of nuclear tRNAs. The knockout AthTrzL1 mutant is viable; therefore, a back-up system must exist for maturation of nuclear tRNAs.
Cytoplasmic Location for AthTrzS1
The AthTrzS1:GFP fusion protein showed diffuse fluorescence in the cell, indicating a cytoplasmic location, and the knockout mutant of AthTrzS1 showed no visible phenotype. Since the tRNAs required for cytoplasmic protein biosynthesis are processed in the nucleus, a possible function for a cytoplasmic tRNase Z would be repair of tRNA 3′ ends that have been loaded with only a partial CCA or a mutated CCA sequence, which would prevent interaction with the aminoacyl tRNA synthetases. The cytoplasmic tRNase Z could remove this incorrect CCA sequence to allow the addition of the correct terminal CCA sequence. This potential function of AthTrzS1 in the cytosol either is not essential or other proteins can take over its function, since the T-DNA mutant is viable.
Interestingly, it was not possible to obtain the double mutant AthTrzS1 × AthTrzL1 (G. Canino and A. Marchfelder, unpublished data). This suggests that AthTrzS1 is also localized in the nucleus and that the nucleus needs one of the tRNase Z enzymes: either AthTrzS1 or AthTrzL1.
MATERIALS AND METHODS
In Vitro Processing Assay
Precursor of the mitochondrial tRNATyr from Oenothera berteriana was prepared as described previously (Kunzmann et al., 1998). mRNA templates were prepared by PCR using total Arabidopsis (Arabidopsis thaliana) DNA. The template for the cox1 substrate is 255 bp (from −319 to −64) in length. Templates for ccmC and nad6 substrates are 232 bp (−37/195) and 233 bp (−17/+216) long. Primer sequences are available upon request. In vitro transcripts were prepared as described previously (Marchfelder et al., 1990). For each processing reaction, 200 ng of recombinant protein was used. All of the reactions were carried out in Cyto buffer (40 mm Tris, pH 8.4, 2 mm MgCl2, 2 mm KCl, and 2 mm dithiothreitol) in a total volume of 100 μL at 37°C for 30 min. The reaction was stopped by phenol/chloroform extraction, and the products were separated on denaturant 8% polyacrylamide gels. Gels were analyzed by autoradiography.
Analysis of Signal Sequences
Target prediction was performed using the following prediction servers: WoLF PSORT (http://wolfpsort.org/; Horton et al., 2007), Predotar (urgi.versailles.inra.fr/predotar/predotar.html; Small et al., 2004), and TargetP (www.cbs.dtu.dk/services/TargetP; Emanuelsson et al., 2007).
Expression of Arabidopsis tRNase Z Proteins in Escherichia coli
Cloning and purification of recombinant AthTrZS1 (nuz) was carried out as described previously (Schiffer et al., 2002) with an additional purification step through the mini Q column (GE Healthcare; Späth et al., 2005). For the expression of recombinant AthTrZS2, the corresponding cDNA sequence without the coding sequence for the potential signal peptide was amplified from clone APD12e02 (Kazusa DNA Research Institute) and cloned into the pUC18 vector. The identity of the clone was verified by sequencing. The coding sequence for AthTrZS2 was subcloned into the NcoI/XhoI sites of the expression vector pET32 (Novagen) and expressed in BL21-CodonPlus (DE3)-RIL as a fusion protein with both His and S tags. After purification using the S tag according to the manufacturer's instructions (Novagen), tags were removed by enterokinase digestion. To exclude the signal peptide, the cDNA from AthTrZL1 was amplified without the first 153 bp (coding sequence for the first 51 amino acids) from the cDNA clone RAFL07-09-G16 (RIKEN Bioresource Center) and cloned into pBluescript KS II. After sequencing, the cDNA was subcloned into the NotI/XhoI sites of pET29 (Novagen) and expressed in BL21-AI (Stratagene) cells. The cDNA for AthTrZL2 without the coding sequence for the first 66 amino acids representing the calculated target peptide was amplified from the cDNA clone RAFL16-86-L12, cloned in pBluescript KS II, and, after sequencing, subcloned into the pET29 expression vector (Novagen) previously digested with BamHI/XhoI. AthTrzL2 was expressed in Rosetta cells (Novagen). Both AthTrZL1 and AthTrZL2 proteins were purified using the S tag according to the manufacturer's instructions (Novagen). The concentration of the recombinant proteins was evaluated with the Qubit (Invitrogen).
Cloning of tRNase Z Fusion Proteins with GFP and RFP and Plant Transformation
The complete coding sequences for AthTrZS1 and AthTrZS2 were cloned in frame upstream of the GFP and RFP genes, respectively, into the HindIII/EcoRI sites of the plant transformation vector pBI121 (BD Clontech). The vector was transformed in Agrobacterium tumefaciens GV2260 and introduced into Arabidopsis wild-type plants via floral dip (Clough and Bent, 1998). The transformed plants were selected on Murashige and Skoog medium containing kanamycin, and the presence of the construct was verified by PCR on genomic DNA with specific primers for the T-DNA insertion. For the localization of AthTrZL1, the coding sequence for the first 108 amino acids was cloned into the psmGFP4 vector (Arabidopsis Biological Resource Center) and used to transform tobacco (Nicotiana tabacum ‘Petit Havana’) protoplasts (Koop et al., 1996). For the localization of AthTrZL2, 497 bp of the coding sequence (corresponding to the first 166 amino acids) was cloned in frame with the GFP frame into the plant transformation vector pBI121 and used to stably transform tobacco by leaf disc transformation (Horsch et al., 1985). Arabidopsis protoplasts were prepared according to Koop et al. (1996). The expression of GFP and RFP fusion proteins in Arabidopsis and tobacco was investigated using a Zeiss Axioplan I microscope (Carl Zeiss). Mitochondria were stained with MitoTracker Red CM-H2XROS (Molecular Probes).
Microscopy Analysis of Arabidopsis Seeds
Wild-type and mutant seeds were collected at different stages of development from a plant heterozygous for the insertion of the AthZ2 gene. The seeds were cleared in 1:5 Hoyer's solution (3.75 g of arabic gum, 2.5 mL of glycerin, and 50 g of chloral hydrate in 100 mL of water) for 4 h and analyzed under a Zeiss Axioplan I microscope (Carl Zeiss) provided with Nomarski optics and a 10× objective.
Plant Growth Conditions and Analysis of Arabidopsis T-DNA Insertion Lines
Wild-type and transformed plants were grown at 22°C in a 16/8-h light/dark cycle. T-DNA insertion lines were obtained from the Gabi Kat collection (http://www.gabi-kat.de/), the Signal collection of the Salk Institute (http://signal.salk.edu/), and the Cold Spring Harbor Laboratory collection (http://www.cshl.edu/). The presence of the T-DNA insertion in the gene coding for the tRNase Z proteins was determined using PCR on genomic DNA with primers specific for the insertion and the gene. Primer sequences are available upon request. Total DNA was extracted from 3-week-old leaves with the Phytopure DNA Kit (Amersham). Total RNA was extracted from 3-week-old leaves with the plant RNeasy kit (Qiagen).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Conserved MBL motives of Arabidopsis tRNase Z proteins (A and B), and alignment of all four Arabidopsis tRNase Z proteins (C).
Supplemental Figure S2. Recombinant tRNase Z proteins of Arabidopsis.
Supplemental Table S1. Predicted localization of all four tRNase Z proteins.
Supplemental Table S2. Overview of tRNase Z genes, proteins, and T-DNA insertion mutants used in this study.
Supplementary Material
Acknowledgments
We thank Elli Bruckbauer for expert technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (grant to A.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Anita Marchfelder (anita.marchfelder@uni-ulm.de).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Aravind L (1999) An evolutionary classification of the metallo-beta-lactamase fold proteins. In Silico Biol 1 69–91 [PubMed] [Google Scholar]
- Barbezier N, Canino G, Rodor J, Jobet E, Saez-Vasquez J, Marchfelder A, Echeverría M (2009) Processing of a dicistronic tRNA-snoRNA precursor: Combined analysis in vitro and in vivo reveals alternate pathways and coupling to assembly of snoRNP. Plant Physiol 150 1598–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaoui M, Pelletier G, Budar F (1997) The steady-state level of mRNA from the Ogura cytoplasmic male sterility locus in Brassica cybrids is determined post-transcriptionally by its 3′ region. EMBO J 16 5057–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budziszewski GJ, Lewis SP, Glover LW, Reineke J, Jones G, Ziemnik LS, Lonowski J, Nyfeler B, Aux G, Zhou Q, et al (2001) Arabidopsis genes essential for seedling viability: isolation of insertional mutants and molecular cloning. Genetics 159 1765–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut I, Moshous D, Mornon JP, de Villartay JP (2002) Metallo-beta-lactamase fold within nucleic acids processing enzymes: the beta-CASP family. Nucleic Acids Res 30 3592–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Daiyasu H, Osaka K, Ishino Y, Toh H (2001) Expansion of the zinc metallo-hydrolase family of the beta-lactamase fold. FEBS Lett 503 1–6 [DOI] [PubMed] [Google Scholar]
- Dominski Z (2007) Nucleases of the metallo-beta-lactamase family and their role in DNA and RNA metabolism. Crit Rev Biochem Mol Biol 42 67–93 [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Brunak S, von Heijne G, Nielsen H (2007) Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protocols 2 953–971 [DOI] [PubMed] [Google Scholar]
- Evans D, Marquez SM, Pace NR (2006) RNase P: interface of the RNA and protein worlds. Trends Biochem Sci 31 333–341 [DOI] [PubMed] [Google Scholar]
- Forner J, Weber B, Thuss S, Wildum S, Binder S (2007) Mapping of mitochondrial mRNA termini in Arabidopsis thaliana: t-elements contribute to 5′ and 3′ end formation. Nucleic Acids Res 35 3676–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanic-Joyce PJ, Gray MW (1990) Processing of transfer RNA precursors in a wheat mitochondrial extract. J Biol Chem 265 13782–13791 [PubMed] [Google Scholar]
- Hölzle A, Fischer S, Heyer R, Schütz S, Zacharias M, Walther P, Allers T, Marchfelder A (2008) Maturation of the 5S rRNA 5′ end is catalyzed in vitro by the endonuclease tRNase Z in the archaeon H. volcanii. RNA 14 928–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper AK, Phizicky EM (2003) tRNA transfers to the limelight. Genes Dev 17 162–180 [DOI] [PubMed] [Google Scholar]
- Horsch RB, Rogers SG, Fraley RT (1985) Transgenic plants. Cold Spring Harb Symp Quant Biol 50 433–437 [DOI] [PubMed] [Google Scholar]
- Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K (2007) WoLF PSORT: protein localization predictor. Nucleic Acids Res 35 W585–W587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop HU, Steinmuller K, Wagner H, Rossler C, Eibl C, Sacher L (1996) Integration of foreign sequences into the tobacco plastome via polyethylene glycol-mediated protoplast transformation. Planta 199 193–201 [DOI] [PubMed] [Google Scholar]
- Kruszka K, Barneche F, Guyot R, Ailhas J, Meneau I, Schiffer S, Marchfelder A, Echeverría M (2003) Plant dicistronic tRNA-snoRNA genes: a new mode of expression of the small nucleolar RNAs processed by RNase Z. EMBO J 22 621–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzmann A, Brennicke A, Marchfelder A (1998) 5′ end maturation and RNA editing have to precede tRNA 3′ processing in plant mitochondria. Proc Natl Acad Sci USA 95 108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchfelder A, Schuster W, Brennicke A (1990) In vitro processing of mitochondrial and plastid derived tRNA precursors in a plant mitochondrial extract. Nucleic Acids Res 18 1401–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathy N, Benard L, Pellegrini O, Daou R, Wen T, Condon C (2007) 5′-to-3′ exoribonuclease activity in bacteria: role of RNase J1 in rRNA maturation and 5′ stability of mRNA. Cell 129 681–692 [DOI] [PubMed] [Google Scholar]
- Meinke DW (1991) Embryonic mutants of Arabidopsis thaliana. Dev Biol 12 382–392 [Google Scholar]
- Perwez T, Kushner SR (2006) RNase Z in Escherichia coli plays a significant role in mRNA decay. Mol Microbiol 60 723–737 [DOI] [PubMed] [Google Scholar]
- Redko Y, Li de Lasierra-Gallay I, Condon C (2007) When all's zed and done: the structure and function of RNase Z in prokaryotes. Nat Rev Microbiol 5 278–286 [DOI] [PubMed] [Google Scholar]
- Ruppel NJ, Hangarter RP (2007) Mutations in a plastid-localized elongation factor G alter early stages of plastid development in Arabidopsis thaliana. BMC Plant Biol 7 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K, Calvo O, Manley JL (2004) Evidence that polyadenylation factor CPSF-73 is the mRNA 3′ processing endonuclease. RNA 10 565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer S, Rösch S, Marchfelder A (2002) Assigning a function to a conserved group of proteins: the tRNA 3′-processing enzymes. EMBO J 21 2769–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I, Peeters N, Legeai F, Lurin C (2004) Predotar: a tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 4 1581–1590 [DOI] [PubMed] [Google Scholar]
- Söll D (1993) Transfer RNA: an RNA for all seasons. In RF Gesteland, JF Atkins, eds, The RNA World. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 157–183
- Späth B, Kirchner S, Vogel A, Schubert S, Meinlschmidt P, Aymanns S, Nezzar J, Marchfelder A (2005) Analysis of the functional modules of the tRNA 3′ endonuclease (tRNase Z). J Biol Chem 280 35440–35447 [DOI] [PubMed] [Google Scholar]
- Tsugeki R, Kochieva EZ, Fedoroff NV (1996) A transposon insertion in the Arabidopsis SSR16 gene causes an embryo-defective lethal mutation. Plant J 10 479–489 [DOI] [PubMed] [Google Scholar]
- Uwer U, Willmitzer L, Altmann T (1998) Inactivation of a glycyl-tRNA synthetase leads to an arrest in plant embryo development. Plant Cell 10 1277–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A, Schilling O, Späth B, Marchfelder A (2005) The tRNase Z family of proteins: physiological functions, substrate specificity and structural properties. Biol Chem 386 1253–1264 [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Hennig L, Gruissem W (2005) Gene-expression analysis and network discovery using Genevestigator. Trends Plant Sci 10 407–409 [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.